Abstract
To understand gustatory physiology and associated dysfunctions it is important to know how stimuli placed in the mouth are encoded both in the periphery and in taste-related brain centres. The identification of distinct taste receptors, together with electrophysiological recordings and behavioural assessments in response to taste stimuli, suggest that information about distinct taste modalities (e.g., sweet versus bitter) are transmitted from the periphery to the brain via segregated pathways. In contrast, gustatory neurons throughout the brain are more broadly tuned, indicating that ensembles of neurons encode taste qualities. Recent evidence reviewed here suggests that the coding of gustatory stimuli is not immutable, but is dependant on a variety of factors including appetite regulating molecules and associative learning.
INTRODUCTION
The gustatory system, together with the somatosensory system, is involved in analyzing diverse features of food, such as its chemosensory (modality, intensity), orosensory (texture, temperature, pungency) and rewarding properties. Other senses such as vision and olfaction also contribute [1–2], but their modulating roles in food perception are beyond the scope of this review.
The first goal of this review is to elaborate gustatory coding schemes in the periphery and cortical areas. In particular, the review highlights the increasing complexity along the gustatory neural pathway, as cortical areas also contain information about tastants’ pleasantness or hedonic value (Glossary). The second goal is to show how the gustatory system, at the central level, integrates information from internal signals and changes the tastants’ cortical representation accordingly.
ORGANIZING TASTES: FROM TASTE BUDS TO CORTEX
Gustatory processing is first achieved at the level of taste receptor cells (TRCs) that are assembled into taste buds (TBs) distributed among different papillae of the tongue, palate, larynx, pharynx, and epiglottis. TBs contain about 100 TRCs that protrude through the lingual epithelium into a taste pore (Figure 1a). Upon tastant binding to receptors on microvilli of TRCs, transduction machinery is activated and neurotransmitters are released that cause the excitation of afferent nerve fibres. Two afferent branches of the facial nerve (VIIth) innervate the anterior tongue (chorda tympani nerve, CT) and the palate (greater superior petrosal, GSP). The lingual-tonsilar branch of the glossopharyngeal (GP or IXth) nerve innervates the posterior and lateral tongue areas and the superior laryngeal branch of the vagus nerve (Xth) innervates TRCs located in the larynx, pharynx and on the epiglottis. The CT has two discrete branches– one projecting to the rostral part of nucleus of solitary tract (rNST) that is involved in taste processing and the other to the medullary reticular formation (RF), a caudal brainstem pathway leading to reflexive oromotor functions [3]. The GP and vagus nerves are known to be involved in swallowing, gagging, salivary secretions and motor responses involved in eating [4].
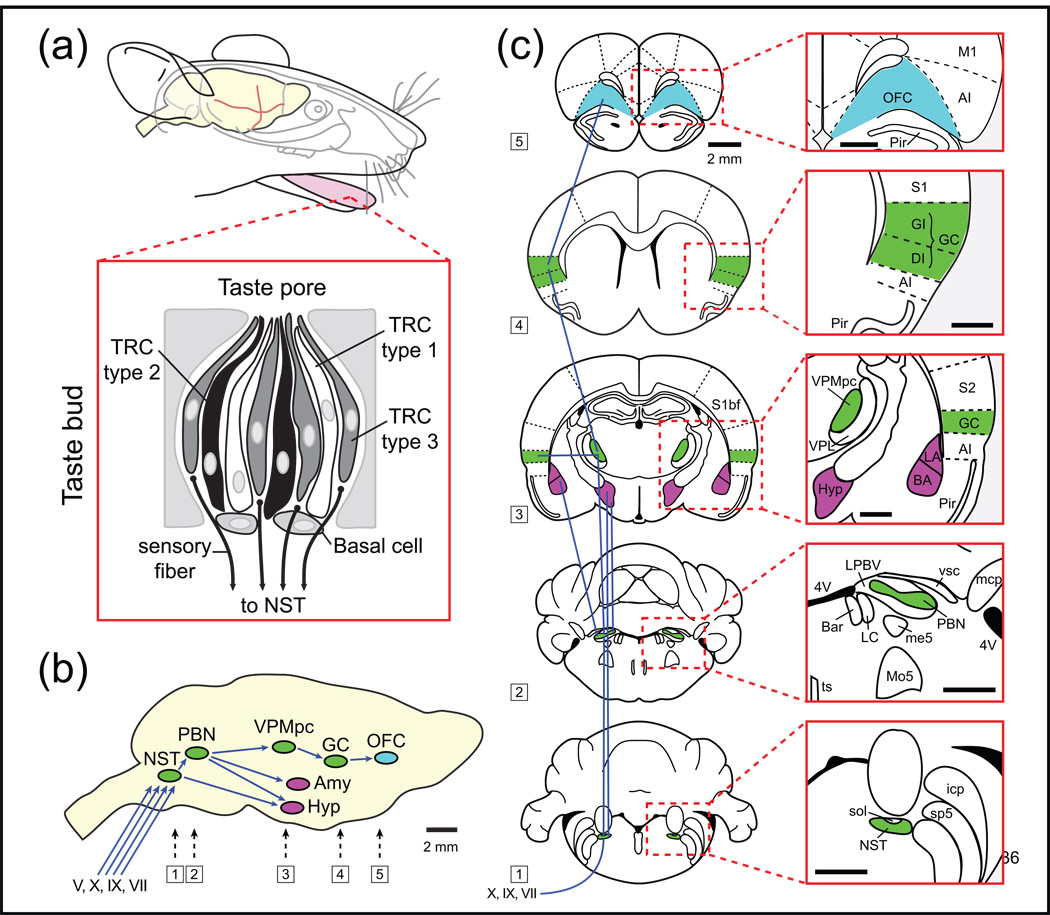
Figure 1. Schematic representation of the rodent taste pathway organization.
(a) Taste receptor cells (TRC) are the chemical sensors and are grouped in anatomical structures called taste buds distributed into different papillae of the tongue and the oral cavity. Each taste bud contains four different types of cells: 3 types of TRCs (types 1, 2 and 3) and basal cells involved in the genesis of new TRCs. (b) Three cranial nerves (VII, IX, X) innervate different parts of the oral cavity and convey taste information to the rNST. Input from the trigeminal nerve (V) also contributes to gustatory processing. The rNST is interconnected with other CNS regions. It receives input from the pontine parabrachial nucleus (PBN), the lateral hypothalamus, the gustatory cortex (GC), the central amygdala and reciprocally from the caudal (visceral) NST. The PBN projects to the VPMpc that in turn projects to the GC, that in turn projects to the OFC. OFC neurons project and receive inputs from the dorsolateral prefrontal cortex (not shown). The medial prefrontal cortex (not shown) appears to function as a sensory–visceromotor link that provides the major cortical output to visceromotor structures in the hypothalamus and brainstem. (c) Anatomical overview of the central taste pathways. Scale bar 1 mm in red boxes. Abbreviations: 4V: fourth ventricle, AI: agranular insular cortex, BA: basolateral amygdala, Bar: Barrington’s nucleus, DI: dysgranular insular cortex, GC: gustatory cortex, GI: granular insular cortex, Hyp: lateral hypothalamus, icp: inferior cerebellar peduncle, LA: lateral amygdala, LC: locus coeruleus, LPBV: lateral parabrachial nucleus ventral, M1: primary motor cortex, mcp: middle cerebellar peduncle, me5: mesencephalic 5 tract, Mo5: motor trigeminal nucleus, NST: nucleus of the solitary tract, OFC: orbitofrontal cortex, PBN: parabrachial nucleus, Pir: piriform cortex, S1: primary somatosensory cortex, S1bf: somatosensory cortex barrel field, S2: secondary somatosensory cortex, sol: solitary tract, sp5: spinal trigeminal tract, ts: tectospinal tract, VPL: ventral osterolateral nucleus of the thalamus, VPMpc: ventral posteromedial nucleus of the thalamus parvocellular division, vsc: ventral spinocerebellar tract.
