Abstract
Bluetongue virus (BTV) is the cause of bluetongue (BT), an emerging, arthropod-transmitted disease of ungulates. The cellular tropism of BTV in ruminants includes macrophages, dendritic cells and endothelial cells (EC), and fulminant infection is characterized by lesions consistent with those of so-called viral hemorrhagic fevers. Specifically, BT is characterized by vascular injury with hemorrhage, tissue infarction and widespread edema. To further investigate the pathogenesis of vascular injury in BT, we evaluated the responses of cultured bovine pulmonary artery EC (bPAEC) and monocyte - derived macrophages (bMDM) to BTV infection by measuring transcript levels of genes encoding molecules important in mediating EC activation and/or endothelial barrier dysregulation. The data confirm that BTV infection of bPAEC resulted in increased transcription of genes encoding chemokine ligand 2 (CCL2) and E-selectin, and BTV-infection of bMDM resulted in increased transcription of genes encoding TNF-α, IL-1β, IL-8, and inducible nitric oxide synthase (iNOS). The data from these in vitro studies provide further evidence that cytokines and other vasoactive substances produced in macrophages potentially contribute to vascular injury in BTV-infected ruminants, along with direct effects of the virus itself on ECs.
Keywords: Bluetongue, Virus, Macrophage, Endothelium
1. Introduction
Bluetongue virus ([BTV]; genus Orbivirus, family Reoviridae) is the etiologic agent of bluetongue (BT), an important and re-emerging arboviral disease of ruminants (Backx et al., 2007; Darpel et al., 2007; MacLachlan et al., 2009; Verwoerd & Erasmus, 2004). BTV is transmitted by Culiciodes insects, thus the global distribution of BTV infection coincides closely with that of competent vector species (Gibbs & Greiner, 1994; Pritchard et al., 2004; Tabachnick, 2004). BT is most commonly described in sheep, although many species of domestic and wild ungulates are susceptible including cattle, but with lower morbidity and mortality (Jauniaux et al., 2008; MacLachlan et al., 2009; Meyer et al., 2009; Verwoerd & Erasmus, 2004). The pathogenesis of BTV infection is apparently similar in all ruminant species (MacLachlan et al., 2009).
Natural BTV infection of ruminants begins with intradermal introduction of the virus during the blood feeding of an infected Culicoides insect. The inoculated virus first interacts with mononuclear inflammatory cells, including dendritic cells, and it then drains to the local lymph node where further replication occurs prior to dissemination to secondary sites of replication such as the lungs, lymph nodes, and spleen (Barratt-Boyes & MacLachlan, 1995; Barratt-Boyes et al., 1992; Hemati et al., 2009; Pini, 1976). Virus replication occurs in mononuclear phagocytic, dendritic, and endothelial cells (EC), thus the infection and response of these cells is critical to the pathogenesis of BT (DeMaula et al., 2002a; Hemati et al., 2009; McLaughlin et al., 2003; Whetter et al., 1989). Whereas BTV infection of the ECs lining small caliber vessels is likely responsible for the thrombosis and subsequent infarction that produce oral ulcers and myonecrosis, the mechanism(s) responsible for the widespread edema that occurs in severely affected animals is less defined (MacLachlan et al., 2008; MacLachlan et al., 2009; Mahrt & Osburn, 1986). It is proposed that inflammatory and vasoactive mediators released by BTV-infected host cells contribute to the increased vascular permeability that characterizes severe BT; specifically, that vasoactive mediators released from BTV-infected cells exert paracrine effects that increase the permeability of adjacent blood vessels (MacLachlan et al., 2009). Consistent with this hypothesis, dendritic cells in lymph draining from the site of BTV infection in sheep express higher levels of genes encoding pro-inflammatory molecules such as interleukin 12 ( IL-12), IL-1β and IL-6 (Hemati et al., 2009).
To further characterize the potential role of BTV-induced host cell-derived mediators and interferon inducible proteins in the pathogenesis of BT, we evaluated the activational response of bovine monocyte derived macrophages (bMDM) and bovine pulmonary artery EC (bPAEC) to BTV infection. The data indicate that BTV infection causes transcriptional activation of both bMDM and bPAEC, suggesting that these responses may be important to the pathogenesis of BT in ruminants.
