Abstract
Objective
This study is to assess the antibacterial activity of omega-6, -7, -9 (n-6, n-7, n-9) fatty acids against various oral microorganisms.
Methods
The n-6, n-7, n-9 fatty acids, such as γ-linoleic acid (GLA), linoleic acid (LA), arachidonic acid (ARA), palmitoleic acid (PA), and oleic acid (OA), their fatty acid ethyl esters, GLA-EE, LA-EE, ARA-EE, PA-EE, OA-EE, and their fatty acid methyl esters, GLA-ME, LA-ME, ARA-ME, PA-ME, OA-ME were investigated for antimicrobial activity against oral pathogens Streptococcus mutans, Candida albicans, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, and Porphyromonas gingivalis. Various concentrations of the fatty acids, their methyl and ethyl esters were tested against various oral pathogens in 96-well plates and blood-agar plate. The plates were incubated anaerobically or aerobically at 37°C for 48 hours, and the colony forming units (CFU) were determined.
Results
The data demonstrated that select n-6, n-7, n-9 fatty acids and their esters exhibited strong antimicrobial activity against these oral microorganisms, demonstrating some specificity for individual microbial species.
Conclusion
The potential use or the combinations of the n-6, n-7, n-9 fatty acids and/or their esters, provided in a local delivery vehicle to infected sites in the oral cavity, could be considered as an additional therapeutic approach to improving oral health.
Keywords: Omega-6, -7, -9 (n-6, n-7, n-9) fatty acids; Antimicrobial activity; Fatty acid ethyl esters; Fatty acid methyl esters
1. Introduction
Omega-6, -7, -9 (n-6, n-7, n-9) fatty acids have been suggested to provide numerous health benefits for humans and are important dietary nutrients.1,2 N-6 fatty acids are essential fatty acids, which the human body cannot produce de novo. The major sources of these n-6 polyunsaturated fatty acids (PUFAs) come from vegetable oils (LA), egg yolk, and meats, particularly organ meat (ARA).3 The n-7 mono-unsaturated fatty acid (MUFA), eg. palmitoleic acid (PA), is a naturally occurring component of healthy skin and a strong antioxidant.4 N-9 MUFA, eg. oleic acid (OA), is a major fatty acid in olive oil. A moderate consumption of OA can lower cholesterol levels and reduce atherosclerosis,5 and has been suggested as an important component of diets describing the “Mediterranean paradox”.
In contrast, American diets are overloaded with various prepared and fast foods, contributing to an unhealthy diet rich in n-6 poly-unsaturated fatty acids, while lacking beneficial amounts of n-3 and n-9 fatty acids.6 An improper balance of these omega fatty acids has been suggested to contribute to various chronic diseases, such as heart disease, cancer, asthma, and arthritis.16,18 Nutritional estimates have suggested that the proper ratio of dietary omega fatty acids for a healthy adult is between 2:1 to 4:1 (n-6:n-3). Both n-6 PUFAs and n-3 PUFAs play multiple roles in cell membrane structure, lipid metabolism, blood clotting, blood pressure, controlling inflammation, that appear to contribute to their healthy benefits.7
It is well known that various fatty acids have anti-inflammatory activities.8–10 For example, clinical studies demonstrate that GLA, primarily found in evening primrose oil, may diminish joint pain, swelling, and morning stiffness that are associated with rheumatoid arthritis.11 Studies show that LA is the major dietary fatty acid in regulating low-density lipoprotein metabolism, thus reducing cholesterol levels and cardiovascular risk.2 Recent studies have shown that n-3 and n-6 PUFAs can also reduce inflammation and alveolar bone resorption in the oral cavity of rats challenged with individual oral bacteria.12–14 It has also been reported that dietary supplementation with fish oil may have potential benefits in modulating destructive host responses, thus contributing to adjunctive management of periodontitis.15–17
We have previously identified that n-3 PUFAs exhibited strong antibacterial activity against various oral bacteria.18 This study examined a range of n-6, n-7, and n-9 fatty acids of various carboxyl lengths for their antimicrobial activities against oral microorganisms to test the hypothesis that selected members of this group of fatty acids would demonstrate genera/species specificity for their microbicidal activity. The data supported that some omega fatty acids and their ester derivatives effectively killed S. mutans, A. actinomycetemcomitans, C. albicans, P. gingivalis, F. nucleatum, and S. gordonii, although this effectiveness varied with the fatty acids. The results suggested a possible use of these fatty acids as adjunctive antimicrobial agents to treat or prevent diseases caused by these oral microorganisms.
