Abstract
The mammalian vestibular epithelium has a limited capacity for spontaneous hair cell regeneration. The mechanism underlying the regeneration is not well understood. Because the Notch signaling pathway mediates the formation of the sensory epithelial mosaic patterning during ear development, it may also play a role in hair cell regeneration in the mature mammalian vestibular epithelium after a lesion. To investigate the process of spontaneous regeneration in the vestibular epithelium vis-à-vis changes in Notch signaling, we induced a unilateral lesion by infusing streptomycin into the mouse posterior semicircular canal, and examined Notch signaling molecules and their mRNA expression levels by immunohistochemistry and quantitative real-time polymerase chain reaction (qRTPCR), respectively. We detected Jagged1 in supporting cells in both normal and lesioned utricles. Atoh1, a marker for early developing hair cells, was absent in the intact mature tissue, but re-appeared after the lesion. Many cells were either positive for both Atoh1 and myosin VIIa, or for one of them. qRTPCR data showed a post trauma decrease of Hes5 and an increase in Atoh1. Atoh1 up-regulation may either be a result of Hes5 down-regulation or mediated by another signaling pathway.
Keywords: Notch, mouse, utricle, regeneration, streptomycin, Atoh1, vestibular
Introduction
Mammalian hair cells are vulnerable to various insults such as aminoglycoside antibiotics, excessive stimulation, infections, and to aging. In contrast to the auditory system, where lost hair cells never regenerate, the mammalian vestibular epithelium exhibits spontaneous hair cell regeneration after trauma (Forge et al., 1993; Kawamoto et al., 2009; Lopez et al., 1997). The extent of regeneration in the vestibular epithelium is limited and depends to a large extent on transdifferentiation of supporting cells with or without proliferation (Forge et al., 1993; Lopez et al., 1998; Rubel et al., 1995). It is also possible that repair of partially damaged hair cells takes place (Zheng et al., 1999). In addition, pluripotent stem cells in the adult mouse utricle may be involved in the regeneration of hair cells (Li et al., 2003). Unlike in mammals, hair cell replacement is effective in nonmammalian vertebrates through direct or indirect transdifferentiation of surviving supporting cells (Adler et al., 1996; Corwin et al., 1988; Roberson et al., 1996; Ryals et al., 1988; Stone et al., 2007; Taylor et al., 2005). The reasons for the lack of effective regeneration mechanisms in the mammalian inner ear are unknown.
Notch signaling plays a crucial role in the differentiation of hair cells and supporting cells in the developing inner ear epithelium in both mammalian and non-mammalian vertebrates (Eddison et al., 2000; Kelley, 2006; Murata et al., 2006; Shailam et al., 1999). Notch signaling is activated by Notch ligands (Delta or Serrate/Jagged family) binding to the Notch receptor in adjacent cells, which leads to γ-secretase mediated cleavage and generation of Notch intracellular domain (NICD) fragments. NICD translocates to the nucleus and stimulates expression of two inhibitory basic helix–loop–helix proteins, hairy and enhancer of split 1 (Hes1) and Hes5, which down-regulate the expression of prosensory genes such as Atoh1, an essential regulator of hair cell development. This in turn leads to the inhibition of hair cell fate and the development of the cell as a supporting cell (Kelley, 2006).
Notch signaling is also involved in maintenance and repair of the hair cell-supporting cell pattern in non-mammalian inner ear epithelia (Daudet et al., 2009; Ma et al., 2008; Stone et al., 1999). In the basilar papilla of chicks, Notch pathway genes are transcribed in the undamaged epithelium, and after hair cell injury, Notch signaling transcription is up-regulated in the regions of highest mitotic activity (Daudet et al., 2009). In the zebrafish lateral line, which spontaneously regenerates after aminoglycoside-induced hair cell death, expression of Notch signaling pathway members notch3, deltaA, and atoh1a transcripts are up-regulated after the insult (Ma et al., 2008).
Notch signaling has been characterized in the normal and drug damaged adult mammalian auditory epithelium. Several Notch pathway molecules, particularly Jagged1, increase after a chemical hair cell lesion in the guinea pig organ of Corti, peak at 2 weeks, decrease at 1 month, and nearly disappear by 2 months (Batts et al., 2009). Local application of a γ-secretase inhibitor in the damaged cochlea generates ectopic hair cells in the mature auditory epithelium (Hori et al., 2007). Hes5-GFP is not up-regulated in the damaged mouse cochlea, which leads to the speculation that the absence of Notch-Hes5 signaling in the traumatized adult mouse organ of Corti is correlated with lack of regeneration in this tissue (Hartman et al., 2009).
Because the Notch signaling pathway mediates the formation of the sensory epithelial mosaic patterning during ear development and is also involved in hair cell regeneration of damaged non-mammalian inner ear epithelium, we speculate that Notch signaling may play a role in the regeneration of the damaged mammalian vestibular epithelium. To determine the status of the Notch signaling pathway in the regeneration of the traumatized mouse utricle, we first induced a severe unilateral hair cell lesion in the mouse utricle by infusing streptomycin into the posterior semicircular canal. We then assayed for Notch signaling molecules in normal utricles, and in traumatized utricles at different time points after the lesion by immunohistochemistry and quantitative real-time polymerase chain reaction (qRTPCR). Using immunohistochemistry, we observed that Jagged1 was located in the supporting cells of the normal utricle and remained in those cells after a lesion. Atoh1 was not detected in the normal tissue but appeared after the trauma. qRTPCR data showed a post trauma decrease for Hes5 and an increase in Atoh1 after the lesion, with no significant change in Notch1, Jagged1 and Hes1.