2. Materials and methods
2.1 Virus
The U.S. prototype strain of BTV serotype 10 (BTV-10) (ATCC, VR-1231) was used for all experiments. Stock virus was prepared by infecting baby hamster kidney (BHK-21) cells (ATCC, CCL-10). The infected cells were pelleted and then sonicated to release cell associated virus after cytopathic effect was advanced. Virus containing cell lysates were centrifuged at 400 g to remove the cellular debris from the virus suspension, prior to ultracentrifugation at 69,000 g through a 5 % sucrose cushion to remove virus from soluble, cell - derived inflammatory mediators. The partially purified virus was re-suspended in phenol red free Dulbeccos minimal essential media (DMEM; Gibco), aliquoted and frozen at −80 °C. Virus titers (TCID50) were determined as previously described (Barratt-Boyes et al., 1992; MacLachlan et al., 1984).
2.2 Bovine pulmonary artery endothelial cells (bPAEC) and monocyte-derived macrophages (bMDM)
The isolation and purification of bPAEC were described previously (DeMaula et al., 2001). Cells were maintained in complete medium (DMEM, 10 % fetal bovine serum, MEM vitamins, non essential amino acids, L- glutamine, sodium pyruvate). Confluent monolayers of bPAEC of similar passage number (9 or 10) were used in all experiments.
The bMDM were also derived and characterized essentially as previously described (Weiss et al., 2002). Briefly, 300 ml of peripheral blood was collected in citrated buffer from the jugular vein of each of five different adult Holstein cows. The blood was centrifuged at 600×g for 20 minutes in 50 ml conical tubes and the buffy coat collected and diluted with an equal volume of PBS. The cell suspension was placed on top of an equal volume of Histopaque 1.083 (Sigma, 10831) and centrifuged at 400 g for 30 minutes. Peripheral blood mononuclear cells (PBMCs) were washed twice in PBS and resuspended in freezing media (90% fetal bovine serum, 10% DMSO) and stored at −80°C. Prior to each experiment PBMCs were thawed and washed twice with cold PBS and their viability was determined using trypan blue staining prior to dilution at 6 × 106 cells ml-1in media containing 7% fetal calf serum, and plating on 24 – well plates. Non-adherent cells were gently removed at 2 and 24 hrs after incubation at 37 °C. Morphology of the adherent cells as macrophages was confirmed by light microscopy, and all studies were performed on bMDM that had been maintained in culture for 7 days.
2.3 Replication of BTV in bPAEC and bMDM
The growth kinetics of BTV in bPAEC and bMDM were determined using both one-step growth curve analysis and sequential immunofluorescence staining for the presence of BTV core protein VP7. Specifically, bPAEC and bMDM were infected with BTV-10 at a multiplicity of infection (MOI) of 1, and individual cultures were harvested at regular intervals thereafter. At each time point the cells were scraped from the culture plate or flask and combined with the supernatant prior to sonication (DeMaula et al., 2001). The cell debris was removed by centrifugation (400 g) and the titer (log 10 TCID50) of the supernatant determined by serial dilution as described (Barratt-Boyes et al., 1992; MacLachlan et al., 1984).
Immunofluorescence staining of BTV-infected bPAEC and bMDM was done at intervals after BTV infection using cells grown on sterile 12 mm coverslips. Individual coverslip cultures were fixed with 4 % paraformaldehyde, permeabilized with 0.01 % Triton X-100, and incubated with 10% bovine serum albumin (BSA) prior to addition of the primary antibody (MAb 290; Whetter et al., 1989) diluted in 1.5 % BSA/PBS. After 1 hour, the coverslips were washed and incubated with the secondary antibody (goat anti-mouse Alexa594) prior to mounting in Prolong gold with DAPI (Invitrogen, P36935). Immunofluorescence staining was visualized with an Olympus BX61 microscope fitted with a xenon lamp and appropriate filters.
