Abstract
Background
Delivery of allergens with bacterial adjuvants has been shown to be a successful immunotherapeutic strategy for food allergy treatment in animal models. How microbial signals, acting through the innate immune system, reshape ongoing allergic responses is poorly understood.
Objective
To investigate the contribution of Toll-like receptors in the response to bacterial adjuvants, we designed an in vitro system to characterize the effect of heat-killed E.coli on peanut-induced responses of dendritic cells (DCs) and T cells.
Methods
Wild-type or Toll-Like Receptor (TLR) signaling-deficient bone-marrow derived DCs were pulsed with crude peanut extract alone (CPE) (50 µg/mL) in the presence of heat-killed E.coli (HKE) (106/mL). DC maturation was analyzed by flow cytometry. Treated DCs were co-cultured with CFSE-labeled CD4+ T cells from sensitized mice. Cytokine production from DCs and T cells was measured by bioplex assays.
Results
Peanut pulsed DCs induced the production of IL-4, IL-5, IL-13 as well as IL-17 and IFN-γ from primed T cells. Adding HKE to CPE-pulsed DCs resulted in a significant decrease in Th2 cytokine production, associated with an increase in IFN-γ and profound attenuation of T cell proliferation. These effects were linked to HKE-induced, TLR-dependent changes in DC reactivity to CPE, especially the production of polarizing cytokines such as IL-12.
Conclusions
TLR signals modulate peanut-induced DC maturation in vitro leading to changes in the T cell response to peanut. These TLR effects must be confirmed in vivo and may constitute another alternative for allergen immunotherapies.
Keywords: Peanut allergy, EMP-123, Dendritic cell, Toll-like receptor, MyD88, IL-12
Introduction
Food allergy is a growing problem in Westernized countries. Peanut allergy is important, because (1) accidental reactions can be severe1, (2) the prevalence has doubled among children and affects approximately 1% of the US population, and (3) it is rarely outgrown2. The only current treatment is strict dietary elimination, but peanut-free environments are difficult to maintain. Since inadvertent exposures are common, patients must scrutinize all foods and carry autoinjectable epinephrine at all times3, impairing their quality of life4.
These data indicate a need for an effective therapy for allergic patients. Previous attempts have been either unsafe or impractical5,6. To enhance the safety of potential immunotherapies, the major peanut allergens Ara h 1, Ara h 2, and Ara h 3 were engineered to disrupt immunodominant IgE binding sites7,8,9. Delivering these modified allergens with a bacterial adjuvant was studied. Using Listeria monocytogenes10 and then Escherichia coli11, Li and colleagues showed that administration of these engineered allergens to C3H/HeJ mice, combined with bacterial adjuvants, protected animals from anaphylaxis upon challenge. This protection was associated with decreased histamine and IgE levels. The shift towards a Th1 response was consistent with previous allergen-based immunotherapies that promote tolerance by reshaping T cell responses12.
Regulation of allergic responses may occur through activation of the innate immune system. Microbial products act on pattern recognition receptors including Toll-like receptors (TLRs) to initiate rapid host immune responses. DCs are professional antigen presenting cells which critically link innate and adaptive immunity. Detection of microbes initiates DC maturation characterized by phenotypic changes and cytokine release. In the gut, commensal flora provides critical immune signals to maintain homeostasis13,14,15, and DCs actively participate in oral tolerance induction16,17.
As part of a larger ongoing effort to understand the role of microbial adjuvants in immunotherapy, we designed an in vitro system to study how TLR signaling in dendritic cells can modify the immune response to peanut. We show that TLR signaling profoundly changes the DC response to peanut, leading to lasting functional changes in the Th2 cytokine response.
Methods
Animals
Four to six week old wild-type C57BL/6 mice were purchased from National Cancer Institute (Bethesda, MD) or Jackson Labs (Bar Harbor, ME). TLR4-, TLR9-, MyD88 [gift from Prof S. Akira, Osaka University, Japan], and MyD88/TRIF-deficient mice were bred at Yale University [Prof R. Medzhitov, Yale University]. All animals were maintained in standard conditions in the Yale Animal Resource Center at Yale University. Experiments were conducted in accordance with the regulatory guidelines established by the Institutional Animal Care and Use Committee at Yale.
Cell preparations
Bone-marrow dendritic cell preparation
Bone-marrow cells were collected from femurs and cultured for 6 days in RPMI 1640 with L-glutamine (Gibco, Auckland, NZ), penicillin/ streptomycin (Cellgro, Hemdon, VA) and 1% GM-CSF (filtered supernatant from J558L cell culture). CD11c expression was analyzed by flow cytometry.
T cell preparation
Defatted crude peanut extract (CPE) was generated as described previously and provided by Dr. Wesley Burks, Duke University. Briefly, ground peanuts were defatted using acetone. After overnight dry, the remaining powder was then dissolved in PBS and filtered.
Mice were immunized by 2 intraperitoneal injections [Day 0 and Day 7] of 200 µg CPE and 2 mg of aluminum hydroxide (Sigma-Aldrich, St-Louis, MO).
After Day 14, CD4+ T cells were isolated from splenocytes using EasySep Negative Mouse CD4 kit (StemCell Technologies, Vancouver, Canada). 95–96% purity was verified by CD4 expression (BD Biosciences, San Jose, CA) using flow cytometry.
Bacteria preparation
Escherichia coli BL21 (DE3) carrying the pET24(a)+ vector11 were provided by Dr. Alexander Grishin, Mount Sinai School of Medicine. LB medium containing kanamycin (30 µg/ml) (Shelton Scientific, Shelton, CT) was inoculated and placed at 37°C in a shaking incubator overnight. Bacteria were washed, collected in PBS supplemented with 20% glycerol and stored at −80C. Bacterial concentration was determined by plating serial dilutions. E.coli were heat-inactivated for 30 min at 65° C and viability was checked by culture.
BMDC culture
At Day 6, BMDC were gently collected, washed and resuspended in complete medium without GMCSF at final concentration 5×105 cells/ml [2ml]. Cells were incubated for 24 hours with either CPE [at optimized concentration 50 µg/ml], or HKE [ratio 1 bacteria per BMDC], or both stimuli.
