Abstract
Diffusion tensor imaging is widely used to evaluate the development of white matter. Information about how alterations in major neurotransmitter systems, such as the dopamine (DA) system, influence this development in healthy children, however, is lacking. Catechol-O-metyltransferase (COMT) is the major enzyme responsible for DA degradation in prefrontal brain structures, for which there is a corresponding genetic polymorphism (val158met) that confers either a more or less efficient version of this enzyme. The result of this common genetic variation is that children may have more or less available synaptic DA in prefrontal brain regions. In the present study we examined the relation between diffusion properties of frontal white matter structures and the COMT val158met polymorphism in 40 children ages 9–15. We found that the val allele was associated with significantly elevated fractional anisotropy values and reduced axial and radial diffusivities. These results indicate that the development of white matter in healthy children is related to COMT genotype and that alterations in white matter may be related to the differential availability of prefrontal DA. This investigation paves the way for further studies of how common functional variants in the genome might influence the development of brain white matter.
Keywords: COMT, children, diffusion tensor imaging, DTI, white matter, genotype, tractography, fractional anisotrophy
Introduction
By increasing action potential conduction speed for axons, the white matter (or myelin) of the brain facilitates the rapid exchange of signals among different brain regions. In normal development, the volume of white matter increases linearly through the second decade of life (Barnea-Goraly et al., 2005b; Eluvathingal et al., 2007; Hasan et al., 2007; Lenroot and Giedd, 2006; Sowell et al., 2003; Toga et al., 2006), a period during which there are also significant gains in cognitive capacity. The proliferation and differentiation of developing oligiodendrocytes that extend myelinating projections that encircle axons are controlled, in part, by neurotransmitters (Belachew et al., 1999; Bongarzone et al., 1998; Karadottir and Attwell, 2007). Little is known, however, about how neurochemical effects might modulate human brain structural development. Studies of genes that affect neurotransmitter systems, such as catechol-O-methyltransferase (COMT), can provide insight into the neurochemical modulation of the development of brain white matter. The present investigation applied diffusion tensor imaging (DTI) to examine for the first time the effects of the COMT polymorphism on white matter structure in 40 healthy children between 9 and 15 years of age.
COMT is a gene that encodes a key enzyme in the metabolism of dopamine (DA). A single nucleotide polymorphism (SNP; G → A transition at codon 158) leading to a valine (val) to methionine (met) substitution in a coding region of COMT has been found to be associated with a greater than two-fold decrease in COMT enzyme activity and DA catabolism (Chen et al., 2004; Lachman et al., 1996; Lotta et al., 1995). Consequently, the met allele of this polymorphism confers reduced enzymatic activity and subsequently increased DA availability (Chen et al., 2004; Tenhunen et al., 1994; Tunbridge et al., 2004), especially in the prefrontal cortex (PFC), in which COMT enzyme activity is the primary factor that determines synaptic levels of DA (Garris and Wightman, 1994; Karoum et al., 1994).
A useful but simplistic description of the COMT behavioral phenotype is that met allelic loading confers a cognitive processing advantage but concomitant difficulties in affective processing. Moreover, there appears to be specificity to the met-allele cognitive advantage: individuals who carry the met allele perform better on higher-order cognitive processing (i.e., tasks requiring mental manipulation) (Bilder et al., 2002; Bruder et al., 2005; Diaz-Asper et al., 2008; Egan et al., 2001; Goldberg et al., 2003; Joober et al., 2002; Malhotra et al., 2002; Rosa et al., 2004), but do not outperform their val-allele homozygous counterparts in the foundations of these operations (i.e., storage, updating, temporal order, maintenance, planning) (Bilder et al., 2002; Bruder et al., 2005; Goldberg et al., 2003; Williams-Gray et al., 2007). Met-allele carriers have also been found to be characterized by a form of cognitive inflexibility (Drabant et al., 2006; Nolan et al., 2004); consequently, investigators have begun to question the cognitive advantage of carrying the met allele (Barnett et al., 2008; Ho et al., 2005). In the domain of emotional functioning, individuals with a met allele have been found to show higher endocrine and subjective responses to stress (Jabbi et al., 2007), higher harm avoidance (Enoch et al., 2003), increased neuroticism (Enoch et al., 2003; Stein et al., 2005), higher trait anxiety (Woo et al., 2004), lower extraversion (Stein et al., 2005), higher pain sensitivity along with reduced μ-opioid receptor response (Zubieta et al., 2003), potentiated startle reflex (Montag et al., 2008), and increased aggression/hostility (Han et al., 2006; Lachman et al., 1998; Rujescu et al., 2003; Volavka et al., 2004). This pattern of findings suggests that met-allele carriers are more emotionally reactive than are their val-allele homozygous counterparts. Given the extensive descriptions of the behavioral phenotype associated with the COMT gene, investigators have suggested that the COMT gene plays a critical role in an apparent evolutionary trade-off between cognitive and affective functions (Papaleo et al., 2008). Thus, it appears that neither polymorphism is clearly advantaged; instead, we may need to rely on brain endophenotypes, like those measured by DTI, to better delineate the function and role of this gene.
DTI is a non-invasive in vivo method of measuring the diffusion of water as it probes tissue microstructure (Basser et al., 1994b; Basser and Pierpaoli, 1996; Moseley et al., 1990). In areas of densely packed neural fibers, the many axonal membranes will restrict diffusion perpendicular to the fiber orientation, resulting in anisotropic diffusion (Beaulieu, 2002; Le Bihan, 2003; Sen and Basser, 2005). Similarly, in areas with small cell composition, reduced intracellular space will cause restriction of diffusion (Sehy et al., 2002). DTI has been used effectively to obtain information about white matter structure, even in compromised populations such as infants (Berman et al., 2005; Gao et al., 2009; McGraw et al., 2002; Morriss et al., 1999; Mukherjee et al., 2002; Neil et al., 1998; Partridge et al., 2005; Sakuma et al., 1991; Schneider et al., 2004), and children with psychiatric disorders (Ashtari et al., 2005; Barnea-Goraly et al., 2005a; Barnea-Goraly et al., 2004; Eluvathingal et al., 2006; Engelbrecht et al., 2002; Ewing-Cobbs et al., 2006; Hermoye et al., 2006; Lebel et al., 2008a; Nagy et al., 2003; Ono et al., 1997; Zimmerman et al., 1998). Moreover, DTI allows investigators to characterize neural endophenotypes in developmental disorders, particularly those that may involve different brain networks (Muller, 2007). For example, in studies of autism researchers have used DTI to document anomalous values in diffusion and anisotropy (e.g., Barnea-Goraly et al., 2004; Bashat et al., 2007; Sundaram et al., 2008).