2.4 RNA extraction and cDNA preparation
Total RNA was collected and purified from BTV-infected and uninfected (control) bPAEC and bMDM using the RNAeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions, and cDNA was synthesized using a reverse transcription kit (Qiagen, Valencia, CA).
2.5 Quantitation of cytokine transcripts and analysis of gene expression in bMDM
Gene expression quantification of the cDNA was carried out using the QuantiTect SYBR Green PCR kit (Qiagen, Valencia, CA) following the manufacturer’s protocol and with the primer pair sets listed in Table 1 for IL-1β, TNF-α, IL-8, iNOS, MX-1, and ribosomal protein S9 (reference gene). Melting curve analysis was performed at the conclusion of the PCR assay. All assays were performed using the ABI 7500 instrument, and quantification of gene expression was performed using the 2−ΔΔCt method as described by the manufacturer (“Guide to performing relative quantitation of gene expression using real-time quantitative PCR”).
Table 1.
Nucleotide identity of primers used to amplify mRNA by quantitative RT-PC
| Target | Sense primer | Antisense primer |
|---|---|---|
| IL-1b | 5 - ggc tta cta cag tga cga gaa tga g - 3 | 5 - aac cga ggt cca ggt gtt g - 3 |
| IL-8 | 5 - gaa gag agc tga gaa gca aga tcc - 3 | 5 - acc cac aca gaa cat gag gc - 3 |
| TNF - α | 5 - ctt ctg cct gct gca ctt cg - 3 | 5 - gag ttg atg tcg gct aca ac - 3 |
| iNOS | 5 - gca gcg gag tga ctt tcc aa - 3 | 5 - gga tgc cag gca aga ctt g - 3 |
| MX1 | 5 - atc ttt caa cac ctg acc gcg - 3 | 5 - gga gca cga aca acg gga tga t - 3 |
| S9 | 5 - gaa gct gat cgg cga gta tg - 3 | 5 - cgc aac agg gca tta cct tc - 3 |
2.6 Quantitation of cytokine transcripts and analysis of gene expression in bPAEC
Gene expression in BTV-infected bPAEC was quantitated using the TaqMan® Gene Expression Assay system (ABI) with the following primer and probe sets: Beta-2-microglobulin (Bt03251628_m1), chemokine ligand 2 (CCL2) (Bt03212322_m1), intercellular adhesion molecule 1 (ICAM1) (Bt03213907_m1), IL- 8 (Bt03211906), E-selectin (Bt03213082_m1), and vascular cell adhesion molecule 1(VCAM1)(Bt03279189_m1). Assays were validated and expression of individual genes quantitated using the 2−ΔΔCt method, as described by the manufacturer.
2.7 Statistical analysis
Results are presented as mean ± SD. Repeated measures ANOVA, followed by the Bonferonni post hoc was used for analysis of differences between infected and uninfected samples. A p-value of < 0.05 was considered significant.
3. Results and discussion
3.1 Replication of BTV in bMDM and bPAEC
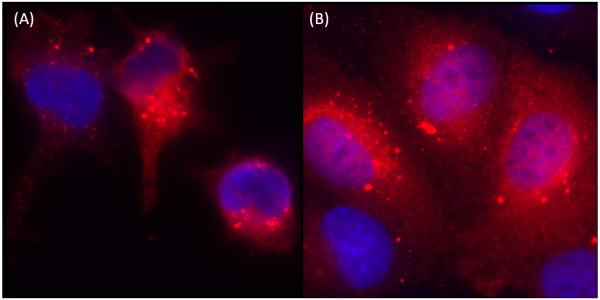
One-step growth curve analysis and immunofluorescence staining for the presence of BTV core protein VP7 indicate that productive replication of BTV occurs in both bPAEC and bMDM, consistent with our previous findings (DeMaula et al., 2001; DeMaula et al., 2002a; Whetter et al., 1989). Specifically, there was an approximately ten-fold increase in virus titer after BTV infection of bMDM, and an approximately thousand-fold (103) increase in bPAEC (data not shown). Similarly, there was intense cytoplasmic immunofluorescence staining of VP7 in both BTV-infected bMDM and bPAEC at 8 hours after infection (Fig. 1).
