Abstract
Spontaneous hyperactivity in the dorsal cochlear nucleus (DCN), particularly in fusiform cells, has been proposed as a neural generator of tinnitus. To determine if sodium salicylate, a reliable tinnitus inducer, could evoke hyperactivity in the DCN, we measured the spontaneous and depolarization-evoked spike rate in fusiform and cartwheel cells during salicylate superfusion. Five minute treatment with 1.4 mM salicylate suppressed spontaneous and evoked firing in fusiform cells; this decrease partially recovered after salicylate washout. Less suppression and greater recovery occurred with 3 minute treatment using 1.4 mM salicylate. In contrast, salicylate had no effect on the spontaneous or evoked firing of cartwheel cells indicating that salicylate’s suppressive effects are specific to fusiform cells. To determine if salicylate’s suppressive effects were a consequence of increased synaptic inhibition, spontaneous inhibitory post-synaptic currents (IPSC) were measured during salicylate treatment. Salicylate unexpectedly reduced IPSC thereby ruling out increased inhibition as a mechanism to explain the depressed firing rates in fusiform cells. The salicylate-induced suppression of fusiform spike rate apparently arises from unidentified changes in the cell’s intrinsic excitability.
Keywords: Salicylate, Tinnitus, Dorsal cochlear nucleus (DCN), Patch Clamp, fusiform cell, cartwheel cell
Introduction
Hearing loss is often accompanied by tinnitus, a phantom auditory sensation whose severity varies from mild to severe. Among adults, the prevalence of tinnitus ranges from 8-15% (Coles, 1984; Henry et al., 2005; Hoffman and Reed, 2004; Nondahl et al., 2002; Snow, 1995) and for approximately 1% the symptoms are severe enough to require medical treatment (Davis and Refaie, 2000; Leske, 1981; Surveys, 1983). While many attempts have been made to identify pharmacological treatments for tinnitus, most drugs have proved ineffective (Dobie, 2004; Salvi et al., 2009). The development of effective drugs therapies has been hindered by a poor understanding of the biological bases of tinnitus.
Although many sites along the auditory pathway have been implicated in tinnitus (Basta and Ernst, 2004; Basta et al., 2008; Chen and Jastreboff, 1995; Eggermont and Kenmochi, 1998; Kenmochi and Eggermont, 1997; Llinas et al., 1999; Lockwood et al., 1998; Ma et al., 2006; Mahlke and Wallhausser-Franke, 2004; Sun et al., 2009; Weisz et al., 2007; Zhang et al., 2003), several lines of evidence suggest that the dorsal cochlear nucleus (DCN) plays a key role in its generation (Brozoski et al., 2002; Kaltenbach and Godfrey, 2008; Shore et al., 2007). Acoustic overstimulation, one of the most frequent causes of tinnitus, elevates spontaneous rates in tonotopic regions of the DCN associate with hearing loss (Axelsson and Ringdahl, 1989; Kaltenbach and McCaslin, 1996). The spectral profile and magnitude of the spontaneous rate increase is correlated with behavioral measures of tinnitus (Kaltenbach et al., 2004; Kaltenbach et al., 1998). Since acoustic trauma generally depress spontaneous activity in the auditory nerve, the hyperactivity observed in the DCN does not appear to originate in the cochlea (Liberman and Dodds, 1984). Moreover, DCN hyperactivity persists after cochlear ablation reinforcing the notion that the hyperactivity is not of cochlear origin (Zacharek et al., 2002). DCN hyperactivity is correlated with the amount of outer hair cell damage; however, the hyperactivity tend to be less when both inner and outer hair cells are damaged (Kaltenbach et al., 2002).
More recent experiments have linked noise-induced tinnitus with hyperactivity in DCN fusiform cells that have best frequencies tuned to the pitch of the tinnitus (Brozoski et al., 2002). Fusiform cells might represent a specific cell type involved in tinnitus initiation and a pharmacologic target for drug therapy. Fusiform cells receive auditory inputs from the cochlea via auditory nerve fibers as well as vestibular, somatosensory and higher order auditory inputs via parallel fibers originating from granule cells (Golding and Oertel, 1997; Oertel and Young, 2004). Fusiform cells receive glycinergic inhibitory inputs from cartwheel cells. Cartwheel cells receive inputs from parallel fibers and form synapses on other cartwheel cells, giant cells and fusiform cells. Fusiform cells relay their output to the inferior colliculus through dorsal acoustic stria.