These three cranial nerve (CN) branches, together with the lingual branch of the trigeminal nerve (Vth), converge in the medulla to synapse in the rNST (Figure 1b). The rodent and primate taste systems differ in that for rodents, fibres from the rNST projects ipsilaterally to the PBN (Figure 1b,c), whereas the primates rNST fibres project directly to the parvocellular division of the ventroposterior medial nucleus of the thalamus (VPMpc). From the PBN there are reciprocal projections to the ventral forebrain, the bed nucleus of stria terminalis, the lateral hypothalamus (LH) and the basolateral amygdala (BLA) [5]. These structures are involved in the processing of taste-related tasks such as feeding and/or taste memory formation.
The gustatory fibres from the VPMpc terminate in the primary gustatory cortex (GC). GC neurons, in turn, project (reciprocally) to the PBN, the primary somatosensory cortex (S1) and the orbitofrontal cortex (OFC; Figure 1b-c). The orbital network receives sensory inputs from several modalities related to the intake of food, including olfaction, taste, visceral afferents, somatic sensation and vision [6].
Specific information about the tastants is also transmitted from the CNS to the solitary nucleus,where it is distributed among many pathways involved in chemical identification, reward, memory and motor responses. Thus, gustatory pathways in the brain consist of interacting and dynamic feed-forward and top-down pathways that are widely distributed among several brain areas involved in tastant identification, reward and the decision to ingest.
TASTE CODING
Two major hypotheses on how taste information is processed currently exist [7]. The first, called “labelled line” (Glossary) refers to a coding model in which peripheral (or central) neurons that respond the most robustly to a given taste modality carry the totality of the information on segregated pathways. This coding scheme may simply be thought of as a wire that goes from the periphery to the higher areas that signals a particular modality (e.g., sucrose). Intensity increases are indicated by an increase in neuronal activity. The second view affirms that a modality and its quantity (intensity) are encoded by ensembles of broadly tuned neurons (Glossary). This “ensemble code” (Glossary) is also known as “combinatorial” or “across fibre pattern”. There are proponents for both major coding schemes, as well as augmented forms of them involving temporal contributions. Evidence for the use of these different schemes at various locations in the gustatory pathway will be discussed.
Coding at the periphery
Data from studies using a variety of different techniques, including genetic, morphological, and electrophysiological recordings, have shown that several types (and subtypes) of TRCs are present in TBs, cell types I, II and III [8]. Basal cells are progenitors of TRC cells and are found at the base of TBs [9–10]. Type I cells were initially believed to be supporting (or ‘glial like’) cells, as they express enzymes involved in transmitter uptake and degradation. However, recent studies showed that they express an amiloride-sensitive epithelial sodium channel (ENaC) [11–12], which has been demonstrated to be the major sensor for salt (NaCl) perception [13].
There is, however, general agreement regarding the roles of type II TRCs. These cells contain G Protein Coupled Receptors (GPCRs) that selectively respond (at concentrations that can be considered close to physiological) to sweet tastants (T1R2-T1R3), bitter tastants (T2Rs) and amino acids/umami (T1R1-T1R3), since the deletion of the receptors (or of the type II cells that contain them) selectively prevents both whole nerve cell responses and behavioural responses – i.e. ingestion for sweet tastants and rejection for bitter tastants (see Figure 2a and [14]). Critical signalling molecules that have been identified as being downstream from these GPCRs include a phospholipase (PLC-β2) and a transient receptor potential (TRP) channel (TRPM5) that is activated by IP3 induced increases in intracellular calcium [14–15].
Figure 2. Neural responses along the rodent taste pathway.
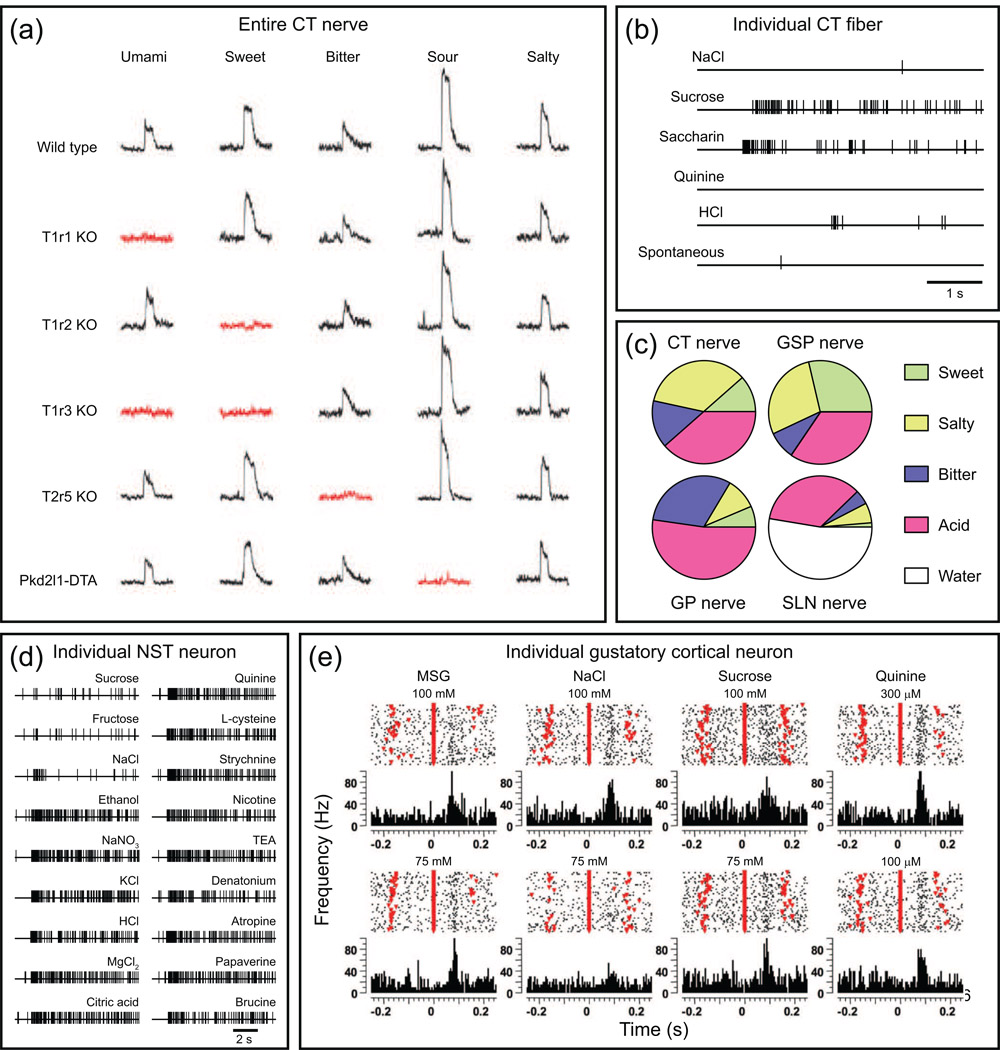
(a) Five perceptually distinct taste qualities, umami, sweet, bitter, sour and salty (not shown but see [13]), are mediated by specific receptors and cells. The traces show whole nerve recordings of tastant-induced activity in the CT nerve of wild type and various gene-knockout (KO) mice or cell ablation studies (Pkd2l1-DTA). T1R1 (and T1R3) functions as a receptor for umami tastants, T1R2 (and T1R3) for sweet tastants, T2R5 for the bitter tastant cycloheximide and PKD2L1 for sour tastants. Pkd2l1-DTA refers to animals expressing diphtheria toxin in Pkd2l1 expressing TRCs cells. Red traces highlight specific taste deficits in each genetically altered mouse line. Adapted, with permission, from Ref [16]. (b) A single unit recording from the hamster chorda tympani (CT) nerve illustrating a neuron that was selectively responsive to super-threshold concentrations of both sweet tastants (sucrose and saccharin) but unresponsive to salty (NaCl), bitter (quinine) or sour (hydrochloric acid, HCl) tastants. Adapted, with permission, from Ref. [91]. (c) Proportional responses to sweet (0.3 M sucrose), salty (0.3 M NaCl), bitter (10 mM quinine) and sour (10 mM HCl) recorded from four different nerve cell types in the hamster gustatory system: CT nerve, greater superior petrosal nerve (GSP- from cranial nerve VII), glossopharyngeal nerve (GP, from CN IX) and from the superior laryngeal nerve (SLN, from CN X) fibres. Each pie represents the response to each stimulus as a proportion of the sum of the responses of that nerve to all stimuli. Adapted, with permission, from Ref [92]. (d) Single unit recording from individual neurons of the rat nucleus of solitary tract (NST) illustrating