Abstract
Application of Single Prolonged Stress (SPS) in rats induces changes in neuroendocrine function and arousal that are characteristic of Post Traumatic Stress Disorder (PTSD). PTSD, in humans, is associated with decreased neural activity in the prefrontal cortex, increased neural activity in the amygdala complex, and reduced neuronal integrity in the hippocampus. However, the extent to which SPS models these aspects of PTSD has not been established. In order to address this, we used high-resolution magic angle spinning proton magnetic resonance spectroscopy (HR-MAS 1H MRS) ex vivo to assay levels of neurochemicals critical for energy metabolism (creatine and lactate), excitatory (glutamate and glutamine) and inhibitory (gamma amino butyric acid (GABA)) neurotransmission, and neuronal integrity (N-acetyl aspartate (NAA)) in the medial prefrontal cortex (mPFC), amygdala complex, and hippocampus of SPS and control rats. Glutamate, glutamine, and creatine levels were decreased in the mPFC of SPS rats when compared to controls, which suggests decreased excitatory tone in this region. SPS did not alter the neurochemical profiles of either the hippocampus or amygdala. These data suggest that SPS selectively attenuates excitatory tone, without a disruption of neuronal integrity, in the mPFC.
Keywords: PTSD, anxiety, emotional regulation, glutamate, GABA, proton magnetic resonance spectroscopy
Introduction
The Single Prolonged Stress (SPS) paradigm refers to the serial application of restraint stress, forced swim, and ether exposure, followed by a quiescent period of seven days. This paradigm has been developed as a rat model of Post Traumatic Stress Disorder (PTSD). PTSD patients show augmented fast negative feedback of the hypothalamic-pituitary-adrenal (HPA) axis [1] and augmented startle reactivity [2–5] and previous reports indicate that SPS models these aspects of PTSD [6, 7]. Recently, a specific deficit in recall of extinguished fear conditioning has been reported in PTSD patients [8] and disrupted extinction recall has also been reported in animals that have undergone SPS treatment [9]. Taken together, these findings further support the validity of SPS in rats as a model of specific neuroendocrine and behavioral changes associated with PTSD.
Changes in neural function in the medial prefrontal cortex (mPFC), amygdala complex, and hippocampus are also characteristic of PTSD. When presented with reminders of traumatic events PTSD patients show attenuated hemodynamic responses in the ventromedial prefrontal cortex [10, 11] and elevated responses in the amygdala complex [12, 13] when compared to control subjects. These alterations in neurocircuitry relating to fear processing and emotion regulation have been proposed as mechanisms for pathological fear in PTSD, where decreased mPFC input to the amygdala fails to appropriately inhibit conditioned fear responses [for a recent review see 14]. Indeed, excitatory projections from glutamatergic neurons in the mPFC have been implicated in the inhibition of principle amygdala neuron firing in rats [15]. In addition, it has been consistently reported that PTSD patients demonstrate reduced hippocampal volume and decreased neuronal integrity (i.e. the destruction of neurons, decreased neuronal density, or aberrant changes in cellular processes within neurons) in the hippocampus [12, 16, 17] in comparison to controls. To date, the effects of SPS on neural activity in the mPFC and amygdala, and neuronal integrity in the hippocampus or hippocampal volume, have not been evaluated.
Magnetic resonance spectroscopy (MRS) can be used to assess the concentrations of multiple neurochemicals within the brain (in vivo) or in a single brain sample (ex vivo), allowing some inference regarding aspects of neural function in the brain. For example, N-acetylaspartate (NAA) is a neurochemical expressed in neurons and neuronal processes, but not glia [18, 19]. NAA is indicative of neuronal death [19], decreases in neural density [19, 20], and aberrant metabolic processes [20, 21]. NAA is reduced in a number of disorders associated with decreased neuronal integrity. These include brain tumors [22], epilepsy [23], and multiple sclerosis [24]. Thus, by measuring levels of NAA one can infer levels of neuronal integrity in the brain [19, 21].
While MRS cannot be used to measure neural activity (i.e. changes in membrane potential over a given period of time) the technique can be used to index the levels of neurochemicals critical to changes in neural activity. For example, by measuring relative concentrations of inhibitory (i.e. gamma amino butyric acid (GABA)) and excitatory (i.e. glutamate) neurotransmitters, and of molecules indicative of energy metabolism which are necessary for changes in membrane potential, the potential for increased neural activity can be inferred (i.e. excitatory tone). MRS technology has been applied to study the neural basis of psychiatric disorders. Changes in hippocampal neuronal integrity in PTSD patients have been reported, in proton (1H) MRS studies, that measure NAA levels in vivo [25–28], suggesting that MRS technology can be useful to further investigate neural processes critical to the etiology of PTSD.
