Abstract
OBJECTIVE
To pilot a randomized controlled trial of OROS-Methylphenidate (MPH) to treat ADHD plus epilepsy.
METHOD
Thirty-three patients, 6–18 years, taking antiepileptic drugs and with a last seizure 1–60 months prior were assigned to a maximum daily dose of 18, 36, or 54mg of OROS-MPH in a double-blind placebo-controlled crossover trial.
RESULTS
There were no serious adverse events and no carry over effects in the crossover trial. OROS-MPH reduced ADHD symptoms more than placebo treatment. There were too few seizures during the active (5) and placebo arms (3) to confidently assess seizure risk; however, considering exposure time, we observed an increased daily risk of seizures with increasing dose of OROS-MPH, suggesting that potential safety concerns need further study.
CONCLUSION
A larger study to assess the effect of OROS-MPH on seizure risk is needed. A crossover design including subjects with frequent seizures could maximize power and address high patient heterogeneity and recruitment difficulties.
Keywords: Epilepsy, Seizures, MPH, ADHD, Stimulant, Methylphenidate, OROS-Methylphenidate, Pharmacotherapy, Childhood seizures
INTRODUCTION
Epilepsy is a highly prevalent neurological disorder estimated to affect 0.5% of children in the developed world [1–5]. Attention Deficit Hyperactivity Disorder (ADHD) affects 12–39% of patients with epilepsy and is a major source of additional impairment in this population [6–9]. The Physician’s Desk Reference contains warnings, stating that stimulants can lower the threshold for having a seizure and that in the face of seizures these agents should be discontinued [10], yet the evidence upon which this warning is based is unclear. Patients with epilepsy were excluded from the clinical trials that established the safety and efficacy of stimulant medications, including MPH, for the treatment of ADHD [11–13]. Consequently, most patients who have ADHD co-morbid with epilepsy are not treated for ADHD due to concerns that stimulants might worsen seizures [7].
Studies examining the potential risk of seizures from stimulants in patients with epilepsy are relatively few and inconclusive. Although there is a limited number of case series of amphetamine used to treat symptoms of ADHD in children with epilepsy [14–16], we have found no prospective or controlled trials of amphetamine compounds in this population. To our knowledge, only immediate release methylphenidate (MPH) has been studied prospectively, with 3 studies reported. In the only double-blind placebo-controlled study, ten children, seizure free at baseline were given MPH 0.6 mg/kg/day in a crossover design with no seizures occurring on active MPH or on placebo [17]. In another study, 30 children were observed for 8 weeks and then given 0.3 mg/kg/day of MPH for 8 weeks. Twenty-five of the 30 patients were seizure-free at baseline and remained so; 5 patients with an average of 1.8 seizures/week during the observation period experienced an average of 3 seizures/week during MPH treatment [18]. This difference in seizure rate was not statistically significant, possibly due to low power. In both studies, 70% of the children significantly improved on MPH. Finally, Gucuyener et al. followed 57 children with epilepsy for 1 year while they took MPH open-label. No increase in seizure frequency was detected [19]. Thus, a total of 97 children with epilepsy have been studied prospectively while taking MPH. Their combined average seizure frequency was 0.65/week prior to, and 0.93/week during MPH treatment [16].
This literature provides some evidence for the short-term efficacy of short-acting MPH in decreasing ADHD symptoms in children with epilepsy. Because of the low baseline seizure rate and small number of patients studied, however, the conclusion that MPH does not lower the seizure threshold to a clinically significant degree remains problematic. Given the prevalence and clinical significance of this dilemma, there is a critical need for prospective, double-blind, placebo-controlled trials of stimulants in children with co-morbid epilepsy and ADHD to develop an evidence base that can guide clinical practice.
Implementing such trials, however, is challenging precisely because of the dilemma itself: trials need to be designed in such a way that they maximize the possibility of detecting benefit while, importantly, not putting children at risk. Moreover, heterogeneity among the epilepsy population, in terms of type and frequency of seizures, further complicates the design of studies and the interpretation of findings. The primary aim of this study, therefore, was to provide pilot data to evaluate the feasibility of clinical trial methods that might be used for a larger study of OROS-MPH in children with co-morbid epilepsy and ADHD while limiting risks to participants. To this end, the feasibility of recruiting for a double-blind placebo controlled crossover trial and the behavior of efficacy and safety measures in the crossover trial were explored. The secondary aim was to obtain preliminary estimates of the efficacy and safety of OROS-MPH to inform power calculations for a larger study.
We chose to examine OROS-MPH because of its popularity and ability to provide consistent MPH plasma levels daily [20–22]. Patients with epilepsy had been excluded from all previous OROS-MPH clinical trials. Also, the sustained release preparation raises a theoretical concern that any adverse effect on seizures might be prolonged relative to immediate release MPH. Thus, prior to exposing study patients with epilepsy to the full range of OROS-MPH doses, the Children’s Hospital Boston Institutional Review Board (IRB) required sufficient exposure to lower doses of OROS-MPH without significant toxicity before any patient was exposed to higher doses. For this reason, the adaptive phase I dosing escalation strategy was incorporated into the pilot study. An additional objective of the phase I design is to look for any very large safety problem in a small number of patients so as to establish a dose range of acceptable risk for subsequent larger clinical trials [23]. Adaptive phase I designs are commonly used in initial oncology trials where dose-limiting toxicities are expected, and finding the maximum acceptable dose within the potential therapeutic dose range is required before undertaking efficacy trials [23, 24]. We hypothesized that we would have no serious adverse effects or increase in seizure risk at any of the tested doses so that the maximum dose, the lesser of 54 mg/day, or 2mg/kg/day, could be designated the target dose for subsequent studies.
METHODS
Patients
Neurologists at Children’s Hospital Boston were queried about patients scheduled in their clinics to identify those with possible ADHD and to obtain permission to approach these families to enroll their children in a randomized-controlled trial of OROS-MPH. Through this process, 279 potential participants were identified and sent an informational letter describing the study. Of the 279 families that were contacted, 239 families declined to participate and 40 completed the informed consent process.
The 40 consented patients then underwent an evaluation to determine if inclusion/exclusion criteria were met. To confirm the epilepsy diagnosis for each patient, the principal investigator (PI), a board-certified child and adolescent psychiatrist (JGH), administered the Seizure Classification Interview [25, 26], reviewed the patient’s medical record, and consulted with the treating neurologist. The epilepsy diagnosis was made according to the International League Against Epilepsy (ILAE) - International Classification of Epileptic Seizures [27]. The PI interviewed each patient and his/her guardian to establish the diagnosis of ADHD and its subtype according to DSM-IV-R criteria [28]. The Kiddie Schedule for Affective Disorders and Schizophrenia - Present and Lifetime or Epidemiologic version was administered to participants by trained raters. The PI reviewed all results to confirm that full DSM-IV-R criteria had been met for ADHD and to determine any additional co-morbid psychiatric disorders [29, 30]. Additional inclusion criteria required: (1) a stable regimen of anti-epileptic drugs, (2) at least one seizure within the past five years, and (3) freedom from seizures for one month prior to starting study medication.
