Abstract
Background & Aims
Autoimmune pancreatitis (AIP) underlies 5%–11% of cases of chronic pancreatitis. An association between AIP and the HLA-DRB1* 0405/DQB1*0401 haplotype has been reported, but linkage disequilibrium has precluded the identification of predisposing HLA gene(s). We studied the role of single HLA genes in the development of AIP in transgenic mice.
Methods
CD4+ T cell-negative I-Aβ chain−/− (Ab0) mice develop AIP spontaneously, likely due to dysregulation of CD8+ T cell responses. We generated Ab0 NOD mice transgenic for HLA-DR*0405, leading to rescue of CD4+ T cells; we compared their susceptibility to AIP with HLA-DQ8 or HLA-DR* 0401 (single) transgenic, or HLA-DR*0405/DQ8 (double) transgenic mice.
Results
CD4+ T cell-competent HLA-DR*0405 transgenic Ab0 NOD mice develop AIP with high prevalence after sublethal irradiation and adoptive transfer of CD90+ T cells, leading to complete pancreatic atrophy. HLA-DR* 0405 transgenic mice can also develop unprovoked AIP, whereas HLA-DR* 0401, HLA-DQ8 and HLA-DR*0405/DQ8 transgenic Ab0 NOD controls all remained normal, even after irradiation and adoptive transfer of CD90+ T cells. Pancreas histology in HLA-DR*0405 transgenic mice was characterized by destructive infiltration of the exocrine tissue with CD4+ and CD8+ T cells, B cells and macrophages. Mice with complete pancreatic atrophy lost weight, developed fat stools, and had reduced levels of serum lipase activity.
Conclusions
Since HLA-DR*0405 expression fails to protect mice from AIP, the HLA-DRB1*0405 allele appears to be an important risk factor for AIP on the HLA-DRB1*0405/DQB1*0401 haplotype. This humanized mouse model should be useful for studying immunopathogenesis, diagnostic markers, and therapy of human AIP.
Keywords: Autoimmune pancreatitis, HLA-DR*0405, CD4+ T cells, immune regulation
Autoimmune pancreatitis (AIP) is a type of chronic pancreatitis characterized by an autoimmune inflammatory process with prominent lymphocytic infiltration, leading to fibrosis of the pancreas and exocrine secretory dysfunction(1, 2). Although still considered a rare disease, AIP accounts for 5–11% of cases of chronic pancreatitis and has been increasingly recognized over the past 10 years(1). Historically, the disease has been diagnosed more frequently in Japan, where a strong association with the HLA-DRB1* 0405/DQB1*0401 haplotype has been identified(3). In Japan, this haplotype has a high prevalence of >21%(4) and is associated with various other autoimmune disorders, notably Vogt-Koyanagi-Harada disease(5), autoimmune hepatitis(6), type 1 diabetes(7) and rheumatoid arthritis(8). Owing to the strong linkage disequilibrium of genes on common HLA haplotypes, it has been impossible to dissect the relative contribution of individual HLA genes to the overall risk attributed to the HLA region in AIP using population-based immunogenetic analysis(3). As an alternative, however, in vivo studies of the function of single HLA genes can be performed in transgenic rodents. This strategy has led to the development of several humanized animal models of autoimmune diseases (reviewed in(9)), but there are no studies for AIP.
In humans, the typical pathological findings in AIP include a firm and indurated pancreas and narrowing of the pancreatic duct. The histologic hallmark of the disease is intense inflammatory cell infiltration around interlobular ducts, mainly composed of CD4+ and CD8+ T cells, B cells, plasma cells and macrophages(2). In addition, acinar atrophy is almost always seen in AIP(10). Systemic manifestations include infiltration of the gall bladder, bile ducts, kidney, lung and salivary glands with lymphocytes and IgG4+ plasma cells. Clinically, AIP is frequently associated with Sjögren's syndrome, rheumatoid arthritis and inflammatory bowel diseases(1, 2); an association with interstitial pneumonitis has also been reported(10). Increases in IgG4 levels and presence of autoantibodies of various specificities characterize the disease serologically. Treatment of AIP with corticosteroids usually leads to rapid resolution of pancreatic inflammatory lesions(1).
The development of pancreatic inflammatory disease similar to human AIP in mice has been described in autoimmunity-prone MRL/Mp mice(11), in autoimmune regulator (Aire)−/−(12) or CD28−/−(13) NOD mice, and in mouse MHC class II negative I-Aβ chain−/−(Ab0) C57BL/6 mice(14). Although these mouse models share clinical and histologic aspects of human AIP, their value as a tool for medical research is limited. Due to dissimilarities in the highly polymorphic MHC class II alleles of both species, pancreatic epitopes recognized by CD4+ T cells that determine the outcome of (auto)immune responses will likely differ between mouse and man.
Here, we report the spontaneous development of AIP in T cell-competent HLA-DR*0405 transgenic Ab0 NOD mice (and nontransgenic Ab0 NOD mice), but not in HLA-DR*0401, HLA-DQ8 or HLA-DR*0405/DQ8 (double) transgenic mice. Since CD4+ T cells in HLA-DR*0405 transgenic mice are unable to protect from AIP developing in the CD4+ T cell-deficient Ab0 NOD background strain, we suggest that HLA-DR*0405 fails to induce CD4+ regulatory T cells capable of controlling autoreactive CD8+ effector T cells and/or macrophages producing pancreatic damage. We conclude that HLA-DR* 0405 is an important risk factor for AIP on the HLA-DRB1*0405/DQB1*0401 haplotype. Our transgenic mouse model can be used to explore pathogenesis, immunodiagnosis and therapy of AIP in humans, but also to study the role of different HLA class II alleles that are central to T cell crossregulation and protection from disease.
Materials and Methods
Animals and treatments
HLA-DR*0405 transgenic mice were generated using a technique described previously(20). Experimental animals transgenic for HLA-DR*0405, HLA-DR* 0401(20) and/or HLA-DQ8(21) +/− human CD4 (huCD4) were obtained from crossbreedings between the above mentioned mouse lines and a huCD4 transgenic line obtained from Dr. D. Littman(22). All mouse lines had been backcrossed for more than 9 generations onto the NOD genetic background and were deficient in mouse MHC class II expression (Ab0 mice obtained from Drs. D. Mathis and C. Benoist, 23). Peripheral blood mononuclear cells of mice in the litters were analyzed by FACS for transgene expression, as well as expression of mouse CD4 (mCD4), CD8 (mCD8) or MHC class II (I-Ag7). Antibodies used for FACS screening were FITC- or PE-labeled anti-HLA-DR (clone L243), anti-HLA-DQ (SK10), anti-huCD4 (SK3), anti-mCD4 (RM4−5), anti-mCD8 (53-6.7) and anti-I-Ag7 (Ox-6; all BD Biosciences). Homozygous HLA-DR/DQ transgenic mice used in experiments had near normal mCD4+ T cell counts and mCD4+/mCD8+ T cell ratios. Mice were maintained on standardized diet AIN-76A (Research Diets) from birth, except for the use of regular chow (Diet 5K67, LabDiet) in 1 experiment as mentioned in the result section. Groups of mice (6−12 weeks of age) were matched for weight and sex. HuCD4 non-transgenic recipients of adoptive transfers were irradiated with 650rad (RT 250 X-ray machine, Philips) 1 day before the i.v. (tail vein) or i.p. injection of fractionated splenic T cells (see below) in 200µl PBS from huCD4 transgenic HLA-syngenic donor mice, as indicated. All mice were monitored weekly for weight, glucosuria (Chemstrip test strips, Roche) and signs of clinical deterioration (hunching, diarrhea) until sacrificed after 2 to 35 weeks of study. Blood and stool samples were collected, the total pancreas weight recorded and organ samples taken for histology. Mice were housed in autoclaved filtertop cages and fed irradiated diet in the animal facilities at Stanford University School of Medicine. Throughout the study, all colonies tested negative for a panel of routinely screened pathogens. Approval for all procedures had been obtained from the institutional animal care and use committee (Protocol # 9987).