The goal of this study was to determine the effect of SPS on levels of excitatory and inhibitory neurotransmitters, neurochemicals indicative of energy metabolism, and NAA in the mPFC, amygdala, and hippocampus. In order to accomplish this, we used high-resolution magic angle (HR-MAS) 1H MRS ex vivo methodology, studying mPFC, amygdala, and hippocampal tissue. Glutamate, GABA and glutamine (the major metabolite of neuronal glutamate [29–32]); chemicals that are components of biochemical pathways that result in ATP production (e.g. succinate for the Krebs cycle, lactate for glucose metabolism, and creatine for ATP production via creatine phosphate); and NAA levels (indicative of neuronal integrity) were measured. We predicted that SPS would attenuate the levels of neurochemicals indicative of excitatory neurotransmission and energy metabolism in the mPFC (i.e. excitatory tone), augment the levels of chemicals indicative of excitatory neurotransmission and energy metabolism in the amygdala complex, and attenuate NAA levels (i.e. disrupt neuronal integrity) in the hippocampus.
Materials and Methods
Animals
Fifteen male Sprague Dawley (SPS = 8, control = 7) rats (Charles River, Wilmington, MA) were pair-housed at the Veterinary Medical Unit of the Ann Arbor Veterans Affairs medical center and maintained on a 12:12 hour light/dark cycle, 19 – 21°C room temperature, and 50 ± 10% humidity. The animals had ad libitum access to food and water. All experimental procedures were approved by the Ann Arbor Veteran Affairs Institutional Animal Care Usage Committee and were in accordance with the National Institute of Health Guide For The Care and Use of Laboratory Animals. Animals were allowed to acclimatize to the colony room for at least 3 days prior to the initiation of experiments.
Single Prolonged Stress
SPS refers to the application of three stressors (restraint stress, forced swim, and ether exposure) followed by a quiescent period of 7 days [33, 34]. In this study, rats were restrained for 2 hours, followed immediately by 20 minutes of forced swimming in 20 – 24 °C water in a plastic tub (55.6 cm diameter, 45.4 cm height), filled two-thirds from the bottom. Following 15 minutes recuperation, rats were exposed to ether (using a dessicator) until general anesthesia, defined as loss of toe and tail pinch responses, was induced (< 5 minutes). Immediately after the induction of general anesthesia, rats were removed from the dessicator, placed in their home cages, and left undisturbed for 7 days. For the control procedure, rats remained in their home cages for the duration of SPS [34].
Brain Dissections
Previous studies have demonstrated that increases in fast negative feedback of the HPA axis and decreases in the ratio of MR/GR mRNA in the hippocampus are observed up to 14 days after SPS [33, 34]. As a result rat brains were harvested within this time window. Three days after SPS (that is 10 days after the application of three stressors) all rats were decapitated without anesthesia; their brains rapidly removed, frozen on dry ice and stored at −80 °C until being transported to Wayne State University for neurochemical analysis. Whole frozen brains were packed in dry ice, delivered overnight, and once received were stored at −80 °C until further dissection to isolate specific brain regions. In order to prepare samples for HR-MAS 1H-MRS analysis, brains were placed into an ice-cold matrix, allowed to thaw enough to cut with a razor blade, and then sliced into 2 mm coronal sections. Slices containing the mPFC (prelimbic and infralimbic regions), amygdala complex (basolateral complex and central nucleus), and hippocampus (CA1 dentate gyrus region) were obtained and then specific regions were microdissected using a punch technique (2.1 mm diameter). Sample-punches were frozen immediately on dry ice and stored at −80 °C until HR-MAS 1H-MRS analysis.