The following ultrapure TLR ligands were used for stimulation of individual BMDC TLR: lipopolysaccharide (LPS) and CpG oligonucleotide - both at 500ng/mL (InvivoGen, San Diego CA).
Co-culture with primed T cells
First system
1 × 105 stimulated BMDCs were cultured with 1 × 106 primed T cells for 3 days [250 µl] in 48-well culture plates. For the neutralizing experiments, LEAF™ anti-IL-12/23 P40 antibody [Clone C17.8] or LEAF™ anti-IFN-γ antibody [Clone H22] or LEAF™ isotype control antibody were used and maintained at 20µg/ml during the whole time of the culture (Biolegend, San Diego, CA USA).
Second system
1.5 × 105 CPE-pulsed BMDCs were cultured with 1.5 × 106 primed T cells for 6 days [500 µl] in 48-well culture plates. T cells were then isolated, washed, counted [to obtain ratio 1:10] and cocultured with 1 × 105 CPE-pulsed BMDC for 3 more days.
BMDC surface marker analysis
Collected BMDC were incubated for 10 min with FCγR blocking antibody and 30 min at 4°C with FITC-conjugated CD80, IAb [equivalent to human MHC-II]; PE-conjugated CD86, CD40 and APC-conjugated CD11c (BD Biosciences, San Jose, CA, USA). Matching isotype controls were used. After thorough washing, cells were fixed with 1% paraformaldehyde solution and analyzed by FACSCalibur (BD Biosciences, San Jose, CA, USA). Results were expressed as mean fluorescence intensity.
Cytokine Assay
Supernatants from BMDC culture were collected after 24 hours. IL-6, IL-10, IL-12p70, TNF-α, IL1-β were measured. Supernatants from co-cultures were analyzed for the presence of IL-4, IL-5, IL-13, IL-10, IFN-γ, and IL-17 at day 3. All cytokine analyses were performed using a multiplex kit according to manufacturer’s instructions (Upstate, Temecula, CA) and analyzed on a Bioplex system (Bio-Rad, CA).
Proliferation Assay
For proliferation, CD4+ T cells were resuspended in PBS (106 cells / ml) and incubated with CFSE (10 µM) at 37° C for 10 min. The reaction was stopped by adding fetal calf serum and extensive washes were performed.
After culture, CD4+ T cells were labeled with APC-conjugated CD3 (BD Biosciences, San Jose, CA), and proliferation was assessed by flow cytometry.
Statistical analysis
Statistical analysis was performed using Student T tests (Prism for Macintosh, GraphPad Software, La Jolla CA). P values of 0.05 or less were considered significant.
Results
Crude peanut extract (CPE) induces a mixed T cell immune response
In order to study the immune response to peanut, mice were immunized twice with CPE and alum. CD4+ T cells from spleens were cocultured with CPE-stimulated BMDCs. CPE induced a mixed immune response characterized by significant production of IL-4, IL-5, IL-13, IL-17, IFN-γ and an intensive T cell proliferation (Fig 1 and Supp Fig 1). This effect was preserved in cocultures using BMDCs deficient in TLR4 and MyD88/TRIF (Fig 2 and Supp Fig 1), suggesting that the immunogenicity of peanut proteins is TLR-independent.
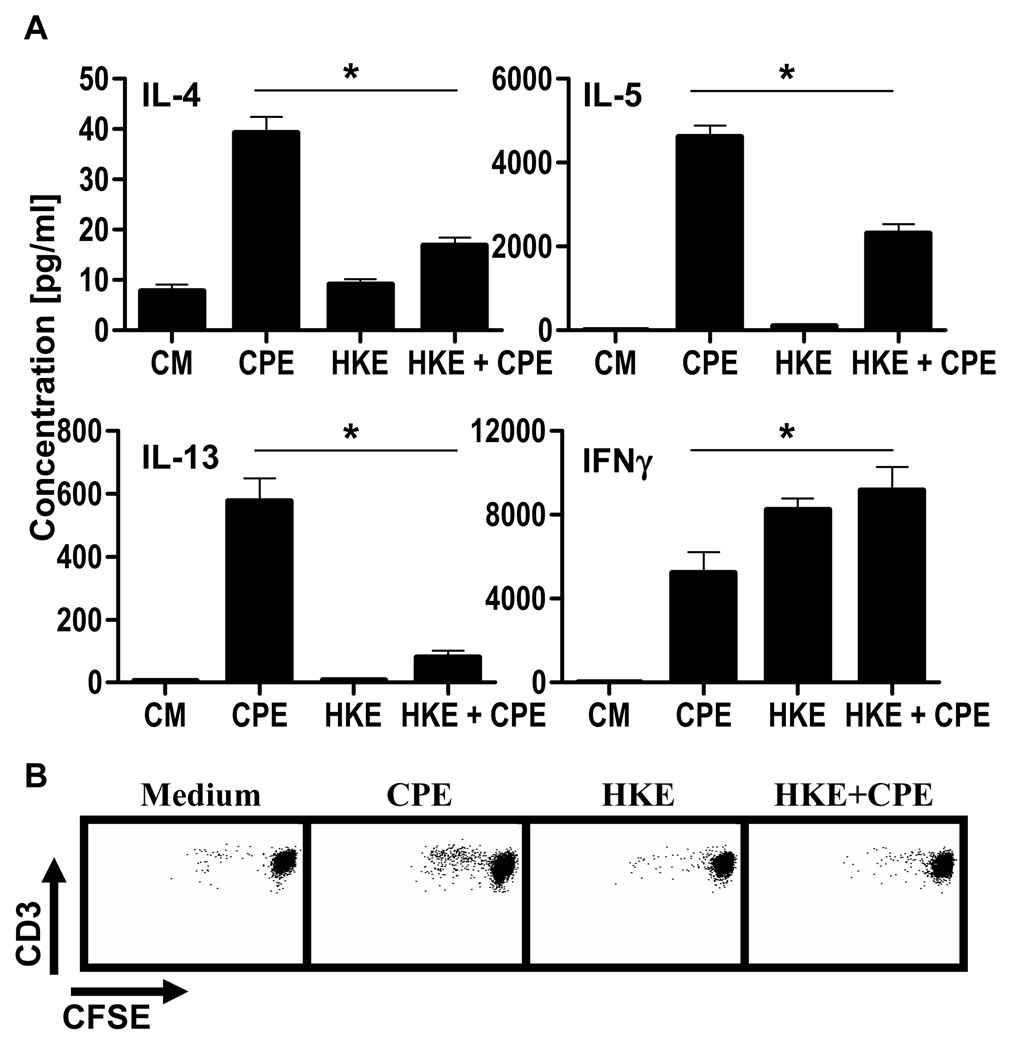
Figure 1. DC treatment with heat-killed E.coli alters T cell immune responses to peanut.