2. Materials and Methods
2.1. Reagents
The n-6 fatty acids arachidonic acid (ARA), γ-linoleic acid (GLA), linoleic acid (LA), the n-7 fatty acid palmitoleic acid (PA), the n-9 fatty acids oleic acid (OA) and the fatty acid ethyl esters, arachidonic acid ethyl ester (ARAEE), linoleic acid ethyl ester (LAEE), palmitoleic acid ethyl ester (PAEE), oleic acid ethyl ester (OAEE) were purchased from Cayman Chemicals (Ann Arbor, MI) and their methyl esters ARAME, GLAME, LAME, PAME were purchased from Sigma (St. Louis, MO). Short-chain and mid-chain fatty acids, acetic acid, butyric acid, octanoic acid, capric acid, lauric acid, myristic acid were also purchased from Sigma (St. Louis, MO). Most of the fatty acids used in this study were obtained as fatty acid stock solutions (in 50%~90% ethyl alcohol) at mg/ml concentrations. Oral microbial species Streptococcus mutans (ATCC 25175), Porphyromonas gingivalis (ATCC 33277), Candida albicans (ATCC 2091), Aggregatibacter actinomycetemcomitans JP2, S. sanguis (ATCC 10556), S. gordonii (ATCC 10558), and Fusobacterium nucleatum (ATCC 25586) were purchased from the American Type Culture Collection (Manassas, VA). TSBYE media and Anaerobe Broth were purchased from Oxoid Ltd. (Cambridge, UK). Growth conditions for most of the bacteria were at 37°C in an anaerobic chamber (Plas-Labs, Lansing, MI) in an atmosphere of 85% N2, 10% H2, and 5% CO2. S. sanguis and S. gordonii were grown at 37°C in 5% CO2 and air, while C. albicans was grown aerobically at 37°C.
2.2. Antimicrobial screening of fatty acid and their esters
Various concentrations (2.5 μg/ml, 25 μg/ml, and 250 μg/ml) of ARA, GLA, LA, PA, OA and their methyl and ethyl esters were prepared in ethanol stock solutions, and antimicrobial activity was tested against the oral microorganism S. mutans by adding 5 μl of the fatty acids, methyl, and ethyl ester solutions to each well of a 96-well plate containing 200 μl of TSBYE medium and a 10% bacterial inoculum from an overnight culture. The plates were then incubated under appropriate growth conditions for the specific microorganism for approximately 16 hours. After overnight incubation, 3 μl of the culture solution was diluted 105 times and plated onto blood agar plates (Remel®). The plates were again incubated under specific environmental conditions for each microorganism for 24~48 hours, at which time colony forming units (CFU) were determined using a stereomicroscope.
The short-chain, medium-chain and long-chain fatty acids (acetic acid, butyric acid, octanoic acid, capric acid, lauric acid, myristic acid, arachidonic acid, linoleic acid, palmitoleic acid, stearic acid, oleic acid, dihomo-γ-linoleic acid, γ-linoleic acid, palmitic acid) were specifically compared for their antimicrobial activity against S. mutans. The fatty acids were tested at a concentration of 25μg/ml using a similar approach to that described above with CFU changes determining the effects of the fatty acids. The concentration of 25μg/ml was used because fatty acids showed good antimicrobial activity at this moderate concentration.