DTI yields measures of anisotropy (a measurement of the directionality of water motion) and diffusivity (a measurement of the magnitude of random water diffusion) (Basser, 1995; Basser et al., 1994b; Pierpaoli et al., 1996). The following parameters can be used to quantify the pattern of diffusion: (i) fractional anisotropy (FA), a measure of the intravoxel preferred directionality of water translational random motion, expressed as a ratio ranging from 0 to 1 (0 = isotropic and 1 = unidirectional); (ii) axial diffusivity (AD), the magnitude of water diffusion along the long axis of the axons, equivalent to the primary eigenvalue of diffusion tensor, λ1; and (iii) radial diffusivity (RD), the magnitude of water diffusion perpendicular to the long axis of the axons, equivalent to the average of the 2nd and 3rd eigenvalues of diffusion tensor, λ2 and λ3. These parameters can be obtained for each voxel within the brain and, combined with diffusion tensor tractography, for each major white matter pathway. Recent work indicates that considering FA in conjunction with directional diffusivity information (e.g., AD and RD) is superior to using FA alone for interpreting white matter features (Dougherty et al., 2007; Gao et al., 2009; Hasan, 2006; Song et al., 2005). A fourth possible parameter, mean diffusivity (MD), was not reported in the present study because it is a simple linear combination of AD and RD [MD=(AD+2RD)/3].
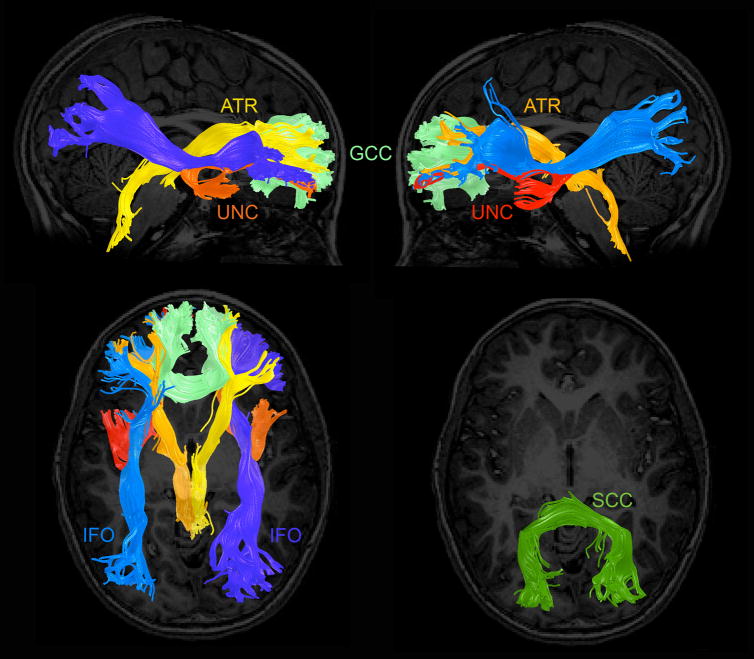
In the present study we examined COMT gene-related differences in four major white matter fiber tracts in a group of children and adolescents. Because the PFC exhibits the greatest alteration in DA function as a result of this SNP (Garris and Wightman, 1994; Gogos et al., 1998; Karoum et al., 1994), we selected four pathways with significant prefrontal terminations as ROIs (Mori et al., 1999). Specifically, we used tractography to delineate genu of the corpus callosum (GCC), which connects the left and right frontal lobes; the anterior thalamic radiation (ATR), which is formed by fibers interconnecting thalamic nuclei and the cerebral cortex of the frontal lobe; the inferior fronto-occipital fasciculus (IFO), which connects anterior frontal regions to the parietal and occipital lobes; and the uncinate fasciculus (UNC), which connects the frontal and temporal lobes. These tracts were traced in each participant and saved as ROIs within which the three DTI parameters of interest (FA, AD, RD) were calculated. We tested these parameters as indicators of differences in white matter microstructure in the three COMT genotype groups (met/met, met/val, and val/val). We hypothesized that altered brain DA levels, influenced by children’s COMT genotype, would be related to altered white matter diffusion properties in these four major prefrontal fiber bundles. In addition, to test the specificity of COMT effects on prefrontal white matter, we also examined a control fiber pathway, the splenuim of the corpus callosum (SCC), that does not have prefrontal terminations, and that was used as a control region in a study examining localized group differences in white matter (Pacheco et al., 2009).
Material and methods
Participants
Participants were 40 children and adolescents (26 females) between the ages of 9 and 15 years (M=11.06, SD= 1.4). They were recruited through their mothers by online advertisements (posted free on the classified-style website: www.craigslist.com) and parent networks (two www.yahoo.com California Bay Area parent groups comprising more than 4000 members). They responded to notices of Stanford University research studies seeking community participants. Each mother-child pair was compensated $25/hour. All participants had no reported history of brain injury, no behavioral indications of possible mental impairment, no past or present Axis I disorder, were right-handed, fluent in English, and reportedly had no learning disorder. Parents and children gave informed consent and assent, respectively, as approved by the Stanford Institutional Review Board.
We are sensitive to the ethical concerns of imaging studies among children, and paid special attention to the unique comfort and information needs of this group. We recently published a set of recommendations for imaging children (Thomason, 2009), and we followed these recommendations in this study.
Procedure
Participants were assessed in two sessions. In the first session, participants were administered structured interviews to assess current and lifetime psychopathology. Trained interviewers assessed the diagnostic status of the adolescents by administering the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL) (Geller et al., 1996; Geller et al., 2001), which has been shown to generate reliable and valid psychiatric diagnoses (Kaufman et al., 1997). Any child/adolescent who received a current or past diagnosis was eliminated from the study. To assess inter-rater reliability, an independent trained rater evaluated 30% of all K-SAD-PL interviews by randomly selecting audiotapes. In all cases, these diagnoses matched the diagnoses made by the original interviewer, κ=1.00, indicating excellent inter-rater reliability. During this session, children and parents also provided saliva samples for genetic testing and viewed a video to prepare them for the MRI scan session. Fourty-six children were recruited for this study and participated in the initial interview session; of these, 40 went on to contribute the MRI data reported in the present study. The remaining 6 were eliminated for technical concerns (movement or acquisition error) or because they did not complete the imaging component of the study; none were eliminated for psychiatric diagnosis. In the second session, brain-imaging data were acquired using a whole-brain MRI scanner.