HR-MAS 1H-MRS
Neurochemical profiles were determined with HR-MAS 1H-MRS as previously described [35–37]. Briefly, frozen intact tissue samples were weighed (~ 3 mg) and placed directly into a Bruker zirconium rotor (2.9-mm diameter, 10 µL capacity) containing 5 µL buffer (pH = 7.4; 100 mM potassium phosphate, 200 mM formate, 1 g/L NaN3 and 3 mM trimethylsilyl-propionate [TSP Sigma; St Louis, MO] diluted with an equal volume of D2O containing 0.75% TSP). TSP serves as an internal chemical shift reference (0.00 ppm), formate (8.44 ppm) for phase corrections, and D2O to lock on the center frequency. The rotor (with sample) was placed into a Bruker magic angle spinning probe maintained at 4 °C in a vertical wide-bore (8.9 cm) Bruker 11.7 T magnet with an AVANCE™ DRX-500 spectrometer (Bruker Biospin Corp., Billerica, MA). Rotors were spun at 4.2 ± 0.002 kHz while positioned at 54.7° relative to the static magnetic field B0. A Carr–Purcell–Meiboom–Gill (CPMG) rotor-synchronized pulse sequence [38] (TR = 3500 ms, bandwidth 8 kHz, 16 k complex points, 32 averages) was used to acquire the spectra with a total acquisition time of 3 minutes 38 s. Spectra were analyzed with a customized Linear-Combination Model (LC Model) software package that uses a linear combination of 27 individual neurochemical model spectra (basis set) as well as non-specific lipid signals to fit the tissue spectrum, and calculates absolute concentration values for neurochemicals with signals between 1.0 – 4.2 ppm [39]. Cramer–Rao bounds estimated the precision with which LCModel fit the data and were typically below 10% indicating excellent fit. Absolute values were corrected for tissue sample weight and expressed as nmol/mg of wet weight. The statistical significance of differences observed between SPS and control was assessed for each neurochemical with a two-tailed Student’s t-test (SPS vs. control) with a 95% confidence interval (p < 0.05).
Results
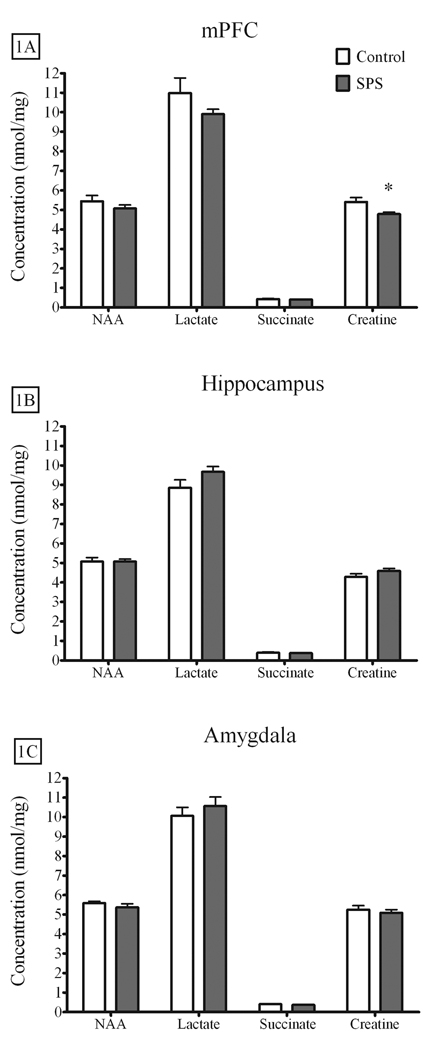
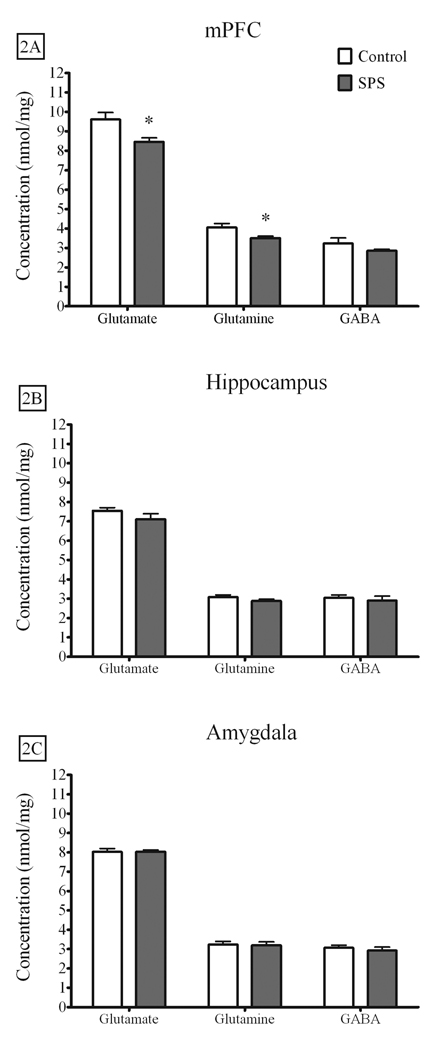
Figures 1A–C illustrates the metabolites used to index neuronal integrity and energy metabolism in the mPFC (SPS = 7, Control = 6), amygdala complex (SPS = 7, Control = 6), and hippocampus (SPS = 8, Control = 7). Of these metabolites, only creatine levels in the mPFC were attenuated by SPS [t(11) = 2.63, p = .023]. Glutamate [t(11) = 2.912, p = .014] and glutamine [t(11) = 2.445, p = .033] levels were also attenuated in the mPFC of SPS rats when compared to controls, while GABA levels were not different [t(11) = 1.404, p = .18]. These results are illustrated in Figure 2A. Neither glutamate, glutamine, nor GABA levels in the hippocampus [glutamate - t(13) = 1.279, p = .223; glutamine - t(13) = 1.335, p = .205; GABA t(13) = .482, p = .638] or amygdala complex [glutamate - t(11) = .044, p = .966; glutamine - t(11) = .198, p = .846; GABA - t(11) = .620, p = .548] were altered by SPS. These results are illustrated in Figures 2B–C.
Figure 1.