Our results present a new ototoxic regimen for a complete elimination of utricular hair cells. We document changes in expression level of Atoh1 and Hes genes and no marked change in Jagged1. We find Atoh1 and myosin VIIa-positive cells early after the lesion suggesting that the process of spontaneous regeneration may start several days after the insult. Our data also suggest that Atoh1 up-regulation leading to hair cell regeneration may be induced by more than one signaling pathway.
Materials and Methods
Animals
For the gender consistency, we used female CD-1 mice at 3 to 4 weeks of age as experimental animals. They were purchased from Charles River Laboratories International Inc. (Wilmington, MA, USA) and were housed at the animal care facility at the University of Michigan. The animals were 4–5 weeks old when the surgery was performed to induce a lesion in the vestibular epithelium. All animal experiments were approved by the University of Michigan Institutional Committee on the Use and Care of Animals (UCUCA) and were performed using acceptable veterinary standards.
Surgery
Prior to all surgical procedures, mice were anesthetized with an intraperitoneal injection of xylazine (7 mg/kg) and ketamine HCl (120 mg/kg). An acesodyne, Ketoprofen (10 mg/kg), was applied subcutaneously before surgery. A chemical lesion was surgically introduced to the posterior semicircular canal of the left ear. The surgical approach and procedures were similar to these described previously (Kawamoto et al., 2009). However, in this study we used a more severely vestibulotoxic aminoglycoside streptomycin instead of gentamicin (Sato et al., 1983), in an attempt to induce a more severe lesion. Briefly, under the operating microscope an incision was made in the left postauricular region, the muscles were separated and the posterior semicircular canal was exposed. A hole was drilled on the posterior semicircular canal using a 26 G needle and 2 μl streptomycin solution (75 mg/ml; X-gen Pharmaceuticals Inc., Northport, NY, USA) was inoculated into the opening with a cannula, as described (Kawamoto et al., 2009). The infusion speed was controlled by a syringe pump (Harvard Apparatus Inc., Holliston, MA, USA) at the rate of 0.5μl/min. After the injection, the hole was sealed by a piece of muscle and the skin was sutured with 6-0 Prolene Suture (Ethicon Inc., Somerville, NJ, USA).
Immunofluorescence staining
Mice that received the vestibular hair cell lesion were sacrificed at the following time points after surgery: 3 days, 1 week, 2 weeks and 4 weeks. At least 3 animals at each time point were used for immunoreactivity analysis for every antibody tested. For normal control material, we used normal mice without manipulation. We elected not to use the contralateral ears because of the possibility that the ototoxic agent could reach these ears via the cochlear aqueduct and CSF route.
Once deeply anesthetized with xylazine and ketamine, animals were decapitated and the temporal bones removed and fixed in 4% paraformaldehyde (PFA) in the phosphate buffered saline (PBS) for 2 hours. Utricles were dissected out and treated with 0.3% Triton X-100 in PBS for 15 minutes, and then blocked using 10% normal donkey serum (Jackson Immunoresearch, West Grove, PA, USA) and 0.1% bovine serum albumin in PBS for 1 hour at room temperature. Tissues were then incubated overnight at 4 °C in a primary antibody. The following primary antibodies were used: goat anti-Jagged 1 antibody (diluted 1:200, Santa Cruz, sc6011), goat anti-Notch1 antibody (diluted 1:100, Santa Cruz, sc6015), rabbit anti-Hes1 antibody (diluted 1:300, Chemicon, AB5702), rabbit anti-Hes5 antibody (diluted 1:200, Chemicon, AB5708), rabbit anti-myosin VIIa antibody (diluted 1:300, Proteus BioSciences Inc., 25–6790), mouse anti-myosin VIIa (diluted 1:500, Hybridoma Bank) and rabbit anti-Atoh1 antibody (diluted 1:100, a kind gift from Dr. Jane Johnson, University of Texas Southwestern Medical Centre)(Helms et al., 1998). After rinsing in PBS, samples were incubated in fluorescence-labeled secondary antibodies (Alexa Fluor 488 or 594, Invitrogen/Molecular Probes, Carlsbad, CA, USA) at a dilution of 1:300 for 45 minutes at room temperature. For actin labeling, samples were additionally incubated with Alexa Fluor 594-conjugated phalloidin (diluted 1:200, Invitrogen/Molecular Probes, Carlsbad, CA, USA) for 30 minutes. After staining, specimens were mounted on glass slides with Fluoro-Gel mounting media (Electron Microscopy Sciences, Hatfield, PA, USA), and examined with a Zeiss LSM 510-META Laser Scanning Confocal Microscope using a 40× objective lens. Confocal images were cropped, labeled, and spaced with Zeiss LSM Image Browser software and Adobe Photoshop software.
Cell counts and data analysis
Quantitative analysis was conducted in whole-mount preparations of utricles doubled stained for Atoh1 and myosin VIIa, using Z-series images taken by confocal microscopy with a 40× objective lens. A group of Z-series images was taken at a random square area (212 μm × 212 μm) of each quadrant of the utricle, beginning (at the Z plane) from the hair cell layer and extending down to the supporting cell layer. Thus, four distinct groups of Z-series images were obtained for each utricle and approximately 75% of the sensory area of the utricle was sampled. The software, tpsDig (Version 2.12, F. James Rohlf, Ecology & Evolution, SUNY at Stony Brook) was used to count numbers of three kinds of cells: Atoh1-positive/myosin VIIa-negative, Atoh1-negative/myosin VIIa-positive and cells positive for both. Those three kinds of cells were counted in each group of images and summed for each utricle. Four utricles were analyzed for each of the following time-point groups after surgery: 1 week, 2 weeks and 4 weeks.
To estimate the changes of Atoh1 and myosin VIIa in the regenerating utricular epithelia, we compared mean proportions of Atoh1-positive cells and myosin VIIa-positive cells at different time points after surgery. For both quantitative measures, statistical differences were examined using ANOVA (Prism 5, GraphPad Software, Inc.). Differences with p-values of 0.05 or less after sequential Bonferroni adjustment for multiple tests were considered significant.