Exclusion criteria were: (1) severe developmental delays or mental retardation, (2) inability to speak English, (3) history of psychosis, (4) current major depression, or (5) for the first seven patients only, a history of bipolar disorder or medication treatment for a mood disorder. After the seventh patient was enrolled, the exclusion criteria were amended to include children with a past history of mood disorder that had remitted and patients in stable condition on an antidepressant or mood stabilizer regimen. This was done to make the sample more representative of the population of children with ADHD co-morbid with epilepsy; however, only two patients taking these agents enrolled, one patient taking risperidone and one taking sertraline. Additionally, patients were excluded at the first visit if their Clinical Global Impressions for ADHD-Severity (CGI-ADHD-S) rating was not 4 or higher (moderate or greater severity) or if the score on the ADHD Rating Scale-IV Home Version was below the 90 percentile on the Inattentive, Hyperactive-Impulsive, and Total score.
Of the 40 patients who consented and were evaluated, one patient did not meet formal criteria for ADHD, three dropped out due to inability to swallow pills, and 3 families changed their minds about participation before taking any study medication. Thirty-three patients took at least one dose of study medication and were included in the data analysis. These 33 patients were between the ages of 6 years, 0 months and 17 years, 10 months.
Design
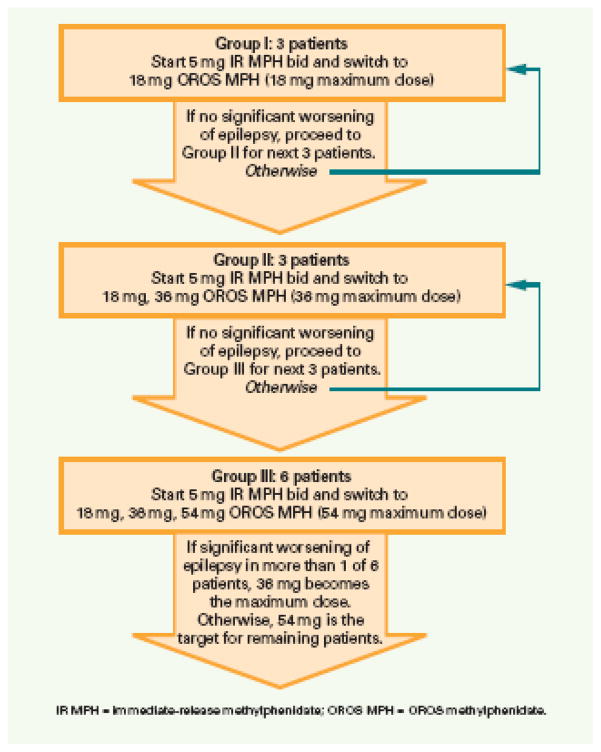
A double-blind placebo-controlled crossover study design was used. Randomization lists for each maximum dose group were prepared by a statistician and maintained by the research pharmacist. Patients were randomized to take either OROS-MPH or placebo for the first arm of the crossover. After completing this arm, they were taken off the study medication for one week then crossed over to the other condition. The adaptive Phase I trial design used is illustrated in Figure 1. Patients were assigned to one of 3 maximum OROS-MPH dose groups. Each patient was given a single day of 5 mg of Immediate Release (IR) MPH in the morning and at noontime. If the child tolerated this dose, 18 mg of OROS-MPH was administered for the remaining 6 days of the first week.
Figure 1. Adaptive Phase I Design.
A randomized double-blind placebo-controlled crossover trial. Each patient remained at the maximum dose for up to 1 week before endpoint measures for that arm were taken and the patient was crossed-over to the other arm of the study or was referred to clinical care outside the study. If 1 child had a significant worsening of epilepsy on active OROS-MPH at least 3 more patients would be tested at that dose level. If at any dose level 2 patients had significant worsening of epilepsy during the active arm, the dose level just below would be fixed as the maximum dose for the rest of the study. As patients enrolled they were assigned to the highest dose group recruiting at the time such that the daily dose of OROS-MPH did not exceed 2mg/kg/day. Thus more than 3 patients could be assigned to each group even if no patient experienced a significant worsening of epilepsy.
For Group I the maximum dose was 18 mg in the morning, and each arm of the crossover lasted one week (5 mg IR MPH for 1 day and 18 mg of OROS-MPH for 6 days). For Group II the maximum dose was 36 mg in the morning and each arm of the crossover lasted two weeks (if the 18 mg dose was tolerated, 36 mg each morning was administered for the second week). For Group III the maximum dose was 54 mg in the morning, and each arm of the crossover lasted 3 weeks (sequentially the 18 mg week, 36 mg week, and 54 mg week). The number of weeks for each arm of the crossover was selected so that patients could be assessed after a week at the maximum dose. At the request of the IRB, patients did not remain on the maximum dose longer than a week in order to minimize the amount of time patients remained on placebo.
Patients were assigned to Groups I, II, and III in ascending order with no patients assigned to higher dose levels until a minimum of 3 patients assigned to the lower dose had completed the crossover trial without a serious adverse event or significant worsening of epilepsy. No patient met either of these conditions so the adaptations described in Figure 1 were never invoked. No second set of three patients had to be tested for any of the groups before recruitment could start for the next higher group. As patients enrolled, all were assigned to group I until 3 of them had completed the crossover. Subsequent patients could be assigned to Group II, or, if 36 mg was more than 2mg/kg, the patient could be assigned to Group I. Once 3 Group II patients had completed the crossover without a significant worsening of epilepsy, subsequent patients could be assigned to Group III or to a lower group in order to keep the maximum dose below 2mg/kg/day.
Detailed descriptions were obtained of seizures during the trial. These seizures were compared to the seizures described during the Seizure Classification Interview, and the treating epilepsy clinician was consulted to determine whether these were consistent with the patient’s typical seizures. A significant worsening of epilepsy was defined as: 1) a doubling of the highest 14-day or highest 2-day seizure rate observed during the 12 months before the trial, 2) a generalized tonic-clonic seizure if none had been experienced in the previous 2 years, or 3) a clinically meaningful intensification in seizure duration or severity. If a seizure occurred but did not meet the criteria for a significant worsening of epilepsy (e.g. the patient had one of his or her typical seizures), the patient discontinued that arm of the crossover. If this occurred during the first arm, the patient waited until he or she was seizure free for one month then continued to the second arm. The Data and Safety Monitoring Board (DSMB) for the study and the IRB were informed of these seizures. Study personnel, the DSMB, and the IRB were not un-blinded through this process since the patient would not be exposed again to same condition (placebo or OROS-MPH).
Assessments
Baseline measures were administered to characterize the sample as follows:
Seizure Classification Interview to gather comprehensive information on seizure history from the primary caregiver [25, 26].
Wechsler Abbreviated Scale of Intelligence (WASI) to estimate IQ [31].
Scales of Independent Behavior-Revised Short Form (SIB-R) administered at baseline to one primary caregiver of each patient to estimate level of adaptive functioning [32].
ADHD Rating Scale-IV Home Version (ADHD RS) [33, 34] is an 18-item scale with one item for each of the DSM-IV ADHD criteria. The PI read each item to the patient’s guardian and then assigned a rating of symptom severity over the preceding week.
Clinical Global Impressions for ADHD-Severity (CGI-ADHD-S) [35] is a single-item rating reflecting the clinician’s assessment of the global severity of the child’s ADHD symptoms in relation to total experience with children with ADHD. It is derived from the CGI Severity scale and is rated on a 7 point scale with 1=normal, not at all and 7= among the most extremely ill patients.