Preparation of T cell fractions for adoptive transfer experiments
Donor T cell fractions were prepared from splenocyte suspensions using red cell lysis buffer (Sigma), reagent kits for the negative selection of CD90+ T cells or the positive selection of CD4+ T cells, LS columns and a MidiMACS separator (all Miltenyi Biotec), following manufacturer's instructions. Numbers of T cells injected per mouse are indicated in the results section.
Histological analyses and scores; immunohistochemistry
Formalin-fixed organ samples were paraffin-embedded and sections (6 µm) stained with hematoxylin and eosin (H/E) for histology. For immunohistochemistry, paraffin-embedded pancreas sections (4 µm) were heat-treated in a steam pressure cooker for antigen demasking. Frozen, OCT-embedded pancreas sections (4 µm) were acetone-fixed. Sections were pre-treated with Peroxidase Block (Dako) and stained using polyclonal rabbit anti-murine CD3 (dilution 1:1500 for 1h; cat#CMC363; Cell Marque), rat anti-murine B220 (1:200, clone RA3–6B2; BD Pharmingen), rat anti-murine Mac-2 (1:20000, clone M3/38; Cedarlane), rat anti-murine CD4 (1:200, clone 4B12; Vector Labs) or rat anti-murine CD8b (1:200, clone H35-17.2; BD Pharmingen), rabbit anti-rat Ig secondary antibody if applicable, anti-rabbit Envision+ kit as per manufacturer's instructions, DAB chromogen (all Dako) and hematoxylin for counterstaining. Pancreas atrophy (loss of pancreatic acinar tissue and replacement by degenerative fat, inflammatory infiltrates or fibrotic tissue), pancreas inflammatory cell infiltration and lung inflammatory cell infiltration ("pneumonitis") scores were assigned to individual mice in a blinded fashion by consensus between 2 observers (R.T.B., T.F.). Scores indicate the percentage of affected areas in whole organ sections, similar to the histological score for chronic pancreatitis described by Demols et al.(24).
Determination of serum lipase activity and stool fat concentration
Lipase activity in serum sampes (1:5 dilution) was measured with the QuantiChrom lipase assay kit (BioAssay Systems) following the manufacturer's instructions, based on the dimercaptopropanol tributyrate method using 5,5'-dithiobis (2-nitrobenzoic acid) as color reagent. Changes in optical density (OD) at 412nm were determined after 10 and 15 min and referred to the calibrator. Stool fat was extracted using the method described by Schwarz et al.(25). Standard triolein concentrations (µg/100mg stool) were measured using a serum triglyceride determination kit and glycerol standard solution (all Sigma), based on the enzymatic measurement of glycerol released from triglycerides. Briefly, stool samples were dried at 50°C for 30 min. 100mg stool was added to 4ml chloroform/methanol (2:1) and homogenized. 1ml H20 was added and 1.7ml of the lower phase was recovered after vigorous mixing and overnight separation. Chloroform was evaporated under N2 at RT. Dry samples were dissolved in Trition X-100 0.05% (Sigma) and 10µl samples were assayed following the manufacturer's instructions.
Statistical analysis
Data were analyzed with Prism 5 software (GraphPad). For body weight data, indicated p values (*p<0.05; **p<0.01; ***p<0.001) were calculated using paired t-tests. Pancreas weights, serum lipase activity and stool fat concentrations were analysed using unpaired t-tests (2 groups) or one-way analysis of variance (ANOVA) and Tukey's mutiple comparison tests (>2 groups). Non-parametric data from histological observations was analysed using Mann-Whitney U tests (2 groups) or Kruskal-Wallis and Dunn's multiple comparison tests (>2 groups). Correlation was analysed using Spearman's r. In all graphs, error bars depict standard errors of the mean.
Results
Pancreatic atrophy in sublethally irradiated HLA-DR*0405 transgenic Ab0 NOD recipients of adoptive T cell transfers
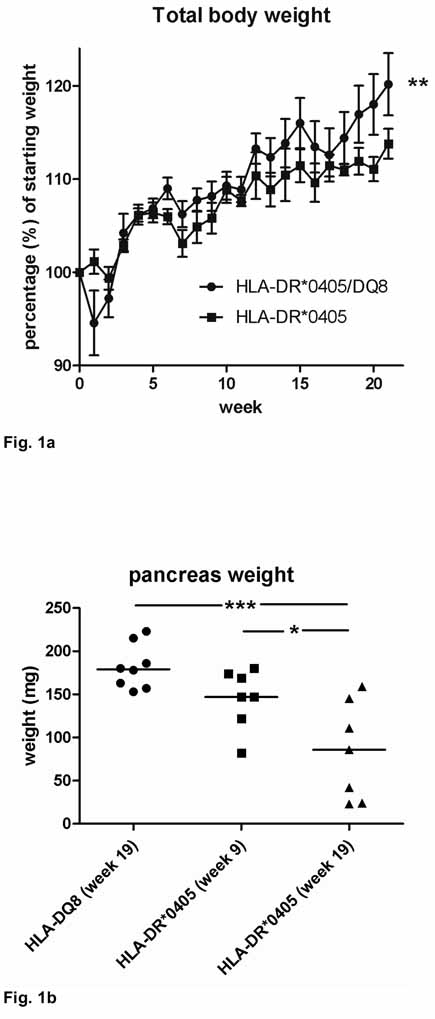
The development of pancreatic atrophy in HLA-DR*0405 transgenic Ab0 NOD mice was first noticed in unrelated experiments after adoptive transfer of 2 × 107 CD90+ T cells/mouse (i.v.) from HLA-DR*0405 transgenic or HLA-DR* 0405/HLA-DQ8 (double) transgenic donor mice (littermate controls) into sublethally irradiated, syngenic recipients (n=6–10 per group; male and female) maintained on standardized diet. Mice were monitored for 21 weeks until sacrifice, and organs were retrieved. One hundred % of HLA-DR*0405 (single) transgenic Ab0 NOD recipients had developed severe pancreatic atrophy at necropsy, whereas all HLA-DR*0405/DQ8 Ab0 NOD recipients had a normal pancreas. In addition, HLA-DR*0405 transgenic recipients had bulky stools and had failed to gain weight compared to controls starting from week 10 (**p=0.002; Fig. 1a).
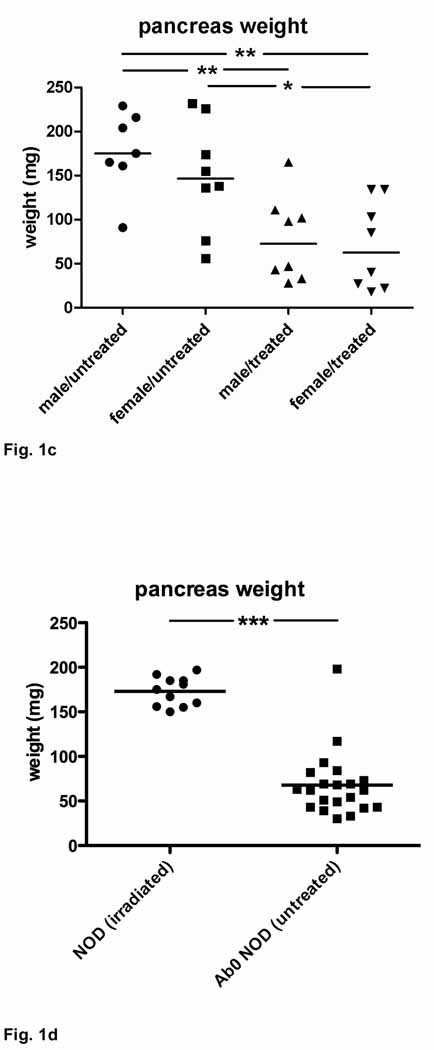
Figure 1. Clinical and macroscopic changes in HLA-DR*0405 transgenic Ab0 NOD mice.
a, b) Progressive loss of a) body weight and b) pancreas weight in HLA-DR*0405 transgenic vs. a) HLA-DR*0405/DQ8 transgenic, or b) HLA-DQ8 transgenic Ab0 NOD mice sublethally irradiated and transferred with CD90+ T cells from syngenic donor mice. Mice were monitored for 9–21 weeks post-treatment (n=6–10, weight- and sex-matched groups). c) Pancreas weights in 4 groups of HLA-DR*0405 Ab0 NOD mice (male vs. female, n=7–8) treated with sublethal irradiation and adoptive CD4+ T cell transfer, compared to animals that were left untreated. Mice were sacrificed after 18 weeks. d) Pancreas weights in sublethally irradiated normal NOD mice (n=11) analyzed 9 weeks post-treatment, or in untreated Ab0 NOD mice (n=21) at 35 weeks of age (***p<0.001, **p<0.01, *p<0.05).
