Abstract
Filopodia sense the extracellular environment and direct movement in many cell types, including neurons. Recent reports suggest that the transmembrane form of the widely- expressed proteoglycan agrin (TM-agrin) regulates formation and stability of neuronal filopodia. In order to elucidate the mechanism by which TM-agrin regulates filopodia, we investigated the role of agrin’s glycosaminoglycan (GAG) chains in the induction of filopodia formation by TM-agrin over-expression in hippocampal neurons, and in the induction of filopodia-like processes in COS7 cells. Deletion of the GAG chains of TM-agrin sharply reduced formation of filopodia-like branched retraction fibers (BRFs) in COS7 cells, with deletion of the heparan sulfate GAG chains being most effective, and eliminated filopodia induction in hippocampal neurons. GAG chain deletion also reduced the activation of Cdc42 and Rac1 resulting from TM-agrin over-expression. Moreover, dominant negative Cdc42 and Rac1 inhibited BRF formation. Lastly, over-expression of TM-agrin increased the adhesiveness of COS7 cells and this increase was reduced by deletion of the GAG chains. Our results suggest that TM-agrin regulates actin-based protrusions in large part through interaction of its GAG chains with extracellular or transmembrane proteins, leading to the activation of Cdc42 and Rac1.
INTRODUCTION
Agrin is a proteoglycan with a 220-kDa protein core that is expressed in secreted and transmembrane forms [1, 2]. Agrin secreted by motor neurons is required for postsynaptic development at the neuromuscular junction [3] and secreted forms of agrin are found in the basement membranes of various tissues [4], [5] where its function is largely unknown. Agrin is also expressed by T-lymphocytes, where it plays a role in regulation of the immunological synapse [6, 7]. The transmembrane form of agrin (TM-agrin) is widely expressed in the developing central nervous system (CNS) and in regions of the adult CNS that show extensive plasticity [8]. The functions of agrin in the CNS are not fully understood, but there is evidence for roles in regulation of neurite outgrowth and branching [9], and synapse formation [10, 11]. We previously reported that over-expression of TM-agrin in myotubes caused extensive formation of filopodia [12]. Moreover, we found that TM-agrin over-expression caused extensive formation of filopodia in immature hippocampal neurons, while suppression of endogenous agrin expression by siRNA reduced the number of filopodia [13]. In a parallel study [14], it was shown that clustering of membrane agrin in various types of neurons resulted in the formation of filopodia-like microprocesses. Both of these studies support the hypothesis that TM-agrin regulates filopodia in developing neurons. Recently, we showed that suppression of endogenous agrin expression by an siRNA lentivirus reduced the number of filopodia on the dendrites of mature hippocampal neurons during the formation of synapses in culture, and reduced synapse formation to a similar extent [15]. In as much as dendritic filopodia have been implicated in synapse formation (reviewed in [16]) these results were consistent with a role of TM-agrin in promoting synaptogenesis through positive regulation of dendritic filopodia.
Several functional effects of agrin, including the above-mentioned regulation of filopodia, involve membrane-cytoskeletal interactions, for example, aggregation of acetylcholine receptors on skeletal muscle cells [17][18], modification of sarcolemmal structure [19], regulation of the immunological synapse [20][7], and enhanced activation of T-lymphocytes [21]. The actin cytoskeleton plays a crucial role in molecular organization of the plasma membrane and in the regulation of cell architecture, including the dynamics of cellular protrusions [22][23]. Organization of the actin cytoskeleton is typically regulated by activation of Rho-family small GTPases and their downstream effectors [24][25]. Whereas Rac1 and Cdc42 have been implicated in protrusion of lamellipodia and filopodia, respectively, RhoA has been implicated in stress fiber formation and in retraction of the trailing edge of motile cells [26]. Recently, we have provided evidence that activation of Cdc42 is important for filopodia formation induced by transfection with TM-agrin in SHSY-5Y neuronal cells [13].
The mechanism by which TM-agrin regulates filopodia is not well understood. It has been suggested that TM-agrin may act as a receptor or co-receptor to trigger an intracellular signaling cascade [27]. On the other hand, a proteolytic fragment of TM-agrin has been shown to act as a ligand to regulate dendritic filopodia during activity-dependent plasticity in mouse hippocampal CA1 neurons [28]. We previously showed [13] that the extracellular N-terminal portion of agrin is required for the induction of filopodia in neurons, while the extracellular C-terminal domains are not. The extracellular N-terminal domains of agrin contain both heparan sulfate (HS) and chondroitin sulfate (CS) glycosaminoglycan (GAG) chains attached at two separate sites [29, 30]. GAG chains have a broad range of ligands, which include protease inhibitors, growth factors, extracellular matrix components, enzymes and viral proteins [31]. The binding of heparan sulfate proteoglycans (HSPGs) to cell adhesion proteins is thought to augment their adhesiveness [32]. It also has been suggested that heparan sulfate binding to neural cell adhesion molecule (NCAM) is required for NCAM-mediated homophilic binding, thus serving as a mechanism for modulating the adhesiveness of NCAM during development [33]. Moreover, the role of agrin in enhancing the function of NCAM has been well documented [34]. There is evidence for multiple functions of the GAG chains of agrin. Agrin GAG chains are involved in the binding of other extracellular matrix components, amyloid peptides, growth factors and receptors [35, 36]. GAG chains of agrin shield agrin and amyloid peptides from proteolytic degradation and may be responsible for the slow turnover of amyloid aggregates [37]. Agrin’s GAG chains also contribute to inhibition of neurite outgrowth on over- expressing cells [38] but, on the other hand, the HS GAG chains are required for potentiation of the stimulation of neurite outgrowth by basic fibroblast growth factor [39].
In the present study we have investigated the roles of the GAG chains of agrin and the activation of Rho-family GTPases in the induction of filopodia in neurons and related actin-based protrusions in COS7 cells. We show that the GAG chains are important for the induction of these protrusions in both neurons and COS7 cells. Moreover, the GAG chains are involved in regulation of Rho-family GTPase activation, which in turn, influences TM-agrin induced formation of actin-based protrusions.
MATERIALS AND METHODS
Constructs
All agrin constructs were based on the rat agrin cDNA sequence [40] [41]. Wild type TM-agrin 4,19-GFP (TM-agrin) as well as the constructs with the N-terminal or C-terminal extracellular domains deleted were constructed as previously described [42]. All GAG-chain attachment site mutation constructs were made by polymerase chain reaction (PCR) using appropriate pairs of forward and reverse synthetic oligonucleotide primers (GenScript Corporation, Scotch Plains, NJ). Mutagenesis was performed using the Quick-Change site-directed mutagenesis kit (Stratagene, San Diego, CA). Primers: 5′-GAG TGT GGC gCA GGG GGC gCT GGT gCT GGG GAG GAC GAC G and 5′--C GTC GTC CTC CCC AGC ACC AGC GCC CCC TGC GCC ACA CTC were used to mutagenize Serine 566, 569 and 571 to Alanine in the heparan sulfate GAG chain binding sites (SI). For mutagenesis of the chondroitin sulfate GAG chain binding sites (SII), we used 5′-GCA ACA TCA ATC TTC AGT GAA gCC GGC gcC GCC AAT GGG and 5′-CCC ATT GGC GGC GCC GGC TTC ACT GAA GAT TGA TGT TGC to mutagenize Serine 953 and 955 to Alanine; 5′-GGC GCC GCC AAT GGG gcT GGC GAC GAG GAA CTG and 5′-CAG TTC CTC GTC GCC AGC CCC ATT GGC GGC GCC to mutagenize Serine 959 to Alanine; 5′-GGC GAC GAG GAA CTG gcT GGA GAT GAG GAG G and 5′-C CTC CTC ATC TCC AGC CAG TTC CTC GTC GCC to mutagenize Serine 965 to Alanine; 5′-GAT GAG GAG GCC gcT GGG GGC GGG gCT GGG GG and 5′-CC CCC AGC CCC GCC CCC AGC GGC CTC CTC ATC to mutagenize Serine 971 and 975 to Alanine. The double GAG chain binding site mutant (SI+II) was generated by sequential mutagenesis using the same sets of primers. In order to increase the expression level in hippocampal neurons, we cloned wild-type agrin GFP and mutants into a mammalian expression vector with a β-actin promoter (gift from Dr. A. Matus, Friedrich Miescher Institute, Switzerland).
DsRed-Agrin 4,19 (TM-agrin) was generated from the GFP(N0)-agrin4,19 construct [42] by cloning in-frame into the pDsRed-Monomer-C1 Vector (Clontech Laboratories, Inc. Mountain View, CA). AU5-tagged Cdc42 wild type and dominant-negative constructs as well as Rac1 wild type and dominant-negative were obtained from Dr. J. Donaldson (NHLBI, NIH, Bethesda, MD). AU5-tagged RhoA wild- type and dominant-negative constructs were provided by Dr. S. Gutkind (NIDCR, NIH, Bethesda, MD). GFP-tagged Myosin10 and GFP-Myo10-HMM constructs were obtained from Dr. R. Cheney (UNC, Chapel Hill, NC).
Cell and culture and transfection
COS7 and HEK293 cells were maintained in high glucose Dulbecco’s Modified Eagle medium (DMEM) with 10% fetal bovine serum, 50 U/ml penicillin and 50 μg/ml streptomycin under 5% CO2 at 37°C. PC12 cells were cultured for 24 h in RPMI 1640 medium supplemented with 10% horse serum plus 5% fetal bovine serum and GlutaMax (Invitrogen). This medium was then replaced for 24 h with differentiation medium, which contained RPMI 1640, 1% horse serum, 50 ng/ml of nerve growth factor and GlutaMax. Cells were transfected at 80–90% confluency with the various DNA plasmids using Fugene 6 (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions.
Rat hippocampal neuron cultures and transfection
Hippocampal neuron cultures were prepared from E19–20 rat embryos as previously described [13, 42]. For transfection, 4×106 hippocampal cells were resuspended in 100 μl of rat neuron Nucleofector® solution (Amaxa, Gaithersburg, MD) with 10 μg of TM-agrin, GFP control or its mutants, electroporated using the O-03 program, then plated on poly-L-lysine (Sigma, St. Louis, MO) coated coverslips in Minimum Essential Medium (MEM) (Invitrogen) supplemented with 10% horse serum, penicillin and streptomycin in a 100 mm Petri dish. After 4 h, the medium was replaced with Neurobasal medium plus B27 supplements (Invitrogen), penicillin and streptomycin. Cultures were maintained at 37°C with 10% CO2.