Clinical Global Impressions for ADHD-Improvement (CGI-ADHD-I) [35] is a single-item rating reflecting the clinician’s assessment of the global change in the child’s ADHD symptoms. A score of 1=very much improved, or 2= much improved at the final visit of each arm was defined as response.
Additionally, at each study visit, the PI, who was blinded to medication status, evaluated the patient for adverse events in two ways. The PI asked the patient and guardian if the patient had experienced any adverse effects or illnesses. If reported, the PI would rate the adverse event from mild to life threatening. Adverse events were also assessed by the administration of the Barkley Side Effects Checklist-Modified (BSECM) [36]. The BSECM is a checklist that assesses 24 side-effects associated with stimulant medications, including insomnia and irritability. A dose-limiting adverse event occurred if one of the BSECM items was elevated above baseline at a moderate or higher level. Patients who experienced a dose-limiting adverse event were discontinued from that arm of the crossover without breaking the blind.
Anti-epileptic drug (AED) levels were drawn just before starting the first arm of the crossover to ensure that a therapeutic level was present before exposure to MPH. Despite the low power to detect an effect of MPH on the plasma levels of the variety of AEDs patients were taking, an attempt was made to draw AED levels again at the last visit of each arm of the crossover if the patient was still taking the study medication. This was done in an effort to detect any large effects on AED levels. Since many patients lived far from the hospital, if the patient discontinued an arm of the crossover trial, the AED levels for that arm could not be obtained, further limiting power to detect changes due to MPH exposure.
Statistical Methods
All statistical tests and all reported confidence intervals (CI) are two-tailed. Descriptive statistics and frequencies were computed for seizure rates and adverse events. McNemar’s test was used to compare the number of patients who discontinued each arm of the crossover. The number of days of exposure to placebo and OROS-MPH were compared using the Student’s t-test. Then, within each treatment arm, the risk for a seizure was computed using raw rates of seizure events divided by days of exposure at different doses. The study design called for patients to stop taking OROS-MPH or placebo as soon as a seizure occurred. Thus the odds of a seizure on each day of exposure were used to estimate seizure risk and the primary statistical analysis of seizure risk was accomplished using logistic regression. Additionally, the number of days of exposure until a seizure occurred was examined and Cox proportional hazards models were used as an additional statistical analysis of seizure risk. For both techniques, the order of the placebo and OROS-MPH arms was examined as a predictor with the understanding that if this predictor was significant, only the first arm of the crossover would be used in subsequent analyses. For the logistic regression, a repeated measures model with a Poisson link function (SAS, Proc Genmod) was used to estimate the odds of having a seizure on each day of OROS-MPH versus placebo. The logistic model contained a term for number of days on that arm of the crossover to control for length of exposure to MPH or placebo. The model also included the OROS-MPH dose and the interaction between dose and length of exposure. The contribution of OROS-MPH dose was assessed both as an absolute dose and as a mg/kg/day dose. A Cox proportional hazards model was used as an additional method since the study protocol called for doses to be increased at days 1, 2, 8, and 15 of the OROS-MPH arm, but many observations were censored either by design (patient was assigned to groups I or II), because of a dose limiting adverse event, or because of a seizure. The Cox proportional hazards model compared the hazard of having a seizure within each day of each dose (SAS, Proc Phreg). The predictors tested in the Cox model were mg/kg/day, dose, number of days on that arm of the crossover, and the interaction between these predictors. For the Cox models, each week of the trial was treated as a repeated measure for each subject. For example, a patient who was taking OROS-MPH for one week and placebo for one week had 2 measures, whereas a patient who was taking OROS-MPH for 2 weeks and placebo for 3 weeks had five measures. We did not use a more complex model that took into account that on the first day of the first week of the active MPH arm patients were given 10 mg of MPH per day rather than 18mg of OROS-MPH. This is because there were no censoring events during this day for any patient, and the additional complexity of the model would not have yielded more information. Because of the small number of seizure events, there was inadequate power to examine other covariates (e.g. seizure etiology and type, ADHD subtype, gender, and seizure frequency prior to entering the trial) in either the Logistic or Cox models. Robust estimates of standard errors were used in the logistic and proportional hazards models to account for correlation among the repeated measures on a patient (i.e. the sandwich variance estimate for the Cox models).
To examine whether OROS-MPH was associated with adverse effects other than seizures, the effect of OROS-MPH versus placebo on BSECM total score and individual items during each week of treatment was estimated using repeated measures regression (SAS, Proc Genmod). In order to increase sensitivity to adverse events that occurred during OROS-MPH treatment, analysis of individual items was not corrected for multiple comparisons.
To explore whether OROS-MPH was efficacious against ADHD in the study population, we examined responder rates as well as decrease in ADHD symptoms during the OROS-MPH and placebo arms of the crossover. We defined responders as those patients with CGI-ADHD-I scores of ‘much’ or ‘very much improved’ at the last observation on that arm of the crossover. To explore whether OROS-MPH was associated with a greater decrease in ADHD symptoms than placebo, the effect of OROS-MPH versus placebo on ADHD total score for each week of treatment was estimated using repeated measures regression (SAS, Proc Genmod). First, the order of the placebo and OROS-MPH arms was examined as a predictor with the understanding that if this predictor were significant, only the first arm of the crossover would be used in subsequent analyses. The subsequent model contained a term for the week within the crossover arm, the OROS-MPH dose and the interaction between dose and week within the crossover arm. Exploratory analyses were performed in which each of the following covariates was added alone to this model as a main effect and as an interaction with week within the crossover arm, with active dose versus (vs.) placebo, and with each of these predictors: predominately inattentive vs. combined type ADHD diagnosis, idiopathic vs. non-idiopathic epilepsy, generalized vs. focal onset seizures, gender, socioeconomic status, race, age, IQ, functional level on SIB-R, and number of AEDs being taken. The p value for significance was set at <0.005 to account for the multiple comparisons (0.05/10).
Changes in AED plasma levels were examined using the Wilcoxon Signed Rank Test.
RESULTS
Thirty-three patients (mean age: 10.5 ± 3.0 years) were randomized and took at least one dose of study medication (placebo or OROS-MPH). They are all included in all of the analyses. Table 1 describes the sample’s baseline characteristics. Three patients were assigned to a target daily dose of 18 mg of OROS-MPH, 9 to a target dose of 36 mg, and 21 to a target dose of 54 mg. The target dose was less than 1 mg/kg/day for 11 patients, 1 to 1.5 mg/kg/day for 13 patients and 1.5 to 2 mg/kg/day for 9 patients.
Table 1.
Patient Demographics
| Demographic Variable | Patient Data Mean±Std Dev, (median, range) |
|---|---|
| Total number of patients | 33 |
| Male patients, n (%) | 19 (57.6) |
| Age, year | 10.5±3.0, (10.4, 6.4–17.5) |
| Weight, kg | 42.4±16.3, (37.7, 20.9–84.4) |
| WASI IQ | 89.7±16.9, (88, 59–123) |
| Scales of Independent Behavior-Revised (SIB-R), Standard Score | 74.8±24.7, (78, 25–129) |
| Antiepileptic drugs at start | 1.2±0.5, (1, 1–3) |
| Epilepsy Etiology, | n (%) |
| Cryptogenic | 12 (36.4) |
| Idiopathic | 13 (39.4) |
| Symptomatic | 8 (24.2) |
| Seizure Type, | |
| Focal Onset | 26 (78.8) |
| Generalized Onset | 7 (21.2) |
| ADHD Type, | |
| Predominantly Inattentive | 16 (48.5) |
| Combined | 17 (51.1) |
Safety: Seizure Recurrence
Although seizures occurred during the study on both OROS-MPH and placebo, no patient met criteria for a significant worsening of epilepsy. One patient had a cluster of 4–5 myoclonic seizures while taking both OROS-MPH and placebo. Counting these clusters as a single episode of seizures, there were a total of 5 seizures on OROS-MPH and 3 on placebo. This includes one patient who had two distinct seizures on a single day of exposure to active OROS-MPH. Families were instructed to stop the study medication once any seizure occurred.