CD4+ T cells were cocultured (ratio 10:1) with CPE-pulsed BMDC stimulated with CPE (50µg/ml), HKE (1 bacteria per BMDC) or both for 72hrs.
[A] Concentrations in IL-4, IL-5, IL-13 and IFN-γ were determined. Concentrations represent mean ± SEM (n=5). (*=P<0.05).
[B] After 5 days, proliferation of CD3+ cells was analyzed by flow cytometry. One representative experiment out of five is shown.
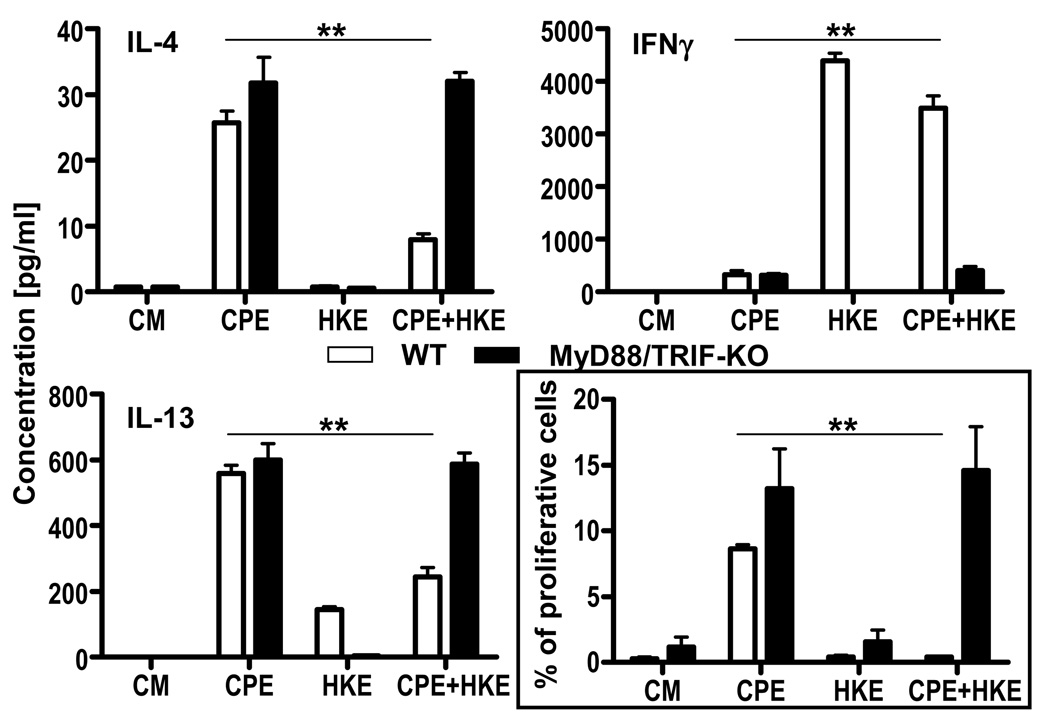
Figure 2. Heat-killed E.coli alters peanut response by triggering TLR signaling in DCs.
WT CD4+ T cells were cocultured (ratio 10:1) with CPE-pulsed BMDC [WT vs. MyD88/TRIF-KO] stimulated with CPE (50µg/ml), HKE (1 bacteria per BMDC) or both for 72hrs. Concentrations in IL-4, IL-13 and IFN-γ were determined. Concentrations represent mean ± SEM (n=5). After 5 days, proliferation of CD3+ cells was analyzed by flow cytometry. Percentage of proliferative cells is indicated by mean ± SEM (n=5). Statistical differences are indicated as **=P<0.01.
Dendritic cell treatment with heat-killed E.coli alters T cell immune responses to peanut
Microbial signals from commensal flora or adjuvants may influence the response to food antigens, and therefore we assessed the effect of E. coli on the peanut response. To prevent outgrowth, we used heat-killed E. coli (HKE) with CPE in our in vitro coculture system. Adding HKE and CPE to BMDCs significantly decreased Th2 cytokine production and increased production of IFN-γ (Fig 1). This change was associated with inhibited T cell proliferation (Fig 1). In order to determine if this bacterial effect was mediated by TLRs, we performed cocultures using BMDCs from MyD88/TRIF double-deficient animals. The absence of MyD88/TRIF signaling in DCs reversed the HKE-induced inhibition of Th2 cytokine production, restoring it to the level induced by CPE alone. IFN-γ was also suppressed. In addition, the inhibition of proliferation by HKE was completely reversed in the absence of MyD88/TRIF (Fig 2 and Supp Fig 2).
Taken together, these results indicate that dendritic cell TLR signaling, in response to microbial stimulation, modifies recall responses to peanut proteins, resulting in a shift towards a Th1 response. This modification is MyD88/TRIF-dependent.