3. Results
3.1. Antimicrobial activity
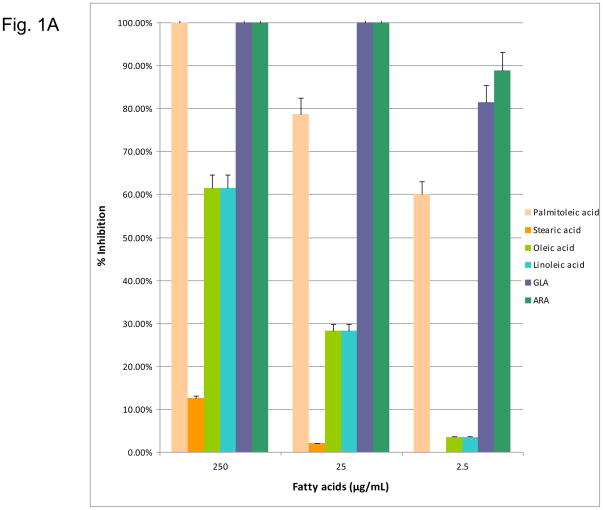
This study examined fifteen of the n-6, n-7, and n-9 fatty acids of various carboxyl lengths for their antimicrobial activities against oral bacteria. After the initial screening, only n-6, -7, -9 fatty acids arachidonic acid (ARA), γ-linoleic acid (GLA), linoleic acid (LA), the n-7 fatty acid palmitoleic acid (PA), the n-9 fatty acids oleic acid (OA) were found with significant antimicrobial activity. The effects of n-6, n-7, and n-9 fatty acids on the growth of S. mutans are depicted in Figure 1A. The data supported that omega-6, -7, -9 fatty acids exhibited significant antimicrobial activity. These fatty acids also exhibited a dosage-dependent inhibition. The n-6 fatty acids, (LA, GLA and ARA) and n-7 fatty acid (PA) were bactericidal at a concentration of 25 μg/mL. However, the n-9 fatty acid, OA, was significantly less effective at inhibiting S. mutans with only a 27.33% decrease in CFU at a concentration 25 μg/mL.
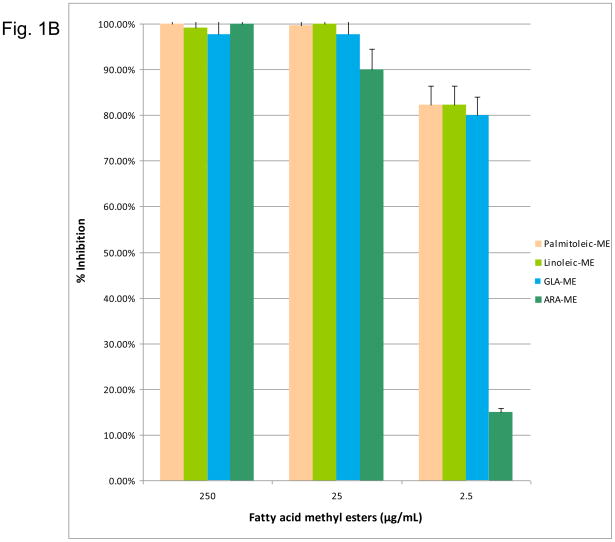
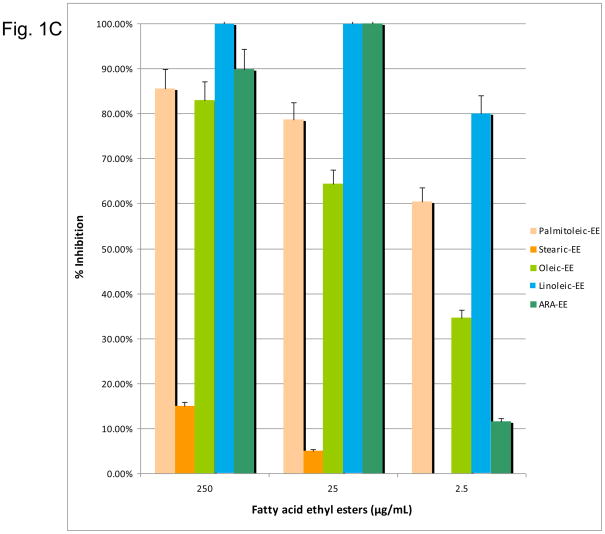
Figure 1.
