Abstract
Neuromodulators including gonadotropin-releasing hormone (GnRH) and sex steroids help integrate an animal's internal physiological state with incoming external cues, and can have profound effects on the processing of behaviorally-relevant information, particularly from the olfactory system. While GnRH and steroid receptors are present in olfactory processing regions across vertebrates, little is known about whether their expression levels change with internal physiological state or external social cues. We used qRT-PCR to measure mRNA levels of two GnRH receptors (GnRH-R1, GnRH-R2), five sex steroid receptors (estrogen receptors: ERα, ERβa, ERβb; androgen receptors: ARα, ARβ), and aromatase in the olfactory bulb of the highly social African cichlid fish Astatotilapia burtoni. We asked whether these receptor levels changed with reproductive condition in females, or with social status, which regulates reproductive capacity in males. Our results reveal that mRNA levels of multiple sex steroid, GnRH receptor subtypes, and aromatase in the olfactory bulb vary with sex, social status in males, and reproductive condition in females, which highlights the potential importance of changing receptor levels in fine-tuning the olfactory system during the reproductive cycle. Further, steroid receptor mRNA levels were positively correlated with circulating steroid levels in males, but negatively correlated in females, suggesting different regulatory control between sexes. These results provide support for the hypothesis that the first-order olfactory relay station is a substrate for both GnRH and sex steroid modulation, and suggest that changes in receptor levels could be an important mechanism for regulating reproductive, social, and seasonal plasticity in olfactory perception observed across vertebrates.
Keywords: androgen receptor, aromatase, Astatotilapia burtoni, estrogen receptor, gonadotropin-releasing hormone, mRNA, olfaction, teleost
1. Introduction
Animals must integrate signals from their external and internal environments to make appropriate and context-dependent behavioral decisions. One key player in this process, the olfactory system, is important for vertebrate behavior, since many taxa use chemical communication in their courtship and dominance displays [1-4]. In many fish species, for example, males determine female reproductive state through compounds released by gravid or pre-ovulatory individuals [5-7], male urine can be used as a form of sexual signaling to denote social rank [3, 8-10], and chemical cues are used in mother-young social bonds [11, 12]. How might the internal physiological state of an individual influence its perception of these chemical cues? Steroid hormones and neuropeptides are known to fine-tune sensory responsiveness to behaviorally relevant cues [4, 13-15], providing a potential link between internal state and external signals, but the mechanisms and site of action for such modulators in the olfactory system are not well understood.
Previous studies in fishes and other vertebrates show that steroids and the modulatory neuropeptide gonadotropin-releasing hormone (GnRH) can influence olfactory processing, and that these effects often vary between sexes or across reproductive seasons [13, 14, 16-21]. For example, more olfactory receptor neurons responded to GnRH application during the breeding season compared to the non-breeding season in the mudpuppy Necturus maculosus [17, 22], olfactory responsiveness differed between sex hormone-treated male and female goldfish Carassius auratus [18], and steroid-related response differences were reported in several mammal species [19]. For any modulator to influence the olfactory system, however, cognate receptors must be present in the tissues implicated, so quantification of receptor levels can be used as a proxy for physiological action. One potential mechanism to explain changes in olfactory responsiveness is variations in steroid and GnRH receptor abundance within processing centers such as the olfactory bulb. However, little is known about how receptor levels change with reproductive state or social status in the olfactory system of any vertebrate. Here, we tested for changes in receptor mRNA levels of key modulators in the first-order olfactory processing center of the brain, the olfactory bulb, using the highly social African cichlid fish Astatotilapia burtoni to test the hypothesis that receptor levels vary with reproductive and social state.
Cichlid fishes use multiple sensory cues (e.g., visual, olfactory, auditory, mechanosensory) to coordinate their complex social behaviors, and show great diversity in reproductive and parental care strategies, which makes them excellent models for discovering how hormones influence sensory function. The African cichlid A. burtoni is endemic to shallow shore pools of Lake Tanganyika and lives in a lek-like social system where males exist in one of two phenotypes: 1) dominant territorial males (~10-30% of population) that are brightly colored, aggressively defend a spawning territory, and actively court and spawn with females; and 2) subordinate non-territorial males that are drab-colored, frequently chased by dominant males, and do not court females [23]. Males can rapidly and reversibly switch between dominant and subordinate phenotypes depending on the composition of the social environment. Importantly, this social transformation in males causes a suite of behavioral and physiological changes along the reproductive axis [24]. Females do not have a similarly organized social system, but typically school with subordinate males and enter the territories of dominant males only to eat and spawn. After spawning, females rear the developing young in their mouths (mouth brooding) for ~2 weeks before releasing them, and then physiologically recover (~25-30 days) before spawning again. While visual cues are important for social behaviors in this species [23, 25, 26], olfactory cues may also provide crucial information on sex, reproductive condition, and dominance status. Previous studies in A. burtoni show that olfactory signals are used for perception of social information [25, 27], and that the olfactory system is responsive to putative pheromone compounds [28-30]. In the closely related tilapia Oreochromis mossambicus, chemical communication is also used by both males and females for reproductive and dominance behaviors [6, 31]. These studies provide support for the importance of olfactory-mediated behaviors in A. burtoni, but whether the olfactory system might be influenced by the animals’ internal physiological state remains unknown.
We do know from previous studies that GnRH receptors and sex steroid receptors are found within the olfactory bulb of several teleosts including A. burtoni [Munchrath and Hofmann, pers. comm. [32-36], and thus the olfactory bulb could be an important substrate for neuropeptide and steroid-mediated olfaction. Here we asked whether social status and/or reproductive state influenced mRNA levels of GnRH receptors, sex steroid receptors, and aromatase in the central olfactory system of male and female A. burtoni, and whether these changes were correlated with circulating steroid levels. Our results show that mRNA levels of multiple sex steroid receptor subtypes, GnRH receptor subtypes, and aromatase in the olfactory bulb of a cichlid fish vary with sex, social status in males, and reproductive condition in females. These results provide support for the hypothesis that the first-order olfactory relay station is a substrate for both GnRH and sex steroid action, and that changing receptor expression may provide a mechanism to influence context-dependent olfactory responsiveness.
2. Methods
2.1. Animals
Laboratory-bred adult male and female Astatotilapia burtoni, derived from wild-caught stock in Lake Tanganyika, Africa, were maintained in aquaria under environmental conditions that mimic their natural equatorial habitat (28°C; pH 8.0; 12h light: 12h dark with full spectrum illumination; constant aeration), and fed cichlid pellets and flakes (AquaDine, Healdsburg, CA, USA) each morning. Aquaria contained gravel-covered bottoms with terra cotta pots cut in half to serve as spawning territories. All experimental procedures were approved by the Stanford Administrative Panel for Laboratory Animal Care.
Stable dominant and subordinate males were established by initially placing two previously dominant territorial males from separate community tanks together in an aquarium with a single terra cotta pot territory and 4 females. In this situation, social interactions between the two males result in one male quickly becoming dominant over the other. The dominant male then defends the territory and uses it to court and spawn with females, while the subordinate male becomes drab-colored, is frequently chased by the newly dominant individual, and becomes reproductively suppressed. Fish were observed daily to verify that the dominant and subordinate males were stable phenotypes and maintained their social status for 4-5 weeks, a time sufficient to ensure reproductive suppression in the subordinate animals [37].
Female A. burtoni breed year round and provide sole parental care to developing young. Females have three distinct reproductive phases, which were selected for analysis: 1) Mouth brooding females had mouths filled with large full-term embryos that they had been brooding for 14 days. Mouth brooding females generally do not eat while carrying developing young; 2) Gravid females had visibly swollen abdomens, numerous large “ready-to-spawn” eggs (some eggs were readily released from the ovary upon dissection in all individuals indicating they were at or near ovulation), and a correspondingly high gonadosomatic index (GSI), and; 3) Recovering females (neither gravid nor mouth brooding) were created by releasing full-term fry from mouth brooders to initiate ovarian recrudescence, and then returning them to their community tanks to recover for 12 days (~equivalent to half of the average ovarian cycle period of 25-30 days) so that they were all approximately in the same state of ovarian growth at the time of sampling.
Fish used in this experiment were those used to compare gene expression in the inner ear as part of a separate study [38]. Gonadosomatic index and plasma steroid concentration data were used here only to test for correlations between receptor mRNA levels in the olfactory bulb and GSI and circulating steroid levels.
2.2. Tissue preparation
All fish used in this study were sacrificed at the same time of day (9:30-10:30 am) to control for any potential diurnal changes in gene expression and were size-matched to account for any differences due to body size (SL = 59.4 ± 4.5 mm SD). Fish were captured from their tanks, anesthetized in ice-cold tank water, and standard length (SL) and total body mass (BM) measured. Immediately before sacrifice by rapid cervical transection, blood samples (50-100 μl) were collected from the caudal vein by caudal severance with heparinized 100 μl capillary tubes within 2 min of capture. Blood was centrifuged for 10 min at 8000 rpm, and the plasma was removed and stored at -80°C until assayed.