E.coli-induced inhibition of Th2 polarization and peanut specific T cell proliferation is long-lasting
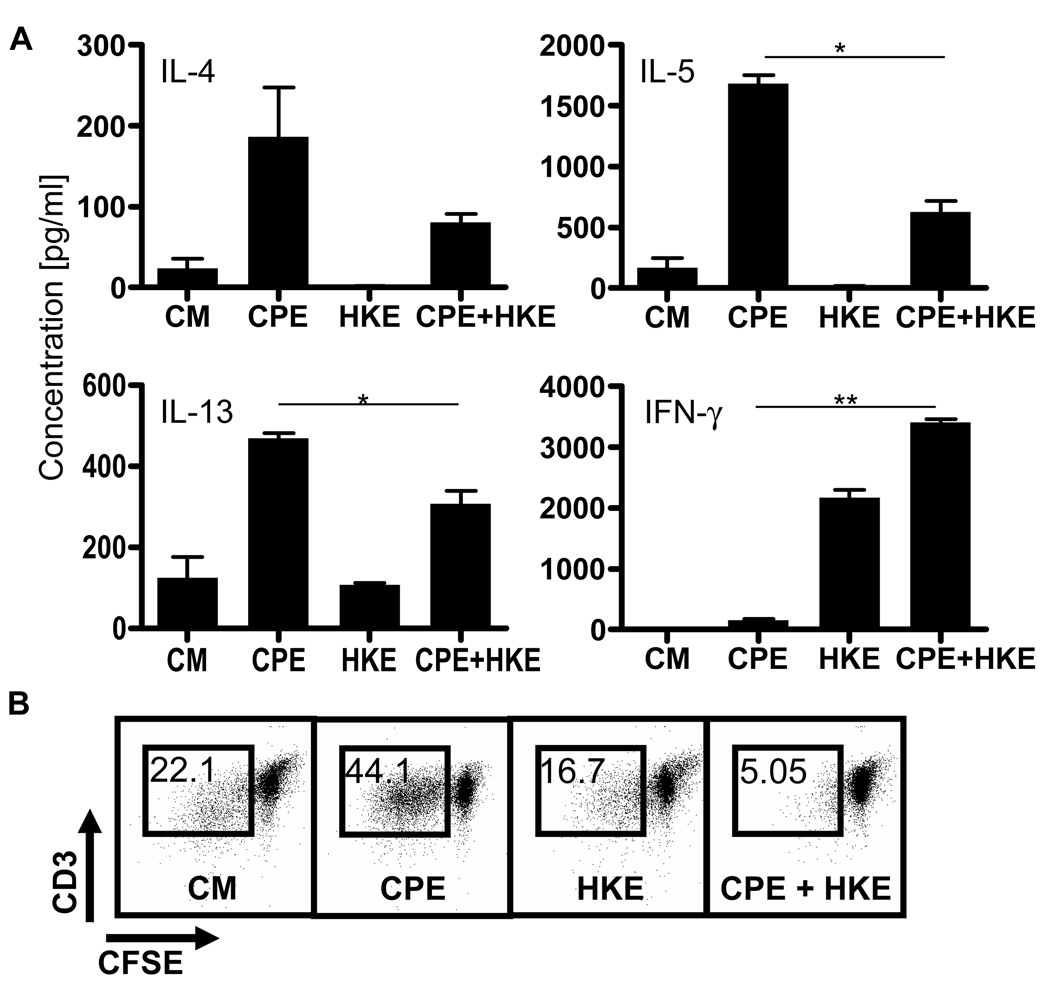
Targeting TLR signaling on BMDCs attenuated the proliferation and Th2 cytokine responses of peanut-specific T cells. In order to investigate if this effect was long lasting, we isolated cocultured T cells which had been initially stimulated with DCs exposed to CPE in the presence of HKE and re-cultured these T cells with DCs exposed to CPE alone. T cells incubated initially with DCs exposed to both CPE and HKE demonstrated impaired production of Th2 cytokines, while the release of IFN-γ (Fig 3A) was unimpaired, compared to T cells initially incubated with DCs exposed to CPE in the absence of HKE. These results correlated with the proliferation of these cells. Peanut-primed T cells initially exposed to DCs stimulated with both HKE and CPE proliferated less [5.05%] than CPE-pulsed BMDCs [44.1%] when re-exposed to DCs stimulated with CPE alone (Fig 3B). In conclusion, the addition of TLR signaling to dendritic cells led to sustained suppression of T cell recall responses to peanut.
Figure 3. Heat-killed E.coli has a long-lasting effect on peanut response.
CD4+ T cells were cocultured (ratio 10:1) with BMDC stimulated with CPE (50µg/ml), HKE (1 bacteria per BMDC) or both. After 7 days, they were washed, counted and cocultured with CPE-pulsed BMDC for 72hrs.
[A] Supernatants were analyzed for the presence of IL-5, IL-13, IL-17, IFN-γ (*=P<0.05, **=P<0.01).
[B] Proliferation of CD3+ cells was determined by flow cytometry. One representative experiment out of five is shown.