RNA isolation and reverse transcription
Three to five independent RNA pools were prepared for each time point after surgery (3 days, 1 week, 2 weeks or 4 weeks) as well as non-treated normal mice as control. For each pool, 6 to 7 utricles were dissected out either in phosphate-buffered saline or RNAlater (Ambion, Austin, TX, USA). Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA, USA) and treated with RNase-free DNase I (Invitrogen, Carlsbad, CA, USA). RNA quality and quantity were assessed with Agilent Bioanalyzer 2100 (Agilent Technologies; New Castle, DE, USA). cDNA was synthesized by SuperScript III first-strand synthesis supermix for qRTPCR (Invitrogen, Carlsbad, CA, USA).
Quantitative real-time polymerase chain reaction
Quantitative real-time PCR (qRTPCR) was performed in StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with TaqMan Gene Expression Assays (Applied Biosystems) by using StepOne Software v2.0 (Applied Biosystems). We examined the following transcripts: Notch1, Jagged1, Hes1, Hes5 and Atoh1. As the invariant control, we used mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a reference. The following assays were used: Notch1 (Assay ID: Mm00435245_m1), Jagged1 (Assay ID: Mm00496902_m1), Hes1 (Assay ID: Mm01342805_m1), Hes5 (Assay ID: Mm00439311_g1), Atoh1 (Assay ID: Mm00476035_s1) and GAPDH (Assay ID: Mm99999915_g1). For each gene, triplicate cDNA samples derived from each RNA pool were assayed.
To estimate changes in mRNA expression levels after the lesion, the 2−ΔΔCT method was used (Daudet et al., 2009; Gong et al., 2006; Livak et al., 2001). For each sample, the difference in the threshold cycle number (CT) between the target gene and GAPDH was defined as ΔCT (CT (target gene) -CT (GAPDH)). These values were averaged across samples in a group (n=3 to 5), and statistical differences were examined using ANOVA (Prism 5). The fold change for each gene after damage was determined by 2−Δ ΔC T
Results
Gross behavior after surgery
We previously reported that a single application of gentamicin into the semicircular canal resulted in a severe to complete depletion of hair cells from mouse utricles and new hair cells started to reappear within 2 weeks (Kawamoto et al., 2009). Herein, we used another vestibulotoxic aminoglycoside, streptomycin, and induced a severe lesion in the mouse vestibular epithelium. After surgery for infusing streptomycin into the posterior semicircular canal, there were no mortalities despite the high dose of the aminoglycoside. Severe vestibular dysfunction was detected after surgery including head tilt, circling, and longitudinal body-axis rotating motion.
Hair cell loss and Jagged1 immunohistochemistry
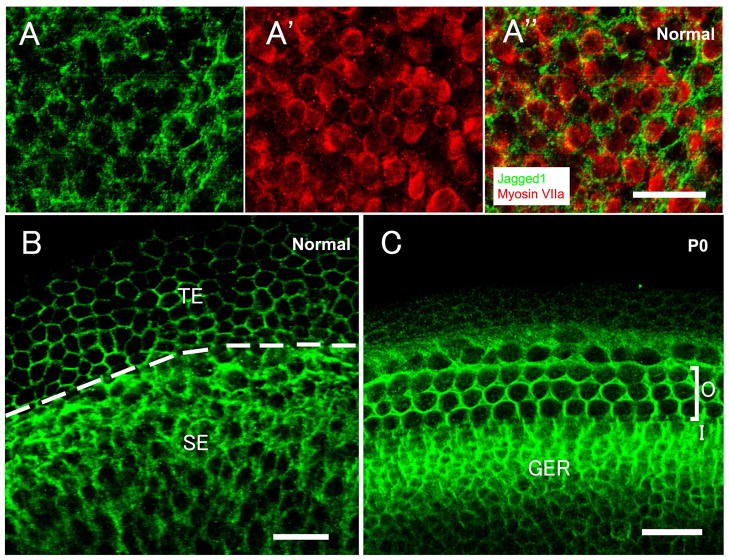
In the normal mature mouse, Jagged1 immunoreactivity was present in the area of supporting cell membrane throughout the utricle. Double-labeling experiments with Jagged1 and the hair cell marker myosin VIIa, revealed that utricular hair cells were devoid of Jagged1 (Fig. 1A-A″). Jagged1 also labeled the membrane of transitional epithelial cells (Fig. 1B), as previously reported (Oesterle et al., 2008). The organ of Corti from neonatal (P0) mice served as positive control for Jagged1 labeling. Jagged1 was restricted to the supporting cell membrane and greater epithelial ridge (GER) area in the organ of Corti of P0 mouse (Fig. 1C).
Fig. 1.
Confocal images of whole mounts of normal mature mouse utricles (A–B) and P0 mouse auditory sensory epithelium (C), stained for Jagged1 (green) with or without myosin VIIa (red). (A-A″) In the sensory epithelium, Jagged1 immunoreactivity is present in the supporting cell membrane, whereas hair cells are devoid of Jagged1 (A: Jagged1 staining; A′: myosin VIIa labeling; A″: the merged image of A and A′). (B) In addition to staining the peripheral areas in supporting cells, Jagged1 also labels the membrane of transitional epithelial cells. The dashed line delineates the boundary of the sensory epithelium (SE) and the transitional epithelium (TE). (C) Jagged1 is expressed in supporting cell membranes in the area of the organ of Corti and the greater epithelium ridge (GER). ‘O’ refers to the outer hair cell area; ‘I’ refers to the inner hair cell area. The scale bar in (A″) applies to (A-A″), and all scale bars represent 20μm.