Table 2 describes the seizures and other AEs experienced by each patient during the crossover trial. Five patients experienced a total of 8 seizures during the trial. The number of patients who experienced a seizure during exposure to OROS-MPH (n=4) did not differ significantly from that during placebo (n=3). The seizures occurred on 7 out of a total 1058 days on which either placebo or active medication was administered. Only 1 child discontinued treatment under placebo but completed the entire active arm, whereas 10 children discontinued that active arm but completed their placebo treatment (p=.01). As a result, exposure to placebo (17.2±7.0 days) was longer than exposure to OROS-MPH (14.9±5.4 days; t32=3.42, p=0.002), and length of exposure needed to be taken into account in calculating seizure risk. Three seizures occurred during the 565 placebo days (rate=0.53 seizures/100days), 1 during the 194 days on which patients were taking either 10 mg or 18 mg active medication (0.52 seizures/100days), 2 during 170 active 36 mg days (1.12 seizures/100days), and 2 during 87 active 54 mg days (2.30 seizures/100days).
Table 2.
Patient Group Assignment, Highest Dose Achieved, and Adverse Events Including Seizures
| Age at enrollment and Gender | Target Dose (mg/day) | 18 mg OROS-MPH (mg/kg) | 36 mg OROS-MPH–day of dose (mg/kg) | 54 mg OROS-MPH -day of dose (mg/kg) | 18 mg placebo | 36 mg placebo-day of dose | 54 mg placebo-day of dose |
|---|---|---|---|---|---|---|---|
| Group 1: Assigned to attempt in random order 1 week 18mg OROS-MPH and 1 week 18mg placebo | |||||||
| 8.3 y.o. F | 18a | C (0.52) | n/a | n/a | C | n/a | n/a |
| 13.8 y.o. M | 18a | C (0.32) | n/a | n/a | C | n/a | n/a |
| 12.5 y.o. M | 18p | C (0.42) | n/a | n/a | C | n/a | n/a |
| Group 2: Assigned to attempt in random order 1 week each 18mg, 36mg OROS-MPH and 1 week each 18mg, 36mg placebo | |||||||
| 17.5 y.o. M | 36p | C (0.22) | nausea - 5 (0.45) | n/a | C | C | n/a |
| 11.0 y.o. F | 36p | C (0.63) | emotional lability - 7 (1.26) | n/a | C | C | n/a |
| 13.6 y.o. F | 36a | C (0.26) | C (0.52) | n/a | C | C | n/a |
| 10.6 y.o. M | 36a | C (0.53) | C (1.06) | n/a | C | C | n/a |
| 6.8 y.o. F | 36a | C (0.86) | overfocus - 5 (1.72) | n/a | C | C | n/a |
| 7.4 y.o. M | 36a | C (0.44) | C (0.88) | n/a | C | C | n/a |
| 10.4 y.o. M | 36p | C (0.33) | C (0.67) | n/a | C | C | n/a |
| 6.8 y.o. M | 36a | C (0.72) | emotional lability - 1 (1.44) | n/a | C | C | n/a |
| 9.0 y.o. M | 36a | C (0.64) | seizure - 4 (1.29) | n/a | C | C | n/a |
| Group 3: Assigned to attempt in random order 1 week each 18mg, 36mg, 54mg OROS-MPH and 1 week each 18mg, 36mg, 54mg placebo | |||||||
| 11.2 y.o. M | 54p | C (0.57) | C (1.14) | tics - 1 (1.71) | C | C | C |
| 12.6 y.o. F | 54p | C (0.32) | C (0.65) | C (0.97) | C | C | C |
| 9.9 y.o. F | 54a | C (0.30) | emotional lability - 6 (0.60) | did not receive | C | C | emotional lability - 2 |
| 6.4 y.o. M | 54a | C (0.61) | insomnia - 6 (1.23) | did not receive | C | C | C |
| 8.3 y.o. F | 54p | C (0.58) | C (1.17) | C (1.75) | C | C | C |
| 17.5 y.o. F | 54a | C (0.26) | seizure - 1 (0.53) | did not receive | C | C seizure - 5 | |
| 16.0 y.o. M | 54p | C (0.21) | C (0.43) | C (0.64) | C | C | C |
| 9.4 y.o. M | 54a | C (0.48) | C (0.95) | C (1.43) | C | C | C |
| 12.4 y.o. F | 54p | C (0.49) | C (0.98) | C (1.47) | C | C | seizure - 5 |
| 8.6 y.o. M | 54a | C (0.66) | C (1.33) | 2 seizures - 1 (2.00) | C | C | seizure - 4 |
| 10.3 y.o. F | 54a | C (0.47) | insomnia - 5 (0.93) | did not receive | C | C | C |
| 7.5 y.o. M | 54a | C (0.62) | C (1.24) | C (1.86) | C | C | C |
| 8.3 y.o. F | 54p | C (0.66) | C (1.33) | C (2.00) | C | C | C |
| 10.5 y.o. M | 54a | C (0.55) | C (1.09) | C (1.64) | C | C | C |
| 12.2 y.o. M | 54p | C (0.41) | C (0.83) | C (1.24) | C | C | C |
| 9.4 y.o. F | 54a | C (0.66) | C (1.32) | C (1.98) | C | C | C |
| 12.2 y.o. M | 54p | C (0.35) | emotional lability -6 (0.69) | did not receive | C | C | C |
| 10.6 y.o. F | 54a | C (0.41) | C (0.81) | C (1.22) | C | C | C |
| 6.4 y.o. M | 54p | seizure - 3 (0.40) | did not receive | did not receive | C | emotional lability - 6 | did not receive |
| 8.2 y.o. M | 54p | C (0.56) | C (1.12) | C (1.68) | C | C | C |
| 12.6 y.o. F | 54p | C (0.33) | insomnia -7 (0.66) | did not receive | C | C | C |
a=first arm of crossover was active OROS-MPH, p=first arm of crossover was placebo, C=completed, n/a=not applicable, AE-X=discontinued due to Adverse Effect (AE) after receiving day X of the OROS-MPH or placebo dose indicated.
Taking patients’ weights into account, 2 seizures occurred during the 369 days of exposure to less than 1.2 mg/kg/day (0.54 seizures/100days) and 3 seizures occurred during 164 days of exposure to doses of 1.2 to 2 mg/kg/day (1.83 seizures/100days). Dividing by the rate during placebo exposure yields raw rate ratios as follows: < 1.2 mg/kg/day = 1.02; 1.2 to 2 mg/kg/day = 3.45.