Heat-killed E.coli modifies the dendritic cell response to peanut
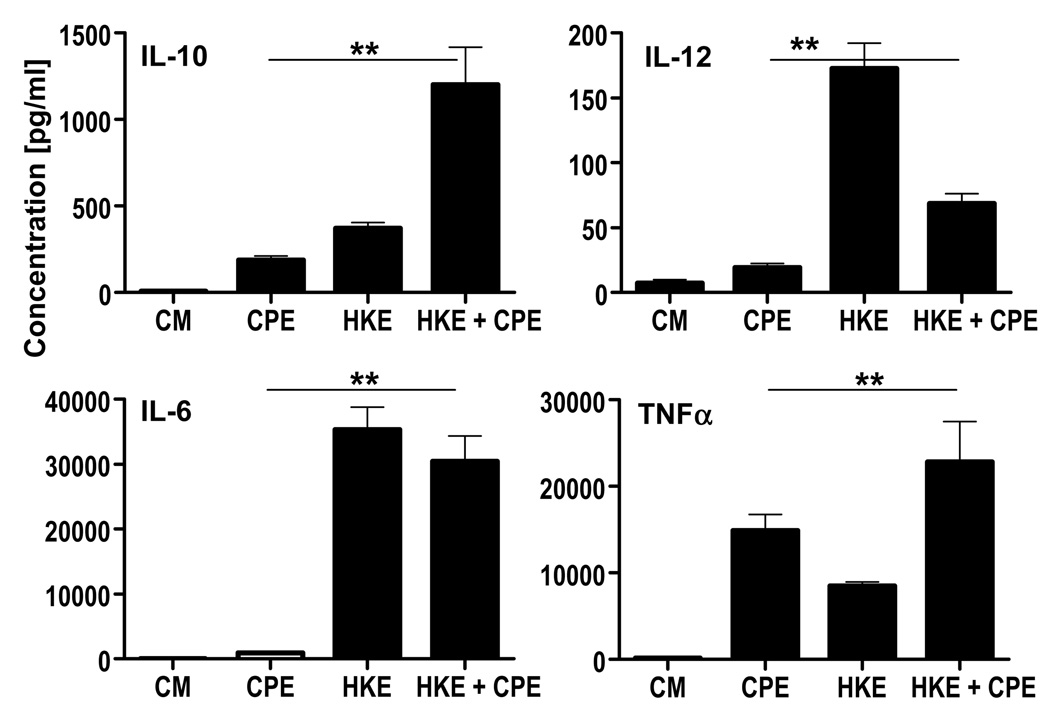
To investigate how dendritic cell recognition of microbial components could alter the T cell response to peanut, we stimulated DCs with HKE and CPE to look for the change in DC maturation. CPE induced TLR-independent maturation of BMDCs, characterized by the upregulation of the class II molecule IAb, and costimulatory molecules CD80 and CD86 but little CD40 (Supp Fig 3). Along with phenotypic changes, peanut stimulation induced production of pro-inflammatory and polarizing cytokines. Compared to LPS and CpG that induce production of tumor necrosis factor alpha (TNF-α), interleukin-10 (IL-10), IL-6 and IL-12, CPE-pulsed DCs released TNF-α and IL-10, but little IL-12 and IL-6 (Fig 4 and Supp Fig 4). In summary, peanut antigens induced TLR-independent dendritic cell maturation and a unique cytokine production profile.
Figure 4. Heat-killed E.coli changes peanut-induced cytokine production by BMDC.
BMDC were stimulated either with CPE (50µg/ml) or HKE (ratio one bacteria per BMDC) or both stimuli for 24hrs. Unstimulated BMDC were used as control. Supernatants were analyzed by ELISA for production of IL-10, IL-12, IL-6, TNFα. Concentrations represent mean ± SEM (n=15) (*=P<0.05, **=P<0.01).
The addition of HKE to CPE stimulation enhanced expression of CD80, CD86, IAb and CD40 on DCs (Supp Fig 3). These phenotypic changes were accompanied by a significant increase in the production of the Th1 polarizing cytokine IL-12. IL-6 and TNF-α were also increased. Interestingly, the IL-10 production was synergistically enhanced by both stimuli (Fig 4). These effects were not observed when TLR- or MYD88/TRIF-deficient BMDCs were used suggesting that TLR pathways are necessary for this synergy.
In conclusion, TLR signaling enhances the peanut-induced dendritic cell maturational program, and has a particular effect on the release of polarizing cytokines such as IL-10 and IL-12.
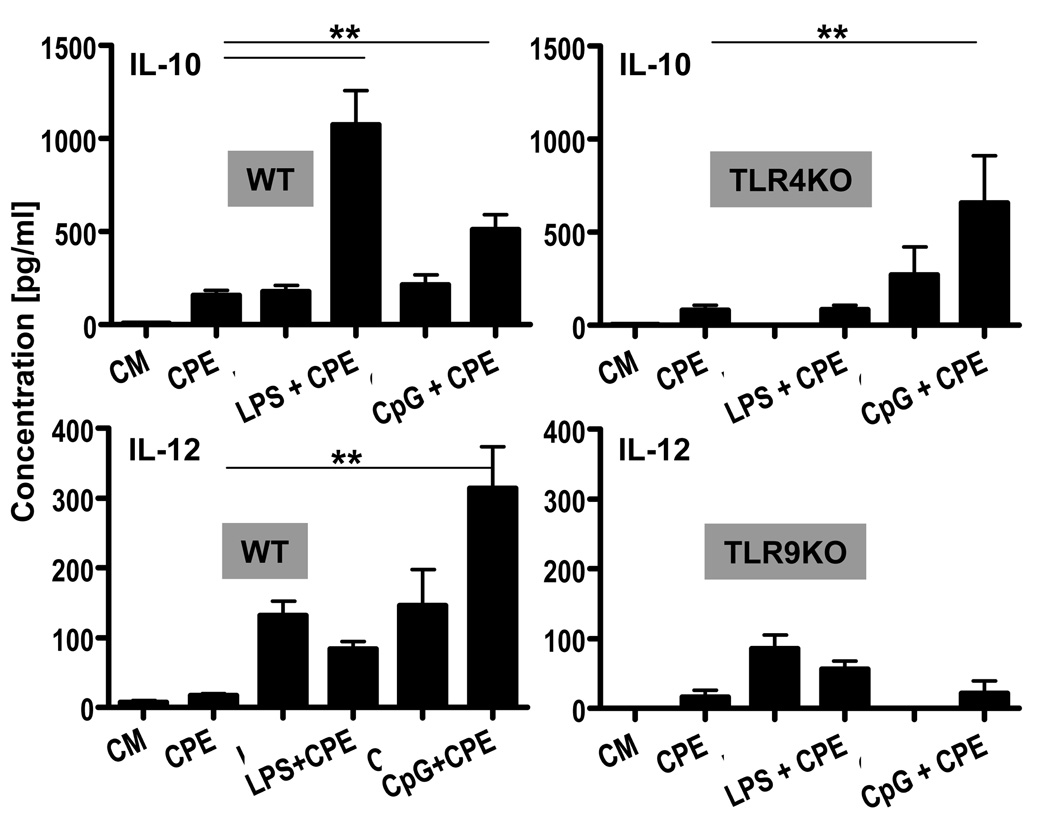
TLR4 stimulation enhances IL-10 production by CPE-stimulated BMDC, whereas TLR9 enhances IL-12 production
To determine the contribution of individual TLRs in the E. coli-induced modification of dendritic cell responses to peanut, we added LPS or CpG to CPE and measured DC cytokine production. TLR4 signaling after stimulation with LPS and CPE triggered synergistic production of IL-10 (Fig 5). Interestingly, CpG signaling through TLR9 had a similar effect on IL-10 production, suggesting that this effect is mediated by common TLR pathways. It is noteworthy that CpG signaling through TLR9 induced synergistic production of IL-12 when added to CPE. These effects were not observed in DCs deficient in the relevant TLR (Fig 5).