The paired olfactory bulbs are the first relay station in the olfactory system that link odorant detection to the brain. In fishes, they receive input from sensory neurons within the olfactory rosette positioned on the snout, and then convey information to higher brain centers. We chose to examine the olfactory bulb because it processes olfactory information prior to integration with other senses or motor systems, and previous studies suggested that many steroid modulatory effects occur here or in higher brain centers rather than at the peripheral olfactory epithelium [18, 28, 39-41]. We also knew from previous studies in A. burtoni that GnRH receptors and steroid receptors were located primarily within the internal cellular layer of the olfactory bulb. Both olfactory bulbs, which included a portion of the olfactory nerves on the distal end but none of the rostral telencephalon on the proximal end, were transected from the brain at the olfactory tracts (connection between the olfactory bulbs and rostral telencephalon) (Fig. 1), flash frozen, and stored at -80°C until analysis. Testes and ovaries were also removed and weighed to calculate the gonadosomatic index [GSI = (gonad mass/body mass) × 100].
Fig. 1.
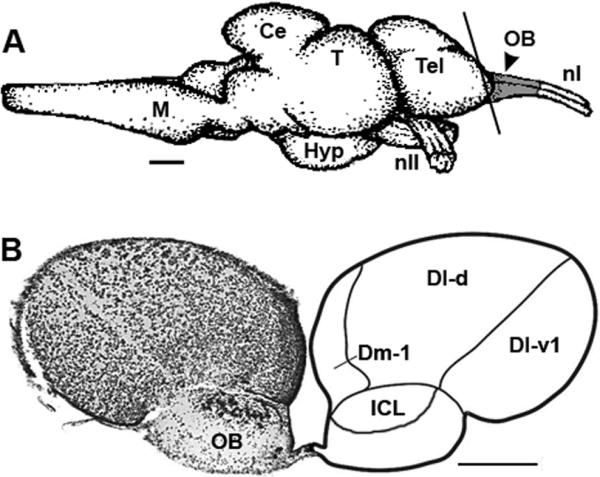
Location and morphology of the olfactory bulb in the African cichlid fish Astatotilapia burtoni. (A) Schematic lateral view of the A. burtoni brain to illustrate the olfactory bulb samples (shaded gray area) used for analysis. Vertical line represents the approximate position and angle of section shown in B. Rostral is to the right. (B) Representative cross section through the rostral telencephalon and olfactory bulbs shows the internal cell layer (ICL) that expresses GnRH receptors and steroid receptors in this species. Abbreviations: Ce, cerebellum; Dl-d, dorsal division of lateral zone of the dorsal telencephalon; Dl-v1, ventral division of the lateral zone of the dorsal telencephalon – subdivision 1; Dm-1, medial part of the dorsal telencephalon – subdivision 1; Hyp, hypothalamus; M, medulla; nI, cranial nerve I or olfactory nerve; nII, cranial nerve II or optic nerve; OB, olfactory bulb; T, tectum; Tel, telencephalon. Scale bars: 1 mm (A), 200 μm (B). Modified from [77] (A) and [78] (B).
Olfactory bulb tissue was homogenized and RNA extracted following standard protocols (RNeasy Micro kit, Qiagen) as previously reported [38]. RNA was treated with DNase (RNase-free DNase set, Qiagen) during the isolation procedure according to kit instructions to remove contaminating genomic DNA. RNA concentration and purity was estimated from spectrophotometric absorbance (260 nm and 280 nm) for all samples. Approximately 0.15 μg of total RNA was reverse transcribed to cDNA (iScript cDNA synthesis kit, Bio-Rad) and samples were diluted 1:10 prior to use as a template for quantitative RT-PCR reactions.
2.3. Quantitative Reverse Transcription-PCR (qRT-PCR)
Astatotilapia burtoni express two androgen receptor (ARα, ARβ), three estrogen receptor (ERα, ERβa, ERβb [32, 42], and two GnRH receptor (GnRH-R1, GnRH-R2) genes [33, 43, 44]. Quantitative RT-PCR was used to measure mRNA expression of these seven different receptor subtypes, plus the aromatase enzyme CYP19a (converts T into E2) from the olfactory bulb of both males and females using previously described protocols [38]. Gene-specific primers, including those for two reference genes, 18s rRNA and glyceraldehyde 3-phosphodehydrogenase (G3PDH), were commercially synthesized (Invitrogen) and identical to those used in previous studies [38, 42, 45, 46]. Each primer pair produced a single melt curve peak in the presence of cDNA template, and showed no amplification when water was used as a template, or when reverse transcriptase was omitted from the cDNA synthesis reaction (negative controls). Quantitative RT-PCR was performed on an iCycler (MyiQ, Bio-Rad, Hercules, CA) and the reaction progress in 30 μl volumes was monitored by fluorescence detection at 490 nm during each annealing step. Reaction parameters were 3 min at 95°C followed by 45 cycles of 95°C, 60°C, and 72°C for 30 s each, and followed by a melting curve analysis over the temperature range of 95°C to 50°C (decrease by 0.5°C increments each cycle). Amplification occurred prior to cycle 32 for all genes and all samples (mean CT values for all 8 target genes ranged from 23.11 – 31.65). All reactions were performed in duplicate and several reaction products per gene were verified by DNA sequencing (Sequetech, Mountain View, CA).
Fluorescence thresholds for each sample were automatically measured (MyiQ software, Bio-Rad) and then Real-Time PCR Miner [46] was used to calculate reaction efficiencies and cycle thresholds from the background subtracted fluorescence readings of individual wells during the reaction. This curve-fitting real-time PCR algorithm objectively calculates reaction efficiency and the fractional cycle number at threshold (CT) of the amplification curve for more accurate computation of mRNA levels. By using the kinetics of individual reactions, estimates of efficiency and CT are independent of the specific equipment used to perform PCR reactions and data can be more reliably compared across plates. The relative amount of target gene mRNA was then normalized to the geometric mean of two housekeeping genes (18s and G3PDH) that were also measured in each sample with the following equation: relative target mRNA levels = [1/(1+Etarget)^CTtarget] / [1/(1+Egeomean)^CTgeomean] × 100, where E is the reaction efficiency and CT is the average cycle threshold of the duplicates [42, 45, 47]. Normalization to multiple reference genes, rather than a single gene, provides a more accurate quantification of mRNA levels [48, 49]. Mean CT values for 18s and G3PDH did not differ between male phenotypes (18s, p = 0.091; G3PDH, p = 0.192) or among female groups (18s, p = 0.082; G3PDH, p = 0.061), nor did they differ between the sexes (18s, p = 0.958; G3PDH, p = 0.689), demonstrating they are appropriate reference genes for this study.
2.4. Steroid assays
Plasma testosterone (T), 11-ketotestosterone (11-KT), and 17β-estradiol (E2) were measured with commercially available reagents (Enzyme ImmunoAssay kits; Cayman Chemical, Inc.). Full details on the extraction protocol, steroid assays, and validation of EIA kits can be found in [38]. Briefly, for each assay, a 5 μl sample of plasma from each subject was extracted three times using 200 μl of ethyl ether and evaporated under a fume hood prior to re-constitution in assay buffer (1:40 dilution; extraction efficiencies 87-89%). EIA kit protocols were then strictly followed, plates were read at 405 nm using a microplate reader (UVmax Microplate Reader, Molecular Devices), and steroid concentrations determined based on standard curves. All samples were assayed in duplicate, and intra-assay coefficients of variation were: T (6.2%, 10.3%); 11-KT (4.9%, 3.1%); E2 (6.0%, 9.1%), while inter-assay coefficients of variation were: T (8.4%); 11-KT (4.2%); E2 (6.2%).
2.5. Statistics
Data sets that were normally distributed with equal variances were analyzed with Student's t-tests or one-way analysis of variance (ANOVA) with post-hoc Tukey's tests for multiple comparisons. Data that did not meet the assumptions of parametric statistics were compared with Mann-Whitney rank sum tests or Kruskal-Wallis tests (KW) with post-hoc Dunn's tests. For consistency, however, all data are plotted as mean ± standard errors (SE) with appropriate statistical test values reported in the text. To test for sex differences in mRNA levels for each receptor subtype, data were also combined within each sex and then compared with Mann-Whitney tests. Correlations were assessed with Pearson product moment or Spearman rank tests. Our results are presented and interpreted without the use of conservative corrections for multiple comparisons such as the Bonferroni correction. While the use of Bonferroni and related procedures may reduce Type I errors, they also reduce statistical power and increase the chance of Type II error, especially in cases of smaller sample sizes (i.e., n < 30) [50]. We therefore chose to report observed effect size (e.g., r values) along with exact p-values to allow reader evaluation of biological importance, rather than utilize the overly conservative Bonferroni correction. However, Bonferroni corrected p-values are also indicated in the correlation tables for reference (Bonferroni corrected p = 0.05/n, where n is the number of hypotheses tested on a set of data). Statistical comparisons were performed with Sigma Plot 11.0 (Systat Software, Inc., San Jose, CA.).