Fig. 1.
Immunofluorescence staining of VP7 in BTV infected (8hr) bMDM and bPAEC. Granular, cytoplasmic staining in (A) bMDM and (B) bPAEC. (400X).
3.2 BTV-infection results in transcriptional activation of bPAEC and bMDM
To evaluate the ability of BTV to induce activation of bPAEC, cellular transcript levels (mRNA) of genes encoding CCL-2, IL-8, VCAM1, ICAM1, and E-selectin were quantified following infection. Only levels of mRNA encoding CCL2 and E-selectin were significantly increased after BTV-infection (Fig. 2A, B; p<0.05). BTV infection of bPAEC has previously been shown to causes increased levels of IL-1, IL-6, and cyclooxygenase-2, which were not evaluated in the current study (DeMaula et al., 2002a). BTV infection of bovine microvascular EC also induced surface expression of E-selectin (DeMaula et al., 2002b). Together these data confirm that BTV infection results in transcriptional activation of bovine EC, both those derived from the pulmonary microvasculature as well as the pulmonary artery, with increased expression of genes encoding cytokines and adhesion molecules. CCL-2 is a potent chemoattractant for macrophages and monocytes, causes firm adhesion of monocytes to vascular endothelium, and is involved in monocyte extravasation (Melgarejo et al., 2009), consistent with the influx of mononuclear inflammatory cells to sites of BTV infection within the skin (Mahrt & Osburn, 1986). Similarly, increased expression of E-selectin on ECs would be expected in the inflammatory response in BTV infection by facilitating and promoting the margination, rolling, and eventual transmigration of leukocytes, including monocytes (Kumar et al., 2005).
Fig. 2.
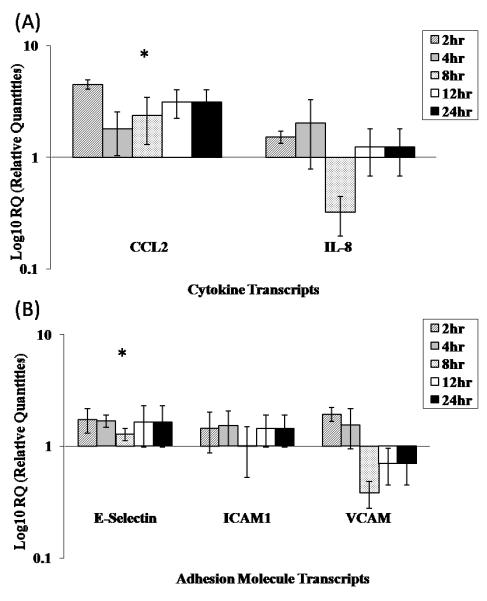
Relative quantification of endothelial cell mRNA of genes encoding (A) proinflammatory mediators CCL2 and IL-8 and (B) adhesion molecules E-selectin, VCAM1 and ICAM1 from BTV infected bPAEC, as measured by real time quantitative PCR. Values are the means ±SD of three replicate experiments performed in duplicate. Expression of genes encoding CCL-2 and E-selectin was significantly increased (p < 0.05). (*) indicates genes with significant increase in mRNA expression.