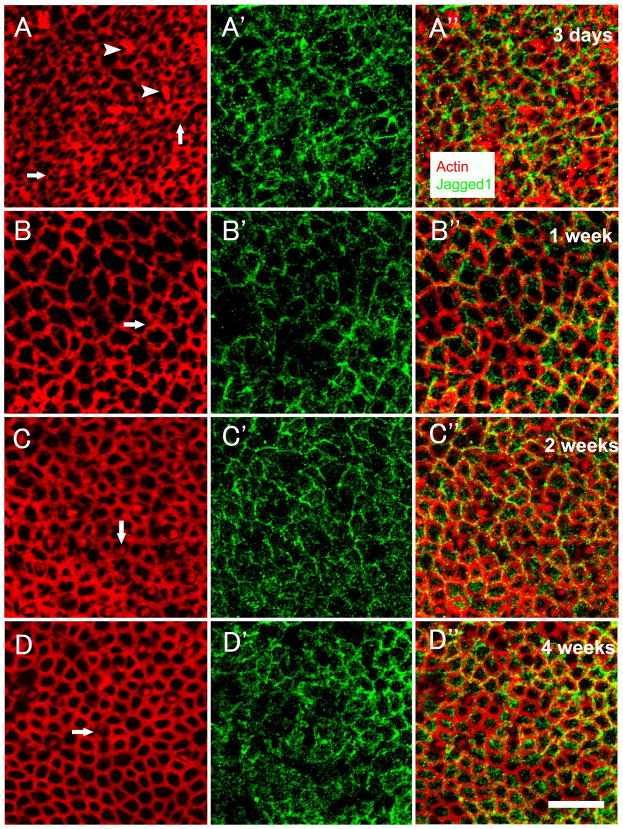
At 3 days (Fig. 2A-A″) after streptomycin infusion, phalloidin staining showed that hair cell bundles in numerous sites were lost (Fig. 2A). Scars were found in areas of hair cell loss as previously shown in mice (Kawamoto et al., 2009) and guinea pigs (Meiteles et al., 1994). Jagged1 staining was evident in the supporting cell membrane (Fig. 2A′). At 1 week (Fig. 2B-B″), a lesion with severe loss of hair cell stereocilia was observed (Fig. 2B). In most animals, the elimination of hair cell stereocilia bundles appeared complete, but in a few cases, a very small number of bundles could be detected. Expanded supporting cells replaced missing hair cells, and numerous scars were found. Jagged1 expression was found in the expanded supporting cell membrane (Fig. 2B′). At 2 weeks (Fig. 2C-C″) and 4 weeks (Fig. 2D-D″), the apical contour of the epithelium appeared reorganized, especially in areas of scars, and new hair cells with short hair bundles were present (Fig. 2C and 2D). Jagged1 remained in the supporting cell membrane (Fig. 2C′ and 2D′). Thus, Jagged1 localization after a lesion resembles that seen in the normal mature ear.
Fig. 2.
Confocal images of whole mounts of utricles obtained at 3 days (A-A″), 1 week (B-B″), 2 weeks (C-C″) or 4 weeks (D-D″) following the lesion, stained with phalloidin (red) and anti-Jagged1 antibody (green). (A-A″) Numerous hair cell stereocilia bundles are lost, resulting in different patterns of scars (arrows in A). A few stereocilia bundles remain (arrowheads in A). Jagged1 signal is evident in the supporting cell membrane (A′). The merged image is shown in (A″). (B-B″) Hair cell loss is more extensive and surviving hair cell stereocilia bundles are not seen. Expanded supporting cells replace missing hair cells, and scars (arrow in B) are found. Jagged1 signal is observed in the expended supporting cells (B′), the merged image is shown in (B″). (C-C″) New hair cells with short hair bundles (arrow in C) are observed throughout the utricle, and Jagged 1 is still present in the supporting cell membrane (C′), the merged image is shown in (C″). (D-D″) The apical contour of the epithelium appears reorganized and new hair cells with short hair bundles (arrow in D) are detected, Jagged1 remains in the supporting cell membrane (D′), the merged image is shown in (D″). Scale bar, 20μm, for all images.
Phalloidin staining used for visualizing stereocilia can be used for detecting hair cells, but the absence of stereocilia may not be always consistent with the loss of hair cell bodies (Zheng et al., 1999). Therefore, as an additional marker for the presence of hair cells, we used a myosin VIIa antibody, a hair cell marker present in both type I and type II utricular hair cells (Hasson et al., 1997). At 3 days after the lesion, some myosin VIIa positive cells are found in the hair cell layer, and numerous aggregates of myosin VIIa, debris of degenerated hair cell, were located above the utricular surface (data not shown). In 1 week tissues, myosin VIIa presented in hair cells with damaged bundles of stereocilia. We also observed some cells that despite being myosin VIIa-positive, their bundles could not be detected by phalloidin labeling (Fig. 3A-A″). These cells did however have an apical domain bordering the luminal membrane. In utricles obtained at 2 weeks (Fig. 3B-B″) and 4 weeks (Fig. 3C-C″) after the trauma, double staining for Jagged1 and myosin VIIa showed that hair cells, surviving or regenerated, were devoid of Jagged1 labeling.
Fig. 3.
Confocal images of whole mounts of utricles obtained at 1 week (A-A″), 2 weeks (B-B″), or 4 weeks (C-C″) following the lesion, stained with anti-myosin VIIa antibody (green in A′ and A″, red in B, B″, C and C″) and phalloidin (red in A and A″) or anti-Jagged1 antibody (green in B, B″, C and C″). (A-A″) A large number of hair cell bundles are lost and some damaged bundles of stereocilia are detected (arrowheads in A). Some cells are myosin VIIa-positive but their bundles are undetectable (arrows in A-A″). The merged image is shown in (A″). (B-B″) and (C-C″) show myosin VIIa positive cells (hair cells) that are negative for Jagged1. Scale bar, 20μm, for all images.