To reproduce these findings in HLA-DR*0405 transgenic mice, we set up an experiment with 2 groups of sex-matched HLA-DR*0405 transgenic Ab0 NOD mice and 1 control group of sex-matched HLA-DQ8 transgenic Ab0 NOD mice maintained on regular chow. Mice were sublethally irradiated 1 day before adoptive transfer of 1 × 107 CD90+ T cells/mouse (i.p.) from syngenic donors (male and female). 1 group of HLA-DR*0405 transgenic mice was monitored for 9 weeks, while the 2 remaining groups (HLA-DR*0405 and HLA-DQ8) were monitored for 19 weeks. As before, progressive weight loss was noticed in HLA-DR*0405 transgenic mice starting from week 10 (***p=0.0003 at week 19, not shown). After sacrifice, pancreas weights were recorded. Pancreatic weights in HLA-DR*0405 vs. HLA-DQ8 transgenic mice were reduced as early as week 9, which became significant at week 19 (***p<0.001; Fig. 1b). Subgroup analysis revealed that female HLA-DR*0405 transgenic mice were more severely affected than their male counterparts (n.s. at week 9, *p<0.05 at week 19), with organs weighing <50mg (as compared to the normal >150mg) in 3 female mice. These mice had bulky stools, indicating severe exocrine pancreatic insufficiency. In contrast, pancreas weights were normal in all HLA-DQ8 transgenic mice, indicating protection from pancreatic disease.
Spontaneous development of AIP in HLA-DR*0405 transgenic Ab0 NOD mice
In order to study whether pancreatic disease develops spontaneously in HLA-DR* 0405 transgenic Ab0 NOD mice, one group of HLA-DR*0405 transgenic mice was monitored for 18 weeks without treatment, while a control group was treated with irradiation and adoptive transfer of 1 × 107 CD4+ T cells i.v. After sacrifice, pancreas weights were reduced in 5/15 untreated HLA-DR* 0405 transgenic mice (<150mg, 4 females, 1 male; Fig. 1c), suggesting spontaneous development of pancreatic disease. Complete pancreatic atrophy (weight <50mg) did not develop in untreated mice within the study period, whereas 8/16 treated mice had pancreas weights <50mg (***p<0.0001, not shown) and displayed signs of exocrine pancreatic insufficiency. These findings suggested acceleration of disease development and progression after sublethal irradiation +/− adoptive CD4+ T cell transfer. Subgroup analysis in this experiment revealed only non-significant trends for reduced pancreas weights in female vs. male mice (both for untreated and treated mice).
Spontaneous development of AIP in Ab0 NOD control mice
Since the development of AIP in Ab0 mice on the C57BL/6 genetic background had previously been reported(14), we asked whether disease was present in mice from the Ab0 NOD strain that represented the genetic background for all HLA class II transgenic lines in this study. Therefore, Ab0 NOD mice (male and female) were left untreated and were monitored for 35 weeks from birth. As additional controls, normal NOD mice (male and female) were sublethally irradiated and monitored for 9 weeks post-treatment. Pancreas weights were significantly reduced in all but one Ab0 NOD mouse at 35 weeks of age, while all normal NOD mice maintained normal pancreas weights ((***p<0.0001, Fig. 1d). Since CD4+ T cells were not required to produce AIP in the Ab0 NOD strain, these results indicated that CD4+ T cells were unlikely to act as pathogenic T cells in HLA-DR*0405 transgenic mice. Rather, HLA-DR*0405-restricted CD4+ T cells failed to protect transgenic mice from the development of AIP, while HLA-DQ8-restricted CD4+ T cells appeared to be fully capable of conferring disease protection (see above).
Pancreatic pathology
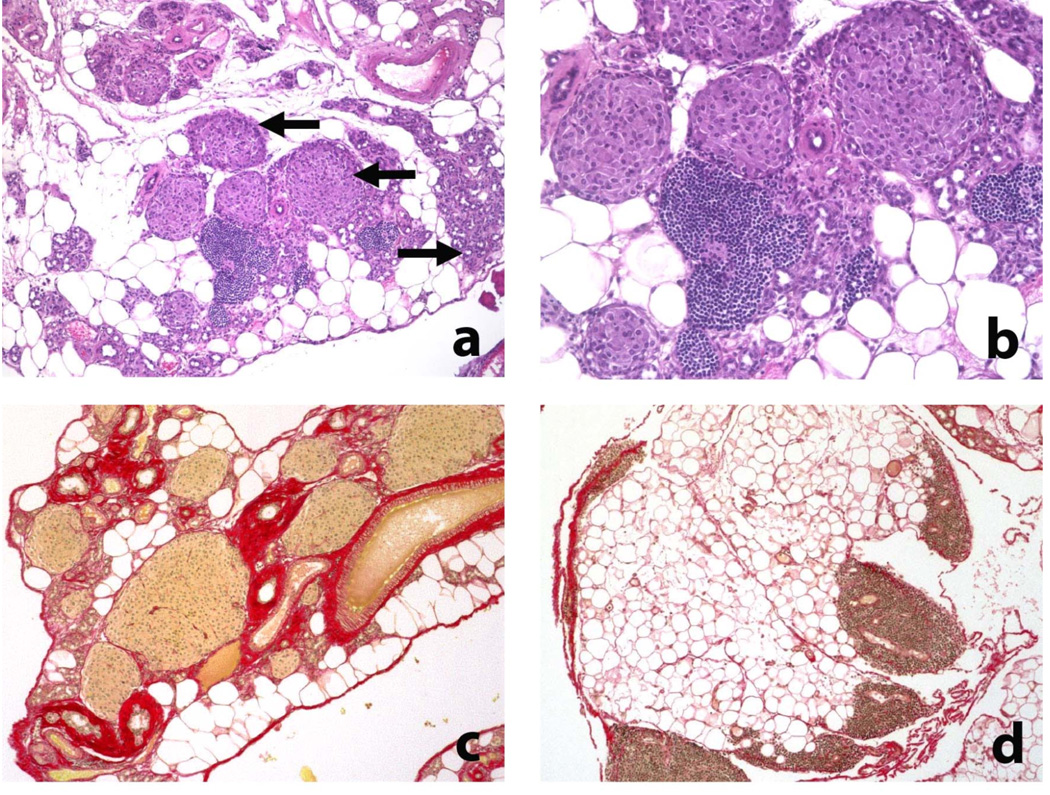
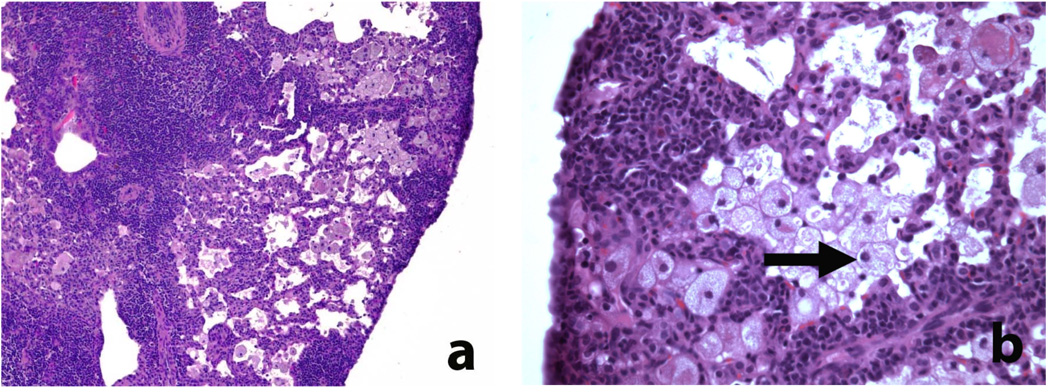
As noted initially in samples collected >20 weeks after sublethal irradiation and adoptive T cell transfer, the most striking pathological finding in HLA-DR* 0405 transgenic Ab0 NOD mice was the development of severe pancreatic atrophy, leading to complete loss of the exocrine tissue, with total pancreas weights as low as 20mg in individual mice. Weight loss in combination with bulky stools, intestinal distention and meteorism were consistent with exocrine pancreatic insufficiency. Histologically, severe pancreatic atrophy was characterized by extensive loss of acinar cell mass and replacement with degenerative fat (Fig. 2a). Loss of zymogen granules in acinar cells and formation of ductular structures were noted in the remaining parenchyma, indicating acinar-to-ductal metaplasia (Fig. 2a,b). In late stages, the entire organ was replaced by degenerative fat, with the exception of the islets of Langerhans that remained normal (Fig. 2b), while scattered infiltrates of inactive lymphocytes were frequently present (Fig. 2a,b). In these samples with end stage pancreatic atrophy, the extent of fibrosis varied greatly, with dense periductal and perivascular deposition of collagen fibers in pancreas samples of some mice (Fig. 2c), but mild fibrosis only in others (Fig. 2d).