Figure 5. Adding TLR4 and TLR9 ligands to CPE stimulation synergizes IL-10 production whereas only TLR9 leads to IL-12 synergy by BMDC.
BMDC (WT vs. TLR4- or TLR9-KO) were either stimulated with CPE (50µg/ml), ultrapure LPS (500 ng/ml), ultrapure CpG (500ng/ml) or combination of both stimuli for 24hrs. Supernatants were analyzed by ELISA for production of IL-10 and IL-12. Concentrations represent mean ± SEM values (n=4). (**=P≤0.01).
Taken together, these observations strongly suggest that the E.coli-induced modification of dendritic cell responses to peanut occurs by the differential triggering of TLRs. The enhancement of the IL-10 and IL-12 production may play a key role in altering T cell responses.
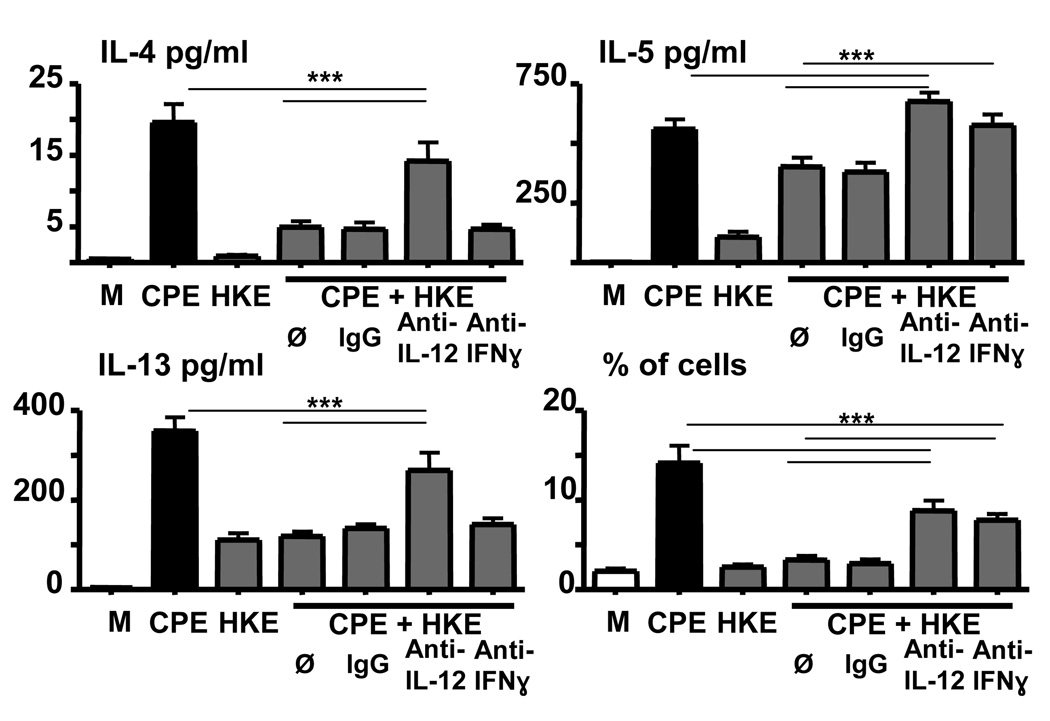
E. coli partially inhibits the peanut-induced immune response via the production of IL-12
In order to investigate the role of polarizing cytokines on the effect of HKE, we performed cocultures using cells from animals deficient in IL-10. As presented in supplementary figure 5, the absence of IL-10 did not impact the effect of HKE. We next performed cocultures using antibodies against IL-12/IL23 p40 and IFN-γ. The use of these two antibodies profoundly inhibited the detection of IFN-γ in the supernatant, while the IL-10 production was unchanged [data not shown], and an isotype control antibody had no effect. As shown in Figure 6, neutralizing IL-12 production partially inhibited the effect of HKE on Th2 cytokines, restoring them to comparable levels observed with CPE alone for IL-4 and IL-13, or to higher level in the case of IL-5. This effect was associated with significant increase in proliferation when compared to CD4 T cell proliferation exposed to CPE and HKE, but to lower level observed with cells exposed to peanut alone. Using a neutralizing antibody for IFN-γ, we were able to make very similar observations for CD4 T cell proliferation and for IL-5 production. Interestingly, IFN-γ did not seem to have much impact on IL-4 and IL-13 production, as neutralization did not significantly change the effect of HKE. These results strongly support the concept that HKE-induced production of IL-12 has direct and indirect [via the release of IFN-γ] effects on Th2 cytokine production and T cell proliferation.
Figure 6. E. coli partially inhibits the peanut-induced immune response via IL-12 production.
CD4+ T cells were cocultured (ratio 10:1) with CPE-pulsed BMDC stimulated with CPE (50µg/ml), HKE (1 bacteria per BMDC) or both for 72hrs. BMDC were preincubated with either an anti-IL-12 antibody or isotype control at 20µg/ml. The anti-IFN-γ antibody 20µg/ml was added with T cells. Antibody concentrations were maintained during the whole time of the coculture. Concentrations in IL-4, IL-5, IL-13 were determined as well as IFN-γ, and IL-10. Concentrations represent mean ± SEM (**=P≤0.01). After 5 days, proliferation of CD3+ cells was analyzed by flow cytometry. 4 combined experiments [involving at least 5 mice] are shown.
In conclusion, bacterial components, acting through dendritic cell TLR signaling, change the production of polarizing cytokines, durably shift the peanut-induced Th2 response towards a Th1 profile, and modify the CD4+ T cell proliferative response.