The effect of SPS on energy metabolites and NAA in the mPFC, hippocampus, and amygdala complex. SPS attenuated creatine levels in the A) mPFC, but had no effect on neurochemical profiles in the B) hippocampus or C) amygdala complex. Data are expressed as nmol/mg tissue, presented as the mean ± standard error of the mean, and analyzed by two-tailed t-test (* p < 0.05). SPS – Single Prolonged Stress, NAA – N-acetyl aspartate, mPFC – medial prefrontal cortex.
Figure 2.
The effect of SPS on glutamate, glutamine, and GABA in the mPFC, hippocampus, and amygdala complex. A) SPS attenuated basal levels of glutamate and glutamine in the mPFC, but had no effect on GABA levels. B) SPS had no effect on any neurochemicals in the hippocampus or C) amygdala complex. Data are expressed as nmol/mg tissue, presented as the mean ± standard error of the mean, and analyzed by two-tailed t-test (* p < 0.05). GABA – gamma amino butyric acid, mPFC – medial prefrontal cortex.
Discussion
In this study SPS attenuated creatine, glutamate, and glutamine, but not NAA levels in the mPFC of the SPS rats. Glutamate is involved in metabolic processes that are not related to neural transmission, which raises the possibility that changes in glutamate observed in this study did not concern glutamate used for neural transmission in the mPFC. However, we observed attenuated glutamine, the precursor metabolite for neuronal glutamate [29, 31, 32, 40], concentrations in the mPFC of SPS rats. In addition, glutamate serves as a precursor molecule for the synthesis of GABA [30, 41, 42], but GABA levels in the mPFC were not affected by SPS. Furthermore, succinate levels, which are indirectly dependent on glutamate levels [30], were not affected by SPS. This change in neurochemical profile is consistent with the assertion that SPS induces a decrease in excitatory tone in the mPFC, without a corresponding change in neuronal integrity, and provides evidence for a SPS-induced deficit in neural activity in the mPFC.
SPS did not alter NAA levels in the hippocampus. Given that decreased hippocampal NAA has been reported in PTSD [17, 25, 26, 28], it appears that SPS does not model this aspect of PTSD. SPS did not alter neurochemical profiles in the amygdala, and amygdala hyperactivity has been linked to PTSD by our group and other investigators [12, 13]. It should be noted, however, that while observed changes in inhibitory and excitatory neurotransmitter levels and energy metabolites can be used to make inferences about excitatory tone in a brain region, the converse is not necessarily true. Thus, it is possible to have changes in neural activity that would not be detected by HR-MAS 1H-MRS. Changes in the sensitivity of glutamate receptors, for instance, could alter the electrochemical gradient of positive ions such as sodium and calcium. Such changes could lead to increased membrane excitability in the amygdala without a detectable change (using HR-MAS 1H-MRS technology) in neurochemical concentrations. It is also possible that the amygdala hyperactivity reported is a functional outcome of diminished prefrontal inhibitory tone, without intrinsic intra-amygdala neurobiological alterations. Thus, further research is needed to examine the effect of SPS on excitatory tone in the amygdala.