3. Results
3.1. GSI and circulating steroid levels
GSI and circulating steroid levels for these animals were previously reported as part of a separate study [38] and those values are summarized here in Table 1. GSI differed among all three reproductive phases in females (KW, H = 28.10, p < 0.001, Dunn's test, p < 0.05), and was two-fold greater in dominant males compared to subordinate males (Student's t-test, t = -6.50, p < 0.001). Serum E2, T, and 11-KT levels differed among all three female reproductive stages (ANOVA or KW, all p < 0.001). In males, dominant individuals had higher serum E2, T, and 11-KT levels compared to subordinate individuals (Mann-Whitney tests, all p < 0.001).
Table 1.
Gonadosomatic index (GSI) and plasma steroid concentrations (ng ml-1) in male and female Astatotilapia burtoni.
| N | GSI | E2 | T | 11-KT | |
|---|---|---|---|---|---|
| Males | |||||
| Dominant | 11 | 1.03 ± 0.07 | 115.2 ± 13.7 | 108.9 ± 17.9 | 4.65 ± 0.8 |
| Subordinate | 11 | 0.43 ± 0.06 | 5.2 ± 2.4 | 4.7 ± 1.5 | 0.47 ± 0.1 |
| Females | |||||
| Brooding | 12 | 0.86 ± 0.12 | 3.7 ± 0.9 | 1.5 ± 0.3 | 0.23 ± 0.01 |
| Recovering | 12 | 2.24 ± 0.30 | 20.7 ± 3.1 | 3.8 ± 0.6 | 0.30 ± 0.03 |
| Gravid | 11 | 8.72 ± 0.75 | 43.6 ± 9.1 | 13.4 ± 3.2 | 0.40 ± 0.03 |
Data are expressed as mean ± SE. E2, estradiol; T, testosterone; 11-KT, 11-ketotestosterone. N = number of animals. These values are a summary of data previously reported as part of a separate study on the same individuals [38].
3.2. GnRH receptor mRNA expression in the olfactory bulb
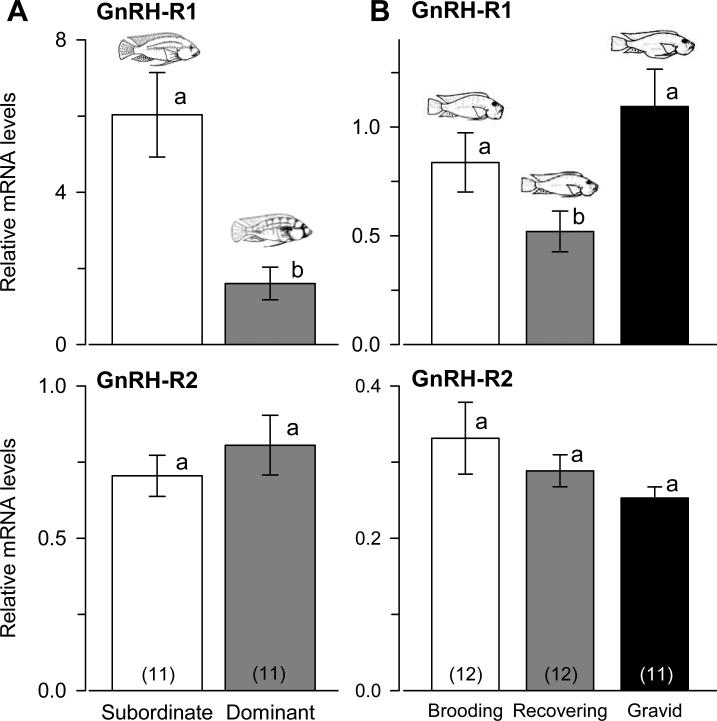
Levels of GnRH-R1 mRNA were approximately 3-fold higher in subordinate as compared to dominant males (Student's t-test, t = 3.724, p = 0.001), but there was no difference in GnRH-R2 mRNA levels between social states (Student's t-test, t = -0.845, p = 0.408) (Fig. 2A). Levels of GnRH-R1 were also positively correlated with GnRH-R2 in subordinate males (r = 0.85, p = 0.001), but not dominant males (r = 0.56, p = 0.077). In females, GnRH-R1 mRNA levels were higher in both brooding and gravid individuals compared to recovering individuals (ANOVA, F = 4.472, p = 0.015) (Fig. 2B). Similar to males, there was no difference in GnRH-R2 mRNA levels across female reproductive states (KW, H = 2.043, p = 0.360). Levels of GnRH-R1 were positively correlated with GnRH-R2 levels in brooding females (r = 0.88, p < 0.001), but not gravid (r = -0.32, p = 0.334) or recovering (r = 0.14, p = 0.657) females. Olfactory bulb mRNA levels of GnRH-R1 were also higher than GnRH-R2 in both sexes (Mann-Whitney rank sum tests, males: U = 70.0, p < 0.001; females: U = 130.0, p < 0.001).
Fig. 2.
GnRH receptor mRNA levels in the olfactory bulb of male and female Astatotilapia burtoni. A) GnRH-R1 mRNA levels were three-fold higher in subordinate compared to dominant males, but GnRH-R2 mRNA levels did not differ between social states. B) Brooding and gravid females had higher GnRH-R1 mRNA levels compared to recovering individuals, but there was no difference in GnRH-R2 levels among reproductive states. Data are plotted as relative mRNA levels (mean ± SE) referenced to the geometric mean of two housekeeping genes (18s and G3PDH). Bars with different letters represent significant differences (p < 0.05) and sample sizes are indicated within each bar.
3.3. Sex steroid receptor mRNA expression in the olfactory bulb
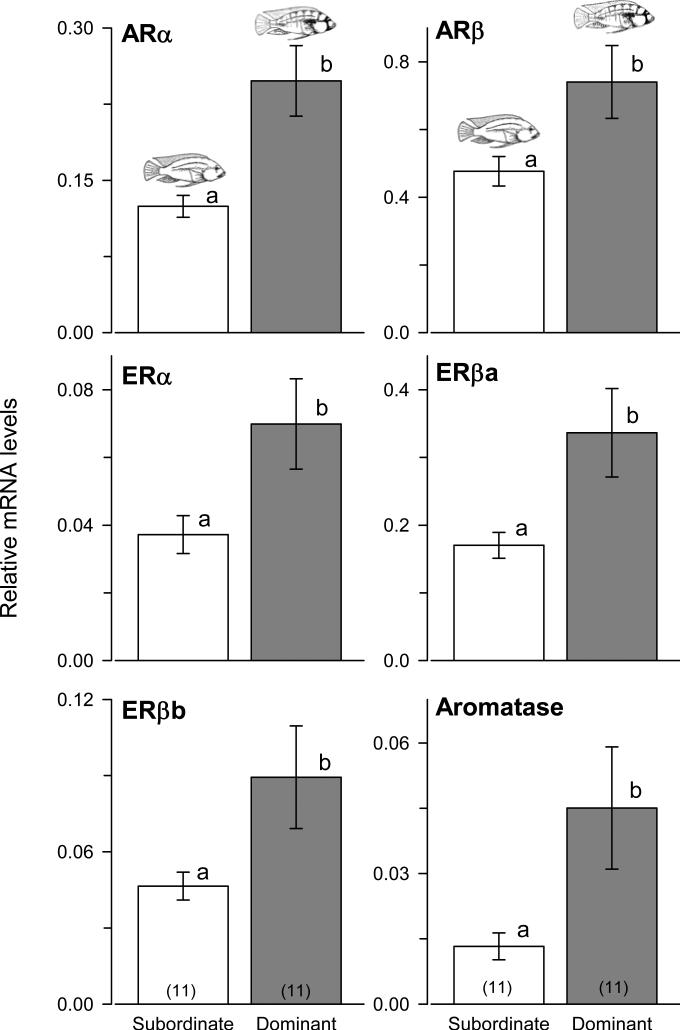
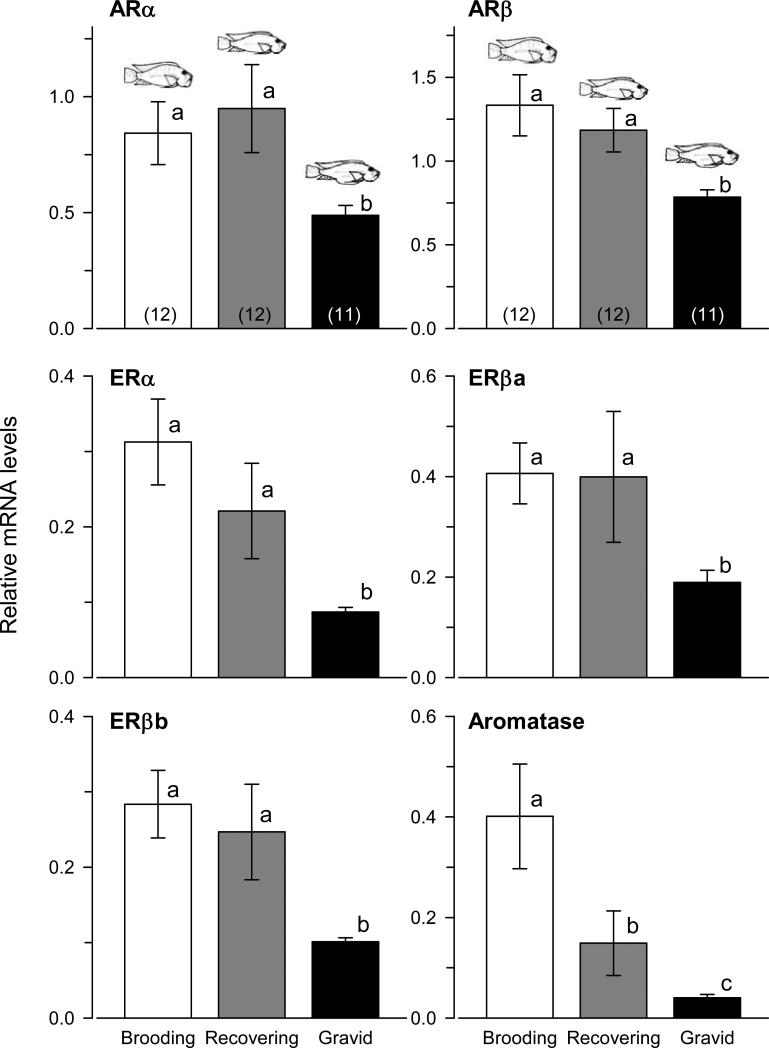
Androgen receptors (Student's t-tests, ARα: t = -3.395, p = 0.003; ARβ: t = 2.271, p = 0.036), estrogen receptors (ERα: t = -2.260, p = 0.035; ERβa: t = 2.438, p = 0.024; ERβb: t = -2.050, p = 0.054), and aromatase (t = -2.209, p = 0.039) mRNA levels were all higher in dominant compared to subordinate males (Fig. 3). Gravid females had lower mRNA levels of androgen receptors (KW, ARα: H = 9.10, p = 0.010; ARβ: H = 12.437, p = 0.002, Dunn's tests, p < 0.05) and estrogen receptors (ERα: H = 17.132, p < 0.001; ERβa: H = 10.864, p = 0.004; ERβb: H = 18.588, p < 0.001) compared to both brooding and recovering females (Fig. 4). Aromatase mRNA expression differed among all three reproductive states where brooding individuals had the highest levels and gravid females the lowest (H = 13.201, p = 0.001).