Atoh1
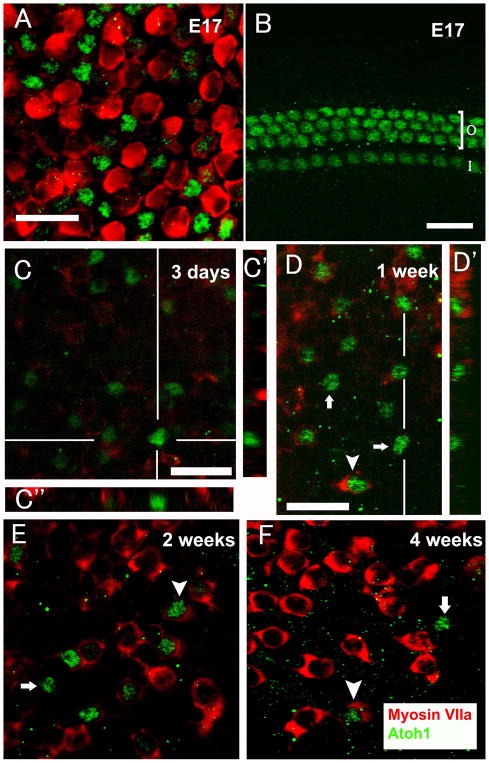
We used the vestibular and auditory sensory epithelia from embryonic (E17) mouse as a positive control for Atoh1 immunoreactivity (Murata et al., 2006). Atoh1 protein was restricted to nuclei of some utricular hair cells and all inner hair cell and outer hair cell nuclei (Fig. 4A and B), consistent with Atoh1 mRNA expression determined by in situ hybridization (Lanford et al., 2000).
Fig. 4.
Confocal images of whole mounts of E17 mouse utricle (A), E17 mouse auditory sensory epithelium (B), and utricles obtained at 3 days (C-C″), 1 week (D-D′), 2 weeks (E) and 4 weeks (F) following the lesion, stained for Atoh1 (green) and myosin VIIa (red, except for B). (A) Atoh1 is found in nuclei of E17 mouse utricular hair cells. (B) Atoh1 is evident in inner hair cells and outer hair cells. ‘O’ refers to the outer hair cell area; ‘I’ refers to the inner hair cell area. (C) Atoh1-positive cells are in the supporting cell layer and are devoid of myosin VIIa labeling. (C′) is a cross sectional image at the position indicated by the white vertical line in Z-series images of (C). (C″) is a cross sectional image at the position indicated by the white horizontal line in Z-series images of (C). (D-D′, E and F) Three kinds of cells are found: Atoh1-positive/myosin VIIa-negative (arrows), Atoh1-negative/myosin VIIa-positive, and cells positive for both (arrowheads). (D′) is a cross sectional image at the position indicated by the white vertical line in Z-series images of (D). The scale bar in (D) applies to (D, E and F), and all scale bars represent 20μm.
No Atoh1 expression was observed in any region of the normal utricle of mature mice. However, as early as 3 days after the lesion, some low-intensity Atoh1 labeled cells were detected in 5 of 8 mouse utricles. To determine whether these Atoh1-positive cells were hair cells or supporting cells, a myosin VIIa antibody was used. Z-series of confocal images showed that Atoh1-positive cells were in the supporting cell layer and were devoid of myosin VIIa labeling (Fig. 4C-C″). One week post-lesion, numerous Atoh1-positive cells were evident throughout the utricles. Double-labeling experiments with Atoh1 and myosin VIIa identified cells which were: Atoh1-positive/myosin VIIa-negative, Atoh1-negative/myosin VIIa-positive and positive for both (Fig. 4D-D′). In cells positive for both Atoh1 and myosin VIIa, Atoh1 located in the nuclei and myosin VIIa presented in the cytoplasm. At 2 weeks (Fig. 4E) and 4 weeks (Fig. 4F) after the lesion, those three kinds of cells were still present in the utricles.
As time advanced from 1 to 4 weeks after the lesion, the average number of Atoh1 positive cells decreased significantly between 1 week and 4 weeks (231±63 vs. 54±26, p<0.01) and that of myosin VIIa positive cells increased (264±40 vs. 1058±131, p<0.01). We calculated the proportion of myosin VIIa positive cells among cells that were Atoh1-positive and the percentage of Atoh1-negative cells among all myosin VIIa-positive cells at the following time points after lesion: 1 week, 2 weeks and 4 weeks. As time advanced, more Atoh1 positive cells were myosin VIIa positive (Fig. 5A). In addition, a significantly higher proportion of myosin VIIa-positive and Atoh1-negative cells at 2 weeks and 4 weeks than at 1 week (Fig. 5B). The cytoarchitecture of the vestibular epithelium and the changes of Atoh1 and myosin VIIa in regenerating hair cells are shown schematically in Figure 5C.
Fig. 5.
Changes of Atoh1 and myosin VIIa in the regenerating utricular epithelia. (A) The mean proportion of Atoh1-positive/myosin VIIa positive cells in Atoh1-positive cells. (B) The mean proportion of myosin VIIa positive/Atoh1-negative cells in myosin VIIa-positive cells. Asterisks in (A and B) label significant differences (p≤0.05). (C) Schematic diagrams show the proposed process of changes of Atoh1 and myosin VIIa in the regenerating utricular epithelium.