Genetic data
DNA was analyzed through saliva collected using the Oragene Kit (DNA Genotek, Inc. Ottawa, Ontario, Canada), an all-in-one system for the collection, preservation, transportation and purification of DNA from saliva. This is a minimally invasive procedure. DNA extracted by this method is of high quality and allows for genotyping with a high success rate (Rylander-Rudqvist et al., 2006). Participants were asked to refrain from eating 30 minutes before saliva collection, to rinse their mouth with water and wait for five minutes before spitting saliva into the collection container, and then to spit sufficient saliva into the container. A research assistant then screwed the cap of the container and shook it vigorously for at least 10–20 seconds. Samples were sent to the lab at room temperature.
Saliva samples were visually inspected in the laboratory for any noticeable food debris or phlegm. DNA was extracted only from clean and clear saliva samples. If there was any evidence of cloudiness or low DNA yield after DNA extraction participants were re-contacted and asked to provide a second sample. DNA was extracted according to the manufacturer’s protocol for manual purification. Briefly, samples were mixed. Following overnight incubation at 50°C the Oragene® purifier was added and centrifuged at 3000 × g for 20 minutes. The supernatant was transferred to a new tube and DNA was precipitated by adding 100% ethanol. The DNA pellet was washed with 70% ethanol, dried, and resuspended in DNA hydration solution (Qiagen, Valencia, CA). To ensure complete rehydration the sample was incubated at 37°C overnight. The amount of DNA was quantified using a spectrophotometer and diluted for genotyping.
The target 217bp COMT gene fragment was amplified using sense primer 5′-TCG TGG ACG CCG TGA TTC AGG-3′ and the antisense primer 5′-AGG TCT GAC AAC GGG TCA GGC-3′. The PCR reaction was carried out in a final volume of 15μl consisting of 50ng of genomic DNA, 50ng each of sense and antisense primers, 7.5ul of Taq PCR Master mix (Qiagen, Cat.#201445) and 10% DMSO. The PCR conditions included an initial denaturation step at 95C for 3 min, followed by 35 cycles of denaturation at 95C for 30s, annealing at 55C for 45 s and extension at 72C for 1 min, with a final extension of 10 min at 72C. The PCR products were digested at 37C for 3 hours with 5 U of the restriction enzyme NIa III (New England Biolabs, Cat#R0125S). The products were electrophoresed through 10% Polyacrylamide gel (Acrylamide/bis-Acrylamide ratio 19:1) at 150 V for 40 min. 10bp marker was used to measure the fragments size. The H allele (high activity val-108) showed 2 bands, at 136bp and 81bp. The L allele (low activity met-108) showed 3 bands, at 96bp, 81bp and 40bp.
DTI and structural data acquisition
Magnetic resonance imaging was performed on a 3.0 T GE whole-body scanner. Participants were positioned in a purpose-built single channel T/R head coil and stabilized by clamps and a bite bar formed with dental impression wax (made of Impression Compound Type I, Kerr Corporation, Romulus, MI) to reduce motion-related artifacts during scanning.
The DTI acquisition used five 3:38 (min:sec) whole-brain scans that were averaged to improve signal quality (18:10 min:sec total scan time). The pulse sequence was a diffusion-weighted, spin-echo, echo-planar imaging sequence (repetition time = 7.8 sec; FOV = 22; matrix size = 128x128). We acquired 56 axial 2.5-mm-thick slices (no skip) for two b-values, b=0 and b=900 sec/mm2. The high b-value was obtained by applying gradients along 28 different diffusion directions. Frequency encode direction was along the left-right direction and conventional chemical-shift-selective (CHESS) fat suppression was used to preserve a good slice profile.
A high-resolution volume scan (140 slices, 1mm slice thickness) was collected for every participant using a spoiled grass gradient recalled (SPGR) sequence for T1 contrast in axial image orientation (TR = 3000ms, TE = 68ms, TI = 500ms, flip angle = 11°, FOV = 25 cm, 256 × 256).
DTI preprocessing
Eddy current distortions and subject motion in the diffusion-weighted images were removed by a 14-parameter constrained non-linear co-registration based on the expected pattern of eddy-current distortions given the phase-encode direction of the acquired data (Rohde et al., 2004). Each diffusion-weighted image was registered to the mean of the (motion-corrected) non-diffusion-weighted (b = 0) images using a two-stage coarse-to-fine approach that maximized the normalized mutual information. After the T1 image was manually aligned to the anterior commissure - posterior commissure (AC-PC) line, the mean of the non-diffusion-weighted images was automatically aligned to the T1 image using a rigid body mutual information algorithm. All raw images from the diffusion sequence were resampled to 2-mm isotropic voxels by combining the motion correction, eddy-current correction, and anatomical alignment transforms into one omnibus transform and resampling the data using a 7th-order b-spline algorithm based on code from SPM5 (Friston and Ashburner, 2004). An eddy-current intensity correction (Rohde et al., 2004) was applied to the diffusion-weighted images at the resampling stage.
The rotation component of the omnibus coordinate transform was applied to the diffusion-weighting gradient directions to preserve their orientation with respect to the resampled diffusion images. The tensors were then fit using a least-squares algorithm (Basser et al., 1994a). We confirmed that the DTI and T1 images were aligned to within a few millimeters in most brain regions. In regions prone to susceptibility artifacts, such as orbito-frontal and inferior temporal regions, the misalignment was somewhat larger due to uncorrected EPI distortions, but never exceeded 5mm.
All the custom image processing and analysis software is available as part of our open-source mrDiffusion package available for download from http://vistalab.stanford.edu/software/.
DTI tractography
Whole brain fiber tracking was performed on AC-PC aligned tensor maps. The seeds for tractography were selected from a uniform 1mm 3D grid spanning the whole brain mask for voxels with FA>.3. Fiber tracts were estimated using a deterministic streamlines tracking algorithm (Basser et al., 2000; Conturo et al., 1999; Mori et al., 1999) with a fourth-order RungeñKutta path integration method (Press et al., 2002) and 1-mm fixed-step size. A continuous tensor field was estimated with trilinear interpolation of the tensor elements. Path tracing proceeded until the FA fell below 0.15 or until the minimum angle between the current and previous path segments was greater than 30 deg. Starting from the initial seed point, fibers were traced in both directions along the principal diffusion axis.