Fig. 3.
Steroid receptor and aromatase mRNA levels in the olfactory bulb of male A. burtoni. Dominant males had higher mRNA levels of all sex steroid receptors (ARα, ARβ, ERα, ERβa, ERβb) and aromatase compared to subordinate males. Data are plotted as relative mRNA levels (mean ± SE) referenced to the geometric mean of two housekeeping genes (18s and G3PDH). Bars with different letters represent significant differences (p < 0.05) and sample sizes are indicated within each bar.
Fig. 4.
Steroid receptor and aromatase mRNA levels in the olfactory bulb of female A. burtoni. Levels of all androgen receptors (ARα, ARβ) and estrogen receptors (ERα, ERβa, ERβb) were lower in gravid females compared to both brooding and recovering individuals, while aromatase mRNA levels differed among all three reproductive states. Data are plotted as relative mRNA levels (mean ± SE) referenced to the geometric mean of two housekeeping genes (18s and G3PDH). Bars with different letters represent significant differences (p < 0.05) and sample sizes are indicated within each bar.
3.4. Sex differences in receptor mRNA expression in the olfactory bulb
Males had 2-3 fold higher mRNA levels of both GnRH receptor subtypes compared to females (Table 2). In contrast, with the exception of ERβa, females had higher mRNA levels of all steroid receptor subtypes and aromatase compared to males (Table 2).
Table 2.
Sex comparisons of GnRH receptors, steroid receptors, and aromatase mRNA levels in the olfactory bulb of Astatotilapia burtoni.
| Receptor subtype | U statistic | p | Summary |
|---|---|---|---|
| GnRH-R1 | 122.0 | < 0.001 | M > F |
| GnRH-R2 | 18.0 | < 0.001 | M > F |
| ARα | 17.0 | < 0.001 | M < F |
| ARβ | 88.0 | < 0.001 | M < F |
| ERα | 51.0 | < 0.001 | M < F |
| ERβa | 275.0 | 0.073 | M = F |
| ERβb | 75.0 | < 0.001 | M < F |
| Aromatase | 129.0 | < 0.001 | M < F |
Female data are combined brooding, recovering and gravid individuals; male data are combined subordinate and dominant individuals. U statistics and p-values are from Mann-Whitney Rank Sum tests and bold values indicate differences at p < 0.05 (Bonferroni correction p < 0.006).
3.5. Correlations between receptor mRNA levels, GSI and circulating steroid levels
Levels of GnRH-R1 mRNA in males were negatively correlated with E2 and T, but not 11-KT, and were positively correlated only with T in females (Table 3). GnRH-R2 mRNA levels were not correlated with any circulating steroid levels in males or females (all p > 0.10). There were also no correlations between GnRH receptor subtypes and circulating steroid levels when male and female individuals were examined separately by reproductive state (all p > 0.10).
Table 3.
Correlations between GnRH receptors, steroid receptors, and aromatase mRNA levels in the olfactory bulb and circulating steroid concentrations in Astatotilapia burtoni.
| Females | ||||||
|---|---|---|---|---|---|---|
| E2 | T | 11-KT | ||||
| r | p | r | p | r | p | |
| GnRH-R1 | 0.18 | 0.293 | 0.35 | 0.042 | 0.32 | 0.062 |
| GnRH-R2 | -0.24 | 0.170 | -0.23 | 0.182 | -0.26 | 0.139 |
| ARα | -0.17 | 0.341 | -0.19 | 0.277 | -0.19 | 0.264 |
| ARβ | -0.32 | 0.062 | -0.29 | 0.087 | -0.33 | 0.052 |
| ERα | -0.39 | 0.020 | -0.33 | 0.054 | -0.38 | 0.025 |
| ERβa | -0.27 | 0.112 | -0.24 | 0.164 | -0.22 | 0.200 |
| ERβb | -0.35 | 0.039 | -0.31 | 0.073 | -0.35 | 0.041 |
| Aromatase | -0.42 | 0.012 | -0.33 | 0.054 | -0.38 | 0.025 |
| Males | ||||||
|---|---|---|---|---|---|---|
| E2 | T | 11-KT | ||||
| r | p | r | p | r | p | |
| GnRH-R1 | -0.54 | 0.011 | -0.45 | 0.039 | -0.39 | 0.081 |
| GnRH-R2 | 0.26 | 0.264 | 0.33 | 0.139 | 0.30 | 0.192 |
| ARα | 0.49 | 0.025 | 0.85 | <0.001 | 0.77 | <0.001 |
| ARβ | 0.36 | 0.109 | 0.68 | <0.001 | 0.71 | <0.001 |
| ERα | 0.30 | 0.183 | 0.62 | 0.003 | 0.70 | <0.001 |
| ERβa | 0.32 | 0.153 | 0.68 | <0.001 | 0.72 | <0.001 |
| ERβb | 0.26 | 0.250 | 0.64 | 0.002 | 0.70 | <0.001 |
| Aromatase | 0.26 | 0.255 | 0.75 | <0.001 | 0.70 | <0.001 |
E2, estradiol; T, testosterone; 11-KT, 11-ketotestosterone. Correlation coefficients (r) and p-values are from Pearson Product moment tests. P-values in bold indicate significant relationships at p < 0.05 (within sex Bonferroni correction p < 0.002).
In males, all steroid receptor subtypes and aromatase were positively correlated with circulating levels of both T and 11-KT (Table 3). In contrast to the androgens, circulating E2 levels were only positively correlated with ARα in males. When dominant and subordinate males were examined separately, there were no correlations between mRNA levels and any plasma steroid levels in subordinate males (all p > 0.05), while in dominant males there were positive correlations between some mRNA levels and both T (ARα, ARβ, aromatase) and 11-KT (ARα, ARβ, ERα, ERβa, ERβb, aromatase), but not E2 (p > 0.05).
There were fewer relationships between circulating steroid levels and mRNA levels in the olfactory bulb of females, but in contrast to data from males, all correlations were negative (Table 3). There was a negative correlation between mRNA levels of ERα and aromatase and circulating levels of E2, T, and 11-KT. There was also a negative correlation between ERβb and both circulating E2 and 11-KT, but not T. When females within each reproductive state were examined separately, there were no correlations between mRNA levels and any circulating steroids in gravid or recovering animals (all p > 0.05), and a negative correlation only between ERβa and T in brooding individuals (p = 0.034).