Figure 2. Histological changes in the pancreas of HLA-DR*0405 transgenic Ab0 NOD mice (I).
a–d) Severe pancreatic atrophy characterized by extensive loss of acinar cell mass and replacement by degenerative fat, scattered infiltrates of lymphocytes and fibrosis (examples taken from HLA-DR* 0405 transgenic mice sacrificed 20 weeks after sublethal irradiation and adoptive CD90+ T cell transfer). a, b) H/E staining demonstating loss of zymogen granules in acinar cells and formation of ductular structures (→), indicating acinar-to-ductal metaplasia. Note preservation of the islets of Langerhans (←; original magn. a) 100×, b) 200×). c, d) Sirius red staining demonstrating variable degrees of fibrosis (mainly periductal, perivascular) in 2 samples with severe atrophy (magn. 200×).
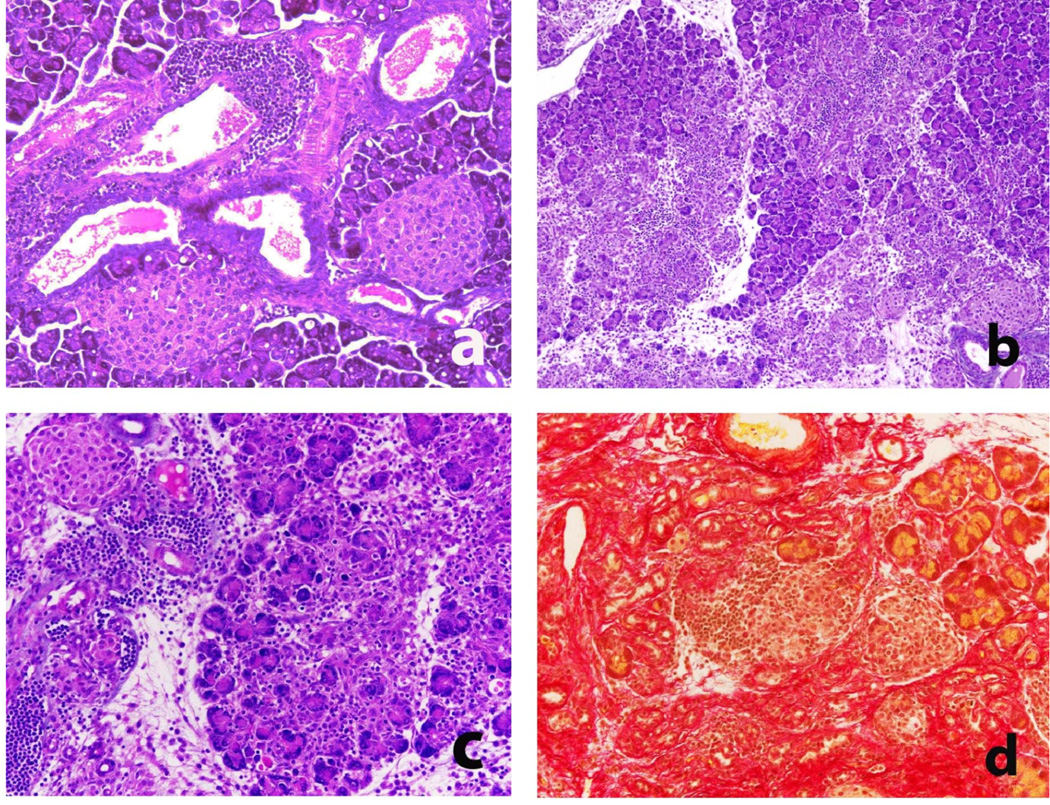
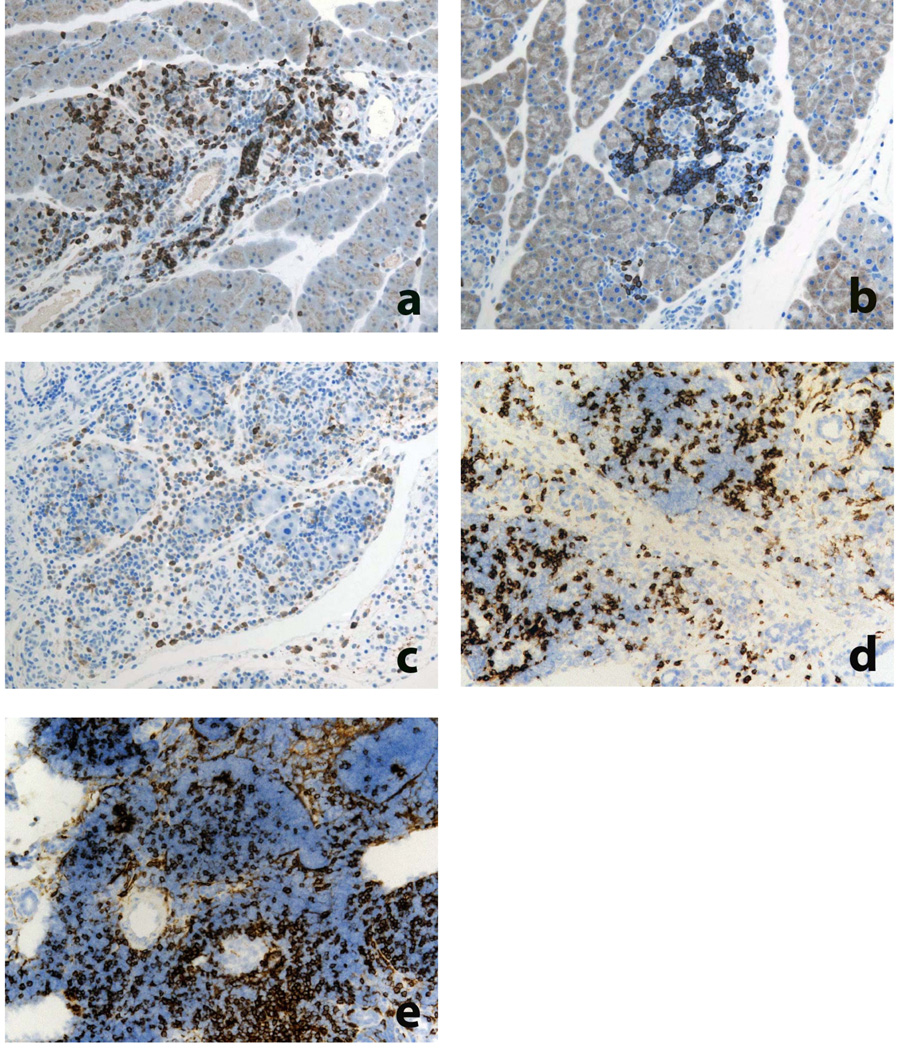
In samples taken from HLA-DR*0405 trangenic mice at 9 weeks, and in additional samples taken as early as 6–8 weeks after sublethal irradiation and adoptive T cell transfer, periductal and perivascular infiltration with lymphocytes and macrophages was prominent (Fig. 3a) and gave rise to multifocal, destructive invasion of the acinar tissue (Fig. 3b,c). Inflammatory changes in organ histology were pathognomonic for AIP as described in humans(2, 10) and mice(11–14). In pancreatitis, sirius red staining revealed dense deposition of collagen fibers in highly infiltrated lobuli (Fig. 3d), clearly demonstrating fibrosis as a result of the inflammatory process. The celluar components involved were further characterized using immunohistochemistry, demonstrating that the inflammatory infiltrates consisted mainly of T cells (CD3+), B cells (B220+) and macrophages (Mac-2+) (Fig. 4a–c). Frequently, pancreatic acini were surrounded by inflammatory cells while other areas were unaffected, underlining the multifocal aspect of disease progession. Within the T cell compartment, the CD4+ and CD8+ T cell subpopulations were equally represented (Fig. 4d,e). Only few plasma cells (Ig+) were found in inflammatory infiltrates (not shown).