a broadly tuned neuron that responded to a variety of perceptually distinct tastants (ethanol, NaNO3, KCl, HCl, MgCl2 and citric acid) but apparently not to NaCl, sucrose or fructose. Adapted, with permission, from Ref. [50]. (e) Raster plots and peri-stimulus time histograms [PSTHs, which are the sum of the responses (action potentials) of individual trials for a given bin size aligned relatively to a stimulus onset] of a broadly tuned neuron from the rat gustatory cortex obtained while a rat was licking to receive a tastant at time 0 ms. Umami (MSG), salty (NaCl), sweet (sucrose) and bitter (quinine) tastants were delivered at two different concentrations for each. Other licks in which no tastants were delivered are indicated by inverted red triangles. Action potentials are indicated by dots. Although difficult to see at this scale, there are clear temporal differences in the responses to tastants. Adapted, with permission, from Ref. [73].
For responses to acid it was found that type III TRCs contain TRP channels called PKD2L1. Deletion of these PKD2L1 expressing cells selectively eliminates whole nerve responses to acid (Figure 2a and [16]). In addition, taste cells containing PKD2L1 also express CAR4 [17], an extracellular glycosylphosphatidylinositol (GPI)-anchored carbonic anhydrase, along with intracellular forms of carbonic anhydrase [17–18]. Both forms play essential roles in modulating the conversion of CO2 to bicarbonate (HCO3−) and hydrogen, which in principle, could produce a sour taste perception (note: CO2 is also a trigeminal nerve stimulant, giving rise to painful and tingling sensations [19]). As noted, deletion of TRCs containing the alpha subunit of ENaCs produced animals exhibiting a complete loss of salt (NaCl) attraction and taste response [13].
These data provide very strong evidence for the labelled lines model, in which separate pathways link the activation of taste cells with particular receptors and to predictable behavioural responses [20]. Further experiments using genetically modified mice with altered taste sensing cells support this idea. For example, mice in which a receptor that was activated by a synthetic and normally tasteless ligand was expressed in bitter responsive cells, induced avoidance behaviour, whereas the same receptor expressed in sweet-responsive cells provoked acceptance behaviour [21–22]. Finally, expressing a bitter taste receptor in a sweet-sensing cell generated mice that were attracted by a bitter tasting compound [21].
As groundbreaking as these experiments are, it would be useful to also record from single units, as the whole-cell nerve recordings would predict that there should largely (or exclusively) be neurons that exhibit selectivity to particular taste qualities, like the example shown in Figure 1b for sweet tastants. However, neurons that are directly activated in response to TRC activation are often broadly tuned [23]. Thus, in the context of the labelled line coding model, it is not clear what information these broadly tuned neurons transmit. In addition, other types of stimuli should be tested to see if the tastant exclusivity is retained. Such stimuli may include divalent and trivalent salts [24], water [25–26], fatty acids [27], nicotine [28] and capsaicin and other compounds that activate TRP vanilloid 1 (TRPV1) channels found in TRCs [29].
Despite the strong evidence outlined above for a labelled line scheme for encoding taste, other studies have indicated that the coding scheme used in the periphery may be more complex. One reason for this rationale is that voltage-dependent calcium channels (VGCCs), whose opening can cause the pre-synaptic vesicular release of transmitters onto primary nerve terminals, are only expressed in type III cells [30]. That is, only type III cells are believed to form conventional synapses with primary neurons, allowing for a major output pathway to the rNST. Moreover, type III cells are broadly tuned to respond widely to a range of stimuli, implying that the afferent fibres that synapse with them should also be broadly tuned.
How then is taste information processed in a model in which type III cells are the only cells releasing transmitters on nerve terminals? One possibility is that other TRC types contact intragemmal fibres without apparently forming synapses [8] and these fibres are activated by the release of transmitters (and/or peptides) from TRCs and then may form a parallel pathway to the rNST [3, 31]. In addition, tastants activating type II cells depolarize them, causing a release of ATP via connexins and/or pannexins [32–33] onto P2X receptors [34] (i.e., ion channels that open in response to the binding of extracellular ATP) expressed on type III cells. This, in turn, depolarizes type III cells, activating VGCCs that subsequently cause the release of serotonin (5-HT) [35] onto nerve terminals. As suggested, ATP (as well as other transmitters) could also activate the non-synapsed intragemmal neurons [36]. In this coding scheme, NaCl or acid would directly activate distinct subpopulations of type III cells that would then directly transmit information to the CNS via primary neurons that are synapsed with them. Since ~80% of type III cells are broadly tuned [37], it follows that this scheme would predict that ensembles of primary neurons would encode tastant identity. Although this model has several attractive features, it has not consolidated the results obtained on taste cells with either recordings from primary neurons or to behavioural experiments. One useful experiment would be to knock out all type cells with VGCCs and determine from single neuron recordings if this affects the neural responses to tastants known to activate type II cells, since deleting PKD2L1 expressing type III cells does not impair whole nerve responses to taste modalities other than sour (Figure 2a and [38]).