Western blot analysis
Transfected COS7 cells were washed twice with ice-cold Dulbecco’s phosphate buffered saline (DPBS), lysed in cold lysis buffer containing 1% Nonidet P40, 20 mM Tris (pH7.5), 150 mM NaCl and protease inhibitors, then sonicated 5 times for 5 seconds each, and incubated for 20 min on ice. The lysate was centrifuged at 15,000 × g for 20 min at 4°C, and the protein concentration of the supernatant was measured by the BCA assay (Pierce Biotechnology, Rockford, IL). Equal amounts of protein were separated by electrophoresis on 3–8% Tris-acetate SDS poly-acrylamide gels (Invitrogen, Carlsbad, California) and transferred to nitrocellulose membranes. Equal protein loading was confirmed by Western blots for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) on a separate gel. Blots were blocked with PBS containing 5% fat-free milk powder for 1 h at room temperature and incubated overnight at 4°C with rabbit anti-GFP (80 ng IgG/ml, A6455, Invitrogen) or rabbit anti-GAPDH (1:5000, NB300–322, Novus Biologicals, Littleton, CO), then with anti-rabbit Alexa Fluor 680 or 800 (1:10,000, Molecular Probes/Invitrogen). Immunoreactivity was detected with the Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, Nebraska). For detection of chondroitin sulfate GAG chains, blots were incubated with monoclonal anti-chondroitin sulfate antibody CS-56 (1:500, C8035, Sigma), then with anti-mouse IgM conjugated with horseradish peroxidase (HRP) (1:10,000, Abcam Inc, Cambridge, MA). Immunoreactivity was detected with the ECL Plus western blotting detection system (GE Healthcare Life Science, Piscataway, NJ).
Immunohistochemistry
For quantification of the morphology of COS7 cells transfected with TM-agrin-GFP and other GFP-tagged constructs, cells were fixed 24 h after transfection in 4% paraformaldehyde, 4% sucrose, and 0.1 M phosphate buffer. The fixative was pre-warmed to 37°C and was allowed to reach room temperature during the 30-min fixation. Cells were blocked and permeabilized with 10% normal goat serum, 1% bovine serum albumin (BSA) and 0.05% saponin in DPBS for 30 min, immunostained with rabbit anti-GFP (0.4 μg IgG/ml, A6455, Invitrogen) for 1 h at 37°C, washed with PBS containing 1% BSA and 0.05% saponin, and incubated with Alexa Fluor 488-conjugated goat anti-rabbit (1:800, Invitrogen) for 1 h at 37 °C. We used rabbit anti-DsRed (1:5000 Clontech Laboratories, Inc. Mountain View, CA), and Alexa Fluor 555-conjugated goat anti-rabbit (1:3000, Invitrogen) to immunostain TM-agrin-DsRed. AU5-tagged Cdc42, Rac1 and RhoA were labeled with monoclonal anti-AU5 antibody (1:1000, Covance Research Products, Berkeley, CA) and Alexa Fluor 555-conjugated goat anti-mouse (1:500, Invitrogen). Some cells were counterstained with rhodamine-phalloidin (1:500, Invitrogen) to visualize actin filaments. Cells were imaged with a Zeiss Axioplan 2 microscope (Carl Zeiss Inc, Oberkochen, Germany) or a Zeiss LSM510 confocal microscope (Carl Zeiss, Germany) using a 63X objective with a 1.4 numerical aperture.
To detect HS and CS in the COS7 cells over-expressing the respective GAG chain mutants, we immunostained with mouse monoclonal anti-heparan sulfate antibody 10E4 (1:100, 370255, Associates of Cape Cod, Inc. East Falmouth, MA), and anti-chondroitin sulfate antibody CS-56 (1:100, C8035, Sigma) followed by Cy3-conjugated anti-mouse (1:500, Jackson ImmunoResearch, Inc., West Grove, PA).
For immunolocalization of fascin and myosin II-B, COS7 cells were fixed using a cytoskeleton preserving fixative containing 60 mM PIPES, pH 7.0, 25 mM HEPES pH 7.0, 10 mM EGTA pH 8.0, 2 mM MgCl2, 0.12 M sucrose and 4% paraformaldehyde (Dr. T. Svitkina, unpublished). The fixative was pre-warmed to 37°C, added to the cells then allowed to cool to room temperature for 30 min. Cells were then permeabilized using 0.5% Triton X-100 for 5 min at room temperature, blocked for 30 min and immuno-stained with a monoclonal antibody to fascin (0.3 μg IgG/ml) (DakoCytomation, Inc. Carpinteria, CA) or with a rabbit antibody to myosin II-B (1:500) (Convance, Berkeley, CA). Primary antibodies were detected with anti-mouse or anti-rabbit Alexa Fluor 555 (1:200, Molecular Probes). All fixed cell preparations for fluorescence were mounted on glass slides with Vectashield mounting medium (Vector Laboratories, Burlingame, CA).
Enzyme-linked immunoassay of cell surface TM-agrin
Cell surface TM-agrin was assayed essentially as described [43]. COS7 cells in 12-well dishes (5×105 cells per well) were transfected with TM-agrin-GFP, one of the three GAG-chain mutant plasmids, or GFP. One day after transfection, the cells were washed with cold DPBS and blocked with DPBS containing 2% BSA, then incubated for 1 h with anti-GFP (1:5000) in blocking solution at room temperature. After four washes with DPBS, the cells were fixed with 3% paraformaldehyde in DPBS for 10 min and washed three times, then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:30,000; Vector Laboratories, Burlingame CA) for 1 h and washed 6 times. Cells were then incubated for 1 h with horseradish peroxidase substrate solution (Sigma) containing 3,3′,5,5′-tetramethylbenzidine and H2O2, and the optical density of the supernatant was measured at 655 nm.
Imaging and Measurement of Cell Protrusions
Digital images of fixed, immunostained cells were acquired and analysed with a CCD camera (C4742–95, Hamamatsu Photonics, Hamamatsu, Japan) and MetaMorph software (Universal Imaging Corp, Downingtown, PA). In order to quantify branched retraction fibers (BRFs), we traced the periphery of the cell, including the area occupied by BRFs (A) then traced the periphery of the cell excluding BRFs and filopodia (B) (see Figure 6G). The two areas were calculated and the latter area was subtracted from the former to give the area occupied by the BRFs. This area was divided by the length of periphery B to give the BRF index (see Figure 6F). For hippocampal neurons, all neurites were measured and the number of filopodia per 10 μm neurite length was calculated. Mean values for 60–80 cells from three experiments were calculated. Statistical significance of differences between mean values was determined by a two-tailed t-test.
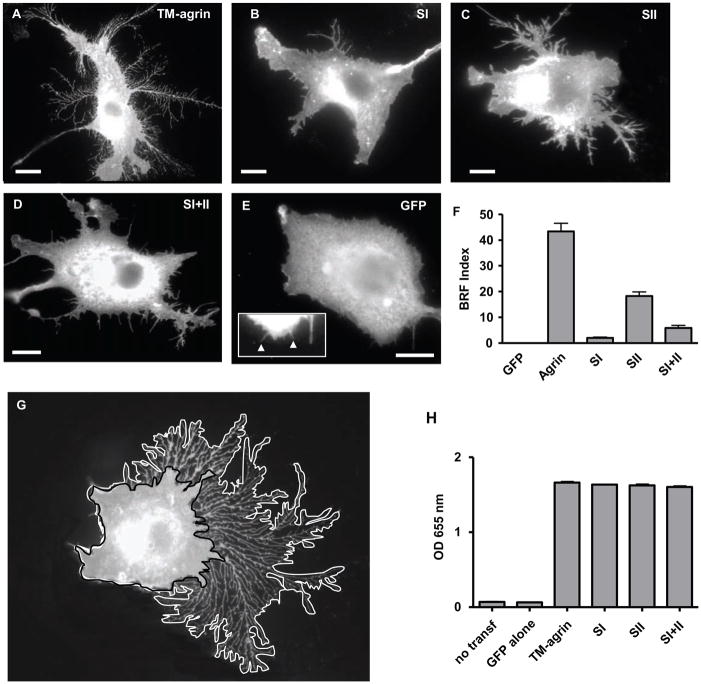
Figure 6.
Mutation of agrin GAG chain binding sites results in reduced induction of BRFs in COS7 cells. Cells were transfected with TM-agrin-GFP (A), SI (B), SII (C), SI+II (D) or GFP (E) and immunostained with GFP antibodies. The higher magnification inset in (E) shows that very small, fine processes could be detected in cells transfected with GFP, which is expressed in the cytoplasm. The BRF index was significantly reduced in cells transfected with all three mutants compared to TM-agrin-GFP (P<0.001), with the greatest reduction in the SI mutant (F). Graph shows mean BRF indices and standard errors from 3 experiments (n = 60 cells for each condition). Reference bar = 10 μm. (G) Example of a TM-agrin-GFP transfected cell showing delineation of the overall area including that occupied by BRFs (white line) and the inner area (black line) that was subtracted from the former in calculating the BRF index (see Methods). (H) Enzyme-linked immuno-assays show that the GAG-chain mutants of TM-agrin were expressed at similar levels to wild-type on the cell surface. Graph shows the mean optical density (OD) at 655 nm and standard error from 3 separate experiments.
To make time-lapse sequences, 2–3 DIV hippocampal neuron or COS7 cell cultures on 30-mm diameter coverslips were transferred to a microscope incubator (XL-3, Carl Zeiss Inc.) on a Zeiss Axiovert 200M microscope (Carl Zeiss, Inc.) and maintained at 37°C under humidified 10% CO2. Time-lapse phase contrast sequences were acquired with a CCD camera (C4742–95–12ER, Hamamatsu Photonics) using a 63X, 1.4 numerical aperture oil immersion objective. The sequences contained 120 frames taken at 30-sec intervals. All images were acquired and analyzed with MetaMorph software.