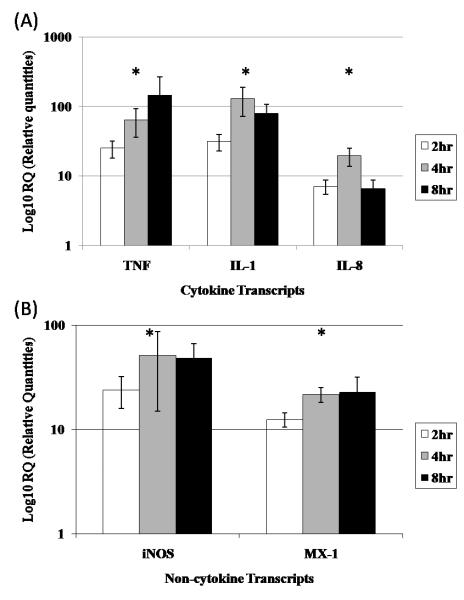
To determine the potential role of host cells other than ECs in the pathogenesis of BT, and macrophages in particular, transcript levels (mRNA) of genes encoding several key proinflammatory mediators, and a single interferon inducible protein, were quantified in BTV infected bMDM. Transcripts encoding TNF-α, IL-1β,IL-8, iNOS, and MX1 were all significantly increased in BTV-infected bMDM, as compared to levels in uninfected cells (Fig. 3A, B; p<0.05). Maximal expression of mRNA encoding TNF- α, IL-1 β, and IL-8, iNOS, and MX1 occurred at 4 – 8 hrs. BTV is a potent inducer of interferon-α, and there is a close temporal relationship between onset of interferon-α production and viremia in BTV-infected ruminants (Foster et al., 1991; Fulton et al., 1982; Jameson & Grossberg, 1978; MacLachlan & Thompson, 1985). MX proteins are interferon inducible, have antiviral activity and are expressed in a variety of cells, including peripheral blood mononuclear cells (Melgarejo et al., 2009). Our data suggests that interferon inducible proteins may be involved in modulating viral replication in vitro, and, by extension, perhaps in vivo. Similarly, production of TNF, IL-1β, and IL-8 by macrophages has been shown to contribute to the pathogenesis of the human viral hemorrhagic fevers (Basu & Chaturvedi, 2008; Bray, 2005; Marty et al., 2006). These cytokines exert a profound effect on ECs leading to increased vascular permeability with subsequent tissue edema and vascular collapse (shock), either by direct injury or by receptor mediated increases in paracellular permeability (Lentsch & Ward, 2000). In particular, IL-8 can be a useful indicator of severe outcome with Dengue virus infection (Basu & Chaturvedi, 2008). Nitric oxide causes vasodilation, increased leukocyte adhesion, and thrombus formation (Tesfamariam & DeFelice, 2007). It is logical to speculate, therefore, that production of these potent vasoactive mediators in BTV-infected macrophages, EC and dendritic cells contributes to the edema and vascular collapse observed in fulminant cases of BT in ruminants. However, in vivo studies will clearly be required to precisely characterize the pathogenesis of BT, specifically the relative roles and importance of direct virus injury and indirect paracrine effects of proinflammatory and chemokine mediators therein. Similarly, although the pathogenesis of BTV infection is apparently similar in all species of ruminants and vascular injury occurs in both BTV-infected sheep and cattle, the change is more pronounced in sheep (DeMaula et al, 2002a; MacLachlan et al., 2009). Thus, to confirm the data we obtained using bMDM and bPAEC, additional in vitro studies should be undertaken in the future utilizing macrophages and ECs derived from sheep and perhaps other species that are highly susceptible to BTV infection.
Fig. 3.
Relative quantification of macrophage mRNA of genes encoding (A) proinflammatory and chemokine mediators TNF-α, IL-1 and IL-8 and (B) immune related and anti-viral molecules (iNOS and MX1) from BTV infected bMDM, as measured by real time quantitative PCR. Values are the means ±SD of 5 replicate experiments (5 individual cows) performed in duplicate. Expression of genes encoding TNF-α, IL-1β, IL-8, iNOS, and MX-1 was significantly increased (p < 0.05). (*) indicates genes with significant increase in mRNA expression.
Acknowledgments
The authors thank Elana Chu for technical assistance.