In addition to the noted ATP-dependent processing in the taste bud, TRCs also contain receptors for leptin, a peptide that is released from adipose cells in response to eating. Single unit CT recordings have shown that leptin caused a selective reduction in responses to sweet tastants [39] whereas endocannabinoids, such as anandamide, bind to CB1 receptors on Type II cells and selectively increase nerve and behavioural responses to sweet tastants [40]. This means that neurons classified as “best responders” in the labelled line scheme may change their tastant selectivity during or after a meal or after ingestion of cannabinoids. “Best” is interpreted to mean that at comparable intensity or response levels, the average activity (usually taken over several seconds and few trials) is greater for one tastant than for the others tested. Thus, depending on the particular context, even at the periphery, the tastant selectivity of a particular neuron may change.
Moreover, peptidergic modulators of appetite such as neuropeptide Y (NPY), cholecystokinin (CCK), galanin and glucagon-like peptide-1 (GLP-1) may alter tastant processing through autocrine or paracrine mechanisms in the taste bud [41–43] and at various levels in the CNS [44]. Finally, in addition to ATP, TRCs also contain other neurotransmitters including noradrenaline, serotonin, acetylcholine, and γ-aminobutyric acid (GABA) whose receptors may be in TRCs and/or nerve terminals [28, 35, 45]. How all these transmitters and peptides function to modulate the encoding of tastants at all levels of the gustatory pathway will be, in the coming years, an important area of research.
Taken together, the present evidence suggests that the peripheral taste system-at least for the five perceptually distinct taste modalities-uses a labelled line coding scheme. The issue of whether this scheme is conserved and utilized within the higher nervous system levels of the pathway is discussed below.
Coding in the Brainstem and Thalamus
Before discussing tastant responses in the brainstem and higher brain regions, when considering gustatory coding, it is important to mention four factors that may influence the interpretation of experimental results. First, a large majority of the electrophysiological recordings are performed in anesthetized animals. The anaesthetic agent used may limit or modify inputs from other brain areas and hence alter and/or modify the neuron’s selectivity to tastants [46]. Second, in nearly all of the studies mentioned, tastants do not activate the entire oral cavity. This is important since inputs from the three taste nerves have very different chemical selectivities (see Figure 2c), which may alter or modify the selectivity of the responses in which only one or two inputs were activated. Third, tastants were generally delivered in a manner that was not in the animal’s control (i.e. passively) as opposed to goal-directed (i.e. active and voluntary). It is known that under these conditions responses to sensory stimuli are markedly different throughout the brain [47–49]. Thus, the manner of delivery of the tastant, by passive delivery over the tongue and palate in anesthetized subjects, or by hand delivery, intraoral cannula or by active licking, can dramatically alter the observed response. Finally, in many (if not most) studies of taste coding the subjects do not usually ingest the stimuli, even though post-ingestive effects are known to alter neural responses (see below).
With respect to gustatory encoding in the CNS, there have been many studies of taste responses in NST, PBN and thalamic (VPN) neurons. Because of space limitations, only a limited number of key aspects will be highlighted from these studies. Figure 2d shows a representative rNST response obtained from an anesthetized rat in which both the anterior tongue and palate were exposed to a variety of tastants for 10 seconds [50]. While this neuron was unresponsive to sucrose or fructose, it was responsive to perceptually different salts, ethanol, acids, and many bitter tasting compounds. It is evident that this broadly tuned neuron is not part of a labelled line, but may be a neural element of an ensemble. However, there are other subpopulations of cells within the NST, PBN and VPN that are tastant selective, insofar as they respond “best” to a particular stimulus. Thus, the extent of selectivity within these brain regions is variable; with some neurons being quite selective and others more broadly tuned [50–54]. As a general statement, higher order neurons appear to be more broadly tuned than those at the periphery. In addition, as in the periphery, a neuron’s selectivity in the CNS can be modified or changed depending on a variety of factors such as circulating hormones, glucose and temperature, as well as input from cortical and visceral areas [55–58].
With respect to a role for spike timing in taste coding, it is clear from experimental evidence that although responses can be evoked by all stimuli in a broadly-tuned neuron, their temporal responses may differ, even among the same taste stimulus category (e.g. compare quinine and denatonium in Figure 2d). From this example, it is evident that for these two stimuli, even if the average spike rate was the same, temporal information could be used to distinguish between them. In general, dynamic features of neuronal activity are the result of a balance of excitatory and inhibitory influences that can arise from intra-area networks as well as input from other cortical and sub-cortical areas. This activity can affect the neuronal spike timing in a manner that may improve discrimination among tastants [59–64].
Coding in the primary gustatory cortex (GC)
The GC is a multimodal area that responds to tastants as well as to thermal, mechanical, visceral and nociceptive stimuli [65–67]. Basically, the responses of individual GC neurons to tastants exhibit the same pattern of activity as those described for brainstem and thalamic neurons in that some have been reported to be quite selective to tastants, whereas others are more broadly tuned (Figure 2e). With few exceptions, the GC responses were measured as the average activity over several seconds and the tastants were delivered passively to the animal.
The voluntary intake and active processing of sensory stimuli differs from passive processing, not only for differences cited above, but also because there is a temporal component that involves licking, swallowing or chewing that is either absent or different under passive processing. The point of integrating the evoked taste response over several seconds has some associated issues including the possibility that other information such as hedonic value and somatosensory input may be included [68–69].
However, trained animals can discriminate among tastants and determine their hedonic value in ~200 ms for rat and ~400 ms for humans [62, 70]. In this regard, recordings from the GC obtained from rodents that lick for food revealed rapid responses ( ~150–200 ms) that were broadly tuned (Figure 2e). They also showed that neuronal ensembles are better predictors of the tastants and concentrations than are individual neurons, suggesting a combinatorial coding scheme [71–73]. These studies also found that it is possible to discriminate among the tastants (and concentrations) on a single trial, and that both rate and temporal information provide better predictions than rate alone [72].
SPATIAL MAPS OF TASTE MODALITIES IN THE GUSTATORY CORTEX
In the mammalian brain, cells which perform a given function, or share common functional properties, are often grouped together anatomically. Striking examples come from the primary visual, auditory and somatosensory neocortices that are organized in spatial maps according to specific features of the sensory stimulus. Following the same organization principles, in the GC one may also expect to find a chemotopic organization, i.e. topographical regions in which neurons respond to a preferred taste modality. Recent studies using optical imaging of intrinsic signals (see Glossary) identified four distinctive spatial patterns representing the four distinct taste modalities (sweet, bitter, salty and sour), but no region was clearly specific to a single modality (Figure 3a,b) [74–75]. In humans, a functional magnetic resonance imaging (fMRI) study showed, despite a considerable inter-individual variability, that the five taste modalities evoke specific patterns with some overlap [76]. Although neither imaging technique currently has the resolution power to resolve single neurons, it is reasonable to assume that these common regions might contain a higher number of broadly tuned neurons, whereas regions responding to either one or two modalities might contain more narrowly tuned neurons. To determine if the regional selectivity would remain narrowly tuned, it would be important to measure responses if all taste sensitive areas (Figure 2c) were activated and also to test other taste and somatosensory stimuli.