(1A) Dose-response inhibition of S. mutans by n-6, 7, 9 fatty acids (palmitoleic acid, stearic acid, oleic acid, linoleic acid, GLA, ARA). (1B) Dose response inhibition by n-6, 7, 9 fatty acid methyl esters (palmitoleic acid-ME, linoleic acid-ME, GLA-ME, ARA-ME). (1C) Dose response inhibition by n-6, 7, 9 fatty acid ethyl esters (palmitoleic acid-EE, stearic acid-EE, linoleic acid-EE, GLA-EE, ARA-EE). Three different concentrations (250 μg/ml, 25 μg/ml, and 2.5 μg/ml) of the fatty acids were used. The percent inhibition is based upon the ratio of CFU at 24 hr between triplicate plates treated with fatty acids and the negative control.
In addition, the n-6, n-7, n-9 fatty acid methyl esters and ethyl esters (excluding OA-ME and GLA-EE due to unavailability) were tested against S. mutans. The n-6, n-7, n-9 fatty acid methyl esters exhibited significant antimicrobial activity. LA-ME PA-ME, GLA-ME, and ALA-ME showed 90–100% inhibition at 25 μg/mL (Figure 1B). However, only the methyl esters of LA, PA, and GLA retained significant killing activity at 2.5 μg/mL. Of the ethyl esters, LA-EE and ARA-EE were bactericidal at a concentration of 25 μg/mL (Figure 1C). OA-EE was the least effective ethyl ester with only 64% inhibition, while LA-EE demonstrated the greatest effect at 2.5 μg/mL. Overall the fatty acid methyl esters were better inhibitors than their ethyl ester counterparts. For all fatty acids, excluding OA, the native fatty acid form showed significantly greater inhibition at a concentration of 25 μg/mL than either ethyl or methyl ester chemical modifications.
3.2 Specificity of fatty acids
To test the specificity of the fatty acid characteristics on inhibitory activities, the antimicrobial activity of the short-chain, mid-chain and long-chain fatty acids was compared. Short-chain FAs, such as acetic acid and butyric acid, exhibited no antimicrobial activity, while the medium- and long-chain fatty acids had significant antimicrobial activity against S. mutans (Figure 2). Among the medium-chain fatty acid acids, caproic acid and myristic acid had significantly higher antimicrobial activity than lauric or octanoic acids. Among the long-chain fatty acids, arachidonic acid, linoleic acid, γ-linoleic acid, palmitoleic acid, and palmitic acid had the greatest antimicrobial activity, with neglibible effects detected for stearic, oleic, dihomo- γ-linoleic acids (Figure 2).
Figure 2.
Effects of small-, medium-, and long- chain fatty acids on S. mutans growth. A concentration 25 μg/mL of small-chain fatty acids (acetic acid, butyric acid), medium-chain fatty acids (capric acid, myristic acid, lauric acid and octanoic acid), and long-chain fatty acids (arachidonic acids, linoleic acid, γ-linoleic acid, palmitoleic acid, palmitic, stearic acid, oleic acid, and dihomo- γ-linoleic acid) were used. The percent inhibition is based upon the ratio of CFU at 24 hr between triplicate plates treated with small-, medium-, and long- chain fatty acids and the negative control.
3.3 Antimicrobial specificity
The antimicrobial specificity of the n-6, n-7, n-9 fatty acids and esters were then tested against various oral microorganisms at a concentration of 25 μg/mL, selected based upon the initial findings using S. mutans (Figure 1). The fatty acids, PA, GLA, LA, and ARA, appeared to be the most active against these oral microorganisms, with the ethyl esters generally being less effective (Table 1). However, the level of inhibition was substantially different across the various microbial species that were evaluated. In contrast, the ethyl esters were frequently the most active antifungal agents in inhibiting C. albicans. The n-6, n-7, n-9 fatty acids and their esters also showed antimicrobial activity against the periodontopathogens A. actinomycetemcomitans, and P. gingivalis, although they were generally most active against the oral streptococci (Table 1). However, even within this genera there exists substantial differences in effectiveness, eg. OA and OA-EE, treated S. gordonii versus the other streptococci.
Table 1.