High doses of sodium salicylate, the active ingredient in aspirin, induce temporary tinnitus in humans and this effect has been exploited in animal models to investigate the neural correlates of tinnitus at different sites along the auditory pathway (Basta et al., 2008; Lobarinas et al., 2004; Lobarinas et al., 2006; Myers and Bernstein, 1965; Yang et al., 2007). In a brain slice preparation of the cochlear nucleus, salicylate treatment caused spontaneous activity to increase in roughly a third of the units; decrease in another third and had no effect on the remaining third (Basta et al., 2008); however, no information was provided on the changes that occurred in specific cell types or region of the cochlear nucleus where the changes occurred. If tinnitus emerges from hyperactivity in DCN fusiform cells, then it would be important to determine exactly what effect salicylate has on fusiform cells and other major DCN cell types, especially cartwheel cells which make glycinergic inhibitory contacts on fusiform cells. Recent studies indicate that salicylate inhibits the current mediated by glycine receptors containing alpha1-subunits (Lu et al., 2009). These results suggest that salicylate might suppress glycinergic inhibitory inputs to fusiform cells thereby increasing the firing rate of fusiform cells and suppressing IPSC. To address this issue, we used the whole cell patch clamp technique to record from fusiform cells and cartwheel cells while perfusing salicylate onto brain slice preparation of the rat cochlear nucleus.
Methods
Slice preparation
SASCO Sprague Dawley rats (aged P13 – P20) were anesthetized with isoflurane and decapitated. The brainstem containing the cochlear nucleus with adjacent structures and cerebellum was cut and then glued to a cutting platform. Pseudosagittal slices (200 μm) were cut into pre-warmed (34 °C) high glucose artificial cerebral spinal fluid (HG-ACSF) containing (in mM; chemicals purchased from Sigma): 75 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 0.1 MgCl2, 100 glucose, 1.36 CaCl2, 4 Na L-lactate, 2 Na-pyruvate, 0.4 Na L-ascorbate, bubbled with 95% O2 and 5% CO2. Slices were incubated in HG-ACSF solution for at least 40 minutes, bubbled with 95% O2 and 5% CO2. Afterwards the temperature of the solution was gradually decreased to room temperature (~25 °C). Slices were transferred into a recording chamber with a continuous flow of fresh ACSF solution containing (in mM): 125 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 25 glucose, 1.36 CaCl2 and bubbled with 95% O2 and 5% CO2. The recording chamber was placed under a differential interference contrast microscope (BX51WI, Olympus). Fusiform cells and cartwheel cells were identified by location within the DCN, soma shape and size, and physiological criteria. Fusiform cell were identified from their location within fusiform layer, where they are the only large cell type, apart from cartwheel cells. Fusiform cells typically have a large, spindle-shaped soma located in the deeper part of the fusiform cell layer; their apical and basal processes project towards the molecular layer and deep layer, respectively. In contrast, the cell bodies of cartwheel cells are round, generally smaller than fusiform cells, and lie close to the surface of fusiform cell layer (Golding et al., 1997). Physiologically, cartwheel cells fire unique ‘complex spikes’ that reliably distinguish them from fusiform cells, which fire simple, uniform spikes (Golding et al., 1997; Manis et al., 1994; Zhang and Oertel, 1993).
Salicylate treatment
Our standard dose of salicylate was 1.4 mM delivered for 5 minutes. This concentration was selected because it matches the level of salicylate found in cerebrospinal fluid of animals injected with 460 mg/kg (i.p.) of sodium salicylate (Jastreboff et al., 1986b); previous studies have shown that salicylate doses of 150 mg/kg (i.p.) or higher reliably produce behavioral evidence of tinnitus (Jastreboff et al., 1997; Lobarinas et al., 2006). In some experiment, 1.4 mM salicylate was applied for only 3 minutes to evaluate the extent of recovery shorter duration treatments. In a few studies, 5 mM salicylate was applied for 5 minutes to assess the effect of a higher dose.
Electrophysiology
Electrodes were pulled from glass capillary tubes (Drummond Scientific, O.D. 0.0565 inches) on a micropipette puller (Sutter, PC-84), fired polished, and wrapped with Parafilm near the tip to minimize pipette capacitance. Electrodes were filled with a potassium gluconate based internal solution containing (in mM): K-gluconate 122, NaCl 9, MgCl2 2, EGTA 0.5, HEPES 9, Tris-creatine PO4 14, MgATP 4, Na-GTP 0.3. Whole-cell current-clamp recordings were made with an Axopatch 200B amplifier (Axon Instruments). Signals were digitized by a 16-bit data acquisition system (DIGIDATA 1320A, AXON), low-pass filtered at 5 kHz and sampled at 10 kHz. Spontaneous spikes were recorded in current clamp without applying any current. Depolarization-evoked spikes were recorded in current clamp during injection of positive current (126 pA).