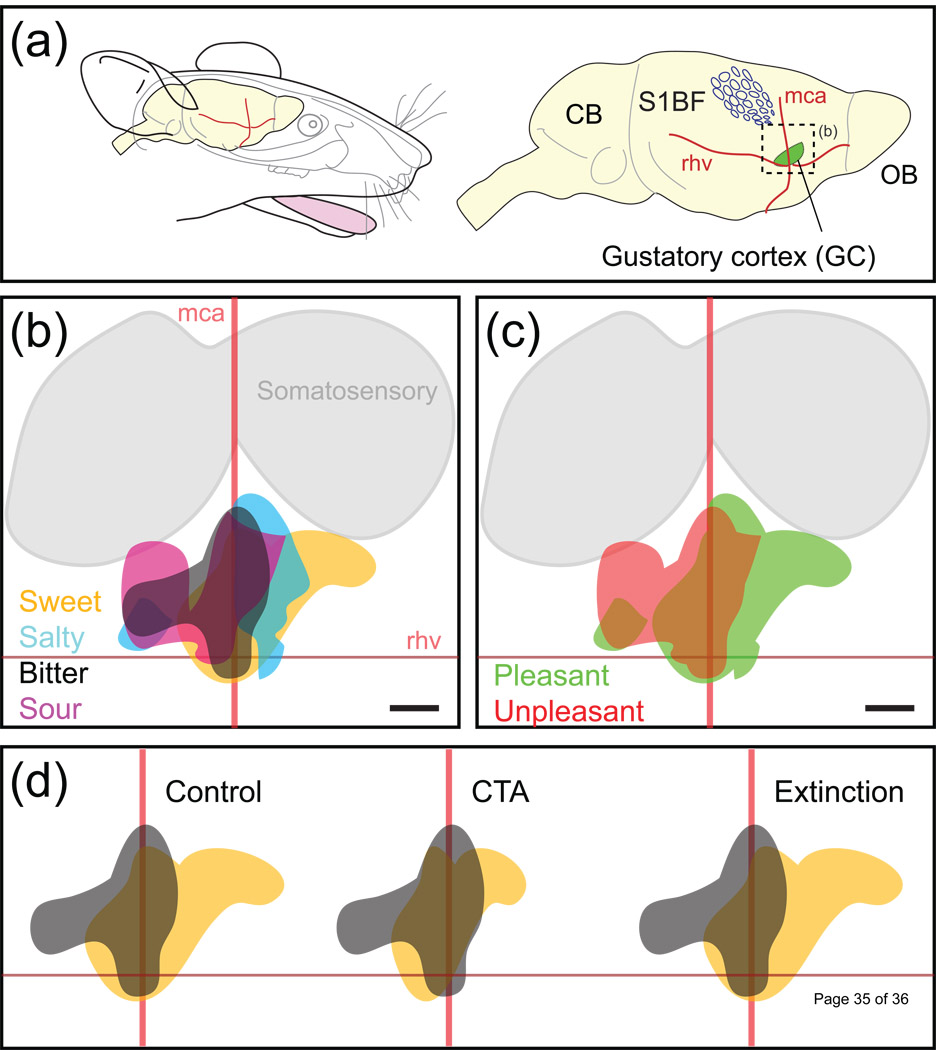
Figure 3. Topographical representations in the rat gustatory cortex.
(a) Approximate size and location of the GC with respect to anatomical landmarks (blood vessels: middle cerebral artery, mca; rhv, rhinal veins) and other sensory areas of the brain (olfactory bulb, OB; primary somatosensory cortex barrel field, S1BF). (b) Schematic representation of the cortical territories activated following stimulation with four stimuli representing four different taste modalities (sweet, salty, bitter, sour). Same orientation as in (a). (c) Pleasant (sweet and salty) and unpleasant (bitter and sour) regions appear to be temporally distinguishable. Responses to pleasant stimuli seem to be represented more rostrally than responses to unpleasant stimuli. (d) Relationship between behavioural state and cortical state in the gustatory cortex. In a naïve (i.e. control) rat, cortical representations of the hedonically positive (saccharin, orange) and negative (quinine, gray) tastants are quite different, though commonly activated cortical territories exist. After conditioned taste aversive (CTA) training, in which the malaise inducing agent lithium chloride (LiCl) is paired with the ingestion of saccharin, the normally positive stimulus of the latter becomes aversive and the pattern changes accordingly to become more similar (highly correlative) to the quinine response. After saccharin aversion extinction (Glossary), the hedonic value of saccharin reverts to a positive response, and its cortical map is again less similar (low correlation) to the quinine pattern. Note that the new representation of saccharin after extinction may not be a simple return to the same one that existed prior to conditioning.
Gustatory perception also possesses an important affective aspect, described as the hedonic value, with a positive value indicating pleasantness and a negative value an unpleasant response. Depending on the concentration, bitter and sour tastants are generally unpleasant, whereas sweet and salty tastants are pleasant. Electrophysiological studies in rats [68, 77], neuroimaging studies in humans [78–79] and intrinsic imaging experiments in rats (Figure 3c) all found that the hedonic value of tastants is represented in the GC. In the intrinsic imaging studies, taste stimuli with similar hedonic values activated more similar regions than stimuli with different hedonic values [74]. fMRI studies indicated that the hedonic value may also be represented in the amygdala [79]. An imaging technique with high temporal resolution such as voltage sensitive dye imaging [80] might be used to individuate different dynamic phases of the response. In summary, with regard to coding schemes, intrinsic imaging data from the GC indicate that the responses to tastants may be represented topographically. Higher spatial resolution calcium imaging studies of single neurons should be performed to see if this topographical representation is maintained at a finer scale.
TASTE ASSOCIATIVE ENCODING
Having explored GC responses under conditions where the taste stimuli does not have any intrinsic meaning to the animal, other than their inherent hedonic value, we now review what happens when the response to a tastant is associated with a salient event. If taste processing were immutable, it follows that the behavioural response should be invariant. However, in a conditioned taste aversion (CTA, see Glossary) paradigm, electrophysiological studies in rats found that some units change their response profile before and after coupling the taste stimulus (usually saccharin) with visceral malaise (usually LiCl) [81–82]. Over a larger scale, using intrinsic imaging in the rat GC, the researchers induced the aversion to a pleasant stimulus (saccharin) and compared its cortical representation to the response elicited by a reference aversive bitter tastant, quinine (Figure 3c and [75]). Their results show that the taste maps for saccharin are plastic and that they rearrange according to the shift of its hedonic value, both in the CTA acquisition (where saccharin becomes aversive) and extinction (saccharin is attractive again) learning phase. As the taste modality remains unchanged (i.e. saccharin interacts with the same peripheral receptors), changes in correlation are directly related to shifts of the perceived hedonic value of the compound. These results provide strong evidence that the GC activation patterns carry information not only for the stimulus modality but also on the palatability of the tastant.