Effects of n-6, 7, 9 fatty acids and their esters on various oral microorganisms
| Mean % Inhibition | ||||||
|---|---|---|---|---|---|---|
| Fatty acid* | Sm | Aa | Pg | Ca | Sg | Ss |
| PA | 100% | 16.47% | 74.78% | 55.70% | 90.43% | 100% |
| PA-EE | 78.59% | 31.74% | 7.52% | 48.60% | 55.32% | 40.92% |
| PA-ME | 99.62% | 67.83% | 9.95% | 5.53% | 90.43% | 100% |
| GLA | 100% | 87.24% | 100% | 60.30% | 100% | 100% |
| GLA-ME | 97.68% | 80.22% | 21.02% | 48.90% | 45.76% | 90.47% |
| LA | 100% | 88.50% | 0% | 38.30% | 96.81% | 100% |
| LA-EE | 100% | 32.30% | 33.41% | 65.07% | 90.43% | 100% |
| LA-ME | 100% | 62.79% | 54.00% | 43.00% | 97.00% | 100% |
| OA | 27.33% | 0% | 73.00% | 57.10% | 0% | 100% |
| OA-EE | 64.00% | 0% | 40.04% | 39.56% | 0% | 92.38% |
| ARA | 100% | 74.00% | 100% | 39.70% | 100% | 100% |
| ARA-EE | 100% | 38.48% | 21.24% | 57.85% | 100% | 100% |
| ARA-ME | 58.43% | 41.98% | 41.59% | 35.50% | 55.30% | 28.13% |
Palmitoleic acid (PA), γ-linoleic acid (GLA), linoleic acid (LA), oleic acid (OA), and arachidonic acid (ARA) are shown and their methyl (ME) and ethyl (EE) esters. Fatty acid concentrations of 25 μg/mL of fatty acid and esters are used. Sm – S. mutans; Aa – A. actinomycetemcomitans; Pg – P. gingivalis; Ca – C. albicans; Sg – S. gordonii; Ss – S. sanguis.
4. Discussion
Polyunsaturated (PUFA) and monounsaturated (MUFA) fatty acids have been known to provide varied health benefits, most notably related to their activity in minimizing inflammation and/or acting as antioxidants.4,8 Recent reports have noted that PUFA could improve oral health suggested to be via these anti-inflammatory effects.13,14 Moreover, while it has been reported that selected fatty acids exhibit antimicrobial activity against various medical pathogens,21 such Neisseria gonorrhoeae,22,23 the literature is sparse in documenting, more broadly, the antimicrobial activity of n-6, n-7, and n-9 fatty acids. Importantly for this report, it is known that the primary trigger of oral inflammation is oral microorganisms,19; however, little is known about the effect of the n-6, n-7, and n-9 fatty acids on the growth and survival of the oral microorganisms that elicit these destructive responses in the oral cavity. We recently have reported a novel bioactivity of the three major n-3 PUFA, eicosapentanoic acid (EPA), docosahexanoic Acid (DHA), α-linolenic acid (ALA), and their ester derivatives. Specifically, this study demonstrated that n-3 PUFA and their ester derivatives exhibited strong antibacterial activity against various oral pathogens, including S. mutans, C. albicans, A. actinomycetemcomitans, F. nucleatum, and P. gingivalis.18
The present study extended these observations and investigated the antimicrobial activity of n-6, n-7, and n-9 fatty acids. All three of the major n-6 PUFAs, linoleic acid (LA), γ-linolenic acid (GLA), arachidonic acid (ARA), the n-7 MUFA, palmitoleic acid (PA), the n-9 MUFA, oleic acid (OA), and their methyl and ethyl esters were found to have antimicrobial activities against various oral microorganisms, including S. mutans, A. actinomycetemcomitans, C. albicans, P. gingivalis, F. nucleatum, and S. gordonii. Our data indicated that there was specificity among the fatty acids with short-chain fatty acids having negligible bioactivity, and select and medium- and long-chain fatty acids having a focused or broad antimicrobial activity. Among the medium-chain fatty acid acids, caproic and myristic acid had significantly increased antimicrobial activity. Among the long-chain fatty acid arachidonic, linoleic, γ-linoleic, palmitoleic, and palmitic acids had elevated antimicrobial activity within the group of FAs that were tested. Evaluation of the antimicrobial specificity of these effects examined the FAs impact on Gram-positive, and Gram-negative oral bacteria, and a oral fungus, C. albicans. The results suggested that these fatty acids and their esters were more effective against Gram-positive bacteria than the Gram-negative oral bacteria. Overall, these agents were least effective against C. albicans, although certain of the fatty acids did inhibit growth of this microorganism by 50–60%.