Classification of fiber tracts
As noted above, we examined five major fiber tracts described in Johns Hopkins University (JHU) white-matter tractography atlas (Wakana et al., 2004): the GCC; the ATR; the IFO; the UNC; and the control tract, the SCC, defined as the region posterior to the narrowing (isthmus) of the callosum (Witelson, 1989). The SCC was recently used as a control region in a DTI study of the serotonin transporter gene (5-HTTLPR) in adults (Pacheco et al., 2009). Of these, three are bilaterally represented (ATR, IFO, and UNC) and two connect the hemispheres via the corpus callosum (GCC, SCC). These pathways, and their voxel-wise probabilities, are provided by the JHU white mater tractography atlas. Custom software (included in the mrDiffusion package) was used to conduct the automated fiber classification. Using spatial transformation from individual to MNI space, each point in each fiber was mapped to the JHU atlas, determining the probability that that fiber point belonged to each of the JHU atlas groups (there are 20 possible groups). These probabilities were averaged across the points within each fiber. The fiber was then classified as representing the JHU group with the highest average probability. The pathways classified as from JHU atlas fiber groups other than those corresponding to the GCC, the ATR, the IFO, the UNC and the SCC, as well as the fibers with average probability under .1 for the JHU atlas groups of interest, were removed from further analyses. Following classification, we removed redundant fibers within tracts in order to reduce oversampling within a tract, a step we refer to as ‘culling’ of fibers. Culling was executed using an algorithm based on three criteria: the length of a trajectory (minimum = 10.0 mm), the linear anisotropy along a trajectory (minimum = 0.10), and the distance between them (minimum average point-to-curve distance = 2.7mm, distance computed across portions of the fibers that were at least 1 mm apart) (Zhang et al., 2003).
Statistical analyses
For each of the four major fiber tracts and the control tract, mean (i) FA; (ii) AD; and (iii) RD were computed. Because, Pearson correlations were significant for every right and left hemisphere FA tract value (all r > .45; all p < .01), data for these tracts were averaged across the right and left hemispheres before submitting them to the multivariate analyses.
The effects of COMT genotype on measures of anisotrophy and diffusion were examined within a general linear model framework using multivariate analyses. A multivariate analysis of variance (MANOVA) was conducted for each fiber pathway. Three DTI parameters (FA, AD, RD) were entered into each model as dependent variables, and COMT genotype (met/met, val/met, val/val) was entered as a fixed factor. We followed up significant (p<.05) MANOVAs with univariate analyses of variance (ANOVAs) and t-tests where appropriate to determine the source of the significant effects. All statistical analyses were conducted using SPSS.
Results
Participants
Participants were 25 Caucasians (62.5%), 3 Asian Americans (7.5%), 2 Hispanic Americans (5%), 8 participants of multi- or bi-racial descent (20%), and 2 who were not classified (5%). Participants were 9 to 15 years of age, covering a range in which the dynamic processes of brain myelination are still occurring. Genotyping yielded three groups of children: homozygous met (n = 6); homozygous val (n = 13); and met/val (n = 21). These allelic frequencies were in Hardy-Weinberg equilibrium, χ2(2)=0.63, p = .73. Demographic data for the three COMT genotype groups are presented in Table 1. The three groups did not differ significantly with respect to age, F(2,39) = 1.2, p = .30, or gender, χ2(2) = 3.5, p = .18.1
Table 1.
Participant demographics and summary statistics
| Val/Val | Met/Val | Met/Met | Statistic | |
|---|---|---|---|---|
| N | 13 | 21 | 6 | χ2(2) = .63, p = .73 |
| Gender (F:M) | 10:3 | 14:7 | 4:2 | χ2(2) = 3.48, p = .18 |
| Age (yr) | 11.14 (1.49) | 11.24 (1.37) | 10.25 (1.18) | F2,39 = 1.22, p = .30 |
Data are expressed as mean (S.D.)
COMT genotype influences white matter microstructure
Separate one-way (by genotype group) MANOVAs with FA, AD, and RD as dependent variables were conducted to examine the effects of gene group for the four fiber pathways with significant prefrontal terminations. These analyses yielded significant main effects for COMT group for three of the four fiber pathways: GCC, F(3,70) = 3.04, p = 0.011; ATR, F(3,70) = 2.79, p = 0.017; and UNC, F(3,70) = 2.47, p = 0.032; the MANOVA conducted on the IFO was not significant, F(3,70) = 1.85, p > .10. In the sections below we present the results of follow-up one-way (by genotype) univariate ANOVAs and t-tests conducted on the three significant tracts in greater detail.
COMT genotype associated with diffusivity in frontal fiber pathways
Genu of the corpus callosum (GCC)
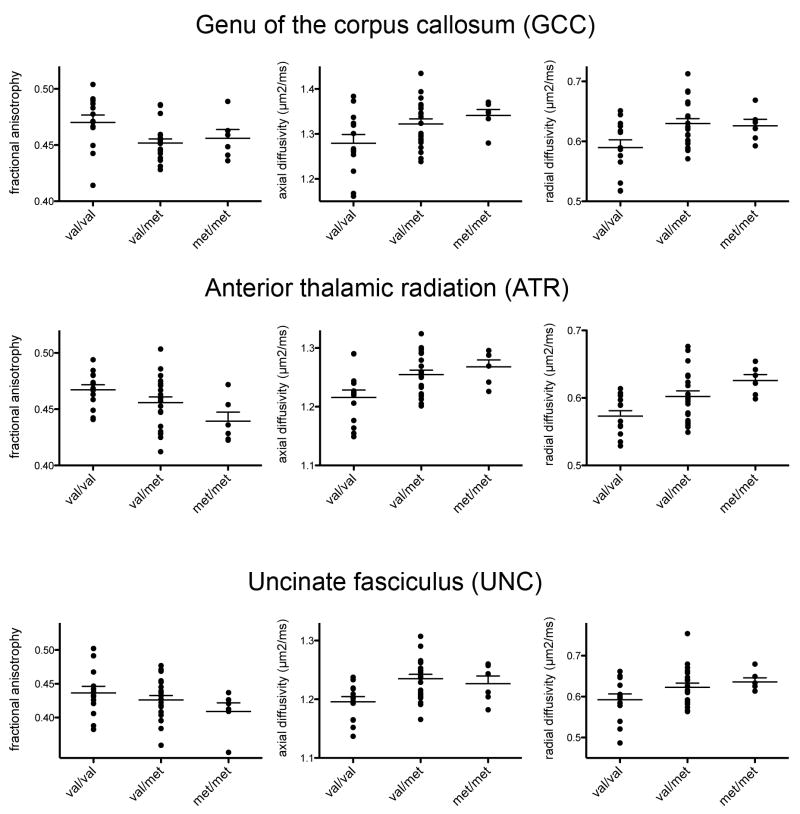
ANOVAs conducted on the GCC yielded significant effects of COMT group on FA, F(2,39) = 3.62; AD, F(2,39) = 3.32; and RD, F(2,39) = 4.28, all ps < .05. Follow up t-tests indicated that the met/val heterozygotes had significantly lower FA and significantly higher AD and RD than did the val-allele homozygotes; the met-allele homozygotes did not differ significantly from either of the other gene groups for any of the three parameters (see Table 2 and Fig. 2).