THE FRONTAL CORTEX AND REWARD
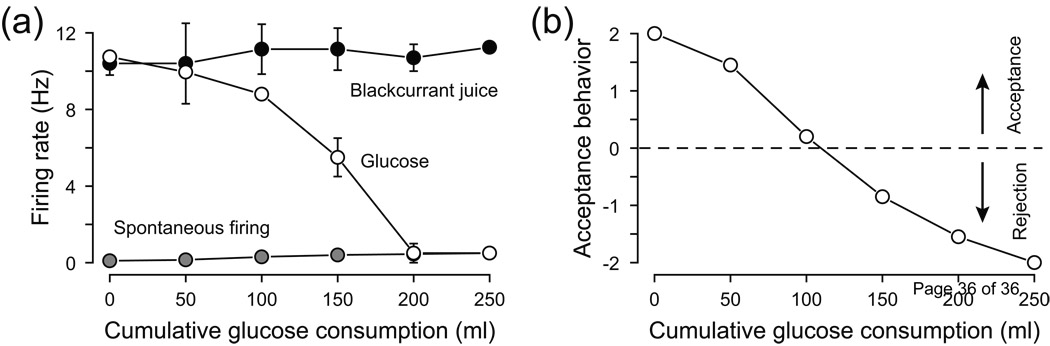
The OFC is often called the secondary taste cortex, as it has direct projections from the GC. The functions of many OFC neurons are involved in decision making, predicting reward, and also encoding the reward value [83–84]. The OFC receives convergent gustatory, somatosensory, visual and olfactory inputs, and consequently, many OFC neurons exhibit multisensory responses that may be important in consolidating the flavour of food. One physiologically interesting gustatory property of the OFC related to gut-brain interactions is called sensory-specific satiety (Glossary). This phenomenon occurs when a particular food eaten to satiety becomes less rewarding, without changing the taste of the food itself [85]. In other words, the relative reward value of that particular food has been diminished while the reward value of other foods may remain unchanged. Sensory specific satiety changes have been observed in both electrophysiological studies from non-human primates [86] and in human fMRI studies [87]. A good example of a neural response associated with sensory specific satiety is shown in Figure 4. Initially the responses to glucose and to blackcurrant juice are comparable. As satiation to glucose proceeds, the response to the sugar dramatically drops, whereas the response to the juice remains unchanged. Such information reveals that throughout the gustatory pathway a neuron’s chemoselectivity (or best neuron category) may change depending on the animal’s internal state. In this regard, recent experiments found that post-ingestive effects will alter taste responses throughout the brain [88–89].
Figure 4. Sensory response is altered by satiety.
(a) The responses of a neuron from the primate orbitofrontal cortex (OFC) that changes from being unselective between glucose and blackcurrant juice to becoming selective to blackcurrant juice as the subject ingested 50 mL of 20% glucose at each point. (b) Behavioural data of acceptance or rejection to the glucose on a rating scale ranging from +2 to −2. Adapted, with permission, from Ref. [86].
OFC neurons also project to the prefrontal cortex (PFC). Studies in non human primates have found that neurons in the dorsolateral prefrontal cortex (DLPFC) encode the reward amount and the rewards forthcoming response, while neurons in the OFC more often encoded the reward amount alone [90]. The authors suggested that reward information entering the PFC via the OFC passes to the DLPFC, where it is involved in controlling behaviour.
CONCLUSION
This review provides evidence that gustatory processing in the periphery uses a labelled line scheme, whereas within the CNS, gustatory processing is contained in a multisensory, distributed, feed-forward and backward, plastic network that includes reward, and whose responses may depend on the animal’s internal state. That is, the processing of information regarding what is ingested must be continually updated, and taken into account as internal states associated with malaise or satiety can greatly modulate the responses. Future studies will necessarily need to delve into more details on the analysis of all the parameters that play a role in gustatory perception (Box 1). First and foremost, one needs to address the nature of the stimulus itself. A food stimulus is characterized not only by its taste, but also by its texture in the mouth, smell and visual appeal. Eating is inherently multisensory and the combined roles of olfaction, somatosensation, vision and audition should be explored at all levels of the gustatory pathway. Furthermore, the parameters that influence the subject need to be analyzed in detail. Satiation, expectation, attention and memory can all strongly influence taste perception. A better understanding of all of these modulating mechanisms in subjects would also pave the way to elucidating the interdependencies of food-related pathologies and altered tastant representation.
TEXT BOX 1
Outstanding questions on gustatory processing
What are the detailed electrophysiological responses and circuitry leading from tastant-specific taste cells to cells in the NST and beyond? Throughout the gustatory system, reward and motor pathways determine the local connectivity and inter-area connectivity.
To what extent do interactions between different taste cells modulate taste responses? It would be interesting to knock-out all taste cells containing VGCCs and then perform both electrophysiological and behavioural measurements. Similarly, this approach would also be of interest to ascertain roles for the numerous transmitters, peptides and their receptors that are expressed in taste buds.
What is the possible role of temporal dynamics and synchrony in the encoding and learning about taste stimuli in and between brain areas?
How are taste stimuli such as fats, oils, water, and metallic tastants processed in taste buds, the gut and in the brain?
How do mixtures and changes in temperatures modify neural responses throughout the taste-reward pathway? These experiments should be performed and compared in both anesthesitized and awake animals. Awake animals should also be given solid foods, not only because it is more natural, but also to determine possible roles of chewing on gustatory processing.
How does making a tastant salient or meaningful to the subject affect gustatory processing? Similarly, how does attention affect gustatory processing?
ACKNOWLEDGEMENTS
We apologize to colleagues whose studies have not been cited in this review due to space constraints. A.C. was supported by the University of Geneva, the Swiss National Science Foundation, the European Research Council (contract number ERC-2009-StG-243344-NEUROCHEMS), EMBO (Young Investigator program) and the European Commission Coordination Action ENINET (contract number LSHM-CT-2005-19063). S.A.S. was supported by the NIH grant DC-001065.
Glossary
- Broadly tuned
describes a neuron or sensory cell that significantly changes its firing discharge to a wide-range of different stimuli (e.g. different taste modalities). This is opposed to narrowly-tuned cells.
- Conditioned taste aversion (CTA)
represents an efficient paradigm of conditioned learning experience where a subject learns to avoid a taste stimulus (conditioned sensory stimulus, CS) paired with visceral malaise (strong unconditioned stimulus, US, such as LiCl) [93].
- Extinction training
occurs when a behavioral response that had previously been reinforced is no longer effective.
- Ensemble code
also named “across fibre pattern”, affirms that the information about a stimulus (e.g. tastant) is extracted by comparing the activity across a neuronal population (or ensemble) that responds with different intensity levels to multiple stimuli [94–95].
- Hedonics
regards the study of pleasant and unpleasant sensations.
- Intrinsic signal imaging
originates from different mechanisms such as changes in the physical properties of the tissue and/or changes of fluorescence or absorption of intrinsic molecules [96]. However, all these signals can be efficiently used for functional mapping and give rise to similar results [97]. This technique offers the best solution to reliably monitor activity of brain activated regions with a very good spatial resolution [98].
- Labelled line
refers to a coding model in which peripheral (or central) neurons that respond the most robustly to a given taste modality carry the totality of the information on segregated pathways to the brain.
- Narrowly tuned
describes a neuron or sensory cell that significantly changes its firing discharge to a very precise subset of stimuli. This is opposed to broadly-tuned cells.