Electron Microscopy
For scanning electron microscopy (SEM), COS7 cells were plated as for light microscopy on coverslips etched with a numbered locator grid (Electron Microscopy Sciences, West Chester PA, Cat. #72265-50). Cultures were fixed 24 h after transfection in 4% paraformaldehyde, 4% sucrose, 0.1 M sodium phosphate buffer prewarmed to 37°C, for 30 min at room temperature. Fields containing transfected cells were recorded at low magnification by both fluorescence and phase contrast microscopy. Cultures were further fixed with 2% glutaraldehyde, postfixed in 1% osmium tetroxide, dehydrated in a graded ethanol series, critical point dried, mounted on SEM stubs and sputter-coated with approximately 5 nm of gold. After coating, the locator grid was visible with a stereomicroscope but not with the SEM, so drops of silver adhesive were applied for orientation and adjacent to grid locations of interest to allow relocation of transfected cells. SEM images of 27 to 36 transfected cells were recorded for each construct, using a Hitachi S-3400N SEM (Hitachi High Technologies Corp., Tokyo, Japan)
Platinum replicas of detergent-extracted COS7 cell cytoskeletons were prepared as described in detail previously [44]. Our procedure differed only in the equipment used for critical point drying and platinum evaporation. The extraction buffer contained 2 μM phalloidin, to stabilize actin filaments, and 2 μM taxol, to stabilize microtubules. The replicas were examined with a JEM 1200EX-II electron microscope (JEOL, USA, Inc., Peabody, MA). The negatives were digitally scanned and image contrast was reversed for publication.
Pull down Assays of Rho Family GTPases
COS7 cells were transfected with control vector, wild type or DN Cdc42, Rac1 or RhoA with TM-agrin or its mutants. After 24 h, cells were lysed in lysis/wash buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Igepal CA-630, 10 mM MgCl2, 1 mM EDTA, 2% glycerol). To measure the active GTP-bound form of Rho family GTPases in the cell lysates, we performed pull down assays (Upstate Cell Signaling Solutions, Lake Placid, NY) using recombinant GST- Pak-1 P21 binding domain (for Cdc42 and Rac1) and Rhotekin-RBD (for RhoA). Equal amounts of the cell lysate were incubated with 10 μg of GST-Pak-1 or 20 μg of GST-RBD agarose for 60 min at 4°C, then sedimented by centrifugation. Complexes were boiled in a Laemmli sample buffer and then separated on 12% SDS-polyacrylamide gels. The separated proteins were immunoblotted using AU5 antibody (1:1000, Covance Research Products, Berkeley, CA) and visualized by anti-mouse Alexa Fluor 680 secondary antibody (1:5000, Molecular Probes/Invitrogen). Immunoreactivity was detected with the Odyssey infrared imaging system (Li-Cor Biosciences.)
Cell Adhesion Assay
Adhesion of COS7 cells to a substratum was assayed as described [45]. Ninety-six-well plates were coated with 0.1 mg/ml poly-L-lysine overnight at 4°C, and then blocked with DPBS containing 2% BSA for 2 h. Transfected cells were harvested with enzyme-free cell dissociation buffer (Invitrogen), and then plated in DMEM with 1% BSA at a density of 60,000 cells/well. Cells were allowed to attach to the wells for 60 min at 37°C, then non-adherent cells were washed away. The remaining adherent cells were fixed with 5% glutaraldehyde for 30min and stained with 5 mg/ml crystal violet in 20% methanol for 30 min, washed with PBS, and extracted with 0.2% Triton X-100 for 1 h. Microplates were colorimetrically evaluated at 562 nm.
RESULTS
Over-expression of full-length TM-agrin-GFP induces formation of filopodia-like processes in cell lines
We previously reported that over-expression of transmembrane agrin (TM-agrin) induces filopodia formation in myotubes, hippocampal neurons and SHSY5Y neuroblastoma cells [12, 13]. In order to develop a convenient model system to study the molecular mechanisms of this induction, we examined the effect of TM-agrin over-expression in various cell lines. Cells were transiently transfected with wild type TM-agrin-GFP. After 24 h, cells were fixed and stained with anti-GFP antibody. Cells over-expressing TM-agrin-GFP presented striking morphological alterations. We found different kinds of filopodia-like processes protruding from the cell periphery in different cell lines (Figure 1A-F). In HEK293 cells (Figure 1A), we found both long filopodia and branched processes attached to the substrate, the latter of which we shall call branched retraction fibers (BRFs). In COS7 cells, we observed an abundance of BRFs but few typical filopodia (Figure 1B). In PC-12 neuronal cells, there were mainly filopodia of typical appearance (Figure 1C). Surprisingly, COS7 cells transfected with TM-agrin-GFP formed fewer filopodia on the unattached surface of the cell than did GFP-transfected controls (Figure 1G–H). Scanning electron microscopy showed that 43% of GFP-transfected cells had 10 or more filopodia on the unattached surface compared to 11% of TM-agrin-GFP transfected cells (p<0.01; Chi Square test). Moreover, 25% of cells transfected with a mutant TM-agrin-GFP lacking heparan sulfate GAG chains (resulting in reduced BRF formation), had 10 or more filopodia on the unattached surface. Thus it appears that the formation of attached BRFs is somehow opposed to the formation of unattached filopodia. Time-lapse sequences of TM-agrin-GFP transfected COS7 cells (Figure 1I) showed that BRFs were formed by retraction of lamellipodia, which were continually extended and retracted by COS7 cells during the normal course of cell migration.
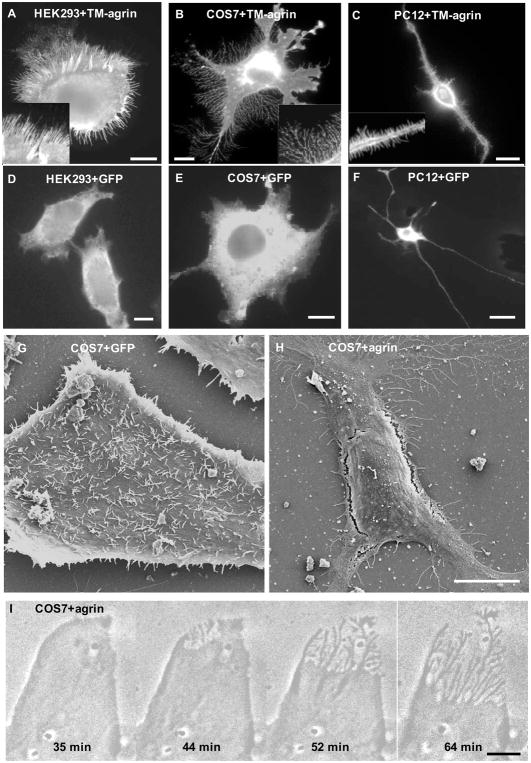
Figure 1.
Over-expression of TM-agrin induces filopodia-like processes in HEK293, COS7 and PC12 cells. Cells were fixed 24 h after transfection with TM-agrin-GFP (A, B and C) or GFP (D, E and F). TM-agrin-GFP was detected by immunofluorescence staining with anti-GFP. All three cell lines showed extensive formation of filopodia-like processes (A–C) compared to control cells transfected with the GFP vector (D–F) but the morphology of the induced processes varied. Insets in A-C show the filopodia-like processes at higher magnification. (G, H) Scanning electron micrographs of COS7 cells fixed 24 h after transfection with GFP (G) or TM-agrin GFP (H). Many of the GFP-transfected cells had many filopodia on the unattached surface, whereas the TM-agrin-GFP transfected cells displayed BRFs and filopodia attached to the substratum but few if any filopodia on the unattached surface. (I) Phase contrast images from a time-lapse sequence of a COS7 cell starting 18 h after transfection with TM-agrin-GFP, showing that BRFs form by retraction of lamellipodia. All reference bars = 10 μm.
Branched retraction fibers of COS7 cells resemble filopodia in protein localization and structure
Cells elaborate filopodia and retraction fibers during cycles of protrusion and withdrawal, and both kinds of processes contain bundles of long actin filaments [44]. To determine whether BRFs contain actin filaments (F-actin), we labeled TM-agrin-GFP transfected cells with rhodamine-phalloidin. F-actin was detected throughout the BRFs (Figure 2A–C). It has been reported that myosin 10 (myo10) localizes to the tips of filopodia and that over-expression of myo10 leads to an increase in the number and length of filopodia. A truncated myo10 lacking the tail domains also localized to the tips of filopodia but did not induce filopodia formation [46]. We found that both of the GFP-tagged myo10 constructs localized to the tips of many of the BRFs (Figure 2D–F, G–I). To further characterize BRFs, we investigated the distribution of two proteins known to be associated with typical actin rich structures. First, fascin, an actin filament bundling protein which is observed in filopodia and other leading edge structures containing actin, was concentrated in BRFs (Figure 2J–L). Second, myosin II-B, which is associated with actin-rich stress fibers but is absent from filopodia, was absent from BRFs (Figure 2M–O). Thus, all of the protein localization results are consistent with a filopodia-like nature of BRFs. Filamentous actin in BRFs occurred in two arrangements, as seen by electron microscopy of extracted COS7 cell cytoskeletons (Figure 3). Parallel to the long axis of the primary fibers and branches we found a core bundle of actin filaments. This further confirmed the filopodia-like nature of BRFs induced by transfection with TM-agrin. At the branching points of BRFs there was a network of actin filaments similar to the network found in the leading edge of lamellipodia [44]. This is consistent with our observation that BRFs are formed by retraction of lamellipodia.
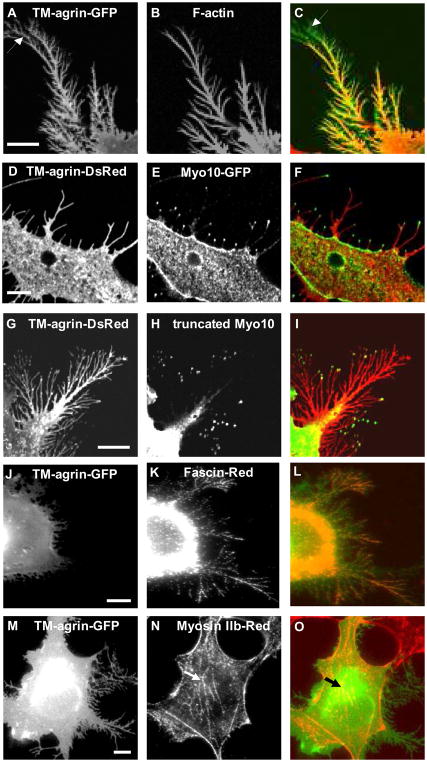
Figure 2.
BRFs induced by over-expression of TM-agrin-GFP in COS7 cells resemble filopodia in protein localization. (A) TM-agrin-GFP visualized by immunofluorescence staining of GFP (green). (B) F-actin labeled with rhodamine-phalloidin (red) is found throughout the BRFs. The distal GFP-positive areas lacking F-actin staining (arrows) represent TM-agrin-GFP shed onto the substratum before the tips of BRFs retracted. (D, G) TM-agrin-Ds-Red (red) visualized by immunofluorescence staining of Ds-Red. (E, H) Over-expressed myo10-GFP and truncated myo10-GFP, visualized by immunofluorescence staining with anti-GFP (green) were localized to the tips of BRFs. (J, M) Over-expressed TM-agrin visualized by immunofluorescence staining of GFP (green), (K) Fascin labeled with fascin antibody (red) is found in BRFs, (N) Myosin II-B labeled with myosin II-B antibody (red) is absent from BRFs, but present in stress fibers (arrows). (C, F, I, L, O) Overlay images. Reference bar = 10 μm.