Correlations between receptor mRNA levels in the olfactory bulb and GSI showed no relationships between GSI and any receptor subtype in all males together (all r < 0.40, p > 0.05) (Table 4), or when dominant and subordinate animals were examined alone (all p > 0.05). In contrast, female GSI was negatively correlated with all steroid receptor subtypes, but positively correlated with GnRH-R1 mRNA levels (Table 4), which may be due in part to the 10-fold differences in female GSI among reproductive states compared to the 2-fold GSI difference in males. When examined within each female reproductive state, there were no correlations between GSI and mRNA levels in the olfactory bulb (all p > 0.05).
Table 4.
Correlations between mRNA levels in the olfactory bulb and gonadosomatic index for male and female Astatotilapia burtoni.
| Receptor subtype | Females | Males | ||
|---|---|---|---|---|
| r | p | r | p | |
| GnRH-R1 | 0.38 | 0.026 | -0.39 | 0.076 |
| GnRH-R2 | -0.28 | 0.104 | 0.18 | 0.430 |
| ARα | -0.37 | 0.030 | 0.39 | 0.072 |
| ARβ | -0.45 | 0.006 | 0.34 | 0.124 |
| ERα | -0.47 | 0.004 | 0.28 | 0.207 |
| ERβa | -0.34 | 0.045 | 0.34 | 0.106 |
| ERβb | -0.47 | 0.005 | 0.38 | 0.080 |
| Aromatase | -0.43 | 0.010 | 0.27 | 0.219 |
Correlation coefficients (r) and p-values are from Pearson Product moment tests. P-values in bold indicate significant relationships at p < 0.05 (within sex Bonferroni correction p < 0.006).
4. Discussion
Our results show that mRNA levels of multiple sex steroid and GnRH receptor subtypes in the olfactory bulb of a cichlid fish vary with sex, social status in males, and reproductive state in females. To our knowledge, this is the first quantification of mRNA levels of GnRH receptors, sex steroid receptors, and aromatase specifically in the olfactory bulb in any non-mammalian vertebrate. While previous studies in many vertebrates showed that both GnRH and steroids can influence olfactory processing, and that these effects often vary with sex and reproductive condition, our results show that changes in receptor mRNA levels within the olfactory bulb may be one potential mechanism to explain this chemosensory plasticity.
4.1. GnRH receptors
GnRH-R1 mRNA levels in the olfactory bulb of A. burtoni differed with sex, social status in males, and reproductive state in females, while GnRH-R2 mRNA levels only differed between sexes with higher levels found in males. These subtype-specific changes in receptor expression associated with reproductive capacity further support the hypothesis that GnRH-R1 and GnRH-R2 serve different functions, even within the same tissue [33]. Similar to other vertebrates [14, 51], varicose GnRH-immunoreactive (-ir) fibers are abundant in the olfactory bulb of A. burtoni and likely originate from GnRH3 neurons in the terminal nerve ganglion, although projections from midbrain GnRH2 and possibly even preoptic area GnRH1 cells cannot be entirely ruled out. A previous study in A. burtoni used in situ hybridization to show that GnRH-R2 mRNA was widely distributed throughout the brain including the olfactory bulbs, but GnRH-R1 mRNA was concentrated in the pituitary, hypothalamus and preoptic area with lower expression in the telencephalon and olfactory bulbs [33]. Since both GnRH receptor subtypes have similar affinities for all GnRH ligands found in A. burtoni (GnRH1, GnRH2, GnRH3) [43, 44], variations in expression levels of receptor subtypes may reflect functional specialization. Since brain levels of the extra-hypothalamic GnRH3 and GnRH2 do not vary between dominant and subordinate males, or among females in different reproductive states [37], any changes in GnRH action in the olfactory bulb may result from either the variations in receptor levels reported here, or possibly via alterations in GnRH release from varicosities, membrane receptor abundance, or other post-transcriptional mechanisms.
The olfactory bulbs in teleosts receive dense GnRH-ir projections and are thought to be prime targets for neuromodulation, but the physiological effects of GnRH at this processing level remain unexamined in any vertebrate [14]. In goldfish, however, GnRH content in the olfactory bulb of males was increased after exposure to pre-ovulatory females, suggesting a role for GnRH in olfactory-mediated reproduction [52]. In several teleost fishes, including A. burtoni, GnRH receptors are localized to the internal granule cellular layer of the olfactory bulb [33, 53, 54] and in mammals GnRH-R1 is also expressed in mitral cells [55]. Our data provide the first quantification of multiple GnRH receptor subtypes in the olfactory bulb of a vertebrate, and suggest that the hypothesized modulatory effects of GnRH on olfactory bulb responsiveness could be regulated by changes in GnRH-R1 expression. Moreover, the differential regulation of the GnRH receptor subtypes supports the hypothesis that these two receptors, which arose from a gene duplication event, may be in the process of subfunctionalization [33].
Previous work suggested that the function of GnRH in the olfactory bulb may be closely associated with physiological condition and reproductive status of the fish [56, 57]. Our results that show differences in GnRH-R1 mRNA levels in the olfactory bulb associated with reproductive state in both males and females provides further support for this hypothesis, as does a study in the bullfrog (bf) that demonstrated differential expression of multiple splice variants of the bfGnRHR-3 transcript in the olfactory lobe between hibernation and pre-breeding seasons [58]. The exact functional significance of GnRH action in the fish olfactory bulb remains unknown, but based on a suite of morphological, physiological and behavioral studies across vertebrates it is hypothesized to enhance detection of reproductive-related odors, enhance general odorant contrast, or modulate olfactory-mediated context-dependent behavioral responses (i.e., feeding versus reproductive) [14].
One unexpected finding in our study was the higher GnRH-R1 mRNA levels found in the olfactory bulb of subordinate compared to dominant males, especially since subordinate males have a significantly suppressed reproductive axis from the brain to the testis and do not hold spawning territories [24]. While it is unknown whether or not GnRH has any effect on olfactory bulb responsiveness in A. burtoni, we can hypothesize about what these changes in GnRH receptor levels might mean to the fish based on what we do know about these distinct male phenotypes. One explanation is that higher GnRH-R1 levels in the olfactory bulb helps to enhance detection of non-reproductive odorant cues, such as food or predators. Subordinate males do not maintain a feeding territory, but often put more energy into growth so that their chances of acquiring and holding a territory in the near future are improved [59]. An alternative hypothesis is that elevated GnRH-R1 levels in subordinate males could improve their perception of olfactory cues released by females and/or males during spawning, which would allow them to better localize spawning pairs for sneak fertilizations. However, while sneaking attempts by subordinate males have been observed in aquaria (personal observations), this does not appear to be a common strategy. It is also possible that GnRH release within the olfactory bulb has inhibitory effects on olfactory processing that are mediated by GnRH-R1 expression on GABA (γ-aminobutyric acid)-containing granule cells in the internal cellular layer. Inhibitory effects of GnRH are also observed in midbrain central gray neurons in rats [60], bullfrog sympathetic neurons [61], and olfactory neurons in the mudpuppy [16, 17]. The functional relationships between GnRH receptor expression and olfactory perception have yet to be described in any taxa, however, and future work is needed to test what role receptors play in GnRH-mediated olfactory modulation.
4.2. Steroid receptors in males
Dominant male A. burtoni had higher mRNA levels of both AR subtypes in the olfactory bulb compared to subordinate males, and AR mRNA levels were positively correlated with circulating androgen levels. These data suggest that olfactory bulbs in dominant reproductively active males could be more sensitive to sex steroids, which may be an adaptation that facilitates olfactory discrimination of conspecifics during territorial and reproductive behaviors. In fishes, gravid females often release pheromones that can influence the behavior and reproductive readiness of males [6, 7], and chemical cues are used by males to signal social rank [3, 9, 31]. Previous work in A. burtoni also indicates that males can discriminate sex and breeding state of conspecifics based on olfactory cues [25]. In the round goby Neogobius melanostomus, male olfactory response thresholds to putative pheromones varied with reproductive stage of the fish [62], and in the cyprinid fish Puntius schwanenfeldi, androgen implants increased the magnitude and sensitivity of electro-olfactogram (EOG) responses to pheromones [13]. While these increases in sensitivity were measured at the level of the olfactory epithelium, suggesting direct hormone effects at the periphery, the authors also acknowledge that the response could be mediated by steroid action in the olfactory bulb and conveyed via efferent projections to the epithelium. Another study showed that neither gender nor reproductive condition had an influence on peripheral EOG responses to female pre-ovulatory pheromones, suggesting that the steroid influence on olfactory-mediated behavioral responsiveness seen in male goldfish occurs at central processing sites [63]. Androgen treatment in several cyprinid species also showed that there were marked sex differences in EOG responses to prostaglandins, but not to steroids [15], while in A. burtoni there is evidence for sex differences in response to some conjugated steroids [28]. These studies suggest that sexual dimorphisms may be compound-specific or differ among species. Another relevant study, however, showed that there are seasonal changes in the number and position of crypt cells (type of olfactory sensory neuron thought to respond to sex pheromones) in the olfactory epithelium of C. auratus [64], suggesting morphological changes in sensory neuron-olfactory bulb wiring could also contribute to changes in olfactory perception. In A. burtoni, the physical and social environment is dynamic such that an individual male might only maintain a spawning territory and reproduce for a few weeks before being usurped [23, 59]. It would be advantageous, therefore, for dominant males to enhance the detection of any sensory signals that would increase reproductive fitness during their territory tenure. Further support of the importance of steroid-mediated olfactory processing is provided by previous findings of ARs within other olfactory processing regions in the brain of A. burtoni (e.g., central nucleus of the dorsal telencephalon, ventral nucleus of the ventral telencephalon) [32], as well as the higher mRNA levels of ARs found in the anterior brain of dominant compared to subordinate males [42].