- Sensory Specific Satiety
refers to a decrease in the reward value of an ingested food while leaving the reward value of other foods unchanged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dalton P, et al. The merging of the senses: integration of subthreshold taste and smell. Nature neuroscience. 2000;3:431–432. doi: 10.1038/74797. [DOI] [PubMed] [Google Scholar]
- 2.Gottfried JA, Dolan RJ. The nose smells what the eye sees: crossmodal visual facilitation of human olfactory perception. Neuron. 2003;39:375–386. doi: 10.1016/s0896-6273(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 3.Zaidi FN, et al. Types of taste circuits synaptically linked to a few geniculate ganglion neurons. J Comp Neurol. 2008;511:753–772. doi: 10.1002/cne.21869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev. 2005;4:143–191. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- 5.Tokita K, et al. Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience. 2009;161:475–488. doi: 10.1016/j.neuroscience.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 7.Smith DV, John SJ. Neural coding of gustatory information. Curr Opin Neurobiol. 1999;9:427–435. doi: 10.1016/S0959-4388(99)80064-6. [DOI] [PubMed] [Google Scholar]
- 8.Kinnamon JC, Yang R. Ultrastructure of Taste Buds. In: Firestein S, Beauchamp GK, editors. The Senses: A Comprehensive Reference. Academic Press; 2008. pp. 135–156. [Google Scholar]
- 9.Finger TE. Cell types and lineages in taste buds. Chemical senses. 2005;30 Suppl 1:54–55. doi: 10.1093/chemse/bjh110. [DOI] [PubMed] [Google Scholar]
- 10.Okubo T, et al. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 2009;27:442–450. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shigemura N, et al. Amiloride-sensitive NaCl taste responses are associated with genetic variation of ENaC {alpha} subunit in mice. Am J Physiol Regul Integr Comp Physiol. 2007 doi: 10.1152/ajpregu.00420.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandenbeuch A, et al. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC neuroscience. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandrashekar J, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandrashekar J, et al. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 15.Perez CA, et al. A transient receptor potential channel expressed in taste receptor cells. Nature neuroscience. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 16.Huang AL, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandrashekar J, et al. The taste of carbonation. Science (New York, N.Y. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyall V, et al. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am J Physiol Cell Physiol. 2001;281:C1005–C1013. doi: 10.1152/ajpcell.2001.281.3.C1005. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, et al. Capsaicin and carbon dioxide act by distinct mechanisms on sensory nerve terminals in the cat cornea. Pain. 1997;70:23–29. doi: 10.1016/s0304-3959(96)03256-3. [DOI] [PubMed] [Google Scholar]
- 20.Yarmolinsky DA, et al. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller KL, et al. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- 22.Zhao GQ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 23.Gilbertson TA, et al. Distribution of gustatory sensitivities in rat taste cells: whole-cell responses to apical chemical stimulation. J Neurosci. 2001;21:4931–4941. doi: 10.1523/JNEUROSCI.21-13-04931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riera CE, et al. Sensory attributes of complex tasting divalent salts are mediated by TRPM5 and TRPV1 channels. J Neurosci. 2009;29:2654–2662. doi: 10.1523/JNEUROSCI.4694-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson KJ, et al. Expression of aquaporin water channels in rat taste buds. Chemical senses. 2007;32:411–421. doi: 10.1093/chemse/bjm006. [DOI] [PubMed] [Google Scholar]
- 26.Cameron P, et al. The molecular basis for water taste in Drosophila. Nature. 2010 doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaillard D, et al. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. Faseb J. 2008;22:1458–1468. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira-Maia AJ, et al. Nicotine activates TRPM5-dependent and independent taste pathways. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1596–1601. doi: 10.1073/pnas.0810184106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyall V, et al. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. The Journal of physiology. 2004;558:147–159. doi: 10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clapp TR, et al. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roper SD. Parallel processing in mammalian taste buds? Physiol Behav. 2009;97:604–608. doi: 10.1016/j.physbeh.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YJ, et al. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romanov RA, et al. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol. 2008;132:731–744. doi: 10.1085/jgp.200810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finger TE, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science (New York, N.Y. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 35.Huang YJ, et al. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohtubo Y, et al. Optical recordings of taste responses from fungiform papillae of mouse in situ. The Journal of physiology. 2001;530:287–293. doi: 10.1111/j.1469-7793.2001.0287l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomchik SM, et al. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang YA, et al. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. The Journal of physiology. 2008;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawai K, et al. Leptin as a modulator of sweet taste sensitivities in mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11044–11049. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida R, et al. Endocannabinoids selectively enhance sweet taste. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:935–939. doi: 10.1073/pnas.0912048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seta Y, et al. Expression of galanin and the galanin receptor in rat taste buds. Arch Histol Cytol. 2006;69:273–280. doi: 10.1679/aohc.69.273. [DOI] [PubMed] [Google Scholar]
- 42.Shin YK, et al. Modulation of taste sensitivity by GLP-1 signaling. Journal of neurochemistry. 2008;106:455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao FL, et al. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11100–11105. doi: 10.1073/pnas.0501988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 45.Cao Y, et al. GABA expression in the mammalian taste bud functions as a route of inhibitory cell-to-cell communication. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4006–4011. doi: 10.1073/pnas.0808672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsurugizawa T, et al. Effects of isoflurane and alpha-chloralose anesthesia on BOLD fMRI responses to ingested L-glutamate in rats. Neuroscience. 2010;165:244–251. doi: 10.1016/j.neuroscience.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Bender G, et al. Neural correlates of evaluative compared with passive tasting. The European journal of neuroscience. 2009;30:327–338. doi: 10.1111/j.1460-9568.2009.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fairhall SL, Macaluso E. Spatial attention can modulate audiovisual integration at multiple cortical and subcortical sites. The European journal of neuroscience. 2009;29:1247–1257. doi: 10.1111/j.1460-9568.2009.06688.x. [DOI] [PubMed] [Google Scholar]
- 49.Krupa DJ, et al. Layer-specific somatosensory cortical activation during active tactile discrimination. Science (New York, N.Y. 2004;304:1989–1992. doi: 10.1126/science.1093318. [DOI] [PubMed] [Google Scholar]
- 50.Lemon CH, Smith DV. Neural representation of bitter taste in the nucleus of the solitary tract. Journal of neurophysiology. 2005;94:3719–3729. doi: 10.1152/jn.00700.2005. [DOI] [PubMed] [Google Scholar]
- 51.Geran LC, Travers SP. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. Journal of neurophysiology. 2006;96:2513–2527. doi: 10.1152/jn.00607.2006. [DOI] [PubMed] [Google Scholar]
- 52.Geran LC, Travers SP. Bitter-responsive gustatory neurons in the rat parabrachial nucleus. Journal of neurophysiology. 2009;101:1598–1612. doi: 10.1152/jn.91168.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Travers SP, Geran LC. Bitter-responsive brainstem neurons: characteristics and functions. Physiol Behav. 2009;97:592–603. doi: 10.1016/j.physbeh.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 54.Verhagen JV, et al. Responses to taste stimulation in the ventroposteromedial nucleus of the thalamus in rats. Journal of neurophysiology. 2003;89:265–275. doi: 10.1152/jn.00870.2001. [DOI] [PubMed] [Google Scholar]