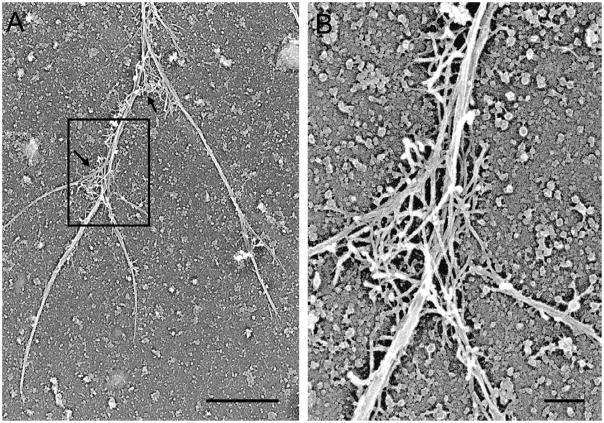
Figure 3.
BRF cytoskeletons consist of long actin filament cables, with actin filament networks primarily at branch points. Platinum replica electron micrograph of a detergent-extracted cytoskeleton shows a segment of a BRF cytoskeleton. Arrows in (A) indicate two branch points with actin filament networks resembling those found in lamellipodia. The marked area in (A) is shown at higher magnification in (B). Reference bars = 1μm and 0.2μm in (A) and (B), respectively.
In view of the robust response of COS7 cells to transfection with TM-agrin and the above observations indicating that BRFs are similar in structure and molecular composition to filopodia, we decided to use this system to study the mechanisms by which TM-agrin may regulate filopodia and other modifications of the actin cytoskeleton.
Deletion of agrin GAG chains reduces induction of branched retraction fibers in COS7 cells and filopodia in hippocampal neurons
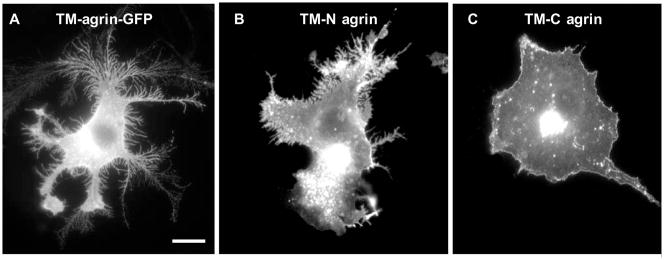
We previously showed that the extracellular N-terminal domains of TM-agrin are required for the induction of filopodia in cultured hippocampal neurons [13]. In the present study, transfection of COS7 cells with a TM-agrin deletion mutant (TM-C agrin) lacking only the N-terminal extracellular domains [42] did not result in formation of BRFs or filopodia. However, transfection with a deletion mutant (TM-N agrin) lacking only the C-terminal domains [42] resulted in the induction of BRFs and substrate-attached filopodia, although to a lesser extent than with wild-type TM-agrin (Figure 4).
Figure 4.
The N-terminal extracellular domains of TM-agrin are required to induce BRF formation in COS7 cells. Cells were transfected with GFP-tagged TM-agrin (A), TM-N agrin, lacking the C-terminal domains (B), or TM-C agrin, lacking the extracellular N-terminal domains (C) and immunostained with GFP antibodies. TM-N agrin induced BRFs to a lesser extent than TM-agrin, but TM-C agrin did not induce BRFs.
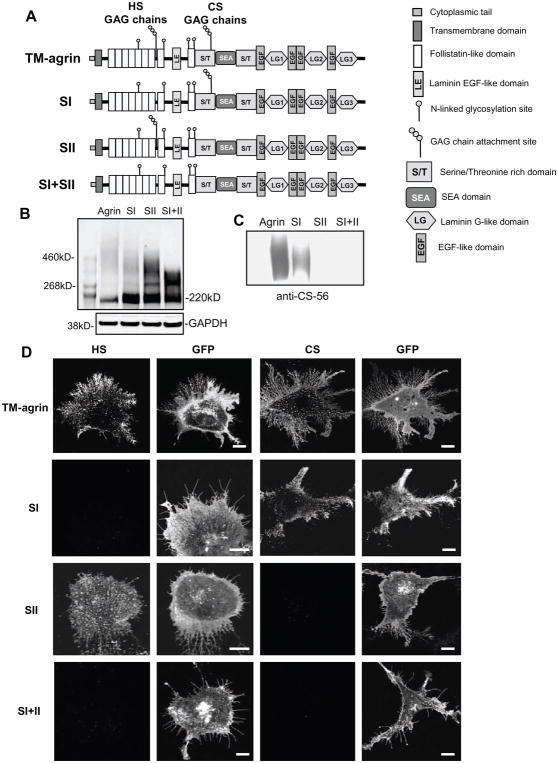
Glycosaminoglycan (GAG) chains are a prominent feature of the N-terminal domains of agrin and are known to interact with various extracellular and transmembrane signaling proteins [47]. Therefore we tested the role of these GAG chains in the induction of filopodia in hippocampal neurons and BRFs in COS7 cells. Agrin contains 20 serine/glycine (SG) sequences as potential GAG chain binding sites. We chose to mutate the two major SG clusters, which are thought to play the most important role in binding GAG chains [30]. The first SG cluster (SI), which binds heparan sulfate (HS) GAG chains, is located between the seventh and eighth follistatin-like domains. The second cluster (SII), which binds chondroitin sulfate (CS) GAG chains, is located in a serine/threonine-rich domain. The TM-agrin-GFP mutants we generated were named SI, SII and SI+II according to the clusters mutated (Figure 5A). In order to ascertain that the expected GAG chains of agrin were eliminated in these mutants, we first over-expressed wild type TM-agrin-GFP or each mutant in COS7 cells and then tested for the presence of the GAG chains 24 h after transfection by Western blot or immunostaining with antibodies to GFP, CS and HS. Western blots using a GFP antibody showed that the high molecular weight smear indicating glycosylation of agrin-GFP was reduced in molecular weight, breadth and density relative to the agrin-GFP core protein band in the GAG chain mutants (Figure 5B). The remaining, narrower smear in the SI+II mutant presumably represented glycosylation of agrin at other sites. Western blots with a CS antibody showed that the SII and SI+II mutants lacked CS GAG chains as expected (Figure 5C). Finally, immunostaining for CS was greatly reduced or absent in cells transfected with the SII and SI+II mutants while immunostaining for HS was greatly reduced or eliminated in the SI and SI+II mutants (Figure 5D). These results confirmed the deletion of HS, CS or both GAG chains in COS7 cells expressing the respective mutants.
Figure 5.
Mutation of the GAG-chain attachment sites results in elimination of the expected GAG chain types in over-expressed TM-agrin. (A) Domain structures of TM-agrin-GFP and the GAG chain mutants used in this study. GFP was attached to the C-terminal end of these constructs. (B) The high molecular weight smear of glycosylated agrin is reduced both in range of molecular weight and in density relative to the agrin core protein in GAG chain mutants. COS7 cells overexpressing TM-agrin-GFP or the mutants were lysed 24 h after transfection and the proteins were separated by SDS-PAGE. The blots were stained with GFP antibody. A separate blot for GAPDH shows that protein loading was equivalent in all lanes, however, total agrin-GFP expression levels in this experiment varied, as indicated by variation in total GFP immunoreactivity. The agrin-GFP core protein band ran at ~ 220 kD. The narrower, lower molecular weight smear in the SI+II mutant is presumed to represent agrin with non-GAG glycosylation. (C) Chondroitin sulfate GAG chains are absent in SII and SI+II mutants. Western blots of wild-type and mutant lysates were stained with chondroitin sulfate antibody CS-56. (D) HS and CS GAGs are lacking in COS7 cells over-expressing the respective GAG chain mutants. Cells transfected with TM-agrin-GFP or mutants (SI, SII and SI+II) were fixed after 24 h and immunostained with mouse monoclonal HS (left-hand column) or CS (3rd column from left) antibodies followed by Cy3-conjugated anti-mouse. TM-agrin and mutants were detected by GFP fluorescence (2nd and 4th column from left). Cells were imaged by confocal microscopy. Cells over-expressing wild-type TM-agrin-GFP showed strong staining for HS and CS but the SI, SII and SI+II mutants lacked immunoreactivity with HS, CS and both antibodies, respectively. Note that GFP fluorescence indicated similar levels of membrane expression for the wild-type and mutant forms of TM-agrin-GFP. Reference bar = 10 μm.
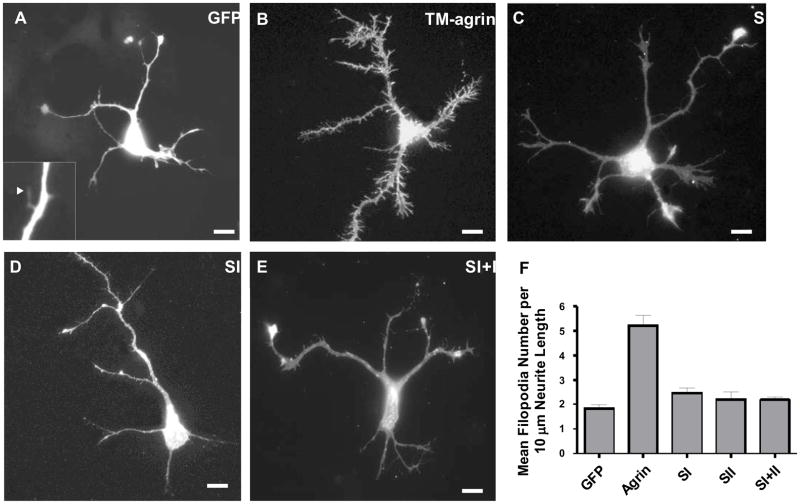
In order to determine whether agrin GAG chains are involved in the induction of BRFs or filopodia, COS7 cells or hippocampal neurons were transfected with GFP alone, TM-agrin-GFP, SI, SII or SI+II. An ELISA assay showed that wild-type TM-agrin-GFP and the GAG chain mutants were expressed at similar levels on the surface of COS7 cells (Figure 6H). Using a quantitative assay of the extent of BRFs formed, we found that elimination of the GAG chains strongly reduced BRF formation in transfected COS7 cells. Branched retraction fiber formation in cells transfected with SI, SII and SI+II was reduced by 95, 58 and 86%, respectively, compared to wild-type TM-agrin (Figure 6A–F). Mutation of the GAG chain initiation sites of agrin had a similar effect on transfected hippocampal neurons. Transfected neurons were fixed after 3 days in culture, and were analyzed for filopodia density (number of filopodia per 10 μm length) along all neurites. Filopodia density in neurons transfected with the SI, SII or SI+II mutants was reduced 50–60% (p<0.001) compared to that of cells transfected with wild-type TM-agrin-GFP (Figure 7). These results indicate that agrin’s GAG chains play an important role in the induction of BRFs in COS7 cells and filopodia in hippocampal neurons.