This publication was made possible by grant number T32 RR07038 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. This publication was also supported by funds provided by the Center for Equine Health at the University of California-Davis and the Bernice Barbour Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backx A, Heutink CG, van Rooij EM, van Rijn PA. Clinical signs of bluetongue virus serotype 8 infection in sheep and goats. Vet Rec. 2007;161:591–592. doi: 10.1136/vr.161.17.591. [DOI] [PubMed] [Google Scholar]
- Barratt-Boyes SM, MacLachlan NJ. Pathogenesis of bluetongue virus infection of cattle. J Am Vet Med Assoc. 1995;206:1322–1329. [PubMed] [Google Scholar]
- Barratt-Boyes SM, Rossitto PV, Stott JL, MacLachlan NJ. Flow cytometric analysis of in vitro bluetongue virus infection of bovine blood mononuclear cells. J Gen Virol. 1992;73:1953–1960. doi: 10.1099/0022-1317-73-8-1953. [DOI] [PubMed] [Google Scholar]
- Basu A, Chaturvedi UC. Vascular endothelium: the battlefield of dengue viruses. FEMS Immunol Med Microbiol. 2008;53:287–299. doi: 10.1111/j.1574-695X.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M. Pathogenesis of viral hemorrhagic fever. Curr Opin Immunol. 2005;17:399–403. doi: 10.1016/j.coi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Darpel KE, Batten CA, Veronesi E, Shaw AE, Anthony S, Bachanek-Bankowska K, Kgosana L, bin-Tarif A, Carpenter S, Muller-Doblies UU, Takamatsu HH, Mellor PS, Mertens PP, Oura CA. Clinical signs and pathology shown by British sheep and cattle infected with bluetongue virus serotype 8 derived from the 2006 outbreak in northern Europe. Vet Rec. 2007;161:253–261. doi: 10.1136/vr.161.8.253. [DOI] [PubMed] [Google Scholar]
- DeMaula CD, Jutila MA, Wilson DW, MacLachlan NJ. Infection kinetics, prostacyclin release and cytokine-mediated modulation of the mechanism of cell death during bluetongue virus infection of cultured ovine and bovine pulmonary artery and lung microvascular endothelial cells. J Gen Virol. 2001;82:787–794. doi: 10.1099/0022-1317-82-4-787. [DOI] [PubMed] [Google Scholar]
- DeMaula CD, Leutenegger CM, Bonneau KR, MacLachlan NJ. The role of endothelial cell-derived inflammatory and vasoactive mediators in the pathogenesis of bluetongue. Virology. 2002a;296:330–337. doi: 10.1006/viro.2002.1476. [DOI] [PubMed] [Google Scholar]
- DeMaula CD, Leutenegger CM, Jutila MA, MacLachlan NJ. Bluetongue virus-induced activation of primary bovine lung microvascular endothelial cells. Vet Immunol Immunopathol. 2002b;86:147–157. doi: 10.1016/s0165-2427(02)00012-0. [DOI] [PubMed] [Google Scholar]
- Foster NM, Luedke AJ, Parsonson IM, Walton TE. Temporal relationships of viremia, interferon activity, and antibody responses of sheep infected with several bluetongue virus strains. Am J Vet Res. 1991;52:192–196. [PubMed] [Google Scholar]
- Fulton RW, Nicholson SS, Pearson NJ, Potter MT, Archbald LF, Pearson JE, Jochim MM. Bluetongue infections in Louisiana cattle. Am J Vet Res. 1982;43:887–891. [PubMed] [Google Scholar]
- Gibbs EP, Greiner EC. The epidemiology of bluetongue. Comp Immunol Microbiol Infect Dis. 1994;17:207–220. doi: 10.1016/0147-9571(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Hemati B, Contreras V, Urien C, Bonneau M, Takamatsu HH, Mertens PP, Breard E, Sailleau C, Zientara S, Schwartz-Cornil I. Bluetongue virus targets conventional dendritic cells in skin lymph. J Virol. 2009;83:8789–8799. doi: 10.1128/JVI.00626-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson P, Grossberg SE. Production of interferon in human cell cultures by a new, potent viral inducer. Adv Exp Med Biol. 1978;110:37–53. doi: 10.1007/978-1-4615-9080-4_4. [DOI] [PubMed] [Google Scholar]