Figure 3. Histological changes in the pancreas of HLA-DR*0405 transgenic Ab0 NOD mice (II).
a) H/E staining demonstrating periductal and perivascular mononuclear cell infiltration of the pancreas as an early finding in HLA-DR*0405 transgenic mice (magn. 200×). b, c) Fulminant AIP in HLA-DR* 0405 transgenic mice at 8 weeks post-treatment, characterized by multifocal, destructive invasion of the acinar tissue with lymphocytes, macrophages and few plasma cells (H/E staining; magn. b) 100×, c) 200×). d) Sirius red staining revealing collagen deposition in infiltrated lobuli in fulminant pancreatitis (representative sample, magn. 200×).
Figure 4. Immunohistochemical characterization of pancreatic inflammatory infiltrates in HLA-DR*0405 transgenic Ab0 NOD mice.
a–e) Immunostainings of pancreas from HLA-DR*0405 mice with fulminant pancreatitis demonstrate inflammatory infiltrates consisting mainly of a) CD3+ T cells, b) B220+ B cells and c) Mac-2+ macrophages. Within the T cell compartment, the d) CD4+ and e) CD8+ subpopulations are equally represented. Stainings performed on a–c) paraffin-embedded or d, e) frozen tissue, using DAB chromogen and hematoxylin for counterstaining (original magn. 200×).
Semiquantitative histological assessment of pancreatic atrophy and inflammatory cell infiltration
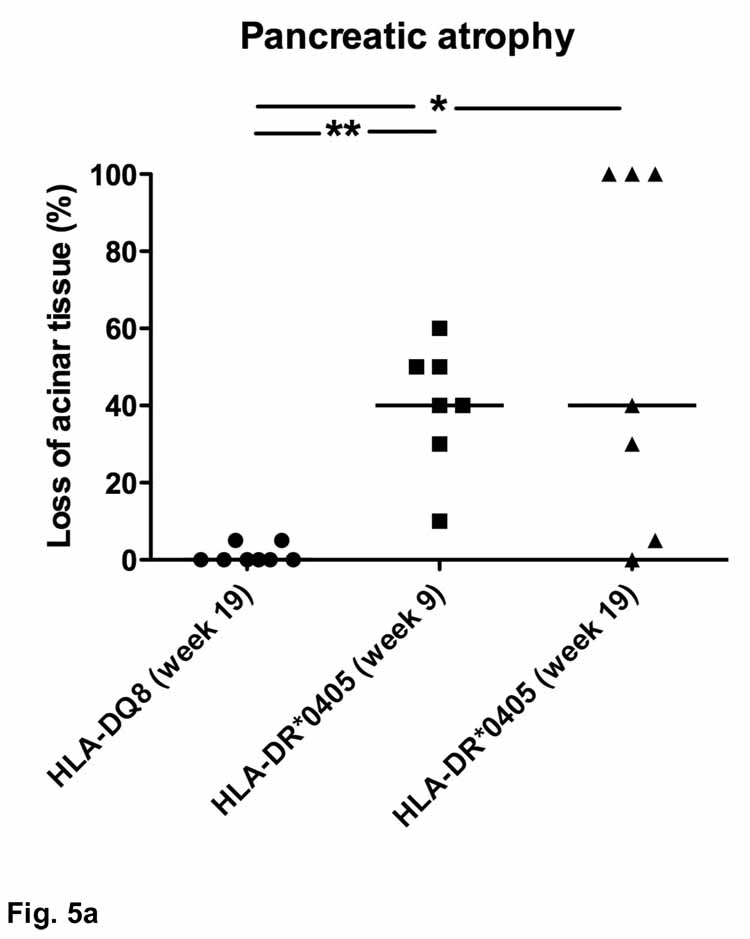
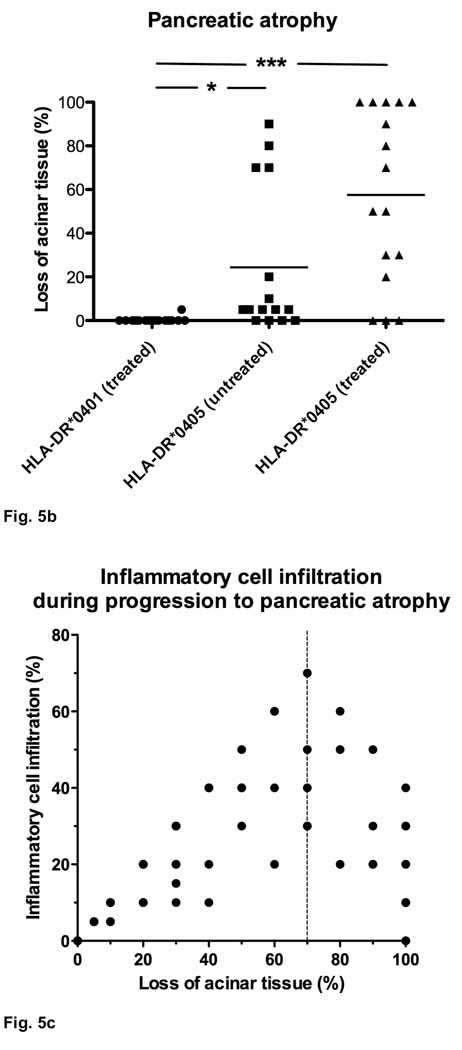
In order to assess the degree of pancreatic damage, we measured atrophy (loss of pancreatic acinar tissue and replacement by degenerative fat, inflammatory infiltrates or fibrotic tissue) and infiltration by inflammatory cells in whole organ sections. Sublethally irradiated HLA-DQ8 transgenic Ab0 NOD recipients of adoptive CD90+ T cell transfers (see above) were completely protected from pancreatic atrophy (Fig. 5a). While mild periductal/perivascular mononuclear infiltrates (similar to Fig. 3a) were occasionally seen, HLA-DQ8 transgenic mice never developed destructive inflammatory lesions. In contrast, 6 out of 7 HLA-DR*0405 transgenic Ab0 NOD mice had lost at least 20% of acinar tissue at 9 weeks post-treatment (**p<0.01), and 3 (female) out of these 7 mice had progressed to complete pancreatic atrophy (100%) at week 19 (*p<0.05 vs. HLA-DQ8 transgenic mice; Fig. 5a). Subanalysis of the results from HLA-DR*0405 transgenic mice at week 19 indicated accelerated progression towards pancreatic atrophy in females vs. males (**p<0.01; not shown). Further, 5 out of 15 untreated HLA-DR*0405 transgenic mice (4 females, 1 male) had lost at least 20% of acinar tissue after 18 weeks of study, demonstrating spontaneous development of pancreatic atrophy (Fig. 5b). The maximal atrophy score reached in an untreated (female) mouse was 90%. In direct comparison, 13 out of 16 mice treated with sublethal irradiation and adoptive CD4+ T cell transfer had atrophy scores of 20% and higher, with 5 (3 females, 2 males) out of 16 mice affected by complete pancreatic atrophy (n.s.; Fig. 5b). Subgroup analysis in this experiment revealed only a non-significant trend for increased pancreatic atrophy scores in untreated female vs. untreated male mice, and no difference for treated mice. An additional HLA-DR control group consisting of sex-matched HLA-DR*0401 transgenic Ab0 NOD mice treated accordingly (sublethal irradiation and CD4+ T cell transfer from syngenic donor mice; n=16) showed no signs of destructive inflammatory lesions, or pancreatic atrophy, at 9 weeks post-treatment (Fig. 5b).