Figure 7.
Mutation of agrin GAG chains leads to decreased induction of filopodia in hippocampal neurons. Neurons were transfected with GFP (A), TM-agrin-GFP (B), SI (C), SII (D) or SI+II (E). After 3 days in culture, the neurons were fixed and visualized by GFP fluorescence. The higher magnification inset in (A) shows that filopodia could be detected in cells transfected with GFP, which is expressed in the cytoplasm. Filopodia density was increased in neurons that over-expressed TM-agrin-GFP compared to the GFP control, while filopodia density of neurons over-expressing SI, SII and SI+II was reduced 50–60% (p<0.001) compared to neurons transfected with TM-agrin-GFP (F). Graph shows mean filopodia densities and standard errors from 3 experiments (n = 60 neurons for each condition). Reference bar = 10 μm.
Effects of transfection with TM-agrin and GAG-chain mutants on activity of Cdc42, Rac1 and RhoA
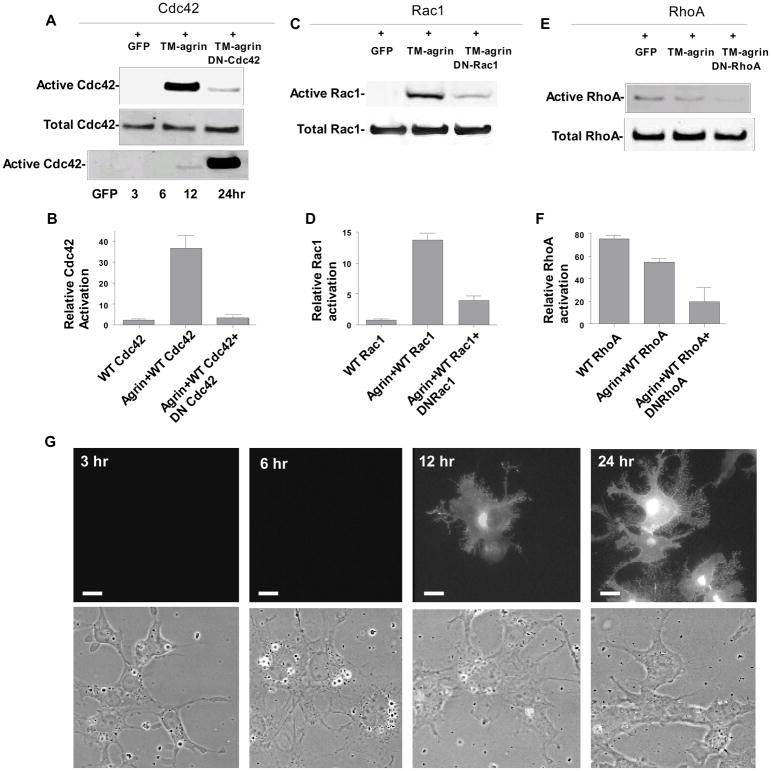
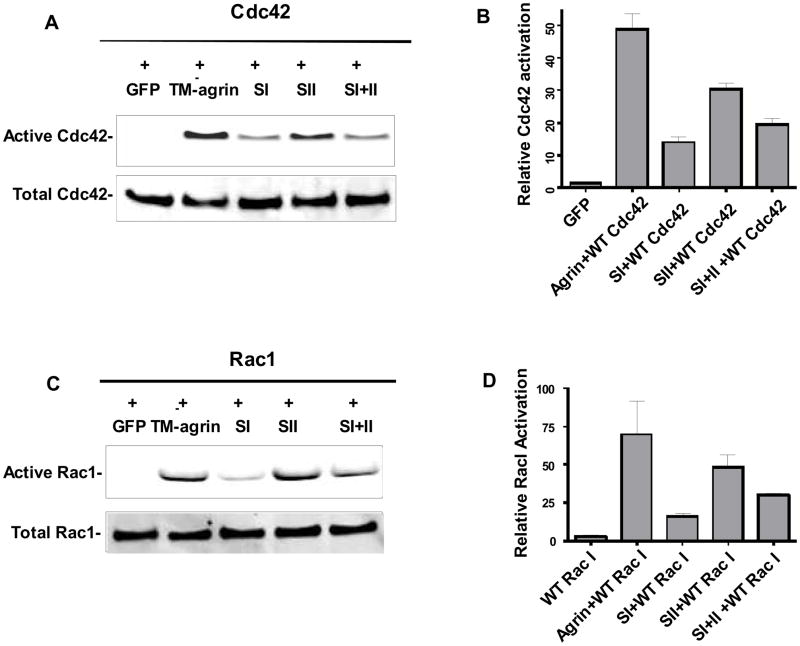
In order to investigate the possible roles of Cdc42, Rac1 and RhoA in the induction of BRFs in TM-agrin-transfected COS7 cells, we co-transfected the cells with TM-agrin-GFP or GFP alone, and wild type Rho-family GTPase constructs, then measured activation of over-expressed GTPase with a pull-down assay. In some experiments we additionally co-transfected with dominant negative Rho-family GTPase constructs. Analysis of the time course of Cdc42 activation revealed a low degree of activation at 12 h after TM-agrin-GFP transfection (Figure 8A), the time point when GFP fluorescence first became visible at the cell membrane and BRFs first appeared (Figure 8G). At 24 h after transfection, Cdc42 activation was increased 16-fold compared to the GFP control (Figure 8A and B). Similarly, Rac1 activation was increased 23-fold at 24 h (Figure 8C and D). The increases in activation of Cdc42 and Rac1 were inhibited by the additional co-expression of their respective dominant negative mutants. In contrast to the results with Cdc42 and Rac1, we found that TM-agrin-GFP reduced the activation of RhoA by about 26% (Figure 8E and F). All three GAG chain mutants evoked Rac1 and Cdc42 activation, but to a lower degree than wild type agrin (Figure 9). Cdc42 activation was reduced by 72, 38 and 60% in cells transfected with SI, SII and SI+II, respectively, compared to activation in cells transfected with wild-type TM-agrin-GFP, and similar reductions in activation were observed for Rac1 (Figure 9B and D). Interestingly, the reductions in activation of Cdc42 and Rac1 paralleled the reductions in BRF formation with the same mutants (Figure 6). The results suggest that Rac1 and Cdc42 are located downstream of agrin in the signaling pathway that leads to BRF formation.
Figure 8.
Transfection of COS7 cells with TM-agrin-GFP results in strong activation of Cdc42 and Rac1 but moderate inhibition of RhoA. Cells were co-transfected with wild- type Rho-family GTPases and GFP (control), or TM-agrin-GFP and the wild-type GTPases, or agrin, wild-type GTPases and dominant negative GTPases. After 24 h, cells were lysed and activation of Rac1, Cdc42 or RhoA was measured with a pull-down assay. Over-expressed Cdc42, Rac1 and RhoA were visualized by Western blotting using an anti-AU5 antibody (A–F). Each graph shows mean activation and standard error (n=3 experiments). (G) TM-agrin-GFP in the cell membrane and BRFs first appear 12 h after co-transfection with TM-agrin-GFP, at the same time that activation of Cdc42 is first detected (A).
Figure 9.
Cdc42 (A,B) and Rac1 (C, D) are activated at lower levels in cells transfected with agrin GAG chain mutants than with wild-type agrin. Cells were co-transfected with wild type Cdc42 or Rac1 and GFP, or TM-agrin-GFP, SI, SII or SI+II. After 24 h, cells were lysed and the activation of Rac1 and Cdc42 were measured by a pull-down assay. Total over-expressed Cdc42 or Rac1 protein was visualized by Western blotting using an AU5 antibody. Graphs show mean activation level and standard error (n = 3 experiments).
Effects of dominant-negative mutants of Cdc42, Rac1 and RhoA on TM-agrin-induced BRF formation
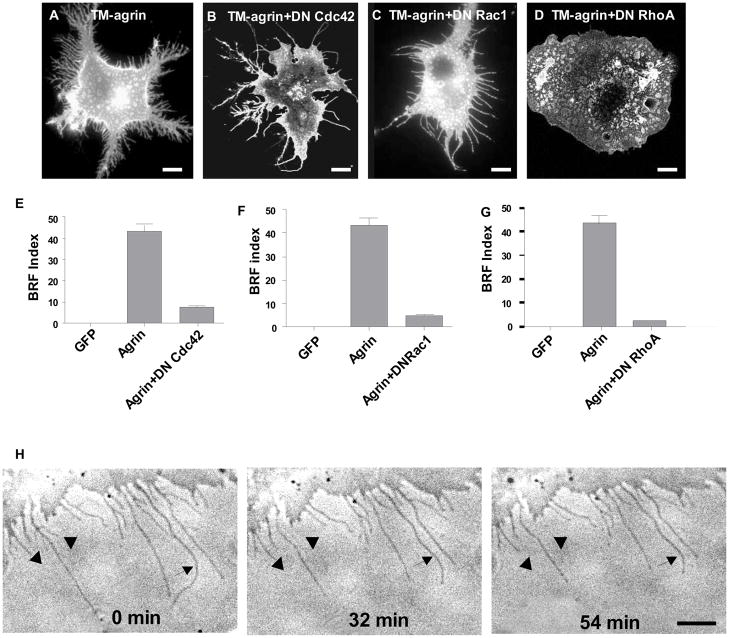
Whereas the actin cytoskeleton rearrangements of lamellipodia and membrane ruffles have been associated with Rac1 activation, filopodia and stress fibers have been associated with Cdc42 and RhoA activity, respectively [48] [49]. To determine whether activation of any of these Rho GTPases is required for the formation of BRFs in cells transfected with TM-agrin-GFP, we co-transfected COS7 cells with TM-agrin-GFP and either the dominant negative mutant of Cdc42 (N17Cdc42), Rac1 (N17Rac1), or RhoA (N19RhoA). In the presence of N17Cdc42, BRFs were virtually eliminated (Figure 10A, B), but a few long filopodia-like processes remained. Time-lapse observations starting 24 h after transfection showed that these processes could be formed during the retraction of BRFs. The few BRFs present lost branches and became single long filopodia-like processes, while long filopodia-like processes became shorter (Figure 10H). In contrast, co-transfection of TM-agrin-GFP with N17Rac1 abolished lamellipodia and membrane ruffles (Figure 10A, C) instead leaving long filopodia-like processes in greater numbers than with dominant negative Cdc42. These processes formed by the retraction of lamellipodia (not shown) but no BRFs were observed. Branched retraction fibers were totally abolished in COS7 cells co-transfected with N19RhoA and TM-agrin-GFP (Figure 10A and D) with only lamellipodia and ruffles present. The lamellipodia observed under these conditions were more extensive than in control cells, consistent with the requirement for RhoA activity in lamellipodium retraction. These results indicate that agrin-induced BRF formation requires the activity of Cdc42, Rac1 and RhoA.