Discussion
The prevalence of food allergies has significantly increased in Westernized countries recently and no treatment is available. Peanut allergy is rarely outgrown, and accidental exposures are common and severe. Relatively little is known about the immunology of peanut proteins. Although our model does not reproduce the complexity of the gastrointestinal immune system of a living animal, our in vitro system has enabled us to make a number of novel observations about the cellular and molecular processes involved in the dendritic cell response to peanut and bacterial adjuvants.
In our in vitro system, peanut proteins induced a mixed Th1, Th2, and Th17 recall response. We show that peanut was able to induce TLR-independent dendritic cell activation. Shreffler et. al. have shown that DC-SIGN was involved in the recognition of glycosylated Ara h 1 by human dendritic cells, leading to Th2 priming18. Homologues of DC-SIGN have been recently described and may play a role in this CPE-mediated effect19.
To assess how TLR signaling affects the T-cell immune response to peanut, we stimulated dendritic cells with peanut and heat-killed E. coli and cocultured them with primed T cells. Addition of E. coli to peanut resulted in skewing the response towards Th1 by decreasing Th2 cytokine production as well as increasing IFN-γ. HKE also affected T cell proliferation, which was largely inhibited. Using MyD88/TRIF-double deficient DCs, we showed that these changes were attributable to TLRs. Interestingly, we found that the suppression of proliferation and Th2 cytokine production mediated by E. coli is sustained when T cells were reexposed to peanut alone.
This TLR-mediated reshaping of the immune response to peanut appears to occur through the enhancement of Th1 responses. IFN-γ production occurs in a STAT-4 dependent manner following the binding of IL-12 to its receptor. This IL-12-driven Th1 response was described to down-regulate Th2 responses. IL-12 has been shown to play a key role in both the prevention20,21 and treatment21 of food allergy in murine models. In our study, adding bacteria to CPE resulted in increasing IL-12, followed by IFN-γ production. We were specifically interested in the possible role of unmethylated CpG motifs in this response since it is well established that TLR9 signaling induces an increase in IL-12 production. We report that adding CpG to peanut extract produces IL-12 synergistically, suggesting that the bacteria-induced Th1 response was attributed to TLR9. Indeed, the effect of HKE was partially dependent upon IL-12 in our system. This is consistent with the results obtained in peanut-allergic mice successfully treated with EMP-12311 and CpG13,22. Since IL-12 and IFN-γ regulate each other, we performed experiments to investigate if the IL-12 effect was mediated via IFN-γ. Neutralizing this cytokine only restored IL-5 production and partially restored CD4 T cell proliferation, suggesting a direct effect of IL-12 on IL-4 and IL-13 production. These results cannot totally exclude the role of other cytokines such as IL-18. Our data show that TLR activation leads to Th1 skewing, which is able to reduce Th2 responses. Future immunotherapeutic strategies to redirect the Th2 response to peanut or other allergens may target Th1 enhancement through the use of microbial compounds. Indeed, clinical trials using TLR9 agonists as adjuvants for aeroallergen immunotherapy have produced encouraging preliminary results23,24, but subsequent trials have been halted due to a lack of efficacy.
We looked at other polarizing cytokines and we observed that adding E.coli to peanut proteins synergistically enhanced IL-10 production through TLR4 signaling. Gringhuis et. al. demonstrated that DC-SIGN modulates TLR4 function leading to increased IL-10 production25. It is tempting to speculate that through DC-SIGN homologues or other lectins, CPE in presence of TLR agonists will lead to an increased IL-10 production. By using DCs and T cells that cannot produce this cytokine, we demonstrated that the inhibitory effect of HKE on the peanut response was not driven by IL-10 in our system. However, this observation does not exclude totally that IL-10, through its immunoregulatory properties, may play a role in vivo.
DC maturation was also associated with IL-6 production. IL-6 induces IL-426, inhibits Th1 differentiation and regulatory T cell activity by blocking FoxP3,27 and plays a role in Th2 differentiation in the lungs of asthmatic patients28.Indeed, primed CD4+ T cells restimulated with CPE produce IL-4, IL-5 and IL-13. These data suggest that IL-6 may participate in the development of Th2 responses to peanut.
In conclusion, peanut antigens stimulate a TLR-independent pro-inflammatory and pro-allergic dendritic cell maturational program. Peanut-stimulated dendritic cells activate primed T cells, inducing proliferation and mixed Th1/Th2/Th17 cytokine responses. Dendritic cell TLR signaling in response to microbial products induces IL-12 production and modifies the recall response to peanut antigens by robustly and durably suppressing T cell proliferation and Th2 cytokine production. The ability of TLR signals to induce the synergistic production of IL-12 as well as a shift towards a Th1 responder phenotype suggests that co-stimulation of innate receptors may be an important approach to immunotherapeutic strategies for food allergy. We are currently conducting further studies to analyze the role of the innate immune system in the response to bacterial adjuvants in vivo.
Key Messages
Peanut proteins uniquely activate dendritic cells independent of TLR pathways.
Peanut-induced dendritic cell signals can be altered by the presence of Toll-like receptor ligands, which redirect T cell responses.
These concepts may be useful in the design and application of immunotherapeutic strategies towards peanut allergy.
Supplementary Material
Acknowledgments
Source of Funding:
NIH-NIAID U19AI066738
PI: Hugh A. Sampson, MD
Abbreviations used
- BMDC
Bone marrow-derived dendritic cell
- CPE
Crude peanut extract
- EMP-123
Heat-killed E.coli expressing mutated recombinant peanut allergens
- HKE
Heat-killed E.coli vector
- IFN
Interferon
- LPS
Lipopolysaccharide
- MHC
Major histocompatibility complex
- MyD88
Myeloid differentiation factor-88
- STAT
Signal transduction and activator of transcription
- TLR
Toll-like receptor
- TRIF
TIR-domain-containing adapter-inducing interferon-β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Capsule Summary:
Enhancing allergen immunotherapy with bacterial adjuvants is a promising experimental approach. In vitro, microbial products signal through dendritic cell Toll-like receptors and reshape the immune response to peanut.