Table 2.
Genetic group means for DTI parameters
| GCC | ATR | UNC | IFO | SCC | ||||
|---|---|---|---|---|---|---|---|---|
| Fractional anisotrophy | met/met | 0.46 a,b | 0.44 a | 0.41 | 0.46 | 0.54 | ||
| met/val | 0.45 a | 0.46 a,b | 0.43 | 0.48 | 0.57 | |||
| val/val | 0.47 b | 0.47 b | 0.44 | 0.49 | 0.58 | |||
| Axial diffusivity (μm2/ms) | met/met | 1.34 a,b | 1.27 a | 1.23 a,b | 1.29 | 1.51 | ||
| met/val | 1.32 a | 1.25 a | 1.24 a | 1.28 | 1.48 | |||
| val/val | 1.28 b | 1.22 b | 1.2 b | 1.26 | 1.46 | |||
| Radial diffusivity (μm2/ms) | met/met | 0.63 a,b | 0.63 a | 0.64 | 0.60 | 0.59 | ||
| met/val | 0.63 a | 0.60 a | 0.62 | 0.58 | 0.55 | |||
| val/val | 0.59 b | 0.57 b | 0.59 | 0.55 | 0.52 | |||
GCC = genu of the corpus callosum; ATR = anterior thalamic radiation; UNC = uncinate fasciculus; IFO = inferior frontal occipital fasciculus; SCC = splenium of the corpus callosum. Multivariate analyses of variance conducted on IFO and SCC were not significant at p < .05; consequently, follow-up tests were not conducted for these tracts. Within GCC, ATR, and UNC, for each parameter, values with different subscripts differ significantly at p < .05.
Figure 2.
Aligned dot plots for Fractional Anisotropy (FA), Axial Diffusivity (AD), and Radial Diffusivity (RD) by gene group. Larger horizontal bars indicate group means, and smaller horizontal bars above means indicate standard error of the mean. Val-allele homozygotes were significantly different from one or both of the other gene groups (for more detail refer to Table 2) except FA in the GCC, for which the three groups did not differ significantly.
Anterior thalamic radiation (ATR)
For the ATR, too, ANOVAs yielded significant main effects of COMT genotype for FA, F(2,39) = 3.80, p < .05; AD, F(2,39) = 5.72, p < .01; and RD, F(2,39) = 5.82, p < .01. Follow up t-tests showed that val-allele homozygotes had significantly higher FA than did met-allele homozygotes; val/met heterozygotes did not differ significantly from either of the homozygote groups. For the other two parameters, AD and RD, the val-allele homozygotes had significantly lower diffusivity than did met-allele carriers (met/met or val/met) (see Table 2 and Fig. 2).
Uncinate fasciculus (UNC)
One of the three univariate ANOVAs conducted on the DTI parameters was significant within this tract: AD, F(2,39) = 5.57, p < .01. T-tests indicated that the met/val heterozygotes had significantly higher AD than did the val-allele homozygotes; the met-homozygotes did not differ significantly from either of the other gene groups (see Table 2 and Fig. 2).
Specificity of COMT associations with DTI parameters
To examine specificity of the genotype-based differences in major frontal pathways, FA, AD, and RD values were extracted for the SCC. Although the pattern of the main effect of genetic group was similar in this ROI to the effects obtained in the PFC ROIs (i.e., higher FA in val-allele carriers), the MANOVA conducted on this tract did not yield a significant effect for COMT genotype for the three dependent variables (FA, AD, RD), F(3,70) = 1.73, p > .10.
Discussion
The present study was designed to examine COMT gene-related differences in major white matter fiber tracts with prefrontal terminations in children and adolescents. Our results indicate that the COMT genotype is associated with altered diffusion parameters in subcortical white matter in a sample of children and adolescents. These findings support the formulation that variants in COMT genotype affect brain development in major prefrontal pathways. White matter microstructural variations may be one of several antecedents to later life alterations in cognitive and affective processing that have been found to differ among COMT val158met gene groups (Barnett et al., 2007; Bruder et al., 2005; Diaz-Asper et al., 2008; Egan et al., 2001; Enoch et al., 2003; Goldberg et al., 2003; Joober et al., 2002; Malhotra et al., 2002; Montag et al., 2008; Nolan et al., 2004; Rosa et al., 2004; Rujescu et al., 2003; Stein et al., 2006; Stein et al., 2005).
A study currently in press examined the relation between COMT genotype and mean FA in a sample of healthy adults and obtained results similar to those reported here (Liu et al., 2009). Liu and colleagues examined other haplotypes in the COMT gene that cover a wider range of protein expression and found that the groups with lower enzymatic activity (i.e., higher DA levels) had lower FA in PFC white matter tracts. Therefore, their study and ours both report lower FA values in participants with COMT alleles that correspond to higher brain DA levels. In contrast to these genetic imaging studies that are reliant on inferences about participants’ brain neurotransmitter levels, previous in vitro work has shown direct correspondence between DA and brain myelination. In vitro works supports what we have shown here by demonstrating that DA receptor activation decreases differentiation in immature oligiodendrocytes and inhibits myelination (Bongarzone et al., 1998; Karadottir and Attwell, 2007). Therefore, the studies we are recounting converge on one conclusion: that high levels of brain DA are associated with reduced human brain myelination.