While the relationship between structure and antimicrobial activity of the n-6, n-7, n-9 fatty acids and their ester derivatives is not clear, it appears that the number of double bonds and the position of the double bonds could greatly influence the antimicrobial activity of these fatty acids. Furthermore, modifying the carboxyl group of the fatty acids appeared to significantly influence their antimicrobial activities. This is evident since the methyl and ethyl esters provided very different inhibitory profiles both in concentration and microbial specificity. In general, these derivatives weakly inhibited the oral bacteria, albeit the ethyl ester derivatives, particularly LA-EE and ARA-EE, had higher activity against the C. albicans. The modified PUFAs and MUFAs demonstrated varied antibacterial patterns that were not limited to the principal differences related to the Gram-positive or Gram-negative nature of the species.
While the antibacterial mechanisms of n-6, n-7, n-9 fatty acids and their esters are unknown, these compounds resemble the bipolar membrane of the bacterial cell wall due to having both a hydrophilic head and hydrophobic tail. This similarity suggests that the fatty acids could possibly target the bacterial and fungal cell membranes, thus, killing by penetrating and disrupting normal function of the cellular membranes.20
To our knowledge, this is the first study demonstrating a significant antimicrobial activity for these fatty acids and their esters against oral microorganisms. Additional in vivo studies are needed to demonstrate that n-6, n-7, n-9 fatty acids could be adjunctive biomolecules that would be incorporated into various delivery approaches for affecting oral infections and their associated diseases, eg. dental caries, periodontal disease. Clearly, there are existing methods that could be considered, eg. chewing gum, toothpaste, mouthrinse, and/or tray delivery (similar to fluoride) that would focus on the local oral delivery of these compounds. However, it must also be recognized that a large number of the population is already taking fatty acid dietary supplements that are marketed to enhance cardiovascular and brain health, often focusing on the anti-inflammatory nature of these compounds. This daily enteral administration results in significant changes in levels of the FAs in serum and cells, and would be expected to be altered in the gingival crevicular fluids. Thus, one could imagine a systemic delivery option with a more targeted profile of FAs. Finally, there is substantial marketing occurring in modifying various foodstuffs (eg. juices, yogurt, milk etc.) to deliver biomolecules, including fatty acids and even for probiotics that would pass through the oral cavity. Thus, one could consider “packaging” an array of FAs into foods that could impact the oral microbiota. Future studies will be required to explore the range of delivery options of these fatty acids to enable them to function as antimicrobial agents in situ.
Acknowledgments
This work was supported by grant R41DE17265-01 from the NIH/NIDCR.
Footnotes
Competing Interests:
No conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harris W. Omega-6 and omega-3 fatty acids: partners in prevention. Curr Opin Clin Nutr Metab Care. 2010;13:125–9. doi: 10.1097/MCO.0b013e3283357242. [DOI] [PubMed] [Google Scholar]
- 2.Santos MJ, López-Jurado M, Llopis J, Urbano G, Mataix FJ. Influence of dietary supplementation with fish on plasma fatty acid composition in coronary heart disease patients. Ann Nutr Metab. 1995;39:52–62. doi: 10.1159/000177842. [DOI] [PubMed] [Google Scholar]
- 3.Whelan J, McEntee Michael F. Dietary (n-6) PUFA and Intestinal Tumorigenesis. J Nutr. 2004;134:3421S–3426S. doi: 10.1093/jn/134.12.3421S. [DOI] [PubMed] [Google Scholar]