Since these raw rate ratios do not take into account any correlation between patients, logistic regression was used to calculate the odds ratio (OR) for a seizure during active treatment. Whether a patient underwent the placebo or OROS-MPH arm first in the crossover was examined and found not to be a significant predictor, arguing against a carryover effect. Subsequent models included a predictor term for dose (examined either as mg/kg/day or as ordinal absolute dose level, that is 18, 36, and 54 mg per day), for days of exposure within each arm (placebo arm and OROS-MPH arm), and the interaction betweens days of exposure and dose. In all of these models, number of days of exposure to placebo or OROS-MPH was significant (p<0.005), as was dose (both mg/kg/day dose and ordinal absolute dose, p<0.005). The interaction between days of exposure and dose was significant whether dose was examined as mg/kg/day (p=0.02) or as the ordinal absolute dose (p<0.001).
As an additional analysis of seizure risk, Cox proportional hazard models were used to evaluate time to a seizure within the exposure to a given dose and to calculate the hazard of a seizure at each dose. As with the logistic models, whether a patient underwent the placebo or OROS-MPH arm first in the crossover was examined as a predictor, found not to be significant, and dropped from the next analysis. This consisted of a Cox model that included a term for dose, days of exposure within the placebo or OROS-MPH arm, and the interaction between them. This analysis indicated that a higher mg/kg/dose predicted a greater hazard of a seizure (p<0.01). While the term representing days of exposure within each arm was not significant, the interaction between days of exposure and dose was (p<0.05).
Tolerability
There were no serious adverse events. Five patients discontinued treatment while taking placebo and 14 while taking OROS-MPH (p=0.007). Dose-limiting adverse events occurred earlier and more frequently during the OROS-MPH arm than the placebo arm, accounting for the longer exposure to placebo. The most common adverse events reported on both study arms were seizures and emotional lability. Emotional lability worsened for 4 patients on OROS-MPH compared with 2 on placebo. All adverse events resolved within 18 hours of discontinuing medication, except for tics (n=1), which resolved within 48 hours.
Analysis of the BSECM total score at each dose revealed a significant main effect of week of treatment, though there was no interaction between treatment and week. On average, the BSECM score decreased with each week of treatment and at about the same rate, regardless of whether the patient was on placebo or OROS-MPH. Given the heterogeneity of BSECM items, the same analysis was carried out for each of the measure’s 24 individual items. In order to better identify potential tolerability problems there was no correction made for the multiple comparisons. Patients were more likely to have trouble falling asleep on OROS-MPH than placebo (χ2=10.60, p = 0.01). The average magnitude of the difference for this item was one point on a ten-point Likert scale at the 36 and 54 mg doses. The effect sizes (difference between active and placebo effect/pooled standard deviation) for this item for each OROS-MPH daily dose were −0.29 for the 18 mg, 2.25 for the 36 mg, and 1.79 for the 54 mg.
Efficacy
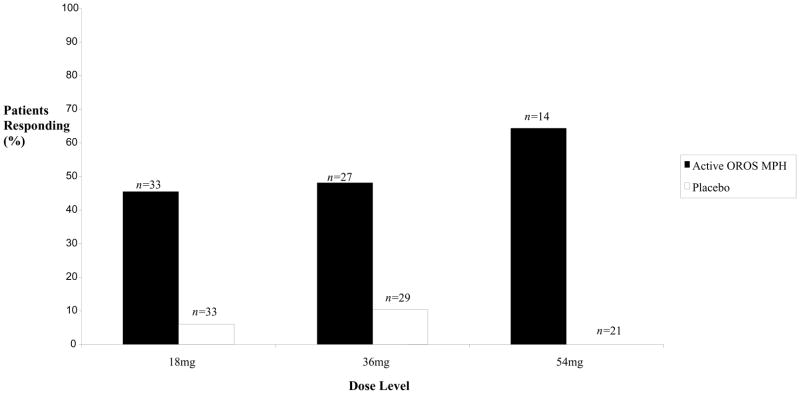
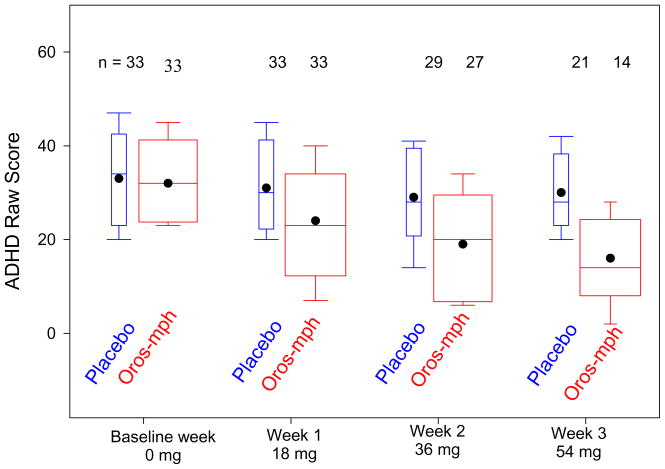
There was a higher rate of response to OROS-MPH than to placebo at each dose (Figure 2). The total ADHD RS scores at each week of the crossover are described in Figure 3. Analysis of the ADHD RS total score at each dose revealed no effect from whether the OROS-MPH or placebo arm came first, arguing against any carryover effect, and so this term was dropped from subsequent models. There was a significant main effect of the baseline ADHD RS total score at the start of each arm of the crossover, so this was included. A significant main effect of week of treatment in the crossover arm (p<0.0001) as well as significant interaction between this variable and whether patients were taking OROS-MPH or placebo was found (p<0.0001). This indicated that the ADHD RS total score dropped each week of treatment for both placebo and OROS-MPH, but dropped more rapidly during the OROS-MPH arm of the crossover.
Figure 2.
Percentage of Patients Responding to Treatment for Active OROS-MPH and Placebo at Each Dose Level
Figure 3. Clinician Rated ADHD Rating Scale IV Total Score by Dose*.
* Boxes show the 25th, 50th, the 75th percentiles. Whiskers extend to the 10th and 90th percentiles. Dots indicate mean level. Oros-mph means are lower than placebo means for doses 18, 36, and 54 mg (each p<.002).
An exploratory analysis to determine the main effect and interaction terms for the covariates listed in the statistical methods section was performed. The only one for which the interaction with week and treatment arm reached the preset alpha level of p<0.005 was seizure type. This yielded the exploratory finding that in this small study the 7 patients with generalized onset epilepsy did not have a difference between the active and placebo arms that was as robust as the difference seen in the 26 patients with focal onset epilepsy (p=0.004). There was also a non-significant trend for patients with predominately inattentive subtype ADHD to have a less robust response to OROS-MPH (p=0.03).
Antiepileptic Drug (AED) Plasma Levels
The 33 patients enrolled were taking a total of 10 different AEDs (Table 3). We were not able to get AED plasma levels for all of these patients’ AEDs. Thirty-four baseline AED plasma levels were obtained. Twenty-five AED levels were obtained while on active MPH, and 26 while on placebo. There were no significant differences in AED levels between OROS-MPH and either placebo or baseline measurements.
Table 3.