Figure 10.
Effects of dominant negative mutants of Cdc42 (N17Cdc42), Rac1 (N17Rac1), or RhoA (N19RhoA) on BRF formation induced by TM-agrin. COS7 cells were co-transfected with TM-agrin-GFP and either GFP or a dominant negative mutant of Cdc42, Rac1 or RhoA. After culture for 24 h, the cells were immunostained with GFP and AU5 antibodies (to detect co-transfected cells) and BRFs were assayed (A–G). BRFs were sharply reduced or eliminated with all the dominant negative mutants compared to TM-agrin-GFP (p<0.001). Graphs show mean BRF index and standard error from 3 experiments (n = 60 cells for each condition). Reference bar = 10 μm. (H) Three frames from a 120 min time-lapse sequence starting 18 h after transfection of a cell with TM-agrin-GFP and DN Cdc42. Numbers indicate time in minutes after the sequence began. Arrowheads indicate branches that were lost during this part of the sequence. Arrows indicate long filopodia-like structures that became shorter. Reference bar = 10 μm.
HS GAG chains of agrin are important for the stability of filopodia induced by agrin in hippocampal neurons
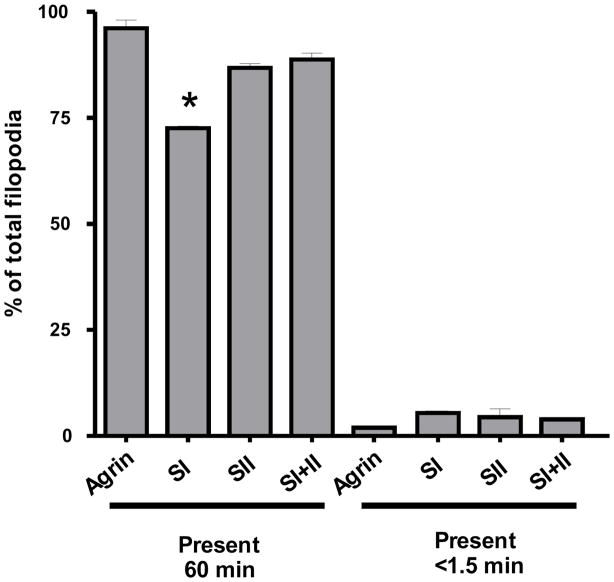
We previously reported that the filopodia of TM-agrin-transfected hippocampal neurons were more stable than controls transfected with GFP and that knocking down endogenous agrin expression decreased the stability of filopodia [13]. Here we examined the role of agrin’s HS and CS GAG chains in stabilization of filopodia in these neurons. We analyzed phase contrast time-lapse sequences of transfected neurons identified by GFP fluorescence in living 2–3 day old cultures, which were plated at the same cell density as those used to analyze filopodia density. For consistency, we only analyzed the stability of filopodia along axons, as we did in the previous study [13]. In agreement with our previous results, the mean lifetime of filopodia along axons was 43.0 min in GFP control transfected neurons compared to 56.5 min in TM-agrin-GFP transfected neurons (p<0.001). Filopodia in SI-transfected neurons had a mean lifetime of 49.6 min compared to 56.5 min, 53.6 min and 55.7 min in TM-agrin-, SII- and SI+II-transfected neurons (p<0.01, 0.05 and 0.01), respectively. Mean lifetimes of filopodia did not differ significantly between TM-agrin-, SII- and SI+II-transfected neurons. From 77.5 to 98% of filopodia in TM-agrin-GFP and mutant-transfected neurons were either very stable (present for 60 min or more) or very labile (present for ≤ 1.5 min). As shown in Figure 11, the percentage of very stable filopodia was significantly reduced in SI-transfected neurons compared to wild-type TM-agrin-GFP and the other GAG-chain mutants. These results indicate that the HS GAG chains play an important role in stabilizing filopodia induced by over-expressed TM-agrin in hippocampal neurons. The observation that filopodia stability of neurons transfected with the SI+II mutant was not significantly different from that of neurons transfected with wild-type TM-agrin-GFP suggests that the CS GAG chains may have an opposite effect to HS GAG chains with respect to filopodia stability.
Figure 11.
Filopodia of hippocampal neurons transfected with the SI mutant (lacking the HS GAG-chain attachment sites) are less stable than those of neurons transfected with wild-type TM-agrin. The lifetimes of filopodia along axons were assayed in one-hour-long phase contrast time-lapse sequences of transfected neurons (n = 8 neurons for each condition). The graph shows the percentage of very stable (lifetime at least 60 min) and very labile (lifetime ≤ 1.5 min) filopodia for wild-type TM-agrin and each of the GAG-chain mutants. The asterisk indicates a value significantly different from that of wild-type TM agrin.
Inhibition of cell adhesion by mutation of agrin GAG chains in COS7 cells
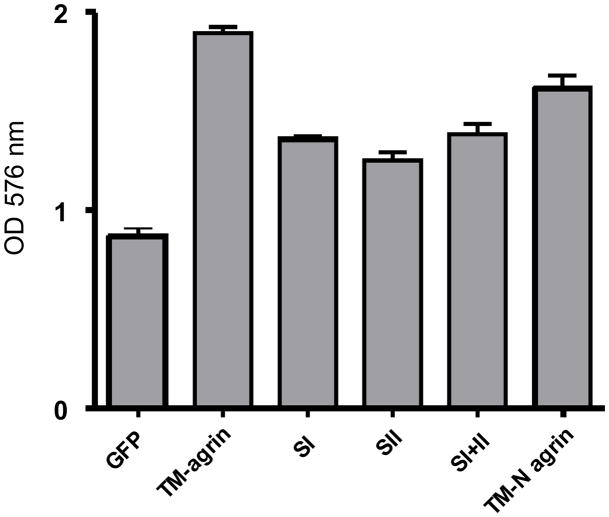
Since BRFs are attached to the culture substratum, and agrin is known to interact with extracellular matrix and other cell adhesion molecules (see Introduction and Discussion), we investigated the effects of TM-agrin-GFP transfection and the deletion of agrin’s GAG chains on COS7 cell adhesion to the culture substratum. In addition, since deletion of the C-terminal domains of TM-agrin, which contain domains that interact with integrins, reduced but did not eliminate BRF induction, we examined the effects of this deletion on adhesion as well. We found that transfection with wild-type TM-agrin-GFP enhanced cell adhesion about two fold compared to the control cells transfected with GFP (Figure 12). Adhesion of cells transfected with the three GAG chain mutants was reduced 27–30% (p<0.001) and deletion of the C-terminal domains (TM-N agrin) resulted in a reduction of 15% (p<0.01) compared to wild type TM-agrin (Figure 12). These results suggest that the GAG chains and C-terminal domains of TM-agrin all have roles in promoting cell adhesion to the substratum.
Figure 12.
Effects of GAG chain deletion and deletion of the C-terminal domains on adhesion of COS7 cells transfected with TM-agrin. Transfected COS7 cells were plated on 96-well dishes coated with poly-L-lysine for 1 h. Non-adherent cells were washed off and adherent cells were assayed by crystal violet staining. Over-expression of TM-agrin significantly increased COS7 cell adhesion to the substratum compared to the control cells (P<0.001). Cells transfected with GAG chain and N-terminal mutants of agrin had significantly less adhesion compared to TM-agrin transfected cells (P<0.01). Graphs show mean OD and standard error (n = 3 experiments).
DISCUSSION
Previous studies have indicated a role for TM-agrin in the regulation of neuronal filopodia [13][14], including the dendritic filopodia implicated in synapse formation [15]. To understand better how TM-agrin regulates actin-based cellular protrusions, we investigated the role of GAG chains of TM-agrin in the formation of filopodia-like processes in COS7 cells and filopodia in hippocampal neurons.
Branched retraction fibers (BRFs) and filopodia induced by TM-agrin
In this study, we observed the induction of various filopodia-like processes in cell lines with over-expression of TM-agrin, in agreement with our previous results in muscle cells. In COS7 cells, formation of branched retraction fibers (BRFs) predominated. Similar protrusions were observed in COS-1 cells overexpressing the proteoglycan syndecan-2 [50]. Recently, formation of filopodia-like processes identical in appearance to BRFs was described in TM-agrin transfected COS7 and HEK293 cells [51]. Filopodia, microspikes and retraction fibers contain actin filament bundles and are regarded as a continuum of structures distinguished by the protrusive activity of the surrounding lamellipodia [44]. In view of the filopodia-like nature of BRFs confirmed by analysis of ultrastructure and protein localization, the abundant formation of BRFs in TM-agrin-transfected COS7 cells and the high transfection efficiency of this cell line, we used this system to study mechanisms by which TM-agrin may regulate filopodia as well as other modifications of the actin cytoskeleton.
Role of agrin GAG chains in the induction of neuronal filopodia and BRFs
We previously showed that the N-terminal half of TM-agrin is required to increase filopodia density in hippocampal neurons [13]. Here, we found that this portion of TM-agrin was sufficient to induce BRF formation in COS7 cells, although less strongly than full-length TM-agrin. Mutation of the GAG-chain binding sites located in the N-terminal half of agrin greatly reduced BRF formation and eliminated induction of neuronal filopodia in transfected cells. This suggests an important role for the GAG chains in agrin-induced remodeling of the actin cytoskeleton. In COS7 cells, elimination of the HS GAG chains had a much more potent effect on BRF induction than elimination of the CS GAG chains. The HS GAG chains are involved in binding of NCAM, fibroblast growth factor-2 (FGF2) and other heparin-binding growth factors to agrin [36]. Moreover, HS GAG chains are important for the ability of agrin to potentiate FGF2-stimulated neurite outgrowth [9]. Heparan sulfate GAG chains are generally thought to play a key role in interactions with extracellular ligands [52]. However, consistent with our observation that deletion of the CS GAG chains sharply reduced BRF formation, a transmembrane CS proteoglycan (NG2) interacts with FGF2 and regulates retraction fiber formation in a GAG-chain dependent manner [53].