Higher FA and lower diffusivity are functionally significant (Deutsch et al., 2005; Dougherty et al., 2007; Olesen et al., 2003) and have been described as markers of developmental progress (Barnea-Goraly et al., 2005b; Eluvathingal et al., 2007; Lebel et al., 2008b; McGraw et al., 2002; Morriss et al., 1999; Sakuma et al., 1991; Schneider et al., 2004; Snook et al., 2005). Recent studies in rodents (Zahrt et al., 1997), children (Wahlstrom et al., 2007), and adults (Mattay et al., 2003; Meyer-Lindenberg et al., 2005; Williams-Gray et al., 2007) have indicated that an intermediate amount of DA is optimal for PFC function. The present findings suggest that in terms of PFC white matter fiber pathways, children are shifted to the right on this theoretical curve (Fig. 3). We posit that val/val represents the optimal position, a position ‘overshot’ by heterozygotes and homozygous met carriers by virtue of possessing too much prefrontal DA. This formulation is tentative because it is based on the assumption that higher FA during development is optimal. Recent work, however, may qualify this postulation; Hoeft and colleagues demonstrated that increased FA is inversely associated with visiospatial ability in a sample of children with Williams Syndrome (Hoeft et al., 2007). Thus, the functional significance of reduced PFC DA levels for the development of white matter is not yet clear and should be a focus for future research.
Figure 3.
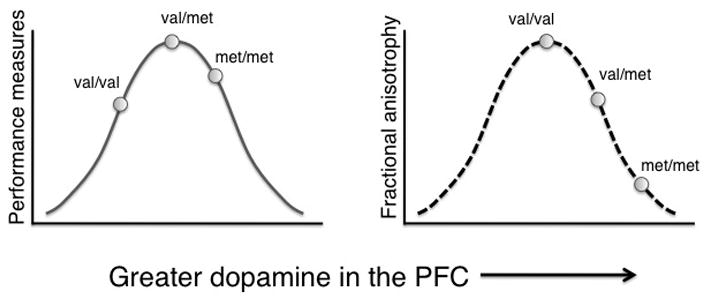
Past research supports an inverted U-shaped relation between performance measures and dopamine level in the PFC (Goldman-Rakic et al., 2000; Williams-Gray et al., 2007). In the present study we found that fractional anisotropy (FA) has an inverse relation with expected dopamine level. Because higher FA is correlated with developmental maturation 1, 2 (see Discussion), this result suggests that val-allele homozygotes are in the optimal position on this curve.
It is important to consider the strengths and limitations of the methods used in the present study. One strength of this study is use of DTI tractography for deriving subject specific ROIs; tractography relies on tracing the path of greatest diffusion across voxels (direction of the eigenvector with the largest eigenvalue of the diffusion tensor) (Basser et al., 2000; Conturo et al., 1999; Jones et al., 1999; Mori et al., 1999; Pajevic et al., 2002) and in the present study, bases derivations on trajectories that are both anatomically plausible and conform to well-characterized major fiber bundles. Moreover, tract-based-ROIs permit quantification of specific white matter pathways across their entire trajectory and should have less contamination from adjacent fiber tracts and non-white matter tissue than do manually drawn ROIs (Snook et al., 2007). Still, tractography methods utilize various acquisition schemes and algorithms for construction of fiber tracts and, like any neuroimaging technique, the derived results can be influenced by methodological details. It is important to note, however, that the three metrics that we selected for quantification of white matter microstructure – FA, AD, and RD – are derived in a straightforward manner from eigenvalue decomposition of the diffusion tensor itself, and are relatively robust with respect to methodological choices. A limitation of the present study is that there are biological and environmental factors that we did not assess in our sample that may affect brain morphology. For example, substance use by pregnant mothers can have adverse effects on brain maturation (Jones et al., 1973; Norman et al., 2009), and we did not assess this factor in our sample.
Identifying significant differences in white matter fiber pathways as they relate to COMT genotype in children is only a starting point for characterizing the putative role that altered COMT enzymatic function may play in affecting the structure and development of white matter. The quadratic age effects of white matter organizational metrics, namely FA and MD (which is derived from AD and RD), are well documented; FA increases and MD decreases until approximately age 30, after which they reverse direction (Hasan et al., 2007; Moseley, 2002). In this context, it is not clear what influence the COMT genotype exerts on white matter organizational metrics in adulthood. It will be important to investigate whether carriers of the met allele ‘catch up’ to their val-allele homozygous counterparts, or whether the observed differences in childhood persist, ultimately altering the trajectory of these parameters through the aging process.
Figure 1.
3D Visualization of the targeted fiber tracts. The top and bottom-left panels show, in a randomly selected participant, the major fiber tracts of interest with strong projections to prefrontal cortical regions. ATR = anterior thalamic radiation (left: light orange; right: yellow); GCC = genu of the corpus callosum (light green); IFO = inferior frontal occipital fasciculus (left: blue; right: purple); and UNC = uncinate fasciculus (left: red; right: orange). The bottom-right panel shows the control fiber tract tested to examine the regional specificity of COMT genotype differences: SCC = splenuim of the corpus callosum (dark green).
Acknowledgments
This project was supported by awards from the National Institute of Mental Health [MH081583 to MET, RR P41–009874 to GHG, MH074849 to IHG, and NIH EY-15000 to RFD], and by a NARSAD Young Investigator Award to MET. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Melissa L. Henry, Sarah Victor, Emily Dennis, and Rebecca Johnson for their assistance in acquiring the scan data, and Yamanda Wright and Lindsey Sherdell for their assistance in participant recruitment, screening, and conducting structured behavioral interviews.