- 4.Spahis S, Vanasse M, Bélanger SA, Ghadirian P, Grenier E, Levy E. Lipid profile, fatty acid composition and pro- and anti-oxidant status in pediatric patients with attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2008;79:47–53. doi: 10.1016/j.plefa.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Nicolosi RJ, Woolfrey B, Wilson TA, Scollin P, Handelman G, Fisher R. Decreased aortic early atherosclerosis and associated risk factors in hypercholesterolemic hamsters fed a high- or mid-oleic acid oil compared to a high-linoleic acid oil. J Nutr Biochem. 2004;15:540–547. doi: 10.1016/j.jnutbio.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine & Pharmacotherapy. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 7.Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annual Review of Nutrition. 2004;24:597–615. doi: 10.1146/annurev.nutr.24.012003.132106. [DOI] [PubMed] [Google Scholar]
- 8.Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann Nutr Metab. 2009;55:123–39. doi: 10.1159/000228999. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor R, Huang YS. Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol. 2006;7:531–4. doi: 10.2174/138920106779116874. [DOI] [PubMed] [Google Scholar]
- 10.Poudel-Tandukar K, Nanri A, Matsushita Y, Sasaki S, Ohta M, Sato M, Mizoue T. Dietary intakes of alpha-linolenic and linoleic acids are inversely associated with serum C-reactive protein levels among Japanese men. Nutr. 2009;29:363–70. doi: 10.1016/j.nutres.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Allayee H, Roth N, Hodis HN. Polyunsaturated fatty acids and disease: implications for nutrigenetics. J Nutrigenet. 2009;2:140–148. doi: 10.1159/000235562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raffaelli L, Serini S, Piccioni E, Manicone PF, Berardi D, Perfetti G, Calviello G. N-3 polyunsaturated fatty acid effect in periodontal disease: state of art and possible mechanisms involved. Int J Immunopathol Pharmacol. 2008;21:261–6. doi: 10.1177/039463200802100202. [DOI] [PubMed] [Google Scholar]
- 13.Campan P, Planchand PO, Duran D. Pilot study on n-3 polyunsaturated fatty acids in the treatment of human experimental gingivitis. J Clin Periodontol. 1997;24:907–913. doi: 10.1111/j.1600-051x.1997.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 14.Kesavalu L, Bakthavatchalu V, Rahman MM, Su J, Raghu B, Dawson D, Fernandes G, Ebersole JL. Omega-3 fatty acid regulates inflammatory cytokine/mediator messenger RNA expression in Porphyromonas gingivalis-induced experimental periodontal disease. Oral Microbiol Immunol. 2007;22:232–239. doi: 10.1111/j.1399-302X.2007.00346.x. [DOI] [PubMed] [Google Scholar]
- 15.Bendyk A, Marino V, Zilm PS, Howe P, Bartold PM. Effect of dietary omega-3 polyunsaturated fatty acids on experimental periodontitis in the mouse. J Periodontal Res. 2009;44:211–216. doi: 10.1111/j.1600-0765.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- 16.Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis. Periodontol. 2000;14:12–32. doi: 10.1111/j.1600-0757.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 17.Eberhard J, Heilmann F, Acil Y, Albers HK, Jepsen S. Local application of n-3 or n-6 polyunsaturated fatty acids in the treatment of human experimental gingivitis. J Clin Periodontol. 2002;29:364–369. doi: 10.1034/j.1600-051x.2002.290413.x. [DOI] [PubMed] [Google Scholar]
- 18.Huang CB, Ebersole JL. A novel bioactivity of omega-3 polyunsaturated acids (n-3 PUFA) and their ester derivatives. Molecular Oral Microbiology. 2010;25:75–80. doi: 10.1111/j.2041-1014.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 19.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J Clin Perio. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 20.Lunde CS, Hartouni SR, Janc JW, Mammen M, Humphrey PP, Benton BM. Telavancin Disrupts the Functional Integrity of the Bacterial Membrane through Targeted Interaction with the Cell Wall Precursor Lipid II. Antimicrobial Agents and Chemotherapy. 2009;53:3375–3383. doi: 10.1128/AAC.01710-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629–42. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- 22.Bergsson, Steingrímsson, Thormar Microbicidal effect of lipids against gram-positive and gram-negative cocci. Icelandic Med J. 1998;84(Suppl 37):118. [Google Scholar]
- 23.Bergsson, Steingrímsson, Thormar In Vitro Susceptibilities of Neisseria gonorrhoeae to Fatty Acids and Monoglycerides. Antimicrob Agents Chemother. 1999;11:2790–2792. doi: 10.1128/aac.43.11.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]