Similar to AR expression, dominant male A. burtoni also had higher mRNA levels of all ERs and aromatase in the olfactory bulb compared to subordinate males. However, there were no relationships between ER mRNA levels and circulating E2 levels in males, but there was a positive correlation between ERs, aromatase, and circulating androgens. This suggests that circulating T may be converted to E2 within the olfactory bulb and then acts on ERs, which is supported by the abundant aromatase expression seen in the olfactory bulb of other teleosts [36, 65-67]. However, it is important to note that we only measured CYP19a aromatase here and future work is needed to test whether the predominant brain aromatase form (CYP19b) shows a similar pattern. Estradiol was shown to enhance olfactory perception at multiple processing levels including the olfactory bulbs in male mammals [19], and E2 also increased olfactory bulb sensitivity to NaCl in goldfish, where the effects were much greater in males compared to females [18]. E2 can also reduce levels of inhibitory GABA and GABA receptors in the olfactory bulb of some mammals to facilitate the responses of mitral cells to conspecific signals [19], but similar experiments have not yet been performed in fishes. Regardless of the mechanisms, the elevated levels of ERs in the olfactory bulb of dominant male A. burtoni suggest E2 may also be an important modulator of olfactory processing in males.
4.3. Steroid receptors in females
Another surprising finding of our study was that reproductive gravid female A. burtoni had lower mRNA levels of ARs and ERs in the olfactory bulb compared to both mouth brooding and recovering females. Females may benefit from greater olfactory sensitivity when they are ready to spawn in order to facilitate detection of any chemicals released by dominant courting males. We therefore predicted that gravid females would have higher steroid receptor levels compared to the other groups, suggesting greater steroid sensitivity of the olfactory bulbs. However, this hypothesis was not supported by our results. It is possible that the changes in steroid receptor mRNA levels in females may simply be a homeostatic mechanism to maintain constant steroid sensitivity of the olfactory bulbs as the circulating hormone levels fluctuate across the reproductive cycle. Interestingly, in the brain (remainder of brain without olfactory bulbs) of the same females used in this study, mRNA levels of ARs and ERs were higher in gravid females compared to recovering and mouth brooding individuals [38]. This indicates that steroid receptor levels are regulated by diverse mechanisms in different regions of the brain, and that changes in receptors could also contribute to functional specialization.
In many fishes, female perception of male odors is also important for reproductive success [1]. For example, reproductive female round gobies [68] and tilapia [9, 69] are behaviorally and physiologically sensitive to compounds released into the water from reproductive males. As noted above for males, the olfactory responsiveness of females often depends on reproductive state, a phenomenon also true for mammals which may be regulated by neuromodulators [19]. In addition to a role in reproduction, chemical cues may also be important in brooding females for recognition of their young. In another maternal mouth brooding cichlid, Cichlasoma nigrofasciatum, mothers were shown to prefer water from their own young [12, 70], suggesting that larval odors and olfactory perception play a role in mother-young parental associations. Olfaction is also critical for maternal behaviors in many mammals, and steroids are thought to play a role in the fine-adjustment of mother-infant interactions [2]. The higher mRNA levels of all steroid receptors in the olfactory bulb of female A. burtoni compared to males suggests steroid modulation is also important for female olfactory processing, but the context may vary across the reproductive cycle.
Levels of AR and ER mRNA in the olfactory bulb of female A. burtoni were also negatively correlated with GSI, which suggests ligand down-regulation of steroid receptors in the olfactory bulb of females rather than the up-regulation seen in males. Few studies have examined reproductive-related changes in steroid receptor expression specifically in the olfactory bulbs of females, so it is unclear whether similar variations exist in other taxa. In female rats, age-dependent differences in AR and ER expression were found in the olfactory bulbs [71-73], but there were no changes in ERs within the olfactory bulb across different stages of pregnancy [74]. Nevertheless, the changes in AR, ER, and aromatase mRNA levels in the olfactory bulb associated with reproductive condition in female A. burtoni suggest that steroids play some important role in olfactory processing or maintenance during reproduction or parental care.
4.4. Sex differences in receptor mRNA levels
The tight correlations between plasma hormone levels and steroid receptor mRNA levels in the olfactory bulb of males, but not females, raises the possibility that modulation of olfactory cues by steroids is more important for males than for females. It is also possible, however, that the correlations between circulating steroid levels and receptor mRNA levels in the olfactory bulb of males result from the greater difference in plasma steroid levels between male phenotypes compared to that of females of different reproductive states (e.g., ~10-20 fold in males; ~2-11 fold in females). A more finely-tuned male olfactory system, however, was also suggested in cyprinids due to the crucial importance of male recognition of female ovulatory status, a cue that might hold less relevance for females [75]. Dominant male A. burtoni may also need a keen olfactory sense for both successful establishment of dominance and for reproduction that might require enhanced odorant contrast detection or discrimination abilities. Comparatively, since females do not hold territories or have a strict dominance hierarchy, their olfactory discrimination may be most important during reproductive and brooding activities. This idea is supported by studies in rats that show odor preference in males is more susceptible to internal hormonal state than it is in females [76]. In the tilapia Oreochromis mossambicus, which has a similar social system to A. burtoni, females have high olfactory sensitivity to substances released in male urine [69], and males increase the frequency of urination as part of their courtship display, especially in the presence of pre-ovulatory females [6, 31]. Male A. burtoni can also distinguish female reproductive state from olfactory cues [25], and there is some evidence for higher EOG responses to certain conjugated steroids in males compared to females [28]. Collectively, these studies show that pre-ovulatory females release compounds that can be used by males to discern reproductive status, and that dominant males also send chemical signals to females via urine release. Thus it is possible that context-dependent olfactory communication is important for both sexes in A. burtoni. There are examples in the literature of sex steroids both enhancing and attenuating olfactory sensitivity, so future studies are needed to determine how changes in steroid receptor levels might influence olfactory acuity in this and other fishes.
In summary, our results show that mRNA levels of multiple sex steroid receptors, GnRH receptor subtypes, and aromatase in the olfactory bulb of a cichlid fish vary with sex, social status, and reproductive state. Future studies are needed to directly test how changes in GnRH and steroid receptor expression might influence olfactory processing, whether changes in receptor mRNA expression are localized to specific olfactory bulb cell types and whether they are reflected in functional protein abundance, and what effects these modulators have on the perception of dominance and reproductive-related olfactory information. Our results indicate that the olfactory bulb is likely a substrate for both GnRH and sex steroid-mediated neuromodulation, and suggest that changes in modulatory receptor levels could be one mechanism to regulate the plasticity in olfactory responsiveness observed across vertebrates.