Footnotes
Multivariate and regression analyses yielded no significant main effects or interactions involving either age or gender on FA for any of the tracts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, Rhinewine J, Kane JM, Adesman A, Milanaik R, Maytal J, Diamond A, Szeszko P, Ardekani BA. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry. 2005;57:448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Eliez S, Menon V, Bammer R, Reiss AL. Arithmetic ability and parietal alterations: a diffusion tensor imaging study in velocardiofacial syndrome. Brain Res Cogn Brain Res. 2005a;25:735–740. doi: 10.1016/j.cogbrainres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005b;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Muller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafo MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bashat DB, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, Even A, Levy Y, Sira LB. Accelerated maturation of white matter in young children with autism: A high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, Lebihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance Series B. 1994a;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994b;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Belachew S, Rogister B, Rigo JM, Malgrange B, Moonen G. Neurotransmitter-mediated regulation of CNS myelination: a review. Acta Neurologica Belgica. 1999;99:21–31. [PubMed] [Google Scholar]
- Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, Vigneron DB, Henry RG. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27:862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, Goldman RS, Hoptman MJ, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Kunz M, Chakos M, Cooper TB, Lieberman JA. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol Psychiatry. 2002;52:701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- Bongarzone ER, Howard SG, Schonmann V, Campagnoni AT. Identification of the dopamine D3 receptor in oligodendrocyte precursors: Potential role in regulating differentiation and myelin formation. Journal of Neuroscience. 1998;18:5344–5353. doi: 10.1523/JNEUROSCI.18-14-05344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, Gilliam TC. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry. 2005;58:901–907. doi: 10.1016/j.biopsych.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. American Journal of Human Genetics. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JDE, Wandell B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Diaz-Asper CM, Goldberg TE, Kolachana BS, Straub RE, Egan MF, Weinberger DR. Genetic variation in catechol-O-methyltransferase: effects on working memory in schizophrenic patients, their siblings, and healthy controls. Biol Psychiatry. 2008;63:72–79. doi: 10.1016/j.biopsych.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc Natl Acad Sci U S A. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Egan MF, Weinberger DR. Catechol O-methyltransferase ValÂ1-sup-5-sup-8Met Genotype and Neural Mechanisms Related to Affective Arousal and Regulation. Archives of General Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, Chugani DC, Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007;17:2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht V, Scherer A, Rassek M, Witsack HJ, Modder U. Diffusion-weighted MR imaging in the brain in children: findings in the normal brain and in the brain with white matter diseases. Radiology. 2002;222:410–418. doi: 10.1148/radiol.2222010492. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Xu K, Ferro E, Harris CR, Goldman D. Genetic origins of anxiety in women: a role for a functional catechol-O-methyltransferase polymorphism. Psychiatr Genet. 2003;13:33–41. doi: 10.1097/00041444-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Hasan KM, Prasad MR, Kramer L, Bachevalier J. Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins disconcordant for traumatic brain injury. AJNR Am J Neuroradiol. 2006;27:879–881. [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J. Generative and recognition models for neuroanatomy. Neuroimage. 2004;23:21–24. doi: 10.1016/j.neuroimage.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Gao W, Lin W, Chen Y, Gerig G, Smith JK, Jewells V, Gilmore JH. Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. AJNR Am J Neuroradiol. 2009;30:290–296. doi: 10.3174/ajnr.A1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B, Williams M, Zimerman B, Frazier J. WASH-U-KSADS (Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia) St. Louis (Missouri): Washington University; 1996. [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Han DH, Kee BS, Min KJ, Lee YS, Na C, Park DB, Lyoo IK. Effects of catechol-O-methyltransferase Val158Met polymorphism on the cognitive stability and aggression in the first-onset schizophrenic patients. Neuroreport. 2006;17:95–99. doi: 10.1097/01.wnr.0000192740.38653.91. [DOI] [PubMed] [Google Scholar]
- Hasan KM. Diffusion tensor eigenvalues or both mean diffusivity and fractional anisotropy are required in quantitative clinical diffusion tensor MR reports: fractional anisotropy alone is not sufficient. Radiology. 2006;239:611–612. doi: 10.1148/radiol.2392051172. author reply 612–613. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Sankar A, Halphen C, Kramer LA, Brandt ME, Juranek J, Cirino PT, Fletcher JM, Papanicolaou AC, Ewing-Cobbs L. Development and organization of the human brain tissue compartments across the lifespan using diffusion tensor imaging. Neuroreport. 2007;18:1735–1739. doi: 10.1097/WNR.0b013e3282f0d40c. [DOI] [PubMed] [Google Scholar]
- Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, Menten R, Clapuyt P, Donohue PK, Hua K, Wakana S, Jiang H, van Zijl PC, Mori S. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Ho BC, Wassink TH, O’Leary DS, Sheffield VC, Andreasen NC. Catechol-O-methyl transferase Val158Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Mol Psychiatry. 2005;10:229, 287–298. doi: 10.1038/sj.mp.4001616. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Barnea-Goraly N, Haas BW, Golarai G, Ng D, Mills D, Korenberg J, Bellugi U, Galaburda A, Reiss AL. More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J Neurosci. 2007;27:11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Kema IP, van der Pompe G, te Meerman GJ, Ormel J, den Boer JA. Catechol-o-methyltransferase polymorphism and susceptibility to major depressive disorder modulates psychological stress response. Psychiatr Genet. 2007;17:183–193. doi: 10.1097/YPG.0b013e32808374df. [DOI] [PubMed] [Google Scholar]
- Jones DK, Simmons A, Williams SC, Horsfield MA. Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn Reson Med. 1999;42:37–41. doi: 10.1002/(sici)1522-2594(199907)42:1<37::aid-mrm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissg Ap. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Joober R, Gauthier J, Lal S, Bloom D, Lalonde P, Rouleau G, Benkelfat C, Labelle A. Catechol-O-methyltransferase Val-108/158-Met gene variants associated with performance on the Wisconsin Card Sorting Test. Arch Gen Psychiatry. 2002;59:662–663. doi: 10.1001/archpsyc.59.7.662. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Attwell D. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience. 2007;145:1426–1438. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63:972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Nolan KA, Mohr P, Saito T, Volavka J. Association between catechol O-methyltransferase genotype and violence in schizophrenia and schizoaffective disorder. Am J Psychiatry. 1998;155:835–837. doi: 10.1176/ajp.155.6.835. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, Beaulieu C. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2008a;32:1732–1740. doi: 10.1111/j.1530-0277.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008b;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Liu B, Li J, Yu C, Li Y, Liu Y, Song M, Fan M, Li K, Jiang T. Haplotypes of catechol-O-methyltransferase modulate intelligence-related brain white matter integrity. Neuroimage Epub. 2009 doi: 10.1016/j.neuroimage.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw P, Liang L, Provenzale JM. Evaluation of normal age-related changes in anisotropy during infancy and childhood as shown by diffusion tensor imaging. AJR Am J Roentgenol. 2002;179:1515–1522. doi: 10.2214/ajr.179.6.1791515. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Montag C, Buckholtz JW, Hartmann P, Merz M, Burk C, Hennig J, Reuter M. COMT genetic variation affects fear processing: psychophysiological evidence. Behav Neurosci. 2008;122:901–909. doi: 10.1037/0735-7044.122.4.901. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Morriss MC, Zimmerman RA, Bilaniuk LT, Hunter JV, Haselgrove JC. Changes in brain water diffusion during childhood. Neuroradiology. 1999;41:929–934. doi: 10.1007/s002340050869. [DOI] [PubMed] [Google Scholar]