Notch1, Hes1 and Hes5
Using an antibody that was previously shown to react with mouse Notch1 in the developing cochlea (Murata et al., 2006), the Notch receptor for Notch1 was not detectable by immunohistochemistry in normal or lesioned utricles at any of the time point examined. No nuclear staining was found in utricles with anti-Hes1 or anti-Hes5 antibody, but staining did appear in the cytoplasm in both supporting cells and hair cells (data not shown). It is presently unclear whether cytoplasmic staining reflects unusual localization in the cell, or non-specific binding of the antibody. Specificity of antibodies against Hes1 and Hes5 was previously tested (Batts et al., 2009).
qRTPCR
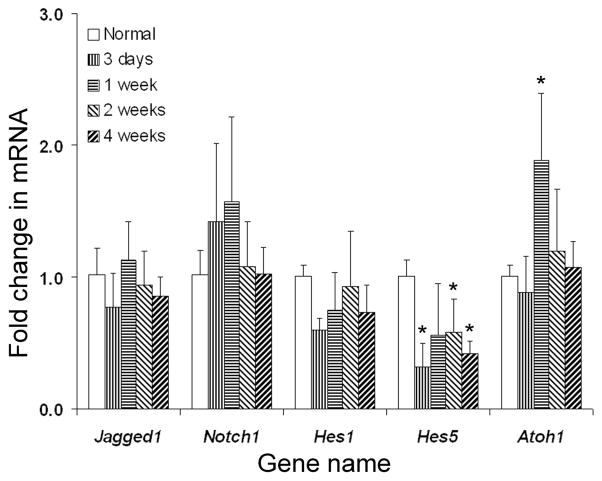
We used qRTPCR to quantify the changes of mRNA expression levels of Notch signaling molecules in the normal and traumatized mouse utricles. All following transcripts: Notch1, Jagged1, Hes1, Hes5 and Atoh1 mRNA were detected in both normal and traumatized utricles. The fold change in mRNA expression (Fig. 6) shows no statistically significant differences in the mRNA expression of Notch1, Jagged1 and Hes1 in traumatized mouse utricle, as compared with normal tissue. However, decreased expression of Hes5 mRNA at 3 days, 2 weeks and 4 weeks after lesion, as well as increased expression of Atoh1 mRNA at 1 week post-streptomycin, were determined.
Fig. 6.
qRTPCR data showing average fold changes in mRNA expression level of Notch signaling genes between each time-point group after lesion and the normal group, with error bars representing standard deviations. Asterisks label significant differences (p≤0.05) in each time-point group compared to the normal group.
Discussion
The role of Notch signaling in the traumatized mouse utricle
Our data show down-regulation of Hes5 and up-regulation of Atoh1 in the hair cell regeneration of traumatized mouse utricle. The signaling cascade and the role of each molecule remain to be elucidated. It is possible that Notch signaling regulates the phenotypic conversion of supporting cells into hair cells, but the involvement of this signaling in supporting cell proliferation in the regenerating mammalian vestibular epithelium is unclear. However, in the damaged avian auditory system, Notch signaling regulates the differentiation of supporting cells into hair cells but has no influence on supporting cell division (Daudet et al., 2009).
Elucidating the role of proliferation in the regenerative process may help design ways to improve and enhance the regeneration. It is also important to determine if Notch signaling has differential involvement in mitotic versus non-mitotic hair cell regeneration. The extent of proliferation contributing to hair cell regeneration in the mammalian vestibular epithelium appears to vary among species (Kawamoto et al., 2009; Lopez et al., 1998; Rubel et al., 1995). Since the regeneration in the mouse has been reported to be mostly based on non-mitotic transdifferentiation (Kawamoto et al., 2009), the change we now report in Hes5 suggests that in mammals involvement of Notch signaling is not necessarily related to a proliferative response. Nevertheless, it would be interesting to test whether other Notch signaling molecules change their expression levels in the vestibular epithelium of chinchillas, where a large number of cells were found to divide during the regenerative process (Lopez et al., 1998).
In addition to generation of new hair cells, it is possible that injured hair cells can undergo repair (Zheng et al., 1999). This would be of clinical importance once means for enhancing the repair are identified. However, the extent of repair is not well known. In utricles examined 1 week after the lesion, we found some myosin VIIa-positive cells in which phalloidin labeling failed to reveal presence of stereocilia, either because none were present, or because stereocilia were extremely degraded. Those cells may be either partially damaged hair cells with stereocilia loss and may undergo self repair, or regenerating hair cells before the appearance of prominent new hair bundles.
It is of interest to compare Notch signaling changes in the damaged mammalian auditory system, which has no capacity for spontaneous regeneration, with the vestibular system, which has partial regenerative potential. After a lesion in the guinea pig cochlea, Notch1 and Jagged1 are up-regulated (Batts et al., 2009; Hori et al., 2007), Hes molecules increase expression in a guinea pig model (Batts et al., 2009) but not in mice (Hartman et al., 2009), and Atoh1 expression is absent at all time points before and after the lesion (Batts et al., 2009). These data in mammals demonstrate that Notch signaling can be reinitiated following auditory hair cell loss but it is repressed downstream its pathway. In contrast, in the mammalian vestibular system, Atoh1 is activated after a lesion is induced. Since Atoh1 activation has been reported to be the impetus for hair cell regeneration in mammals, we speculate that Atoh1 expression is switched off by one or more pathways, which leads to the lack of regeneration potential in the mammalian cochlea. The up-regulation of Hes may be one reason for the inhibition of Atoh1 expression.
The up-regulation of Atoh1
Atoh1 is critical for hair cell development and Atoh1-null mice fail to generate cochlear and vestibular hair cells (Bermingham et al., 1999). Over-expression of Atoh1 in postnatal rat cochlear explants results in ectopic hair cells in the greater epithelial ridge and also facilitates the conversion of postnatal utricular supporting cells into hair cells (Zheng et al., 2000). Virus mediated over-expression of Atoh1 in lesioned mature mammalian inner ears results in hair cell regeneration (Izumikawa et al., 2005; Staecker et al., 2007). In the normal mature auditory epithelium of birds Atoh1 protein is not detected, but the process of spontaneous hair cell regeneration after a lesion involves up-regulation of Atoh1 (Cafaro et al., 2007).