AED Levels at Baseline, on Active Medication, and on Placebo
| AED | Number of Patients | Number of patients with levels at first baseline | Number of patients with levels on active | Number of patients with levels on placebo | Average Baseline Level | Average Active Level | Average Placebo Level | p-value Active vs. Placebo | p-value Baseline vs. Active |
|---|---|---|---|---|---|---|---|---|---|
| Valproate | 14 | 13 | 6 | 9 | 57.7 | 79.5 | 61.9 | 0.5 | 0.893 |
| Carbamazepine | 6 | 6 | 6 | 5 | 9 | 9.4 | 8.1 | 0.144 | 0.6 |
| Lamotrigine | 6 | 4 | 3 | 1 | 3.24 | 2 | 4.1 | * | 0.18 |
| Topiramate | 4 | 3 | 3 | 3 | 7.3 | 8.1 | 6.7 | 0.285 | 0.18 |
| Levitracetam | 4 | 3 | 1 | 2 | 12.3 | 9 | 14.5 | * | * |
| Gabapentin | 1 | 1 | 1 | 1 | 5 | 4.3 | 8.2 | * | * |
| Oxcarbazepine | 2 | 2 | 2 | 2 | 26 | 28.5 | 30 | * | * |
| Ethosuximide | 2 | 2 | 1 | 1 | 80.5 | 87 | 80 | * | * |
| Lorazepam ** | 1 | no levels | |||||||
| Diazepam ** | 1 | no levels |
p-values based on Wilcoxon Signed Rank Test.
Insufficient data to calculate Wilcoxon Signed Rank score
DISCUSSION
The purpose of this pilot study is to test the feasibility of methods for a larger trial of OROS-MPH in patients with ADHD in the context of epilepsy, to look for any large safety problem that, being evident in such a small number of these subjects, would require modification of the dose range for the larger study, and to get some initial estimates of safety and efficacy that might be helpful in planning the larger trial. The first question to be answered is whether the results of the pilot study indicate that performing a larger study of OROS-MPH in this population should be a priority. We believe that the answer is yes. The pilot study’s findings add to the motivation for a larger trial. There was robust evidence of efficacy of OROS-MPH against the symptoms of ADHD in these patients, indicating that there is likely a significant benefit to patients from this treatment.
The safety of this medication for patients with epilepsy, however, is the primary concern motivating a study such as this one, and it is more difficult to evaluate. We did find some preliminary indication of an increase in the daily risk of having a seizure with increasing doses of OROS MPH. It is important to note that the number of seizure events observed in the pilot study (3 during the placebo arm and 5 during the active OROS-MPH arm) is too small to confidently determine whether there is an increase in seizure risk from higher OROS-MPH doses. The small number of seizures that occurred during the trial makes the observation of a possible increase in daily seizure risk potentially unstable. For example, if one patient of the patients with seizures had not enrolled or if the chance waxing and waning of his seizures had been different, we may not have seen a statistically significant signal of increased risk. Moreover, the study was not powered to detect anything but a very large increase in seizure risk. Thus, our observation provides reason for caution, but this study is too small to resolve such an important safety concern. The effect of OROS-MPH on seizures is still unknown and this pilot study only underlines the need for a larger trial. In a disorder as prevalent as ADHD in the context of epilepsy, the findings of robust efficacy and evidence that a potential safety concern needs further study argue forcefully for performing an adequately powered trial of OROS-MPH or other extended release MPH products.
The primary goal of this study was to demonstrate the feasibility of the methods, including the adaptive phase I component, which is appropriate for situations in which safety is a major concern. This adaptive phase I design was found to be feasible to execute and did not preclude a preliminary assessment of both risk and benefit. Most important, no serious adverse events, no cases of status epilepticus, and no instances of significant worsening of epilepsy occurred in this vulnerable patient population. Thus for a similar population this pilot study supports including the full range of OROS-MPH doses tested (up to the lesser of 2mg/kg/day or 54 mg per day) without having to include the adaptive design in the larger trial. However, if an even more vulnerable patient group were included, for example patients with more frequent seizures, consideration can be given for including an adaptive phase I component for those patients. Other methodological considerations include the crossover rather than parallel groups design, the outcome measures for efficacy, tolerability, and safety, the characteristics of the study population, the procedures in response to the occurrence of seizures, and the recruitment strategy.
Regarding the use of a crossover design instead of a parallel groups design, an advantage of a crossover design is that each patient functions as his or her own control and the effects of confounding variables that might obscure seeing a differential response to OROS-MPH versus placebo are minimized. Given the heterogeneity of seizure disorders, this is an important advantage. The short half life of both the plasma level and behavioral effects of MPH have allowed crossover studies to be used successfully to study the effects of MPH on ADHD symptoms [12, 21]. However, crossover designs are still vulnerable to period and carry-over effects, subject drop out, and variability in ADHD symptom severity over time. Thus testing for such period or carryover effects is important. In their absence, the crossover design will have greater power to detect differences in outcomes between the conditions when compared to a parallel groups design. However, if period or carryover effects are detected, usually only the first arm of the crossover can be used in the analysis. In that case, the effort and expense of the second arm would have been largely wasted.
For this pilot study, the crossover design offered an additional advantage; it allowed the study to overcome two important ethical concerns. First, there was more than minimal risk to the participation given the MPH package insert warnings against its use in patients with seizures. Second, for patients assigned to placebo, ADHD treatment was arguably being withheld while they participated and there could be risks if treatment were withheld long enough. It is difficult to justify (and for IRBs to approve) exposing pediatric subjects to more than minimal risk when there is no prospect of direct benefit to the individual patient. The crossover design exposed all subjects to both MPH and placebo so that an unblinded clinician not participating in the study ratings could look at the data generated by the study and tell the patient’s guardian whether there was a more robust response to OROS-MPH than to placebo. The patient’s guardian could then use this information in planning future treatment. The IRB agreed that this information provided a direct benefit to individual patient that balanced the risks of participation.
In this pilot study, all randomized patients entered both arms of the crossover and only dropped out when adverse effects developed. We looked for carryover or period effects for both the efficacy and tolerability measures, as well as the primary safety indicator, seizure occurrence. We found no evidence of carryover or period effects. We were able to document strong evidence for efficacy and identify a potential safety concern, despite the small number of patients and the small number of seizure events. Taken together, the experience gathered during the pilot study indicates that use of a crossover design is feasible and advantageous in this population.
In regards to the efficacy, tolerability and safety outcome measures: Both the ADHD RS and the CGI-ADHD-I showed robust differences favoring OROS-MPH over placebo, even though there was some improvement over time on placebo. They seem appropriately sensitive to change for use in the larger trial. The tolerability measure used, the BSECM (Barkley Side Effects Checklist-Modified), yielded results consistent with the known side effect profile of MPH and thus seems appropriate for the larger trial as well [20–22].