Decreased neural activity in prefrontal cortical regions, without a change in neuronal integrity, is believed to be a salient feature of PTSD [10, 11]. The results of this study suggest that SPS attenuates excitatory tone in the mPFC without a change in neuronal integrity. This suggests that SPS can be used to model prefrontal cortical dysfunction associated with PTSD. Previous research has demonstrated that acute stress-induced changes in glutamate metabolism are caused by stress-induced increases in corticosterone concentrations [43, 44]. SPS does not affect baseline (laboratory observation) or stress-induced increases in corticosterone concentrations [34], which demonstrates that corticosterone-induced changes in glutamate metabolism is not the mechanism by which SPS alters glutamate function in the mPFC. Further research is needed to determine the mechanism by which SPS selectively attenuates glutamate metabolism in the mPFC. The results of this study suggest that SPS decreases neural activity in the mPFC. This assertion is also supported by the finding that SPS induces deficits in extinction recall [50], because decreased neural activity in the mPFC disrupts extinction recall [36, 44]. Further research is needed to investigate the effects of SPS on direct measures of neural activity (e.g. single unit activity) in the mPFC.
Acknowledgements
The research in this manuscript was supported by a Veteran Affairs Merit award and Department of Defense grant W81XWH-08-1-0661 to Dr. Israel Liberzon, NIDA award DA024760 to Dr. Shane Perrine, DA R01-16736 to Dr. Matthew Galloway, the Joe Young Fund for Psychiatry Research, and the Fund for Medical Research in Anesthesiology at Wayne State University School of Medicine. We would like to thank Dr. Farhad Ghoddoussi for his expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yehuda R, et al. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry. 1993;150(1):83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Butler RW, et al. Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. Am J Psychiatry. 1990;147(10):1308–1312. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- 3.Morgan CA, et al. Fear-potentiated startle in posttraumatic stress disorder. Biol Psychiatry. 1995;38(6):378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- 4.Morgan CA, 3rd, et al. Exaggerated acoustic startle reflex in Gulf War veterans with posttraumatic stress disorder. Am J Psychiatry. 1996;153(1):64–68. doi: 10.1176/ajp.153.1.64. [DOI] [PubMed] [Google Scholar]
- 5.Shalev AY, et al. Physiologic responses to loud tones in Israeli patients with posttraumatic stress disorder. Arch Gen Psychiatry. 1992;49(11):870–875. doi: 10.1001/archpsyc.1992.01820110034005. [DOI] [PubMed] [Google Scholar]
- 6.Khan S, Liberzon I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology (Berl) 2004;172(2):225–229. doi: 10.1007/s00213-003-1634-4. [DOI] [PubMed] [Google Scholar]
- 7.Kohda K, et al. Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: a putative post-traumatic stress disorder model. Neuroscience. 2007;148(1):22–33. doi: 10.1016/j.neuroscience.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 8.Milad MR, et al. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42(7):515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto S, et al. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008;33(9):2108–2116. doi: 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]
- 10.Bremner JD, et al. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156(11):1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin LM, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 12.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 13.Liberzon I, et al. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 14.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 15.Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23(35):11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown S, et al. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of former prisoners of war with and without posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci. 2003;15(3):367–370. doi: 10.1176/jnp.15.3.367. [DOI] [PubMed] [Google Scholar]
- 17.Freeman TW, et al. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Med. 1998;40(1):66–71. doi: 10.1002/mrm.1910400110. [DOI] [PubMed] [Google Scholar]
- 18.Urenjak J, et al. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13(3):981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N- acetylaspartate after acute brain injury. Magn Reson Med. 1995;34(5):721–727. doi: 10.1002/mrm.1910340511. [DOI] [PubMed] [Google Scholar]