Our immunohistochemistry data reveal that most Atoh1-positive cells in the utricle 3 days following the lesion are in the supporting cell layer and devoid of myosin VIIa stain, suggesting that supporting cells up-regulate expression of Atoh1 after a lesion to the hair cells. However, myosin VIIa is present in half of Atoh1 positive cells at 1 week after the lesion and the proportion increases at 2 weeks and 4 weeks (Fig. 5A). This sequence of appearance of Atoh1 and myosin VIIa mimics embryogenesis, where Atoh1 expression precedes the appearance of myosin VIIa in a gradient of differentiation within the organ of Corti (Chen et al., 2002). We observe an increasing number of cells that are myosin VIIa positive and Atoh1 negative from 1 to 4 weeks after the lesion, suggesting that regenerating hair cells down-regulate Atoh1 as they mature, similar to developmental sequence during embryogenesis. Similar observations were made in the avian regenerating auditory epithelium (Cafaro et al., 2007). Taken together, as shown in the Fig. 5C, we hypothesize that the Atoh1-positive/myosin VIIa-negative cells are new hair cells at an early stage of regeneration. As for Atoh1-negative/myosin VIIa-positive cells in traumatized utricles, these may be either hair cells that survive the ototoxic trauma or regenerating hair cells which are in an advanced phase of maturation, after Atoh1 has been down-regulated.
Using SEM or epi-fluorescence, no regenerated hair cells with short bundles could be detected as early as 1 week after a lesion in the mammalian vestibular epithelium (Forge et al., 1993; Kawamoto et al., 2009; Li et al., 1997). In contrast, we now show that Atoh1 emerges as early as 3 days after the lesion, suggesting that the process of spontaneous regeneration may be initiated earlier than previously thought, and that hair bundles can only be detected 1 to 2 weeks after the onset of differentiation into the hair cell phenotype (Fig. 5C).
Although our qRTPCR data show that the Atoh1 transcript is up-regulated after a lesion, the level of change of Atoh1 expression between normal and traumatized utricles is not as dramatic as that in the regenerating auditory epithelium in birds (Daudet et al., 2009). It would be important to investigate whether a causative relationship exists between the relatively small increase in Atoh1 in the traumatized mammalian vestibular epithelium, and the limited capacity for regeneration.
The relationship between Notch1 and Jagged1 and the up-regulation of Atoh1 is not well understood. Since Notch1 and Jagged1 levels are not affected by the trauma in the mammalian vestibular epithelium, it is necessary to consider several other Notch signaling pathway components (Baron, 2003; Lai, 2004; Schweisguth, 2004). It is also possible that Atoh1 is regulated through the FGF signaling pathway similar to that in the pillar cells of neonatal and embryonic cochlear explants (Doetzlhofer et al., 2009) and/or by Wnt signaling pathway as in human colon cancer (Tsuchiya et al., 2007).
Implications for designing therapies
Inhibition of Notch signaling gives rise to ectopic hair cells in the developing and damaged organ of Corti (Hori et al., 2007; Yamamoto et al., 2006). In non-mammalian inner ears, inhibition of Notch signaling causes excessive regeneration of hair cells following a lesion, and over-expression of activated Notch in supporting cells inhibits hair cell regeneration (Daudet et al., 2009; Ma et al., 2008). These data raise the possibility that a therapy that would repress Notch signaling may also be applied in the traumatized mammalian vestibular epithelium, in order to enhance hair cell regeneration. Notch inhibition could be accomplished, for instance, by using a γ-secretase inhibitor, or repressing the expression of critical Notch ligands or receptors using specific antagonists.
Virus mediated over-expression of Atoh1 in the damaged mammalian vestibular epithelium is a promising method for enhancing regeneration of hair cells (Staecker et al., 2007). However, non-invasive pathways using small molecules with no side effects may be necessary for clinical applicability. In addition, elimination of Hes5, the repressor of Atoh1, using siRNA at a suitable time point after a lesion may potentially increase expression of Atoh1, which could lead to a larger numbers of new hair cells.
Pathways other than Notch signaling may additionally be involved in regulating proliferation and other aspects of tissue response to trauma in the mammalian vestibular epithelium. Future work should focus on identification of such additional pathways, which control or regulate the regeneration in the mammalian vestibular system after a lesion, so as to manipulate key elements for enhancing the regeneration.
Conclusions
We have shown that the response of cells in the utricle to an ototoxic trauma involve changes of Notch signaling molecules and Atoh1 expression. Using qRTPCR we detected down-regulation of Hes5, up-regulation of Atoh1 and no changes in levels of Notch1, Jagged1 and Hes1. Jagged1 is expressed in the supporting cells of normal utricle and remains in supporting cells after the lesion. Atoh1 immuno-fluorescence, undetectable in the normal mouse utricle, appears after trauma in nuclei with or without myosin VIIa labeling. Our results suggest that the spontaneous regeneration may arise earlier than previously thought and Atoh1 up-regulation may be initiated by more than one signaling pathway.
Acknowledgments
We thank Dr. Jane Johnson for the anti-Atoh1 antibody, and Lisa Beyer and Donald Swiderski for technical assistance. This work was supported by the A. Alfred Taubman Medical Research Institute and NIH-NIDCD Grants R01-DC01634 and P30-DC05188. Guo-Peng Wang received support from the China Scholarship Council (No. 2008616087).