Figure 5. Histological scores for pancreatic atrophy and inflammatory cell infiltration in HLA-DR*0405 transgenic Ab0 NOD mice.
Scores indicating area involved (percentage) in whole organ sections. a) Pancreatic atrophy in HLA-DR*0405 transgenic NOD mice, but not in HLA-DQ8 NOD mice (sublethally irradiated and transfered with CD90+ T cells from syngenic donor mice), monitored for 9–19 weeks post-treatment (n=7–8, weight- and sex-matched groups; **p<0.01, *p<0.05). b) Spontaneous development of pancreatic atrophy in untreated HLA-DR*0405 transgenic mice after 18 weeks of study. HLA-DR*0405 transgenic mice treated with sublethal irradiation and adoptive CD4+ T cell transfer are more severely affected than untreated mice (n.s.), while HLA-DR*0401 transgenic controls monitored for 9 weeks post-treatment do not show any signs of disease (***p<0.001, *p<0.05). c) Close correlation of pancreatic atrophy with inflammatory cell infiltration in samples with atrophy scores ranging from 0–70% (***r=0.96). In advanced stages (>70%; dotted line), infiltration with inflammatory cells diminishes.
Taken together, histological analysis corroborated the macroscopic findings, as pancreas weights had a close negative correlation with pancreatic atrophy scores (***r=−0.71; not shown). In addition, progression to pancreatic atrophy in HLA-DR*0405 transgenic mice was closely correlated with pancreatic inflammatory cell infiltration until approx. 70% of the exocrine tissue was lost (***r=0.96; Fig. 5c). Beyond this stage, inflammatory cell infiltration diminished. In some cases of complete pancreatic atrophy, no residual infiltrates were found in the remaining degenerative fat tissue.
Additional pathology
Pneumonitis was noticed as an additional histological finding in up to 65% of sublethally irradiated HLA-DR*0405 transgenic Ab0 NOD recipients of adoptive T cell transfer, and also -with the same prevalence- in untreated HLA-DR*0405 transgenic mice, but not in HLA-DR*0401, -DQ8 or - DR*0405/DQ8 (double−) transgenic Ab0 NOD mice (treated with irradiation and adoptive T cell transfer). Pneumonitis was characterized by perivascular and peribronchial mononuclear cell infiltration, but also by infiltration of the alveoli with foamy macrophages, predominantly in the periphery of the organ (Fig. 6 a,b). In severe cases, up to 50% of the total area in lung tissue sections was infiltrated by inflammatory cells (using a histological pneumonitis score). There were non-significant trends for more severe pneumonitis in mice monitored for longer periods (18–21 weeks post-irradiation and adoptive transfer), as well as in mice affected by complete pancreatic atrophy (not shown). However, rarely was pneumonitis noted in HLA-DR*0405 transgenic mice in the absence of pancreatic involvement. Silver stains of lung tissue sections from mice with severe pneumonitis were negative for Pneumocystis carinii. Only occasionally, inactive mononuclear infiltrates were noticed in other organs of HLA-DR*0405 transgenic mice (stomach, liver, kidney, heart, thyroid, parotoid, submandibular and lacrimal glands). The gut and skin were normal.
Figure 6. Extrapancreatic pathology in HLA-DR*0405 transgenic Ab0 NOD mice.
a, b) Pneumonitis characterized by perivascular and peribronchial mononuclear cell infiltration, but also by infiltration of the alveoli with foamy macrophages (→), predominantly in the periphery of the organ (original magn. a) 100×, b) 400×; example taken from an untreated HLA-DR*0405 transgenic mouse, age 26 weeks at sacrifice).
Pancreatic functional studies
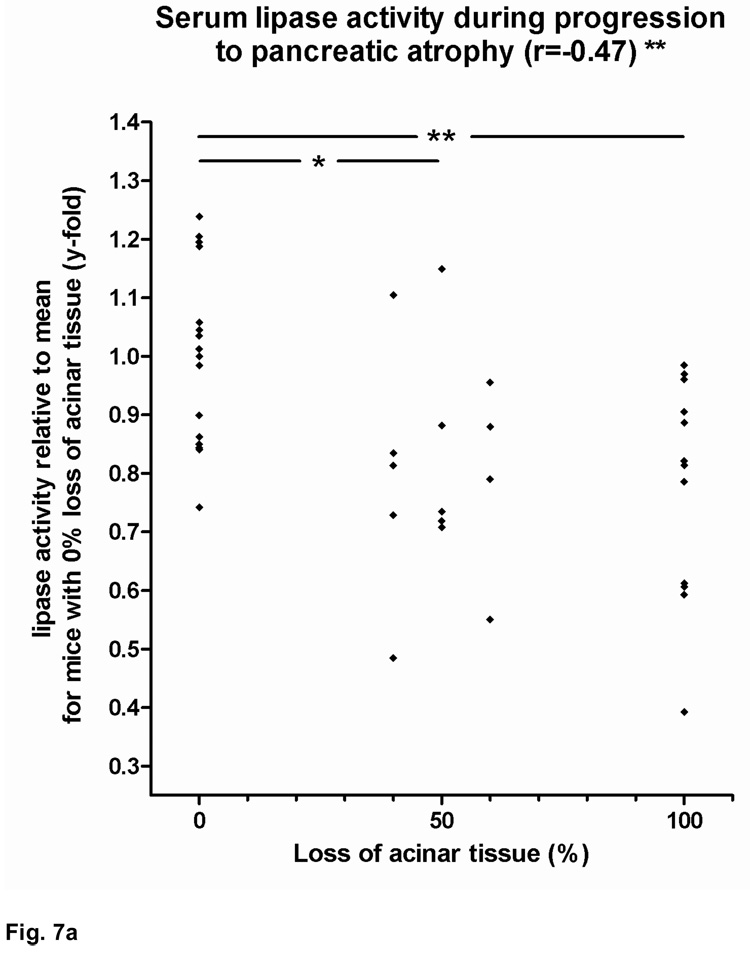
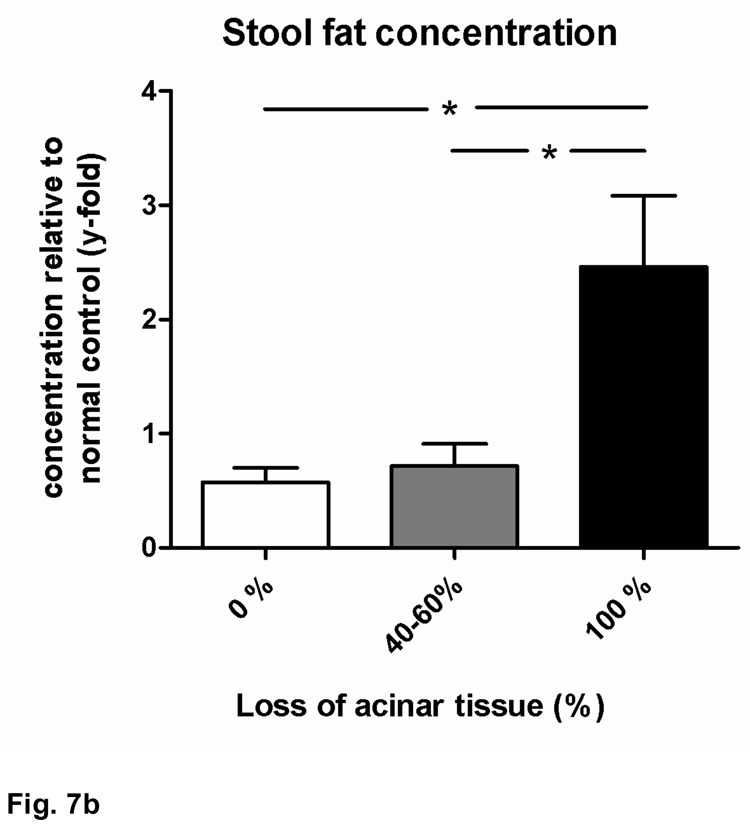
As mentioned above, the development of severe pancreatic atrophy was associated with weight loss and other signs of exocrine pancreatic insufficiency, such as bulky stools, intestinal distention and meteorism. Serum lipase activity in HLA-DR*0405 transgenic Ab0 NOD mice was negatively correlated with the degree of pancreatic atrophy (**r=−0.47). Groups of mice with >40% acinar tissue loss had significant reductions in serum lipase activity when compared to mice with 0% loss (*p<0.05 and **p<0.01; Fig. 7a). In addition, HLA-DR*0405 transgenic mice with complete pancreatic atrophy had increased stool fat concentrations compared to mice with either 0% or 40–60% acinar tissue loss (*p<0.05; Fig. 7b), demonstrating fat maldigestion due to pancreatic insufficiency in end stage disease, similar to the functional consequences of AIP in humans.