In regards to the principal safety concern, whether OROS-MPH increases the risk of seizures: an element of study design, the procedure invoked if a seizure occurred (dropping out of the current arm of the crossover after a seizure), while guarding the safety of patients, reduced the power to detect a difference in seizure rates between OROS-MPH and placebo. By design, patients could only have a single day of seizures in an arm of the crossover. Because of this limitation, differences in safety between the OROS-MPH and placebo arms of the study could be demonstrated by daily risk of a seizure, whether quantified with Logistic or Cox regression, but not by raw seizure counts. The power to detect a difference in daily seizure rate was also constrained by the inclusion/exclusion criteria that were used. Only patients who were seizure free for the previous month could enroll. Most enrolled patients were not expected to, and indeed did not have a seizure while on either arm of the study. Even for a larger trial, the power to detect an increase in seizures from OROS-MPH treatment will be limited unless patients with more frequent seizures are enrolled and they are allowed to remain in the treatment arm even if they have a seizure. The lack of serious adverse effects, episodes of status epilepticus, or clinically significant worsening of epilepsy observed in the pilot study provides the prior experience needed to justify such study procedures ethically with this more vulnerable population. One possible design would be to include subjects with seizures between one per month and one per day and observe their seizure rate prior to randomization long enough to confidently establish the patient’s individual seizure rate prior to starting study drug. Then, with each seizure the patient experienced while taking study drug, a statistician could determine whether there was sufficient evidence that the seizure rate had increased for that patient. If the rate of seizures did increase, the patient would be dropped from that arm of the crossover trial. Such a design would allow the study to observe more seizure events during each arm of the crossover trial, thus affording greater confidence in estimating the effect of OROS-MPH on seizures. It would also necessitate the careful tracking of seizures and other adverse events by a Data and Safety Monitoring Board (DSMB).
Of considerable importance vis-à-vis feasibility, we found it very difficult to recruit patients for this pilot study. Of the 279 families that were contacted, 239 families declined to participate (89%). This raises a significant barrier to success, especially if the goal had been to recruit patients with more frequent seizures. Patients and families will be aware of the potential for increasing seizure rate and thus reluctant to expose the child to this risk. Moreover, so as to not to confound interpretation of findings, it would be desirable to enroll only patients whose AED regimen could remain unchanged during the trial. One possible strategy for improving participation would be to obtain consent for active screening for ADHD symptoms at the time of a clinic appointment rather than approaching families by a letter. Those whose child screens positive for probable ADHD and impairment would then have the opportunity to discuss the study in person. Even with this more direct recruiting method, it is likely that a larger trial would have to be done across several epilepsy centers, and even then there would be risk for selection bias due to the low participation rates. These limitations will need to be acknowledged and balanced against the potential value of a better developed evidence base for physicians and families to make judgments about the potential risks and benefits of pharmacological therapy for patients with co-occurring ADHD and epilepsy.
While reviewing the results of this pilot study it is important to bear in mind several important limitations. The number of patients studied was small, their seizure types were varied, and their baseline seizure frequencies were low. Thus, even the most robust finding of an effect of OROS-MPH improving ADHD symptoms may not generalize to patients with all types of seizures or to those with more frequent seizures. As has been discussed, the actual number of seizure events observed is insufficient to reliably determine an effect of OROS-MPH on seizures. The number of patients taking each AED was very low, and therefore the power to detect an effect of MPH on AED levels was inadequate in this study.
In conclusion, our findings from this pilot study of efficacy, in tandem with preliminary evidence for a safety concern, argue forcefully for an adequately powered trial of OROS-MPH or other extended release MPH products aimed at providing a more comprehensive body of data to document efficacy and especially, risk. Although the findings underline the need for a larger trial, such a trial would have to be designed to safeguard against the potential risk for worsening seizures. If, as is recommended, patients with more frequent seizures are included in subsequent trials, starting the trial with an adaptive phase I component could be considered, as the present pilot study gives good evidence of its feasibility. In addition, our experience highlights the potential difficulty of recruiting patients for such a study, and so the study design would need to be economical in terms of the number of patients required and their characteristics in order to provide meaningful and interpretable findings.
Acknowledgments
This study was supported by NIMH grant K23 MH066835
The authors thank Alka Indurkhya for her expert advice on data analysis, Danielle Guild, Tanya Oken, and Brian Travers for their help in the preparation of this manuscript. Most especially the authors thank the patients and families that participated in this study.
Footnotes
Disclosures: In the past year, Dr. Joseph Gonzalez-Heydrich, has been a consultant to AstraZeneca and received research support from Pfizer, Johnson & Johnson, and GlaxoSmithKline. In previous years, he has served as a consultant to Abbott Laboratories, Pfizer Inc, Johnson & Johnson (Janssen, McNeil Consumer Health), Novartis, Parke-Davis, Glaxo-SmithKline, AstraZeneca, and Seaside Therapeutics; has been a speaker for Abbott Laboratories, Pfizer Inc, Novartis, Bristol-Meyers Squibb; and has received grant support from Abbott Laboratories, Pfizer Inc, Johnson & Johnson (Janssen, McNeil Consumer Health), Akzo-Nobel/Organon and the NIMH. McNeil Consumer Health provided the active OROS-Methylphenidate and matching placebo for this study.
In the past year, Dr. Stephen Faraone has received consulting fees and has been on Advisory Boards for Eli Lilly, McNeil and Shire and has received research support from Eli Lilly, Pfizer, Shire and the National Institutes of Health. In previous years, Dr. Faraone has received consulting fees or has been on Advisory Boards or has been a speaker for the following sources: Shire, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. In previous years he has received research support from Eli Lilly, Shire, Pfizer and the National Institutes of Health.
Dr. Joseph Biederman is currently receiving research support from the following sources: Alza, AstraZeneca, Bristol Myers Squibb, Eli Lilly and Co., Janssen Pharmaceuticals Inc., McNeil, Merck, Organon, Otsuka, Shire, NIMH, and NICHD. In 2009, Dr. Joseph Biederman received a speaker’s fee from the following sources: Fundacion Areces, Medice Pharmaceuticals, and the Spanish Child Psychiatry Association. In previous years, he has received research support, consultation fees, or speaker’s fees for/from the following additional sources: Abbott, AstraZeneca, Celltech, Cephalon, Eli Lilly and Co., Esai, Forest, Glaxo, Gliatech, Janssen, McNeil, NARSAD, NIDA, New River, Novartis, Noven, Neurosearch, Pfizer, Pharmacia, The Prechter Foundation, Shire, The Stanley Foundation, UCB Pharma, Inc. and Wyeth.