Abbreviations
- CSF
cerebrospinal fluid
- GER
greater epithelial ridge
- NICD
Notch intracellular domain
- PBS
phosphate buffered saline
- PFA
paraformaldehyde
- qRTPCR
quantitative real-time polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- Baron M. An overview of the Notch signalling pathway. Semin Cell Dev Biol. 2003;14:113–9. doi: 10.1016/s1084-9521(02)00179-9. [DOI] [PubMed] [Google Scholar]
- Batts SA, Shoemaker CR, Raphael Y. Notch signaling and Hes labeling in the normal and drug-damaged organ of Corti. Hear Res. 2009;249:15–22. doi: 10.1016/j.heares.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–41. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007;236:156–70. doi: 10.1002/dvdy.21023. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–4. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009;326:86–100. doi: 10.1016/j.ydbio.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16:58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddison M, Le Roux I, Lewis J. Notch signaling in the development of the inner ear: lessons from Drosophila. Proc Natl Acad Sci U S A. 2000;97:11692–9. doi: 10.1073/pnas.97.22.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–9. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Gong TW, Karolyi IJ, Macdonald J, Beyer L, Raphael Y, Kohrman DC, Camper SA, Lomax MI. Age-Related Changes in Cochlear Gene Expression In Normal and Shaker 2 Mice. J Assoc Res Otolaryngol. 2006 doi: 10.1007/s10162-006-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman BH, Basak O, Nelson BR, Taylor V, Bermingham-McDonogh O, Reh TA. Hes5 expression in the postnatal and adult mouse inner ear and the drug-damaged cochlea. J Assoc Res Otolaryngol. 2009;10:321–40. doi: 10.1007/s10162-009-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, Yee AG, Mooseker MS, Corey DP. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol. 1997;137:1287–307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 1998;125:919–28. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- Hori R, Nakagawa T, Sakamoto T, Matsuoka Y, Takebayashi S, Ito J. Pharmacological inhibition of Notch signaling in the mature guinea pig cochlea. Neuroreport. 2007;18:1911–4. doi: 10.1097/WNR.0b013e3282f213e0. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Izumikawa M, Beyer LA, Atkin GM, Raphael Y. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hear Res. 2009;247:17–26. doi: 10.1016/j.heares.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–49. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–73. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Shailam R, Norton CR, Gridley T, Kelley MW. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J Assoc Res Otolaryngol. 2000;1:161–71. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–9. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- Li L, Forge A. Morphological evidence for supporting cell to hair cell conversion in the mammalian utricular macula. Int J Dev Neurosci. 1997;15:433–46. doi: 10.1016/s0736-5748(96)00102-5. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez I, Honrubia V, Lee SC, Schoeman G, Beykirch K. Quantification of the process of hair cell loss and recovery in the chinchilla crista ampullaris after gentamicin treatment. Int J Dev Neurosci. 1997;15:447–61. doi: 10.1016/s0736-5748(96)00103-7. [DOI] [PubMed] [Google Scholar]
- Lopez I, Honrubia V, Lee SC, Li G, Beykirch K. Hair cell recovery in the chinchilla crista ampullaris after gentamicin treatment: a quantitative approach. Otolaryngology - Head & Neck Surgery. 1998;119:255–62. doi: 10.1016/S0194-5998(98)70060-9. [DOI] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28:2261–73. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiteles LZ, Raphael Y. Scar formation in the vestibular sensory epithelium after aminoglycoside toxicity. Hear Res. 1994;79:26–38. doi: 10.1016/0378-5955(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Murata J, Tokunaga A, Okano H, Kubo T. Mapping of notch activation during cochlear development in mice: implications for determination of prosensory domain and cell fate diversification. J Comp Neurol. 2006;497:502–18. doi: 10.1002/cne.20997. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9:65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson DW, Kreig CS, Rubel EW. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Auditory Neuroscience. 1996;2:195–205. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Dew LA, Roberson DW. Mammalian vestibular hair cell regeneration. Science. 1995;267:701–7. doi: 10.1126/science.7839150. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–6. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Sato K, Saito T, Matsuhira T. Comparative study by scanning electron microscopy on vestibular toxicities of dibekacin, ribostamycin, and other aminoglycoside antibiotics in guinea pigs. Int J Clin Pharmacol Ther Toxicol. 1983;21:109–14. [PubMed] [Google Scholar]
- Schweisguth F. Regulation of notch signaling activity. Curr Biol. 2004;14:R129–38. [PubMed] [Google Scholar]
- Shailam R, Lanford PJ, Dolinsky CM, Norton CR, Gridley T, Kelley MW. Expression of proneural and neurogenic genes in the embryonic mammalian vestibular system. J Neurocytol. 1999;28:809–19. doi: 10.1023/a:1007009803095. [DOI] [PubMed] [Google Scholar]
- Staecker H, Praetorius M, Baker K, Brough DE. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007;28:223–31. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Delta1 expression during avian hair cell regeneration. Development. 1999;126:961–73. doi: 10.1242/dev.126.5.961. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51:633–47. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- Taylor RR, Forge A. Hair cell regeneration in sensory epithelia from the inner ear of a urodele amphibian. J Comp Neurol. 2005;484:105–20. doi: 10.1002/cne.20450. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Nakamura T, Okamoto R, Kanai T, Watanabe M. Reciprocal targeting of Hath1 and beta-catenin by Wnt glycogen synthase kinase 3beta in human colon cancer. Gastroenterology. 2007;132:208–20. doi: 10.1053/j.gastro.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Tanigaki K, Tsuji M, Yabe D, Ito J, Honjo T. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J Mol Med. 2006;84:37–45. doi: 10.1007/s00109-005-0706-9. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–6. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Keller G, Gao WQ. Immunocytochemical and morphological evidence for intracellular self-repair as an important contributor to mammalian hair cell recovery. J Neurosci. 1999;19:2161–70. doi: 10.1523/JNEUROSCI.19-06-02161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]