- Jauniaux TP, De Clercq KE, Cassart DE, Kennedy S, Vandenbussche FE, Vandemeulebroucke EL, Vanbinst TM, Verheyden BI, Goris NE, Coignoul FL. Bluetongue in Eurasian lynx. Emerg Infect Dis. 2008;14:1496–1498. doi: 10.3201/eid1409.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Robbins and Cotran pathologic basis of disease. Elsevier Saunders. 2005:54. [Google Scholar]
- Lentsch AB, Ward PA. Regulation of inflammatory vascular damage. J Pathol. 2000;190:343–348. doi: 10.1002/(SICI)1096-9896(200002)190:3<343::AID-PATH522>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- MacLachlan NJ, Crafford JE, Vernau W, Gardner IA, Goddard A, Guthrie AJ, Venter EH. Experimental reproduction of severe bluetongue in sheep. Vet Pathol. 2008;45:310–315. doi: 10.1354/vp.45-3-310. [DOI] [PubMed] [Google Scholar]
- MacLachlan NJ, Drew CP, Darpel KE, Worwa G. The pathology and pathogenesis of bluetongue. J Comp Pathol. 2009;141:1–16. doi: 10.1016/j.jcpa.2009.04.003. [DOI] [PubMed] [Google Scholar]
- MacLachlan NJ, Schore CE, Osburn BI. Antiviral responses of bluetongue virus-inoculated bovine fetuses and their dams. Am J Vet Res. 1984;45:1469–1473. [PubMed] [Google Scholar]
- MacLachlan NJ, Thompson J. Bluetongue virus-induced interferon in cattle. Am J Vet Res. 1985;46:1238–1241. [PubMed] [Google Scholar]
- Mahrt CR, Osburn BI. Experimental bluetongue virus infection of sheep; effect of vaccination: pathologic, immunofluorescent, and ultrastructural studies. Am J Vet Res. 1986;47:1198–1203. [PubMed] [Google Scholar]
- Marty AM, Jahrling PB, Geisbert TW. Viral hemorrhagic fevers. Clin Lab Med. 2006;26:345–386. doi: 10.1016/j.cll.2006.05.001. [DOI] [PubMed] [Google Scholar]
- McLaughlin BE, DeMaula CD, Wilson WC, Boyce WM, MacLachlan NJ. Replication of bluetongue virus and epizootic hemorrhagic disease virus in pulmonary artery endothelial cells obtained from cattle, sheep, and deer. Am J Vet Res. 2003;64:860–865. doi: 10.2460/ajvr.2003.64.860. [DOI] [PubMed] [Google Scholar]
- Melgarejo E, Medina MA, Sanchez-Jimenez F, Urdiales JL. Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol. 2009;41:998–1001. doi: 10.1016/j.biocel.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Meyer G, Lacroux C, Leger S, Top S, Goyeau K, Deplanche M, Lemaire M. Lethal bluetongue virus serotype 1 infection in llamas. Emerg Infect Dis. 2009;15:608–610. doi: 10.3201/eid1504.081514. [DOI] [PubMed] [Google Scholar]
- Pini A. Study on the pathogenesis of bluetongue: replication of the virus in the organs of infected sheep. Onderstepoort J Vet Res. 1976;43:159–164. [PubMed] [Google Scholar]
- Pritchard LI, Sendow I, Lunt R, Hassan SH, Kattenbelt J, Gould AR, Daniels PW, Eaton BT. Genetic diversity of bluetongue viruses in south east Asia. Virus Res. 2004;101:193–201. doi: 10.1016/j.virusres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Tabachnick WJ. Culicoides and the global epidemiology of bluetongue virus infection. Veterinaria Italiana. 2004;40:145–150. [PubMed] [Google Scholar]
- Tesfamariam B, DeFelice AF. Endothelial injury in the initiation and progression of vascular disorders. Vascul Pharmacol. 2007;46:229–237. doi: 10.1016/j.vph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Verwoerd DW, Erasmus BJ. Bluetongue. In: Coetzer JAW, Tustin RC, editors. Infectious Diseases of Livestock. 2 nd Ed Oxford University Press; Cape Town: 2004. pp. 1201–1220. [Google Scholar]
- Weiss DJ, Evanson OA, Moritz A, Deng MQ, Abrahamsen MS. Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Infect Immun. 2002;70:5556–5561. doi: 10.1128/IAI.70.10.5556-5561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetter LE, MacLachlan NJ, Gebhard DH, Heidner HW, Moore PF. Bluetongue virus infection of bovine monocytes. J Gen Virol. 1989;70:1663–1676. doi: 10.1099/0022-1317-70-7-1663. [DOI] [PubMed] [Google Scholar]