- 55.Boucher Y, et al. Trigeminal modulation of gustatory neurons in the nucleus of the solitary tract. Brain Res. 2003;973:265–274. doi: 10.1016/s0006-8993(03)02526-5. [DOI] [PubMed] [Google Scholar]
- 56.Di Lorenzo PM. The neural code for taste in the brain stem: response profiles. Physiol Behav. 2000;69:87–96. doi: 10.1016/s0031-9384(00)00191-8. [DOI] [PubMed] [Google Scholar]
- 57.Smith DV, et al. Medullary taste responses are modulated by the bed nucleus of the stria terminalis. Chemical senses. 2005;30:421–434. doi: 10.1093/chemse/bji037. [DOI] [PubMed] [Google Scholar]
- 58.Zhu M, et al. Activation of delta-opioid receptors reduces excitatory input to putative gustatory cells within the nucleus of the solitary tract. Journal of neurophysiology. 2009;101:258–268. doi: 10.1152/jn.90648.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Lorenzo PM, et al. Temporal coding of sensation: mimicking taste quality with electrical stimulation of the brain. Behav Neurosci. 2003;117:1423–1433. doi: 10.1037/0735-7044.117.6.1423. [DOI] [PubMed] [Google Scholar]
- 60.Di Lorenzo PM, et al. Dynamic coding of taste stimuli in the brainstem: effects of brief pulses of taste stimuli on subsequent taste responses. J Neurosci. 2003;23:8893–8902. doi: 10.1523/JNEUROSCI.23-26-08893.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Lorenzo PM, et al. Quality time: representation of a multidimensional sensory domain through temporal coding. J Neurosci. 2009;29:9227–9238. doi: 10.1523/JNEUROSCI.5995-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gutierrez R, et al. Spike timing precision of licking-synchronized neurons in the taste-reward circuit is enhanced upon lerning. J Neurosci. 2010;30:287–303. doi: 10.1523/JNEUROSCI.0855-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hallock RM, Di Lorenzo PM. Temporal coding in the gustatory system. Neuroscience and biobehavioral reviews. 2006;30:1145–1160. doi: 10.1016/j.neubiorev.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Roussin AT, et al. Variability in responses and temporal coding of tastants of similar quality in the nucleus of the solitary tract of the rat. Journal of neurophysiology. 2008;99:644–655. doi: 10.1152/jn.00920.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanamori T, et al. Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. Journal of neurophysiology. 1998;79:2535–2545. doi: 10.1152/jn.1998.79.5.2535. [DOI] [PubMed] [Google Scholar]
- 66.Kadohisa M, et al. Neuronal representations of stimuli in the mouth: the primate insular taste cortex, orbitofrontal cortex and amygdala. Chemical senses. 2005;30:401–419. doi: 10.1093/chemse/bji036. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto T, et al. Gustatory responses of cortical neurons in rats. I. Response characteristics. Journal of neurophysiology. 1984;51:616–635. doi: 10.1152/jn.1984.51.4.616. [DOI] [PubMed] [Google Scholar]
- 68.Katz DB, et al. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci. 2001;21:4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller P, Katz DB. Stochastic transitions between neural states in taste processing and decision-making. J Neurosci. 2010;30:2559–2570. doi: 10.1523/JNEUROSCI.3047-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halpern BP. Constraints imposed on taste physiology by human taste reaction time data. Neuroscience and biobehavioral reviews. 1986;10:135–151. doi: 10.1016/0149-7634(86)90024-2. [DOI] [PubMed] [Google Scholar]
- 71.Jones LM, et al. Natural stimuli evoke dynamic sequences of states in sensory cortical ensembles. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18772–18777. doi: 10.1073/pnas.0705546104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stapleton JR, et al. Ensembles of gustatory cortical neurons anticipate and discriminate between tastants in a single lick. Frontiers in neuroscience. 2007;1:161–174. doi: 10.3389/neuro.01.1.1.012.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stapleton JR, et al. Rapid taste responses in the gustatory cortex during licking. J Neurosci. 2006;26:4126–4138. doi: 10.1523/JNEUROSCI.0092-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Accolla R, et al. Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci. 2007;27:1396–1404. doi: 10.1523/JNEUROSCI.5188-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Accolla R, Carleton A. Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4010–4015. doi: 10.1073/pnas.0708927105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schoenfeld MA, et al. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience. 2004;127:347–353. doi: 10.1016/j.neuroscience.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 77.Katz DB, et al. Gustatory processing is dynamic and distributed. Curr Opin Neurobiol. 2002;12:448–454. doi: 10.1016/s0959-4388(02)00341-0. [DOI] [PubMed] [Google Scholar]
- 78.Nitschke JB, et al. Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nature neuroscience. 2006;9:435–442. doi: 10.1038/nn1645. [DOI] [PubMed] [Google Scholar]
- 79.Small DM, et al. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- 80.Grinvald A, Hildesheim R. VSDI: a new era in functional imaging of cortical dynamics. Nat Rev Neurosci. 2004;5:874–885. doi: 10.1038/nrn1536. [DOI] [PubMed] [Google Scholar]
- 81.Grossman SE, et al. Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. J Neurosci. 2008;28:2864–2873. doi: 10.1523/JNEUROSCI.4063-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yasoshima Y, Yamamoto T. Short-term and long-term excitability changes of the insular cortical neurons after the acquisition of taste aversion learning in behaving rats. Neuroscience. 1998;84:1–5. doi: 10.1016/s0306-4522(97)00636-2. [DOI] [PubMed] [Google Scholar]
- 83.Lara AH, et al. Encoding of gustatory working memory by orbitofrontal neurons. J Neurosci. 2009;29:765–774. doi: 10.1523/JNEUROSCI.4637-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 85.Rolls ET, et al. Sensory-specific and motivation-specific satiety for the sight and taste of food and water in man. Physiol Behav. 1983;30:185–192. doi: 10.1016/0031-9384(83)90003-3. [DOI] [PubMed] [Google Scholar]
- 86.Rolls ET, et al. Hunger Modulates the Responses to Gustatory Stimuli of Single Neurons in the Caudolateral Orbitofrontal Cortex of the Macaque Monkey. The European journal of neuroscience. 1989;1:53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 87.de Araujo IE, et al. Human cortical responses to water in the mouth, and the effects of thirst. Journal of neurophysiology. 2003;90:1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- 88.Wan S, et al. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus Solitarius neurons. Journal of Neuroscience. 2008;28:4957–4966. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Araujo IE, et al. Neural ensemble coding of satiety states. Neuron. 2006;51:483–494. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe M, et al. Behavioral reactions reflecting differential reward expectations in monkeys. Exp Brain Res. 2001;140:511–518. doi: 10.1007/s002210100856. [DOI] [PubMed] [Google Scholar]
- 91.Ogawa H, et al. Multiple sensitivity of chordat typani fibres of the rat and hamster to gustatory and thermal stimuli. The Journal of physiology. 1968;199:223–240. doi: 10.1113/jphysiol.1968.sp008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harada S, Smith DV. Gustatory sensitivities of the hamster’s soft palate. Chemical senses. 1992;17:37–51. [Google Scholar]
- 93.Garcia J, et al. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science (New York, N.Y. 1955;122:157–158. [PubMed] [Google Scholar]
- 94.Bathellier B, et al. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron. 2008;57:586–598. doi: 10.1016/j.neuron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 95.Erickson RP. The evolution of neural coding ideas in the chemical senses. Physiol Behav. 2000;69:3–13. doi: 10.1016/s0031-9384(00)00193-1. [DOI] [PubMed] [Google Scholar]
- 96.Grinvald A, et al. In-vivo Optical Imaging cortical Architecture and Dynamics. In: Windhorst U, Johansson H, editors. Modern Techniques in Neuroscience Research. Springer Verlag; 1999. pp. 893–969. [Google Scholar]
- 97.Frostig RD, et al. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bathellier B, et al. Wavelet-based multi-resolution statistics for optical imaging signals: Application to automated detection of odour activated glomeruli in the mouse olfactory bulb. Neuroimage. 2007;34:1020–1035. doi: 10.1016/j.neuroimage.2006.10.038. [DOI] [PubMed] [Google Scholar]