Dr. Blaise Bourgeois has received grant/research support from Eisai, Ovation and UCB Pharma. In the past two years, he has also received consulting fees from Genzyme and Bayer Material Science.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clinic Proceedings l. 1996;71:576–86. doi: 10.4065/71.6.576. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson KJ, Koivikko MJ. Prevalence, classification, and severity of epilepsy and epileptic syndromes in children. Epilepsia. 1997;38:1275–82. doi: 10.1111/j.1528-1157.1997.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 3.Sidenvall R, Forsgren L, Heijbel J. Prevalence and characteristics of epilepsy in children in northern Sweden. Seizure. 1996;5:139–46. doi: 10.1016/s1059-1311(96)80108-7. [DOI] [PubMed] [Google Scholar]
- 4.Bell GS, Sander JW. The epidemiology of epilepsy: the size of the problem. Seizure. 2001;10:306–14. doi: 10.1053/seiz.2001.0584. quiz 315–6. [DOI] [PubMed] [Google Scholar]
- 5.Waaler PE, Blom BH, Skeidsvoll H, Mykletun A. Prevalence, classification, and severity of epilepsy in children in western Norway. Epilepsia. 2000;41:802–10. doi: 10.1111/j.1528-1157.2000.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 6.Dunn DW, Austin JK, Harezlak J, Ambrosius WT. ADHD and epilepsy in childhood. Developmental medicine and child neurology. 2003;45:50–54. [PubMed] [Google Scholar]
- 7.Tan M, Appleton R. Attention deficit and hyperactivity disorder, methylphenidate, and epilepsy. Arch Dis Child. 2005;90:57–9. doi: 10.1136/adc.2003.048504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldenkamp AP, Arzimanoglou A, Reijs R, Van Mil S. Optimizing therapy of seizures in children and adolescents with ADHD. Neurology. 2006;67:S49–51. doi: 10.1212/wnl.67.12_suppl_4.s49. [DOI] [PubMed] [Google Scholar]
- 9.Thome-Souza S, Kuczynski E, Assumpcao F, Jr, Rzezak P, Fuentes D, Fiore L, Valente KD. Which factors may play a pivotal role on determining the type of psychiatric disorder in children and adolescents with epilepsy? Epilepsy Behav. 2004;5:988–94. doi: 10.1016/j.yebeh.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous. Physician’s Desk Reference. 61. Montvale, NJ: Thomson PDR; 2007. p. 3165. (Adderall), 3167 (Adderall XR), 1883 (Concerta), 3176 (Daytrana), 1420 (Dexedrine), 3176 (DextroStat), 2221 (Focalin), 3325 (Metadate), 2270 (Ritalin), 2272 (Ritalin LA), 2270 (Ritalin SR) [Google Scholar]
- 11.Jadad AR, Boyle M, Cunningham C, Kim M, Schachar R. Treatment of attention-deficit/hyperactivity disorder. Evidence Report: Technology Assessment (Summary) 1999:i–viii. 1–341. [PMC free article] [PubMed] [Google Scholar]
- 12.MTA Cooperative Group. A 14-Month Randomized Clinical Trial of Treatment Strategies for Attention-Deficit/Hyperactivity Disorder. Archives of General Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 13.MTA Cooperative Group. Moderators and Mediators of Treatment Response for Children With Attention-Deficit/Hyperactivity Disorder: The Multimodal Treatment Study of Children With Attention-Deficit/Hyperactivity Disorder. Archives of General Psychiatry. 1999;56:1088–1096. doi: 10.1001/archpsyc.56.12.1088. [DOI] [PubMed] [Google Scholar]
- 14.Baptista-Neto L, Dodds A, Rao S, Whitney J, Torres A, Gonzalez-Heydrich J. An expert opinion on methylphenidate treatment for attention deficit hyperactivity disorder in pediatric patients with epilepsy. Expert Opinion On Investigational Drugs. 2008;17:77–84. doi: 10.1517/13543784.17.1.77. [DOI] [PubMed] [Google Scholar]
- 15.Plioplys S, Dunn DW, Caplan R. 10-year research update review: psychiatric problems in children with epilepsy. Journal Of The American Academy Of Child And Adolescent Psychiatry. 2007;46:1389–1402. doi: 10.1097/chi.0b013e31815597fc. [DOI] [PubMed] [Google Scholar]
- 16.Torres AR, Whitney J, Gonzalez-Heydrich J. Attention-deficit/hyperactivity disorder in pediatric patients with epilepsy: Review of pharmacological treatment. Epilepsy Behav. 2008;12:217–233. doi: 10.1016/j.yebeh.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Feldman H, Crumrine P, Handen BL, Alvin R, Teodori J. Methylphenidate in children with seizures and attention-deficit disorder. American Journal of Diseases of Children. 1989;143:1081–6. doi: 10.1001/archpedi.1989.02150210117030. [DOI] [PubMed] [Google Scholar]
- 18.Gross-Tsur V, Manor O, van der Meere J, Joseph A, Shalev RS. Epilepsy and attention deficit hyperactivity disorder: is methylphenidate safe and effective? J Pediatr. 1997;130:670–4. doi: 10.1016/s0022-3476(97)70258-0. [DOI] [PubMed] [Google Scholar]
- 19.Gucuyener K, Erdemoglu AK, Senol S, Serdaroglu A, Soysal S, Kockar AI. Use of methylphenidate for attention-deficit hyperactivity disorder in patients with epilepsy or electroencephalographic abnormalities. J Child Neurol. 2003;18:109–12. doi: 10.1177/08830738030180020601. [DOI] [PubMed] [Google Scholar]
- 20.McGough JJ, McBurnett K, Bukstein O, Wilens TE, Greenhill L, Lerner M, Stein M. Once-daily OROS methylphenidate is safe and well tolerated in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2006;16:351–6. doi: 10.1089/cap.2006.16.351. [DOI] [PubMed] [Google Scholar]
- 21.Pelham WE, Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, Morrisey SM, Chronis AM, Forehand GL, Nguyen CA, Hoffman MT, Lock TM, Fielbelkorn K, Coles EK, Panahon CJ, Steiner RL, Meichenbaum DL, Onyango AN, Morse GD. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107:E105. doi: 10.1542/peds.107.6.e105. [DOI] [PubMed] [Google Scholar]
- 22.Wilens TE, McBurnett K, Bukstein O, McGough J, Greenhill L, Lerner M, Stein MA, Conners CK, Duby J, Newcorn J, Bailey CE, Kratochvil CJ, Coury D, Casat C, Denisco MJ, Halstead P, Bloom L, Zimmerman BA, Gu J, Cooper KM, Lynch JM. Multisite controlled study of OROS methylphenidate in the treatment of adolescents with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2006;160:82–90. doi: 10.1001/archpedi.160.1.82. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, O’Dwyer PJ, Christian M, Humphrey JS. Phase I clinical trial design in cancer drug development. Journal of Clinical Oncology. 2000;18:684–92. doi: 10.1200/JCO.2000.18.3.684. [DOI] [PubMed] [Google Scholar]
- 24.Thall PF, Lee JJ, Tseng CH, Estey EH. Accrual strategies for phase I trials with delayed patient outcome. Statistics In Medicine. 1999;18:1155–1169. doi: 10.1002/(sici)1097-0258(19990530)18:10<1155::aid-sim114>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Ottman R, Hauser WA, Stallone L. Semistructured interview for seizure classification: agreement with physicians’ diagnoses. Epilepsia. 1990;31:110–5. doi: 10.1111/j.1528-1157.1990.tb05368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ottman R, Lee JH, Hauser WA, Hong S, Hesdorffer D, Schupf N, Pedley TA, Scheuer ML. Reliability of seizure classification using a semistructured interview. Neurology. 1993;43:2526–30. doi: 10.1212/wnl.43.12.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anonymous. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy Epilepsia. 1989;30:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 28.Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 29.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Puig-Antich J, Orvaschel H, Tabrizi M, Chambers W. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Epidemiologic Version. New York, NY: 1980. [Google Scholar]
- 31.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- 32.Bruininks RH, Woodcock RW, Weatherman RE, Hill BK. Scales of Independent Behavior-Revised (SIB-R) Chicago: Riverside; 1996. [Google Scholar]
- 33.DuPaul G. The ADHD Rating Scale: Normative data, reliability, and validity. Worcester, MA: 1990. [Google Scholar]
- 34.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklist, norms and clinical interpretations. New York, NY: The Guilford Press; 1998. [Google Scholar]
- 35.Guy W. Clinical global impression. In: Guy W, editor. ECDEU assessment manual for psychopharmacology. Rockville, Md: National Institute of Mental Health; 1976. pp. 218–21. [Google Scholar]
- 36.Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: A systemic placebo-controlled evaluation. Pediatrics. 1990;86:184–192. [PubMed] [Google Scholar]