Figure 7. Functional changes in HLA-DR*0405 transgenic Ab0 NOD mice.
a) Decrease of serum lipase activity with the progression to pancreatic atrophy (**r=−0.47). Significant reductions in serum lipase activity in groups of HLA-DR*0405 transgenic mice with 100% or 40–60% vs. 0% loss of acinar tissue (**p<0.01 and *p<0.05; data expressed as lipase activity relative to the mean of mice with 0% tissue loss). b) Increased stool fat concentrations in HLA-DR*0405 transgenic mice with complete pancreatic atrophy (100%) vs. mice with either 0% or 40–60% loss of acinar tissue (*p<0.05; data expressed as fat concentration relative to mean for normal wildtype controls), demonstrating fat maldigestion due to pancreatic insufficiency in end stage disease.
Discussion
MHC class II genes are the best characterized predisposing factors for autoimmune diseases. In an effort to advance our understanding of the mechanisms that underlie MHC class II disease associations in humans, a variety of HLA class II transgenic mice have been developed(9). Transgene-encoded HLA class II molecules are functional in murine MHC class II negative mice, where they shape the CD4+ T cell repertoire by controlling positive and negative selection in the thymus. Moreover, disease associated HLA class II molecules are capable of conferring disease susceptibility. Therefore, HLA transgenic mice can manifest aspects of the relevant human disease(s). With a primary interest in the association of HLA-DR*0405 with type 1 diabetes, we generated mice expressing this human MHC class II molecule on professional antigen-presenting cells, but found that this mouse line develops AIP and additional organ manifestations, while the pancreatic islets remain intact. In this report, we describe the characteristics of AIP in HLA-DR*0405 transgenic Ab0 NOD mice. Together with the established association of the HLA-DRB1*0405/DQB1*0401 haplotype with AIP in humans(3), our findings indicate that the HLA-DR*0405 dimer confers genetic susceptibility to autoimmune disease in the pancreas. AIP develops spontaneously, without a requirement for additional inflammatory or co-stimulatory triggers, and progresses to complete pancreatic atrophy. Furthermore, AIP in HLA-transgenic mice closely mimics disease in humans regarding pathology, histology, functional consequences and clinical manifestations. Using sublethal irradiation and adoptive T cell transfer to induce disease, a high penetrance of 60–100% is reached at 20 weeks post-treatment.
In the CD4+ T cell deficient Ab0 C57BL/6 mouse model of AIP(14), a pathogenic role was attributed mainly to CD8+ T cells that were able to transfer disease to athymic recipients. CD8+ T cells may have a similar pathogenic role in the development of AIP in HLA-DR*0405 transgenic Ab0 NOD mice (as well as in humans), as indicated by disease development in the Ab0 NOD background strain, but also by the immunohistochemical findings in HLA-DR*0405 transgenic mice. However, we want to emphasize the clear differences between these models. Since Ab0 mice lack MHC class II expression, they are severely deficient in CD4+ T cells. In comparison, HLA-DR* 0405 transgenic Ab0 mice are CD4+ T cell competent and have restored cellular and humoral immunity. Thus, HLA-DR*0405 transgenic mice have near normal mCD4+ T cell counts, mCD4+/mCD8+ T cell ratios, but also normalized T helper cell function, as demonstrated by adequate cytokine production (e.g. IFNγ, IL-2 and IL-10) upon in vitro restimulation with T cell-dependant antigen (not shown). Further supporting our findings, a second HLA-DR*0405 transgenic Ab0 NOD mouse line generated separately by us also develops AIP (results not shown). Immunosuppressive CD4+ T cell populations, namely CD4+CD25+ regulatory T cells (Treg), are key regulators of CD4+ and CD8+ effector T cell functions and play a decisive role in controlling immune reactions that lead to autoimmune diseases in mice and humans(15, 16). Therefore, a HLA transgenic model enables us to study the protective as well as pathogenic functions of both CD4+ and CD8+ T cells in AIP. We propose a model for the development of AIP in which defects in the adequate priming of regulatory HLA-DR*0405-restricted CD4+ T cells lead to unopposed CD8+ T cell, CD4+ effector T cell and/or macrophage functions. The result of this dysregulated immune response is inflammatory disease of the exocrine pancreas and the lung.
Humanized mice have been used to identify T cell epitopes and immunodominant peptides that trigger autoimmune disease in patients, and to test therapies that target HLA class II-restricted antigen presentation. We are confident that our model in HLA-DR*0405 transgenic mice will facilitate the identification of T cell and B cell epitopes involved in the pathogenesis of AIP. Similar to AIP in humans, characterized by the presence of autoantibodies e.g. against carbonic anhydrase II or lactoferrin, and organ infiltration with B cells and plasma cells(1, 2, 10), B cells featured strongly in pancreatic infiltrates in HLA-DR*0405 transgenic mice. AIP is highly treatable with corticosteroids, but has remained a diagnostic challenge(1, 10). Therefore, the clinical value of improved non-invasive diagnostic tools, i.e. disease-specific serum autoantibodies, would be considerable.
Our finding of additional organ involvement is of particular interest, since pneumonitis in HLA-DR*0405 transgenic mice mimics interstitial pneumonitis associated with AIP in humans(10). This indicates that AIP in HLA-DR*0405 transgenic mice develops as the leading manifestation of systemic autoimmune disease, as is the case with AIP in humans. Other organ manifestations frequently associated with AIP, such as mononuclear infiltration of salivary glands or bile ducts, were absent in HLA-DR*0405 transgenic mice. This may be due to the presence in humans of additional antigenic epitopes not shared with mice. Further, none of the HLA-DR*0405 transgenic mice affected by AIP developed overt diabetes mellitus, as indicated by negative urine glucose status throughout the experiments.
While AIP in humans develops more frequently in males than in females, there was a clear trend in this study towards more severe pancreatic inflammatory disease in female HLA-DR*0405 transgenic mice. This discrepancy may be explained by additional sex-dependent genetic factors not shared between mice and humans. This is further substantiated by a similar preponderance of AIP in female MRL/Mp(11) and female Ab0 C57BL/6 mice(14).
HLA-DR*0405 transgenic mice suffering from AIP lost weight when maintained on regular, natural-ingredient chow. When mice were maintained on the more readily digestible standardized diet AIN-76A instead, weight loss was less severe, while neither the incidence nor the rate of progression of AIP was influenced (not shown). Together with findings of reduced serum lipase activity and increased stool fat concentrations in HLA-DR*0405 transgenic mice, these observations underline the functional and clinical consequences of exocrine pancreatic insufficiency in this model.