We previously showed that the effect of TM-agrin on filopodia density in hippocampal neurons was in part due to stabilization of filopodia [13]. Here, we found that elimination of the HS GAG chains reduced filopodia stability but elimination of the CS GAG chains did not, despite the strong effect on filopodia density. Interestingly, elimination of both HS and CS GAG chains did not reduce filopodia stability. This suggests that the CS GAG chains have an opposing effect on neuronal filopodia stability to that of HS GAG chains. Thus, the stimulatory effect of the CS GAG chains on filopodia density must be explained by a mechanism other than increasing the stability of the filopodia, possibly through their effect on adhesion, discussed below.
Rho-family GTPases Cdc42, Rac1 and RhoA in TM-agrin induced BRF formation
Rho-family small GTPases play essential roles in regulating the organization of the actin cytoskeleton in many cell types [54]. Activation of Cdc42 typically leads to the formation of microspikes and filopodia, whereas activation of Rac1 mediates the generation of membrane ruffles and lamellipodia [55]. Our present results with respect to the activation of Cdc42 and Rac1 and the effect of dominant negative forms are consistent with a role for activation of these GTPases downstream of TM-agrin in regulation of the actin cytoskeleton, although the intermediate signaling molecules remain to be determined. In contrast to the strong activation of Cdc42 and Rac1, RhoA activation was reduced by only 26%, consistent with the inactivation of RhoA coupled with Rac1 and Cdc42 activation during nerve growth factor-stimulated neurite outgrowth [56]. This moderate reduction of RhoA activation left enough activity to induce the retraction necessary for BRF formation. In contrast, co-transfection with DN RhoA strongly reduced RhoA activation, eliminated BRF formation, and resulted in more extensive presence of lamellipodia than in controls, since retraction of lamellipodia was strongly inhibited. Our results are consistent with a model in which Rac1 acts to promote lamellipodia extension and RhoA acts to cause their retraction but Cdc42 promotes formation and extension of the actin filament bundles that form the cores of the BRFs, preventing their retraction. It is also noteworthy that deletion of the HS GAG chains reduced activation of Cdc42 and Rac1 more than did deletion of CS GAG chains. This relative effect was paralleled by the effect on BRF formation. This further supports the idea that specific GAG chain interactions with other proteins are key to modulation of the actin cytoskeleton by TM-agrin.
In a recent article (Porten et al., 2009) [51], it was reported that N-terminal follistatin domains, particularly the 7th follistatin domain of TM-agrin, were necessary and sufficient to induce the formation of filopodia-like processes in HEK293 cells and cultured embryonic chick tectal neurons. When the 3 serines that serve as HSPG GAG chain attachment sites were mutated, the N-terminal construct lost 70% of its process-inducing activity but when the domain containing those attachment sites as well as the 8th follistatin domain was deleted, full activity was restored. The authors interpreted these results as possibly indicating that the HSPG GAG chains serve to prevent an interaction between the 8th and 7th follistatin domain that inhibits the process-inducing activity of the 7th follistatin domain. In this respect, it is interesting that in the present study, the elimination of the HSPG GAG chains, which are inserted between the 7th and 8th follistatin domains, more strongly reduced BRF formation than elimination of the CS GAG chains. However, the following differences between the assays and constructs used by Porten et al [51] and in the present study, leave room for interpretation as to the specific roles of the GAG chains and follistatin domains. First, the present study used an assay in which the portion of the total cell area occupied by BRFs was assayed. Although the filopodia-like processes assayed by Porten et al appear virtually identical to the BRFs that we observed in COS7 cells, these investigators assayed the percentage of cells with 10 or more individual processes or at least 2 very long processes. We would expect the assay used in the present study to be more sensitive to graded changes in the extent of process formation than the assay used by Porten et al.. Consequently, the two assays might yield different interpretations of the results. Second, the present study involved mutations of the serines serving as GAG-chain attachment sites in full-length TM-agrin and the results clearly showed their importance for BRF formation and Rho-family GTPase activation. In contrast, Porten et al. primarily used constructs containing only the transmembrane and follistatin domains, with or without the domains containing the GAG-chain attachment sites. Although those results show the sufficiency of specific follistatin domains in these truncated constructs to induce process formation, the effect of deleting individual follistatin domains from full-length TM-agrin remains unknown. Future experiments may help to elucidate the roles, relative importance and possible interactions between the follistatin domains and the GAG chains.
The identity of the proteins or receptors that interact with the GAG chains of TM-agrin to positively regulate filopodia and similar cell processes remains unknown. However, it has recently been reported that the formation of filopodia-like processes on cultured neurons induced by the clustering of TM-agrin depends on the clustering of TM-agrin in lipid rafts and the subsequent activation of Fyn, leading to downstream activation of MAP kinase [27]. On the basis of these results, Ramseger et al. proposed that TM-agrin acts as a receptor or co-receptor to initiate this signaling cascade. The results of the present study suggest the possibility that the GAG chains of TM-agrin are important for its activity as a co-receptor.
Adhesive effect of agrin GAG chains and the role of cell adhesion in BRF and filopodia formation induced by TM-agrin transfection
Our observation that BRFs are formed by the partial retraction of lamellae attached to the substrate suggested that increased cell-substrate adhesion could be involved in BRF formation. Therefore we investigated the adhesive effects of the GAG chains, whose strong negative charges might promote adhesion to coverslips coated with positively-charged poly-L-lysine. Over-expression of TM-agrin in COS7 cells increased cell-substrate adhesion more than two-fold and this effect was markedly reduced by deletion of the GAG chains. In contrast to the greater effect of HS GAG-chain deletion on BRF formation and the activation of Cdc42 and Rac1, all three GAG-chain mutants showed similar reductions in adhesion. These results suggest that HS-specific mechanisms are at least as important as the charge of GAG chains for BRF formation.
Filopodia density in neurons was reduced to control levels with all three GAG-chain mutants, but neuronal filopodia stability was reduced only by deletion of the HS GAG chains. Thus, the adhesive effect of the GAG chains alone does not fully account for the ability of TM-agrin to regulate filopodia. It is possible that the interaction of the HS GAG chains of agrin with NCAM [29] is involved in the increase of filopodia density induced by TM-agrin transfection or that there is an adhesive interaction between NCAM attached to the cell membrane and the TM-agrin shed onto the substrate [42]. Laminin-G homology domains in the C-terminal half of agrin also mediate cell-substrate adhesion, through their interaction with integrins [57]. Consistent with this, we found that COS7 cells transfected with the TM-agrin mutant lacking the C-terminal half of agrin (TM- N agrin) were less adhesive than wild-type TM-agrin transfected cells. Moreover, BRF formation was reduced in COS7 cells transfected with TM-N agrin, consistent with a possible role of integrin-mediated adhesion. However, TM-C agrin did not induce BRFs in COS7 cells, and filopodia density was not reduced in neurons transfected with TM-agrin-N compared to full-length TM-agrin [13]. Therefore the role of an integrin-dependent mechanism, if any, in neuronal filopodia regulation and BRF formation would appear to be secondary to that of the GAG chains of TM-agrin.
In summary, we have established a role for the GAG chains of TM-agrin in modulation of the actin cytoskeleton in a manner dependent on regulation of Rho-family GTPase activity. Future studies should identify the protein-GAG chain interactions and additional signal transduction events, through which agrin modulates the actin cytoskeleton.
Acknowledgments
We thank Drs. Hiro Katagiri and Herbert Geller and Mr. Tom McCann for suggestions and help with the analysis of cell surface GAG chains. We are also grateful to Lei Jiang, Allison Bailey and Faith Kung for technical assistance and to Dr. Richard Cheney for generously providing myosin 10 constructs.