- Moseley M. Diffusion tensor imaging and aging - a review. NMR Biomed. 2002;15:553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, Tsuruda J, Norman D. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, Conturo TE, Neil JJ, McKinstry RC. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23:1445–1456. [PMC free article] [PubMed] [Google Scholar]
- Muller RA. The study of autism as a distributed disorder. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Skare S, Andersson JL, Lilja A, Flodmark O, Fernell E, Holmberg K, Bohm B, Forssberg H, Lagercrantz H, Klingberg T. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr Res. 2003;54:672–679. doi: 10.1203/01.PDR.0000084083.71422.16. [DOI] [PubMed] [Google Scholar]
- Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, Akbudak E, Aronovitz JA, Miller JP, Lee BC, Conturo TE. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209:57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry. 2004;161:359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Developmental Disabilities Research Reviews. 2009;15:209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Res Cogn Brain Res. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ono J, Harada K, Mano T, Sakurai K, Okada S. Differentiation of dys- and demyelination using diffusional anisotropy. Pediatr Neurol. 1997;16:63–66. doi: 10.1016/s0887-8994(96)00249-4. [DOI] [PubMed] [Google Scholar]
- Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM. Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. J Neurosci. 2009;29:6229–6233. doi: 10.1523/JNEUROSCI.0896-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajevic S, Aldroubi A, Basser PJ. A continuous tensor field approximation of discrete DT-MRI data for extracting microstructural and architectural features of tissue. J Magn Reson. 2002;154:85–100. doi: 10.1006/jmre.2001.2452. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge SC, Mukherjee P, Berman JI, Henry RG, Miller SP, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ, Vigneron DB. Tractography-based quantitation of diffusion tensor imaging parameters in white matter tracts of preterm newborns. J Magn Reson Imaging. 2005;22:467–474. doi: 10.1002/jmri.20410. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Press W, Teukolsky S, Vetterling W, Flannery B. Numerical 2002 [Google Scholar]
- Recipes in C++: The Art of Scientific Computing. Cambridge University Press; Cambridge, U.K: [Google Scholar]
- Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn Reson Med. 2004;51:103–114. doi: 10.1002/mrm.10677. [DOI] [PubMed] [Google Scholar]
- Rosa A, Peralta V, Cuesta MJ, Zarzuela A, Serrano F, Martinez-Larrea A, Fananas L. New evidence of association between COMT gene and prefrontal neurocognitive function in healthy individuals from sibling pairs discordant for psychosis. Am J Psychiatry. 2004;161:1110–1112. doi: 10.1176/appi.ajp.161.6.1110. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Giegling I, Gietl A, Hartmann AM, Moller HJ. A functional single nucleotide polymorphism (V158M) in the COMT gene is associated with aggressive personality traits. Biol Psychiatry. 2003;54:34–39. doi: 10.1016/s0006-3223(02)01831-0. [DOI] [PubMed] [Google Scholar]
- Rylander-Rudqvist T, Hakansson N, Tybring G, Wolk A. Quality and quantity of saliva DNA obtained from the self-administrated oragene method--a pilot study on the cohort of Swedish men. Cancer Epidemiol Biomarkers Prev. 2006;15:1742–1745. doi: 10.1158/1055-9965.EPI-05-0706. [DOI] [PubMed] [Google Scholar]
- Sakuma H, Nomura Y, Takeda K, Tagami T, Nakagawa T, Tamagawa Y, Ishii Y, Tsukamoto T. Adult and neonatal human brain: diffusional anisotropy and myelination with diffusion-weighted MR imaging. Radiology. 1991;180:229–233. doi: 10.1148/radiology.180.1.2052700. [DOI] [PubMed] [Google Scholar]
- Schneider JF, Il’yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46:258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Sehy JV, Ackerman JJ, Neil JJ. Evidence that both fast and slow water ADC components arise from intracellular space. Magn Reson Med. 2002;48:765–770. doi: 10.1002/mrm.10301. [DOI] [PubMed] [Google Scholar]
- Sen PN, Basser PJ. A model for diffusion in white matter in the brain. Biophys J. 2005;89:2927–2938. doi: 10.1529/biophysj.105.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neuro development in children and young adults. Neuroimage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Snook L, Plewes C, Beaulieu C. Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. Neuroimage. 2007;34:243–252. doi: 10.1016/j.neuroimage.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Newman TK, Savitz J, Ramesar R. Warriors Versus Worriers: The Role of COMT Gene Variants. CNS Spectrums. 2006;11:745–748. doi: 10.1017/s1092852900014863. [DOI] [PubMed] [Google Scholar]
- Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology. 2005;30:2092–2102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion Tensor Imaging of Frontal Lobe in Autism Spectrum Disorder. Cerebral cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen J, Salminen M, Lundstrom K, Kiviluoto T, Savolainen R, Ulmanen I. Genomic organization of the human catechol O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem. 1994;223:1049–1059. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- Thomason ME. Children in non-clinical functional magnetic resonance imaging (FMRI) studies give the scan experience a “thumbs up”. Am J Bioeth. 2009;9:25–27. doi: 10.1080/15265160802617928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volavka J, Kennedy JL, Ni X, Czobor P, Nolan K, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Lieberman JA. COMT158 polymorphism and hostility. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:28–29. doi: 10.1002/ajmg.b.20149. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Hooper CJ, Vrshek-Schallhorn S, Oetting WS, Brott MJ, Luciana M. Variations in the Catechol O-methyltransferase Polymorphism and Prefrontally Guided Behaviors in Adolescents. Biological Psychiatry. 2007;61:626–632. doi: 10.1016/j.biopsych.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson’s disease. J Neurosci. 2007;27:4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112 ( Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woo JM, Yoon KS, Choi YH, Oh KS, Lee YS, Yu BH. The association between panic disorder and the L/L genotype of catechol-O-methyltransferase. J Psychiatr Res. 2004;38:365–370. doi: 10.1016/j.jpsychires.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Demiralp C, Laidlaw DH. Visualizing diffusion tensor MR images using streamtubes and streamsurfaces. Visualization and Computer Graphics, IEEE Transactions. 2003;9:454–462. [Google Scholar]
- Zimmerman RA, Haselgrove JC, Wang Z, Hunter JV, Morriss MC, Hoydu A, Bilaniuk LT. Advances in pediatric neuroimaging. Brain Dev. 1998;20:275–289. doi: 10.1016/s0387-7604(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzig MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opiod neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]