Acknowledgements
We thank Brian Grone, Julie Desjardins, Russ Carpenter, and two anonymous reviewers for their insightful comments that improved the manuscript. This research was funded by National Institutes of Health (NIH) NRSA F32NS061431 to KPM and NIH NS 034950 to RDF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Derby CD, Sorensen PW. Neural processing, perception, and behavioral responses to natural chemical stimuli by fish and crustaceans. J Chem Ecol. 2008;34:898–914. doi: 10.1007/s10886-008-9489-0. [DOI] [PubMed] [Google Scholar]
- 2.Levy F, Keller M. Olfactory mediation of maternal behavior in selected mammalian species. Behav Brain Res. 2009;200:336–345. doi: 10.1016/j.bbr.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Barata EN, Hubbard PC, Almeida OG, Miranda A, Canario AV. Male urine signals social rank in the Mozambique tilapia (Oreochromis mossambicus). BMC Biol. 2007;5:54. doi: 10.1186/1741-7007-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillot PV, Chapouthier G. Olfaction, GABAergic neurotransmission in the olfactory bulb, and intermale aggression in mice: modulation by steroids. Behav Genet. 1996;26:497–504. doi: 10.1007/BF02359754. [DOI] [PubMed] [Google Scholar]
- 5.Burnard D, Gozlan RE, Griffiths SW. The role of pheromones in freshwater fishes. J Fish Biol. 2008;73:1–16. [Google Scholar]
- 6.Miranda A, Almeida OG, Hubbard PC, Barata EN, Canario AV. Olfactory discrimination of female reproductive status by male tilapia (Oreochromis mossambicus). J Exp Biol. 2005;208:2037–2043. doi: 10.1242/jeb.01584. [DOI] [PubMed] [Google Scholar]
- 7.Poling KR, Fraser EJ, Sorensen PW. The three steroidal components of the goldfish preovulatory pheromone signal evoke different behaviors in males. Comparative Biochemistry and Physiology. 2001;129:645–651. doi: 10.1016/s1096-4959(01)00361-x. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira RF, Almada V, Canario AV. Social modulation of sex steroid concentrations in the urine of male cichlid fish Oreochromis mossambicus. Horm Behav. 1996;30:2–12. doi: 10.1006/hbeh.1996.0002. [DOI] [PubMed] [Google Scholar]
- 9.Barata EN, Fine JM, Hubbard PC, Almeida OG, Frade P, Sorensen PW, Canario AV. A sterol-like odorant in the urine of Mozambique tilapia males likely signals social dominance to females. J Chem Ecol. 2008;34:438–449. doi: 10.1007/s10886-008-9458-7. [DOI] [PubMed] [Google Scholar]
- 10.Appelt CW, Sorensen PW. Female goldfish signal spawning readiness by altering when and where they release a urinary pheromone. Anim Behav. 2007;74:1329–1338. [Google Scholar]
- 11.Russock HI. The effects of natural chemical stimuli on the preferential behavior of Oreochromis mossambicus (Pisces: Cichlidae) fry to maternal models. Behavior. 1990;115:315–326. [Google Scholar]
- 12.Lutnesky MM. Attraction to larval pheromones in female convict cichlids (Cichlasoma nigrofasciatum). J Comp Psychol. 1989;103:297–305. [Google Scholar]
- 13.Cardwell JR, Stacey NE, Tan ESP, McAdam DSO, Lang SLC. Androgen increases olfactory receptor response to a vertebrate sex pheromone. Journal of Comparative Physiology A. 1995;176:55–61. [Google Scholar]
- 14.Kawai T, Oka Y, Eisthen H. The role of the terminal nerve and GnRH in olfactory system neuromodulation. Zoolog Sci. 2009;26:669–680. doi: 10.2108/zsj.26.669. [DOI] [PubMed] [Google Scholar]
- 15.Belanger RM, Pachkowski MD, Stacey NE. Methyltestosterone-induced changes in electro-olfactogram responses and courtship behaviors of cyprinids. Chem Senses. 2010;35:65–74. doi: 10.1093/chemse/bjp085. [DOI] [PubMed] [Google Scholar]
- 16.Park D, Eisthen HL. Gonadotropin releasing hormone (GnRH) modulates odorant responses in the peripheral olfactory system of axolotls. Journal of Neurophysiology. 2003;90:731–738. doi: 10.1152/jn.01162.2002. [DOI] [PubMed] [Google Scholar]
- 17.Eisthen HL, Delay RJ, Wirsig-Wiechmann CR, Dionne VE. Neuromodulatory effects of gonadotropin-releasing hormone on olfactory receptor neurons. Journal of Neuroscience. 2000;20:3947–3955. doi: 10.1523/JNEUROSCI.20-11-03947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hara TJ. Electrophysiological studies of the olfactory system of the goldfish, Carassius auratus L. - III. Effects of sex hormones on olfactory activity. Comp Biochem Physiol. 1967;22:209–225. doi: 10.1016/0010-406x(67)90182-x. [DOI] [PubMed] [Google Scholar]
- 19.Moffatt CA. Steroid hormone modulation of olfactory processing in the context of socio-sexual behaviors in rodents and humans. Brain Res Brain Res Rev. 2003;43:192–206. doi: 10.1016/s0165-0173(03)00208-x. [DOI] [PubMed] [Google Scholar]
- 20.Doty RL, Cameron EL. Sex differences and reproductive hormone influences on human odor perception. Physiol Behav. 2009;97:213–228. doi: 10.1016/j.physbeh.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodley SK, Baum MJ. Effects of sex hormones and gender on attraction thresholds for volatile anal scent gland odors in ferrets. Horm Behav. 2003;44:110–118. doi: 10.1016/s0018-506x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Delay RJ. Gonadotropin-releasing hormone modulates voltage-activated sodium current and odor responses in Necturus maculosus olfactory sensory neurons. J Neurosci Res. 2007;85:1656–1667. doi: 10.1002/jnr.21297. [DOI] [PubMed] [Google Scholar]
- 23.Fernald RD, Hirata NR. Field study of Haplochromis burtoni: quantitative behavioral observations. Animal Behavior. 1977;25:964–975. [Google Scholar]
- 24.Fernald RD. Social regulation of reproduction: what changes and why? Hormones, Brain and Behavior. 2009;1:683–691. [Google Scholar]
- 25.Crapon de Caprona MD. Olfactory communication in a cichlid fish, Haplochromis burtoni. Z Tierpsychol. 1980;52:113–134. doi: 10.1111/j.1439-0310.1980.tb00706.x. [DOI] [PubMed] [Google Scholar]
- 26.Fernald RD. Quantitative behavioral observations of Haplochromis burtoni under semi-natural conditions. Animal Behavior. 1977;25:643–653. [Google Scholar]
- 27.Crapon de Caprona MD. The effect of chemical stimuli from conspecifics on the behavior of Haplochromis burtoni (Cichlidae, Pisces) Experientia. 1974;30:1394–1395. doi: 10.1007/BF01919654. [DOI] [PubMed] [Google Scholar]
- 28.Cole T, Stacey NE. Olfactory responses to steroids in an African mouth-brooding cichlid, Haplochromis burtoni (Gunther). J Fish Biol. 2006;68:661–680. [Google Scholar]
- 29.Cole T, Stacey NE. Olfactory and endocrine response to steroids in an African cichlid fish, Haplochromis burtoni. Fish Physiol Biochem. 2003;28:265–266. [Google Scholar]
- 30.Robison RR, Fernald RD, Stacey NE. The olfactory system of a cichlid fish responds to steroidal compounds. J Fish Biol. 1998;53:226–229. [Google Scholar]
- 31.Almeida OG, Miranda A, Frade P, Hubbard PC, Barata EN, Canario AV. Urine as a social signal in the mozambique tilapia (Oreochromis mossambicus). Chem Senses. 2005;30(Suppl 1):i309–310. doi: 10.1093/chemse/bjh238. [DOI] [PubMed] [Google Scholar]
- 32.Harbott LK, Burmeister SS, White RB, Vagell M, Fernald RD. Androgen receptors in a cichlid fish, Astatotilapia burtoni: structure, localization, and expression levels. J Comp Neurol. 2007;504:57–73. doi: 10.1002/cne.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C-C, Fernald RD. Distributions of two gonadotropin-releasing hormone receptor types in a cichlid fish suggest functional specialization. The Journal of Comparative Neurology. 2006;495:314–323. doi: 10.1002/cne.20877. [DOI] [PubMed] [Google Scholar]
- 34.Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J Comp Neurol. 2005;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- 35.Forlano PM, Marchaterre M, Deitcher DL, Bass AH. Distribution of androgen receptor mRNA expression in vocal, auditory and neuroendocrine circuits in a teleost fish. J Comp Neurol. 2009 doi: 10.1002/cne.22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelinas D, Callard GV. Immunolocalization of aromatase- and androgen receptor-positive neurons in the goldfish brain. General and Comparative Endocrinology. 1997;106:155–168. doi: 10.1006/gcen.1997.6891. [DOI] [PubMed] [Google Scholar]
- 37.White SA, Nguyen T, Fernald RD. Social regulation of gonadotropin-releasing hormone. J Exp Biol. 2002;205:2567–2581. doi: 10.1242/jeb.205.17.2567. [DOI] [PubMed] [Google Scholar]
- 38.Maruska KP, Fernald RD. Steroid receptor expression in the fish inner ear varies with sex, social status, and reproductive state. BMC Neurosci. 2010 doi: 10.1186/1471-2202-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorensen PW, Hara TJ, Stacey NE, Dulka JG. Extreme olfactory specificity of male goldfish to the preovulatory steroidal pheromone 17(alpha), 20(beta)-dihydroxy-4-pregnen-3-one. Journal of Comparative Physiology A. 1990;166:373–383. [Google Scholar]
- 40.Oshima K, Gorbman A. Influence of thyroxine and steroid hormones on spontaneous and evoked unitary activity in the olfactory bulb of goldfish. Gen Comp Endocrinol. 1966;7:482–491. doi: 10.1016/0016-6480(66)90070-0. [DOI] [PubMed] [Google Scholar]