Abbreviations used
- GAG
glycosaminoglycan
- HSPG
heparan sulfate proteoglycan
- HS
heparan sulfate
- CS
chondroitin sulfate
- BRF
branched retraction fiber
- NCAM
neural cell adhesion molecule
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burgess RW, Skarnes WC, Sanes JR. Agrin isoforms with distinct amino termini: differential expression, localization, and function. J Cell Biol. 2000;151:41–52. doi: 10.1083/jcb.151.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neumann FR, Bittcher G, Annies M, Schumacher B, Kroger S, Ruegg MA. An alternative amino-terminus expressed in the central nervous system converts agrin to a type II transmembrane protein. Mol Cell Neurosci. 2001;17:208–25. doi: 10.1006/mcne.2000.0932. [DOI] [PubMed] [Google Scholar]
- 3.Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey EW, Siebenlist RE, Wallskog PA, Walters LM, Bolender DL, Yorde DE. Basal lamina components are concentrated in premuscle masses and at early acetylcholine receptor clusters in chick embryo hindlimb muscles. Dev Biol. 1988;130:471–486. doi: 10.1016/0012-1606(88)90343-0. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey EW, Nitkin RM, Wallace BG, Rubin LL, McMahan UJ. Components of Torpedo electric organ and muscle that cause aggregation of acetylcholine receptors on cultured muscle cells. J Cell Biol. 1984;99:615–627. doi: 10.1083/jcb.99.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bezakova G, Ruegg MA. New insights into the roles of agrin. Nat Rev Mol Cell Biol. 2003;4:295–308. doi: 10.1038/nrm1074. [DOI] [PubMed] [Google Scholar]
- 7.Khan AA, Bose C, Yam LS, Soloski MJ, Rupp F. Physiological regulation of the immunological synapse by agrin. Science. 2001;292:1681–6. doi: 10.1126/science.1056594. [DOI] [PubMed] [Google Scholar]
- 8.Megeath LJ, Kirber MT, Hopf C, Hoch W, Fallon JR. Calcium-dependent maintenance of agrin-induced postsynaptic specializations. Neuroscience. 2003;122:659–68. doi: 10.1016/s0306-4522(03)00602-x. [DOI] [PubMed] [Google Scholar]
- 9.Kim MJ, Cotman SL, Halfter W, Cole GJ. The heparan sulfate proteoglycan agrin modulates neurite outgrowth mediated by FGF-2. J Neurobiol. 2003;55:261–77. doi: 10.1002/neu.10213. [DOI] [PubMed] [Google Scholar]
- 10.Gingras J, Rassadi S, Cooper E, Ferns M. Agrin plays an organizing role in the formation of sympathetic synapses. J Cell Biol. 2002;158:1109–18. doi: 10.1083/jcb.200203012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ksiazek I, Burkhardt C, Lin S, Seddik R, Maj M, Bezakova G, Jucker M, Arber S, Caroni P, Sanes JR, Bettler B, Ruegg MA. Synapse loss in cortex of agrin-deficient mice after genetic rescue of perinatal death. J Neurosci. 2007;27:7183–95. doi: 10.1523/JNEUROSCI.1609-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhm CS, Neuhuber B, Lowe B, Crocker V, Daniels MP. Synapse-forming axons and recombinant agrin induce microprocess formation on myotubes. J Neurosci. 2001;21:9678–89. doi: 10.1523/JNEUROSCI.21-24-09678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCroskery S, Chaudhry A, Lin L, Daniels MP. Transmembrane agrin regulates filopodia in rat hippocampal neurons in culture. Mol Cell Neurosci. 2006;33:15–28. doi: 10.1016/j.mcn.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Annies M, Bittcher G, Ramseger R, Loschinger J, Woll S, Porten E, Abraham C, Ruegg MA, Kroger S. Clustering transmembrane-agrin induces filopodia-like processes on axons and dendrites. Mol Cell Neurosci. 2006;31:515–24. doi: 10.1016/j.mcn.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 15.McCroskery S, Bailey A, Lin L, Daniels MP. Transmembrane agrin regulates dendritic filopodia and synapse formation in mature hippocampal neuron cultures. Neuroscience. 2009;163:168–79. doi: 10.1016/j.neuroscience.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- 17.Dai Z, Luo X, Xie H, Peng HB. The actin-driven movement and formation of acetylcholine receptor clusters. J Cell Biol. 2000;150:1321–34. doi: 10.1083/jcb.150.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weston C, Yee B, Hod E, Prives J. Agrin-induced acetylcholine receptor clustering is mediated by the small guanosine triphosphatases Rac and Cdc42. J Cell Biol. 2000;150:205–12. doi: 10.1083/jcb.150.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezakova G, Lomo T. Muscle activity and muscle agrin regulate the organization of cytoskeletal proteins and attached acetylcholine receptor (AchR) aggregates in skeletal muscle fibers. J Cell Biol. 2001;153:1453–63. doi: 10.1083/jcb.153.7.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moransard M, Borges LS, Willmann R, Marangi PA, Brenner HR, Ferns MJ, Fuhrer C. Agrin regulates rapsyn interaction with surface acetylcholine receptors, and this underlies cytoskeletal anchoring and clustering. J Biol Chem. 2003;278:7350–9. doi: 10.1074/jbc.M210865200. [DOI] [PubMed] [Google Scholar]
- 21.Jury EC, Flores-Borja F, Kabouridis PS. Lipid rafts in T cell signalling and disease. Semin Cell Dev Biol. 2007;18:608–15. doi: 10.1016/j.semcdb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–9. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 23.Yamada A, Yoshio M, Nakayama H. Bi-directional movement of actin filaments along long bipolar tracks of oriented rabbit skeletal muscle myosin molecules. FEBS Lett. 1997;409:380–4. doi: 10.1016/s0014-5793(97)00558-9. [DOI] [PubMed] [Google Scholar]
- 24.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–5. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 25.Ohta Y, Suzuki N, Nakamura S, Hartwig JH, Stossel TP. The small GTPase RalA targets filamin to induce filopodia. Proc Natl Acad Sci U S A. 1999;96:2122–8. doi: 10.1073/pnas.96.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–86. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 27.Ramseger R, White R, Kroger S. Transmembrane form agrin-induced process formation requires lipid rafts and the activation of Fyn and MAPK. J Biol Chem. 2009;284:7697–705. doi: 10.1074/jbc.M806719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto-Miyai K, Sokolowska E, Zurlinden A, Gee CE, Luscher D, Hettwer S, Wolfel J, Ladner AP, Ster J, Gerber U, Rulicke T, Kunz B, Sonderegger P. Coincident pre- and postsynaptic activation induces dendritic filopodia via neurotrypsin-dependent agrin cleavage. Cell. 2009;136:1161–71. doi: 10.1016/j.cell.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Tsen G, Halfter W, Kröger S, Cole GJ. Agrin is a heparan sulfate proteoglycan. J Biol Chem. 1995;270:3392–3399. doi: 10.1074/jbc.270.7.3392. [DOI] [PubMed] [Google Scholar]
- 30.Winzen U, Cole GJ, Halfter W. Agrin is a chimeric proteoglycan with the attachment sites for heparan sulfate/chondroitin sulfate located in two multiple serine-glycine clusters. J Biol Chem. 2003;278:30106–14. doi: 10.1074/jbc.M212676200. [DOI] [PubMed] [Google Scholar]
- 31.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–77. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 32.Moon JJ, Matsumoto M, Patel S, Lee L, Guan JL, Li S. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J Cell Physiol. 2005;203:166–76. doi: 10.1002/jcp.20220. [DOI] [PubMed] [Google Scholar]
- 33.Kadmon G, Kowitz A, Altevogt P, Schachner M. The neural cell adhesion molecule N-CAM enhances L1-dependent cell-cell interactions. J Cell Biol. 1990;110:193–208. doi: 10.1083/jcb.110.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole GJ, Halfter W. Agrin: an extracellular matrix heparan sulfate proteoglycan involved in cell interactions and synaptogenesis. Perspect Dev Neurobiol. 1996;3:359–71. [PubMed] [Google Scholar]
- 35.Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol Cell Biol. 2002;22:1881–92. doi: 10.1128/MCB.22.6.1881-1892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cotman SL, Halfter W, Cole GJ. Identification of extracellular matrix ligands for the heparan sulfate proteoglycan agrin. Exp Cell Res. 1999;249:54–64. doi: 10.1006/excr.1999.4463. [DOI] [PubMed] [Google Scholar]
- 37.Cotman SL, Halfter W, Cole GJ. Agrin binds to beta-amyloid (Abeta), accelerates abeta fibril formation, and is localized to Abeta deposits in Alzheimer’s disease brain. Mol Cell Neurosci. 2000;15:183–98. doi: 10.1006/mcne.1999.0816. [DOI] [PubMed] [Google Scholar]
- 38.Bixby JL, Baerwald-De la Torre K, Wang C, Rathjen FG, Ruegg MA. A neuronal inhibitory domain in the N-terminal half of agrin. J Neurobiol. 2002;50:164–79. doi: 10.1002/neu.10025. [DOI] [PubMed] [Google Scholar]
- 39.Kim JH, Lee HK, Takamiya K, Huganir RL. The role of synaptic GTPase-activating protein in neuronal development and synaptic plasticity. J Neurosci. 2003;23:1119–24. doi: 10.1523/JNEUROSCI.23-04-01119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rupp F, Payan DG, Magill-Solc C, Cowan DM, Scheller RH. Structure and expression of a rat agrin. Neuron. 1991;6:811–823. doi: 10.1016/0896-6273(91)90177-2. [DOI] [PubMed] [Google Scholar]
- 41.Ferns M, Hoch W, Campanelli JT, Rupp F, Hall ZW, Scheller RH. RNA splicing regulates agrin-mediated acetylcholine receptor clustering activity on cultured myotubes. Neuron. 1992;8:1079–1086. doi: 10.1016/0896-6273(92)90129-2. [DOI] [PubMed] [Google Scholar]
- 42.Neuhuber B, Daniels MP. Targeting of recombinant agrin to axonal growth cones. Mol Cell Neurosci. 2003;24:1180–96. doi: 10.1016/j.mcn.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Lin L, Jeanclos EM, Treuil M, Braunewell KH, Gundelfinger ED, Anand R. The calcium sensor protein visinin-like protein-1 modulates the surface expression and agonist sensitivity of the alpha 4beta 2 nicotinic acetylcholine receptor. J Biol Chem. 2002;277:41872–8. doi: 10.1074/jbc.M206857200. [DOI] [PubMed] [Google Scholar]
- 44.Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003;160:409–21. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tillet E, Gential B, Garrone R, Stallcup WB. NG2 proteoglycan mediates beta1 integrin-independent cell adhesion and spreading on collagen VI. J Cell Biochem. 2002;86:726–36. doi: 10.1002/jcb.10268. [DOI] [PubMed] [Google Scholar]
- 46.Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat Cell Biol. 2002;4:246–50. doi: 10.1038/ncb762. [DOI] [PubMed] [Google Scholar]
- 47.Gallagher BC. Branching morphogenesis in the avian lung: electron microscopic studies using cationic dyes. J Embryol Exp Morphol. 1986;94:189–205. [PubMed] [Google Scholar]
- 48.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 50.Granes F, Garcia R, Casaroli-Marano RP, Castel S, Rocamora N, Reina M, Urena JM, Vilaro S. Syndecan-2 induces filopodia by active cdc42Hs. Exp Cell Res. 1999;248:439–456. doi: 10.1006/excr.1999.4437. [DOI] [PubMed] [Google Scholar]
- 51.Porten E, Seliger B, Schneider VA, Woll S, Stangel D, Ramseger R, Kroger S. The process-inducing activity of transmembrane agrin requires follistatin-like domains. J Biol Chem. 2009;285:3114–25. doi: 10.1074/jbc.M109.039420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hileman RE, Fromm JR, Weiler JM, Linhardt RJ. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays. 1998;20:156–67. doi: 10.1002/(SICI)1521-1878(199802)20:2<156::AID-BIES8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 53.Stallcup WB, Dahlin-Huppe K. Chondroitin sulfate and cytoplasmic domain-dependent membrane targeting of the NG2 proteoglycan promotes retraction fiber formation and cell polarization. J Cell Sci. 2001;114:2315–25. doi: 10.1242/jcs.114.12.2315. [DOI] [PubMed] [Google Scholar]
- 54.Hall A. G proteins and small GTPases: distant relatives keep in touch. Science. 1998;280:2074–5. doi: 10.1126/science.280.5372.2074. [DOI] [PubMed] [Google Scholar]
- 55.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 56.Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 57.Martin PT, Sanes JR. Integrins mediate adhesion to agrin and modulate agrin signaling. Development. 1997;124:3909–3917. doi: 10.1242/dev.124.19.3909. [DOI] [PubMed] [Google Scholar]