- 20.Karl A, Werner A. The use of proton magnetic resonance spectroscopy in PTSD research--meta-analyses of findings and methodological review. Neurosci Biobehav Rev. 2010;34(1):7–22. doi: 10.1016/j.neubiorev.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Martin E, et al. Absence of N-acetylaspartate in the human brain: impact on neurospectroscopy? Ann Neurol. 2001;49(4):518–521. [PubMed] [Google Scholar]
- 22.Tamiya T, et al. Proton magnetic resonance spectroscopy reflects cellular proliferative activity in astrocytomas. Neuroradiology. 2000;42(5):333–338. doi: 10.1007/s002340050894. [DOI] [PubMed] [Google Scholar]
- 23.Hammen T, et al. Clinical applications of 1H-MR spectroscopy in the evaluation of epilepsies--what do pathological spectra stand for with regard to current results and what answers do they give to common clinical questions concerning the treatment of epilepsies? Acta Neurol Scand. 2003;108(4):223–238. doi: 10.1034/j.1600-0404.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 24.Sijens PE, et al. Analysis of the human brain in primary progressive multiple sclerosis with mapping of the spatial distributions using 1H MR spectroscopy and diffusion tensor imaging. Eur Radiol. 2005;15(8):1686–1693. doi: 10.1007/s00330-005-2775-0. [DOI] [PubMed] [Google Scholar]
- 25.Ham BJ, et al. Decreased N-acetyl-aspartate levels in anterior cingulate and hippocampus in subjects with post-traumatic stress disorder: a proton magnetic resonance spectroscopy study. Eur J Neurosci. 2007;25(1):324–329. doi: 10.1111/j.1460-9568.2006.05253.x. [DOI] [PubMed] [Google Scholar]
- 26.Neylan TC, et al. Cortisol levels are positively correlated with hippocampal Nacetylaspartate. Biol Psychiatry. 2003;54(10):1118–1121. doi: 10.1016/S0006-3223(03)01974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuff N, et al. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villarreal G, et al. Proton magnetic resonance spectroscopy of the hippocampus and occipital white matter in PTSD: preliminary results. Can J Psychiatry. 2002;47(7):666–670. doi: 10.1177/070674370204700709. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195(4284):1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- 30.Cooper J, Bloom F, Roth RH. Biochemical Basis of Neuropharmacology. 7th ed. USA: Oxford University Press; 1996. p. 528. [Google Scholar]
- 31.Hogstad S, et al. Glutaminase in neurons and astrocytes cultured from mouse brain: kinetic properties and effects of phosphate, glutamate, and ammonia. Neurochem Res. 1988;13(4):383–388. doi: 10.1007/BF00972489. [DOI] [PubMed] [Google Scholar]
- 32.Erecinska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35(4):245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 33.Liberzon I, et al. Neuroendocrine and psychophysiologic responses in PTSD: a symptom provocation study. Neuropsychopharmacology. 1999;21(1):40–50. doi: 10.1016/S0893-133X(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 34.Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22(6):443–453. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 35.O'Leary-Moore SK, et al. Neurochemical changes after acute binge toluene inhalation in adolescent and adult rats: a high-resolution magnetic resonance spectroscopy study. Neurotoxicol Teratol. 2009;31(6):382–389. doi: 10.1016/j.ntt.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perrine SA, et al. Cardiac effects of MDMA on the metabolic profile determined with 1H-magnetic resonance spectroscopy in the rat. NMR Biomed. 2009;22(4):419–425. doi: 10.1002/nbm.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghoddoussi F, et al. Methionine sulfoximine, an inhibitor of glutamine synthetase, lowers brain glutamine and glutamate in a mouse model of ALS. J Neurol Sci. 2010;290(1–2):41–47. doi: 10.1016/j.jns.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Cheng LL, et al. Quantitative neuropathology by high resolution magic angle spinning proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1997;94(12):6408–6413. doi: 10.1073/pnas.94.12.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 40.Nicholls D. Proteins, Transmitters and Synapses. Cambridge, MA: Blackwell Science; 1994. p. 253. [Google Scholar]
- 41.Erlander MG, Tobin AJ. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res. 1991;16(3):215–226. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- 42.Patel AB, et al. Glutamine is the major precursor for GABA synthesis in rat neocortex in vivo following acute GABA-transaminase inhibition. Brain Res. 2001;919(2):207–220. doi: 10.1016/s0006-8993(01)03015-3. [DOI] [PubMed] [Google Scholar]
- 43.Moghaddam B, et al. Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res. 1994;655(1–2):251–254. doi: 10.1016/0006-8993(94)91622-5. [DOI] [PubMed] [Google Scholar]
- 44.Roy M, Sapolsky RM. The exacerbation of hippocampal excitotoxicity by glucocorticoids is not mediated by apoptosis. Neuroendocrinology. 2003;77(1):24–31. doi: 10.1159/000068337. [DOI] [PubMed] [Google Scholar]