- 41.Oshima K, Gorbman A. Effect of estradiol on NaCl-evoked olfactory bulbar potentials in goldfish: dose-response relationships. Gen Comp Endocrinol. 1969;13:92–97. doi: 10.1016/0016-6480(69)90225-1. [DOI] [PubMed] [Google Scholar]
- 42.Burmeister SS, Kailasanath V, Fernald RD. Social dominance regulates androgen and estrogen receptor gene expression. Horm Behav. 2007;51:164–170. doi: 10.1016/j.yhbeh.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robison RR, White RB, Illing N, Troskie BE, Morley M, Millar RP, Fernald RD. Gonadotropin-releasing hormone receptor in the teleost Haplochromis burtoni: structure, location, and function. Endocrinology. 2001;142:1737–1743. doi: 10.1210/endo.142.5.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flanagan CA, Chen CC, Coetsee M, Mamputha S, Whitlock KE, Bredenkamp N, Grosenick L, Fernald RD, Illing N. Expression, structure, function, and evolution of gonadotropin-releasing hormone (GnRH) receptors GnRH-R1SHS and GnRH-R2PEY in the teleost, Astatotilapia burtoni. Endocrinology. 2007;148:5060–5071. doi: 10.1210/en.2006-1400. [DOI] [PubMed] [Google Scholar]
- 45.Au TM, Greenwood AK, Fernald RD. Differential social regulation of two pituitary gonadotropin-releasing hormone receptors. Behav Brain Res. 2006;170:342–346. doi: 10.1016/j.bbr.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 46.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12:1047–1064. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitter K, Kotoulas G, Magoulas A, Mulero V, Sepulcre P, Figueras A, Novoa B, Sarropoulou E. Evaluation of candidate reference genes for QPCR during ontogenesis and of immune-relevant tissues of European seabass (Dicentrarchus labrax). Comp Biochem Physiol B Biochem Mol Biol. 2009;153:340–347. doi: 10.1016/j.cbpb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR--a perspective. J Mol Endocrinol. 2005;34:597–601. doi: 10.1677/jme.1.01755. [DOI] [PubMed] [Google Scholar]
- 50.Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol. 2004;15:1044–1045. [Google Scholar]
- 51.Wirsig-Wiechmann CR. Function of gonadotropin-releasing hormone in olfaction. Keio J Med. 2001;50:81–85. doi: 10.2302/kjm.50.81. [DOI] [PubMed] [Google Scholar]
- 52.Yu KL, Peter RE. Alterations in gonadotropin-releasing hormone immunoactivities in discrete brain areas of male goldfish during spawning behavior. Brain Res. 1990;512:89–94. doi: 10.1016/0006-8993(90)91174-f. [DOI] [PubMed] [Google Scholar]
- 53.Soga T, Ogawa S, Millar RP, Sakuma Y, Parhar IS. Localization of the three GnRH types and GnRH receptors in the brain of a cichlid fish: Insights into their neuroendocrine and neuromodulator functions. J Comp Neurol. 2005;487:28–41. doi: 10.1002/cne.20519. [DOI] [PubMed] [Google Scholar]
- 54.Peter RE, Prasada Rao PD, Baby SM, Illing N, Millar RP. Differential brain distribution of gonadotropin-releasing hormone receptors in the goldfish. Gen Comp Endocrinol. 2003;132:399–408. doi: 10.1016/s0016-6480(03)00084-4. [DOI] [PubMed] [Google Scholar]
- 55.Albertson AJ, Navratil A, Mignot M, Dufourny L, Cherrington B, Skinner DC. Immunoreactive GnRH type I receptors in the mouse and sheep brain. J Chem Neuroanat. 2008;35:326–333. doi: 10.1016/j.jchemneu.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Onuma T, Higa M, Ando H, Ban M, Urano A. Elevation of gene expression for salmon gonadotropin-releasing hormone in discrete brain loci of prespawning chum salmon during upstream migration. J Neurobiol. 2005;63:126–145. doi: 10.1002/neu.20125. [DOI] [PubMed] [Google Scholar]
- 57.Biju KC, Singru PS, Schreibman MP, Subhedar N. Reproduction phase-related expression of GnRH-like immunoreactivity in the olfactory receptor neurons, their projections to the olfactory bulb and in the nervus terminalis in the female Indian major carp Cirrhinus mrigala (Ham.). Gen Comp Endocrinol. 2003;133:358–367. doi: 10.1016/s0016-6480(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, Oh DY, Bogerd J, Choi HS, Ahn RS, Seong JY, Kwon HB. Inhibitory activity of alternative splice variants of the bullfrog GnRH receptor-3 on wild-type receptor signaling. Endocrinology. 2001;142:4015–4025. doi: 10.1210/endo.142.9.8383. [DOI] [PubMed] [Google Scholar]
- 59.Hofmann HA, Benson ME, Fernald RD. Social status regulates growth rate: consequences for life-history strategies. Proc Natl Acad Sci U S A. 1999;96:14171–14176. doi: 10.1073/pnas.96.24.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan A, Dudley CA, Moss RL. Hormonal modulation of the responsiveness of midbrain central gray neurons to LH-RH. Neuroendocrinology. 1985;41:163–168. doi: 10.1159/000124170. [DOI] [PubMed] [Google Scholar]
- 61.Boland LM, Bean BP. Modulation of N-type calcium channels in bullfrog sympathetic neurons by luteinizing hormone-releasing hormone: kinetics and voltage dependence. . J Neurosci. 1993;13:516–533. doi: 10.1523/JNEUROSCI.13-02-00516.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belanger RM, Corkum LD, Zielinski BS. Differential behavioral responses by reproductive and nonreproductive male round gobies (Neogobius melanostomus) to the putative pheromone estrone. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:77–83. doi: 10.1016/j.cbpa.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Sorensen PW, Hara TJ, Stacey NE. Extreme olfactory sensitivity of mature and gonadally-regressed goldfish to a potent steroidal pheromone, 17α,20β–dihydroxy–4–pregnen–3–one. J Comp Physiol A. 1987;160:305–313. [Google Scholar]
- 64.Hamdani EH, Lastein S, Gregersen F, Doving KB. Seasonal variations in olfactory sensory neurons - fish sensitivity to sex pheromones explained? Chem Senses. 2008;33:119–123. doi: 10.1093/chemse/bjm072. [DOI] [PubMed] [Google Scholar]
- 65.Forlano PM, Schlinger BA, Bass AH. Brain aromatase: new lessons from non-mammalian model systems. Front Neuroendocrinol. 2006;27:247–274. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme an mRNA expression identify glia as source. Journal of Neuroscience. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pellegrini E, Menuet A, Lethimonier C, Adrio F, Gueguen MM, Tascon C, Anglade I, Pakdel F, Kah O. Relationships between aromatase and estrogen receptors in the brain of teleost fish. Gen Comp Endocrinol. 2005;142:60–66. doi: 10.1016/j.ygcen.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Belanger AJ, Arbuckle WJ, Corkum LD, Gammon DB, Li W, Scott AP, Zielinski BS. Behavioral and electrophysiological responses by reproductive female Neogobius melanostomus to odours released by conspecific males. J Fish Biol. 2004;65:933–946. [Google Scholar]
- 69.Frade P, Hubbard PC, Barata EN, Canario AV. Olfactory sensitivity of the Mosambique tilapia to conspecific odours. J Fish Biol. 2002;61:1239–1254. [Google Scholar]
- 70.Myrberg AA., Jr The role of chemical and visual stimuli in the preferential discrimination of young by the cichlid fish Cichlasoma nigrofasciatum (Gunther). Z Tierpsychol. 1975;37:274–297. doi: 10.1111/j.1439-0310.1975.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 71.Wong CC, Poon WH, Tsim TY, Wong EY, Leung MS. Gene expressions during the development and sexual differentiation of the olfactory bulb in rats. Brain Res Dev Brain Res. 2000;119:187–194. doi: 10.1016/s0165-3806(99)00173-x. [DOI] [PubMed] [Google Scholar]
- 72.Guo XZ, Su JD, Sun QW, Jiao BH. Expression of estrogen receptor (ER) -alpha and -beta transcripts in the neonatal and adult rat cerebral cortex, cerebellum, and olfactory bulb. Cell Res. 2001;11:321–324. doi: 10.1038/sj.cr.7290103. [DOI] [PubMed] [Google Scholar]
- 73.Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-beta mRNA in the female rat brain. Brain Res. 2007;1155:34–41. doi: 10.1016/j.brainres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 74.Mann PE, Babb JA. Neural steroid hormone receptor gene expression in pregnant rats. Brain Res Mol Brain Res. 2005;142:39–46. doi: 10.1016/j.molbrainres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Lastein S, Hamdani el H, Doving KB. Gender distinction in neural discrimination of sex pheromones in the olfactory bulb of crucian carp, Carassius carassius. Chem Senses. 2006;31:69–77. doi: 10.1093/chemse/bjj007. [DOI] [PubMed] [Google Scholar]
- 76.Xiao K, Kondo Y, Sakuma Y. Sex-specific effects of gonadal steroids on conspecific odor preference in the rat. Horm Behav. 2004;46:356–361. doi: 10.1016/j.yhbeh.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 77.Fernald RD, Shelton LC. The organization of the diencephalon and the pretectum in the cichlid fish, Haplochromis burtoni. J Comp Neurol. 1985;238:202–217. doi: 10.1002/cne.902380207. [DOI] [PubMed] [Google Scholar]
- 78.Burmeister SS, Munshi RG, Fernald RD. Cytoarchitecture of a cichlid fish telencephalon. Brain Behav Evol. 2009;74:110–120. doi: 10.1159/000235613. [DOI] [PMC free article] [PubMed] [Google Scholar]