Relevant Financial Disclosures:
Sampson, Caplan, Bottomly: Shareholders, Allertein Therapeutics, LLC
Bibliography
- 1.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. Journal of Allergy and Clinical Immunology. 2001;107(1):191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: A 5-year follow-up study. Journal of Allergy and Clinical Immunology. 2003;112(6):1203. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Burks AW, Sampson HA. Clinical Features of Acute Allergic Reactions to Peanut and Tree Nuts in Children. Pediatrics. 1998 Jul 1;102(1):e6. doi: 10.1542/peds.102.1.e6. [DOI] [PubMed] [Google Scholar]
- 4.Avery N, King R, Knight S, Hourihane JOB. Assessment of quality of life in children with peanut allergy. Pediatric Allergy and Immunology. 2003;14(5):378–382. doi: 10.1034/j.1399-3038.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 5.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. Journal of Allergy and Clinical Immunology. 1997 Jun;99(6, Part 1):744–751. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 6.Leung DY, Sampson HA, Yunginger JW, Burks AW, Schneider LC, Wortel CH, et al. Effect of Anti-IgE Therapy in Patients with Peanut Allergy. N Engl J Med. 2003 Mar 13;348(11):986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 7.Burks AW, Cockrell G, Stanley JS, Helm RM, Bannon GA. Recombinant peanut allergen Ara h I expression and IgE binding in patients with peanut hypersensitivity. Journal of Clinical Investigation. 1995;96(4):1715. doi: 10.1172/JCI118216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, et al. Identification and Mutational Analysis of the Immunodominant IgE Binding Epitopes of the Major Peanut Allergen Ara h 2. Archives of Biochemistry and Biophysics. 1997;342(2):244–253. doi: 10.1006/abbi.1997.9998. [DOI] [PubMed] [Google Scholar]
- 9.Rabjohn P, Helm EM, Stanley JS, West CM, Sampson HA, Burks AW, et al. Molecular cloning and epitope analysis of the peanut allergen Ara h 3. Journal of Clinical Investigation. 1999;103(4):535. doi: 10.1172/JCI5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XM, Srivastava K, Huleatt JW, Bottomly K, Burks AW, Sampson HA. Engineered recombinant peanut protein and heat-killed Listeria monocytogenes coadministration protects against peanut-induced anaphylaxis in a murine model. J Immunol. 2003;170(6):3289–3295. doi: 10.4049/jimmunol.170.6.3289. [DOI] [PubMed] [Google Scholar]
- 11.Li XM, Srivastava K, Grishin A, Huang CK, Schofield B, Burks W, et al. Persistent protective effect of heat-killed Escherichia coli producing "engineered," recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol. 2003;112(1):159–167. doi: 10.1067/mai.2003.1622. [DOI] [PubMed] [Google Scholar]
- 12.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6(10):761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 13.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172(11):6978–6987. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 14.Berin MC, Zheng Y, Domaradzki M, Li XM, Sampson HA. Role of TLR4 in allergic sensitization to food proteins in mice. Allergy. 2006;61(1):64–71. doi: 10.1111/j.1398-9995.2006.01012.x. [DOI] [PubMed] [Google Scholar]
- 15.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of Commensal Microflora by Toll-Like Receptors Is Required for Intestinal Homeostasis. Cell. 2004 Jul 23;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunology. 2001:2361–2367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 17.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 2006 Mar 20;203(3):519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, et al. The Major Glycoprotein Allergen from Arachis hypogaea, Ara h 1, Is a Ligand of Dendritic Cell-Specific ICAM-Grabbing Nonintegrin and Acts as a Th2 Adjuvant In Vitro. J Immunol. 2006;177(6):3677–3685. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 19.Powlesland AS, Ward EM, Sadhu SK, Guo Y, Taylor ME, Drickamer K. Widely Divergent Biochemical Properties of the Complete Set of Mouse DC-SIGN-related Proteins. J. Biol. Chem. 2006 July;281(29):20440–20449. doi: 10.1074/jbc.M601925200. [DOI] [PubMed] [Google Scholar]
- 20.Temblay JN, Bertelli E, Arques JL, Regoli M, Nicoletti C. Production of IL-12 by Peyer patch-dendritic cells is critical for the resistance to food allergy. J Allergy Clin Immunology. 2007;120(3):659–665. doi: 10.1016/j.jaci.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 21.Lee SY, Huang CK, Zhang TF, Schofield BH, Burks AW, Bannon GA, et al. Oral administration of IL-12 suppresses anaphylactic reactions in a murine model of peanut hypersensitivity. Clinical Immunology. 2001;101(2):220–228. doi: 10.1006/clim.2001.5122. [DOI] [PubMed] [Google Scholar]
- 22.Zhu F, Kandimalla ER, Yu D, Agrawal S. Oral administration of a synthetic agonist of Toll-like receptor 9 potently modulates peanut-induced allergy in mice. J Allergy Clin Immunology. 2007;120(3):631–637. doi: 10.1016/j.jaci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Simons FER, Shikishima Y, Van Nest G, Eiden JJ, HayGlass KT. Selective immune redirection in humans with ragweed allergy by injecting Amb a 1 linked to immunostimulatory DNA. Journal of Allergy and Clinical Immunology. 2004 Jun;113(6):1144–1151. doi: 10.1016/j.jaci.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, et al. Immunotherapy with a Ragweed-Toll-Like Receptor 9 Agonist Vaccine for Allergic Rhinitis. New England Journal of Medicine. 2006;355(14):1445. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 25.Gringhuis SI, den Dunnen J, Litjens M, van het Hof B, van Kooyk Y, Geijtenbeek TB. C-Type Lectin DC-SIGN Modulates Toll-like Receptor Signaling via Raf-1 Kinase-Dependent Acetylation of Transcription Factor NF-[kappa]B. Immunity. 2007 May 25;26(5):605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Diehl S, Chow C, Weiss L, Palmetshofer A, Twardzik T, Rounds L, et al. Induction of NFATc2 Expression by Interleukin 6 Promotes T Helper Type 2 Differentiation. J. Exp. Med. 2002 Jul 1;196(1):39–49. doi: 10.1084/jem.20020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends in Molecular Medicine. 2008 Mar;14(3):109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006 Aug 1;80(2):227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.