While HLA-DQ4 transgenic mice were not available to us, HLA-DQ8 transgenic controls were chosen following the group set-up in the initial (unrelated) experiment that led to discovery of the syndrome in HLA-DR*0405 transgenic mice (shown in Fig. 1a). In addition, HLA-DR*0401 transgenic mice were used as HLA-DR positive controls in a follow-up experiment. Interestingly, both HLA-DR*0401 transgenic, HLA-DQ8 transgenic, and HLA-DR* 0405 transgenic Ab0 NOD mice co-expressing HLA-DQ8 were completely protected from the development of AIP. This protective effect may be attributed to the role of HLA-DR*0401 or -DQ8-restricted regulatory T cells. In support of this hypothesis, we found AIP in HLA-DR*0401 or -DQ8 transgenic Ab0 NOD mice after blockade of Treg function in vivo using monoclonal anti-CD25 antibody (preliminary results). Modulation of susceptibility to autoimmune disease by co-expression of additional HLA-DQ and -DR molecules has previously been demonstrated in humans (17) and HLA-transgenic mice, where it was attributed to increased Th2 cytokine production(18, 19). Our humanized model may be used to determine the relative roles of HLA-DR and HLA-DQ molecules in the priming of effector vs. regulatory CD4+ T cells in AIP. Meanwhile, our results identify HLA-DR*0405 as a prominent risk factor on the HLA-DRB1*0405/ DQB1*0401 haplotype associated with AIP.
Acknowledgments
We thank Suzanne White and Wendy Dasgupta (Beth Israel Deaconess Medical Center) for assistance with histology and Dr. Scott Rodig and Donna Skinner (Brigham and Women's Hospital, Boston, MA) for support with immunohistochemistry. This work was supported by grants DK55364 and DK72288 to G.S., a Harvard University institutional grant to D.S., as well as Zedira GmbH, Darmstadt, Germany.
Non-standard abbreviations
- AIP
Autoimmune pancreatitis
- Aire
autoimmune regulator gene
- (Ab0) mice
I-Aβ chain−/−
- Treg
CD4+CD25+ regulatory T cells
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest to disclose.
References
- 1.Finkelberg DL, Sahani D, Deshpande V, et al. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670–2676. doi: 10.1056/NEJMra061200. [DOI] [PubMed] [Google Scholar]
- 2.Kloppel G, Luttges J, Lohr M, Zamboni G, et al. Autoimmune pancreatitis: pathological, clinical, and immunological features. Pancreas. 2003;27:14–19. doi: 10.1097/00006676-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kawa S, Ota M, Yoshizawa K, et al. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology. 2002;122:1264–1269. doi: 10.1053/gast.2002.33022. [DOI] [PubMed] [Google Scholar]
- 4.Saito S, Ota S, Yamada E, et al. Allele frequencies and haplotypic associations defined by allelic DNA typing at HLA class I and class II loci in the Japanese population. Tissue Antigens. 2000;56:522–529. doi: 10.1034/j.1399-0039.2000.560606.x. [DOI] [PubMed] [Google Scholar]
- 5.Shindo Y, Inoko H, Yamamoto T, et al. HLA-DRB1 typing of Vogt-Koyanagi-Harada's disease by PCR-RFLP and the strong association with DRB1*0405 and DRB1*0410. Br J Ophthalmol. 1994;78:223–226. doi: 10.1136/bjo.78.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seki T, Ota M, Furuta S, et al. HLA class II molecules and autoimmune hepatitis susceptibility in Japanese patients. Gastroenterology. 1992;103:1041–1047. doi: 10.1016/0016-5085(92)90041-v. [DOI] [PubMed] [Google Scholar]
- 7.Awata T, Kuzuya T, Matsuda A, et al. Genetic analysis of HLA class II alleles and susceptibility to type 1 (insulin-dependent) diabetes mellitus in Japanese subjects. Diabetologia. 1992;35:419–424. doi: 10.1007/BF02342437. [DOI] [PubMed] [Google Scholar]
- 8.Kim HY, Kim TG, Park SH, et al. Predominance of HLA-DRB1*0405 in Korean patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:988–990. doi: 10.1136/ard.54.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregersen JW, Holmes S, Fugger L. Humanized animal models for autoimmune diseases. Tissue Antigens. 2004;63:383–394. doi: 10.1111/j.0001-2815.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande V, Mino-Kenudson M, Brugge W, et al. Autoimmune pancreatitis: more than just a pancreatic disease? A contemporary review of its pathology. Arch Pathol Lab Med. 2005;129:1148–1154. doi: 10.5858/2005-129-1148-APMTJA. [DOI] [PubMed] [Google Scholar]
- 11.Kanno H, Nose M, Itoh J, et al. Spontaneous development of pancreatitis in the MRL/Mp strain of mice in autoimmune mechanism. Clin Exp Immunol. 1992;89:68–73. doi: 10.1111/j.1365-2249.1992.tb06879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang W, Anderson MS, Bronson R, et al. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meagher C, Tang Q, Fife BT, et al. Spontaneous development of a pancreatic exocrine disease in CD28-deficient NOD mice. J Immunol. 2008;180:7793–7803. doi: 10.4049/jimmunol.180.12.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallance BA, Hewlett BR, Snider DP, et al. T cell-mediated exocrine pancreatic damage in major histocompatibility complex class II-deficient mice. Gastroenterology. 1998;115:978–987. doi: 10.1016/s0016-5085(98)70270-7. [DOI] [PubMed] [Google Scholar]
- 15.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 17.Baisch JM, Weeks T, Giles R, et al. Analysis of HLA-DQ genotypes and susceptibility in insulin-dependent diabetes mellitus. N Engl J Med. 1990;322:1836–1841. doi: 10.1056/NEJM199006283222602. [DOI] [PubMed] [Google Scholar]
- 18.Taneja V, Griffiths MM, Luthra H, et al. Modulation of HLA-DQ-restricted collagen-induced arthritis by HLA-DRB1 polymorphism. Int Immunol. 1998;10:1449–1457. doi: 10.1093/intimm/10.10.1449. [DOI] [PubMed] [Google Scholar]
- 19.Wen L, Chen NY, Tang J, et al. The regulatory role of DR4 in a spontaneous diabetes DQ8 transgenic model. J Clin Invest. 2001;107:871–880. doi: 10.1172/JCI11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fugger L, Michie SA, Rulifson I, et al. Expression of HLA-DR4 and human CD4 transgenes in mice determines the variable region beta-chain T-cell repertoire and mediates an HLA-DR-restricted immune response. Proc Natl Acad Sci USA. 1994;91:6151–6155. doi: 10.1073/pnas.91.13.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herman AE, Tisch RM, Patel SD, et al. Determination of glutamic acid decarboxylase 65 peptides presented by the type I diabetes-associated HLA-DQ8 class II molecule identifies an immunogenic peptide motif. J Immunol. 1999;163:6275–6282. [PubMed] [Google Scholar]
- 22.Killeen N, Sawada S, Littman DR. Regulated expression of human CD4 rescues helper T cell development in mice lacking expression of endogenous CD4. EMBO J. 1993;12:1547–1553. doi: 10.1002/j.1460-2075.1993.tb05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosgrove D, Gray D, Dierich A, et al. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 24.Demols A, Van Laethem JL, Quertinmont E, et al. Endogenous interleukin-10 modulates fibrosis and regeneration in experimental chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1105–G1112. doi: 10.1152/ajpgi.00431.2001. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz M, Lund EG, Setchell KD, et al. Disruption of cholesterol 7alpha-hydroxylase gene in mice. II. Bile acid deficiency is overcome by induction of oxysterol 7alpha-hydroxylase. J Biol Chem. 1996;271:18024–18031. doi: 10.1074/jbc.271.30.18024. [DOI] [PMC free article] [PubMed] [Google Scholar]