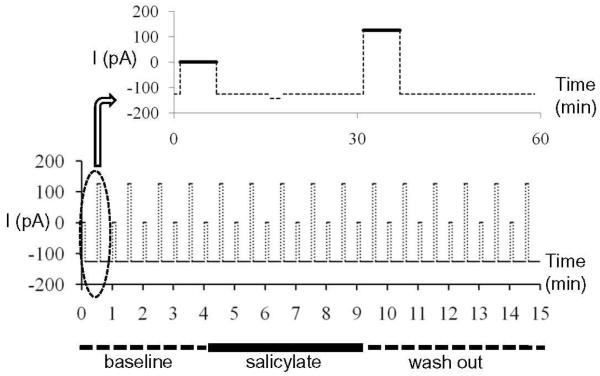
Each recording session lasted 15 minutes and consisted of 15 one-minute epochs (Figure 1). Negative current (−126 pA) was injected through the electrode to keep the cells hyperpolarized except during the acquisition of spontaneous activity (6 s, 0 pA) and depolarization-evoked activity (6 s, +126 pA). Input resistances were monitored through the experiment from the response to a hyperpolarization step between spontaneous and evoked recordings (Figure 1). Only cells with stable input resistance were included in the analysis. Spontaneous and depolarization-evoked spikes rates were calculated from the last 5 s of each of the data acquisition periods. This protocol was employed so that the cartwheel cells produced spikes with relatively uniform amplitude and interspike intervals. Baseline activity was recorded for 4 minutes (Figure 1). Then salicylate in ACSF was perfused on the slice for 3 or 5 minutes. The slice was then washed with normal ACSF until the end of the experiment.
Figure 1.
Protocol for current clamp recording. During current clamp recording, cell injected with −126 pA except during acquisition of spontaneous and evoked spike rate when the current was 0 and +126 pA. A hyperpolarization current step was performed between two recording periods to monitor the input resistance. Salicylate was applied for 5 minutes starting at minute 5, followed by a wash out period.
To record IPSC, a Cs based internal solution was used which contained (in mM): CsF 50, CsCl 90, EGTA 5, QX-314 0.5, and HEPES 10. The pH was adjusted to 7.3 with CsOH. The membrane potential was held at −70 mV. The series resistance was compensated electrically with the amplifier. All currents recorded under these conditions were predominantly if not exclusively IPSC because fusiform cells do not receive noticeable spontaneous EPSC in the absence of stimulation (Zhang and Oertel, 1994). The integral of IPSC was recorded and calculated using custom software written in MATLAB 7.2. The 1.4 mM dose of salicylate was applied to each cell for 2.5 minutes followed by washout with ACSF.
Data analysis
Whole cell recordings of spike rate were evaluated from 31 fusiform cells and 7 cartwheel cells whose physiological properties remained stable over the entire recording interval. In addition, recordings of IPSC were obtained from 8 fusiform cells. Data was collected using Clampex 9.2 software and spikes were counted using custom software written in MATLAB 7.2. To allow for comparison across cells with different spike rates, each cell’s spike rate was normalized to the average of the pre-salicylate baseline spike rate. Some of the fusiform cells had very low spontaneous spike rates (< 2.5 spikes/sec) or no spontaneous activity. Therefore these cells were not used in the spontaneous spike rate analysis. However, all fusiform cells were used for the analysis of evoked spike rate. Averaged values were shown ± 1 standard error.
To determine if the effects of salicylate on the spontaneous and depolarization-evoked spike rates were statistically significant, normalized spike rates recorded before the onset of salicylate application (i.e. minutes 1 to 4) were used as the control values and the values from each minute after that were used as an individual treatment (condition). Statistical analysis software (SigmaStat V3.5) was used to test for statistical significance. A one-way repeated measures ANOVA was performed to determine if there was overall group differences (p<0.05). For the post-hoc analysis, each time point during and salicylate treatment was compared to the control group (Mann-Whitney Rank Sum Test) to determine which values were significantly different from the control.
The experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) and were approved by University at Buffalo Institutional Animal Care and Use Committee.
Results
Fusiform cells
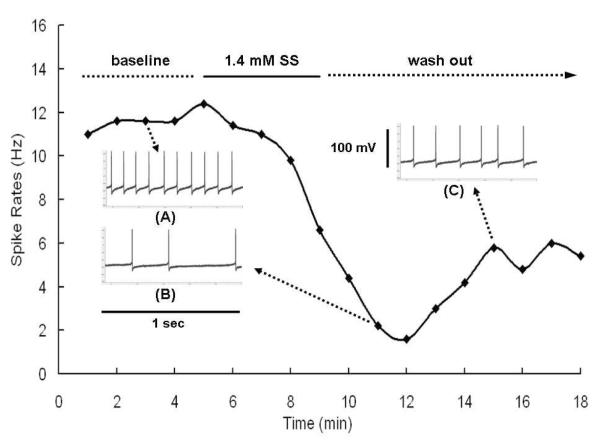
Figure 2 shows the spontaneous rates measured from a typical fusiform cell during the baseline period, salicylate treatment and washout with ACSF. Inserts A, B and C show typical spike waveforms recorded from the cell during baseline, salicylate and washout periods respectively. Approximately two minutes after the start of salicylate application, the spontaneous rate of the fusiform cell declined from roughly 11 Hz to 1-2 Hz. Spontaneous rate partially recovered to 5-6 Hz during the first 2-3 minutes of the washout period and remained stable over the next few minutes. The typical recording period, 15 minutes, was composed of a 4 minute baseline, a 5 minute salicylate application period, and a 6 minute recovery. In some cases, recordings continued for longer periods post-salicylate, but there was very little recovery beyond the 6 minute recovery period.
Figure 2.
A sample plot of spontaneous spike rate of fusiform cell obtained during 5-s intervals over baseline, during 5 minute treatment with 1.4 mM sodium salicylate (SS) and during the washout period. Inserts A, B, and C show typical spiking pattern observed during 1-s intervals during baseline, SS and washout periods.
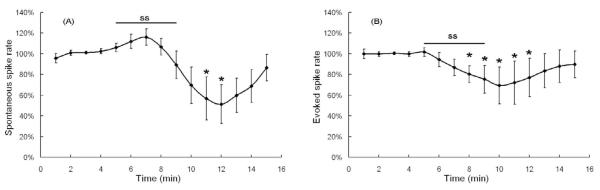
The mean spontaneous spike rate of 6 fusiform cells was 9.95 ± 0.9 spikes/s. Figure 3A shows the mean normalized spontaneous spike rates recorded from fusiform cells (n=6) treated with 1.4 mM salicylate for 5 minutes. Salicylate caused a non-significant increase in spike rates up to minute 7 (116 ± 8%) and then the rate declined over the remainder of salicylate treatment and for the first 3 minutes of the washout period reaching a minimum of 51 ± 19%. Afterwards, spike rate gradually recovered to 86 ± 13% of baseline at 6 minutes post-treatment. Spontaneous spike rates were significantly different over the pre- and post-salicylate recording interval (one-way repeated measure ANOVA, P <0.001). Spontaneous spike rates following salicylate treatment were significantly less than baseline control measures at minute 11 and minute 12 (post-hoc analysis, Mann-Whitney Rank Sum Test, P < 0.05).
Figure 3.
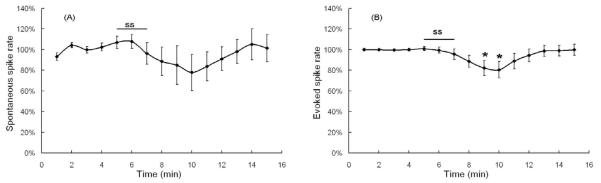
Effect of 5 minute treatment with 1.4 mM salicylate (SS) on fusiform cells. (A) Mean spontaneous spike rates (N=6; +/− SE). (B) Mean rate evoked by a +126 pA depolarization current (N=12; +/− SE). The curve shows the average of normalized spike rates across all cells recorded under this condition. * indicates points significantly different from baseline, which is a set of normalized spike rates during the first 4 minutes of each cell.
To evaluate the effects of salicylate on evoked spike rate, 126 pA of depolarizing current was delivered through the recording electrode. Spike rate was evaluated before, during and after the 5 minute salicylate treatment in 12 fusiform cells. The mean current-evoked spike rate during baseline was 23.2 ± 1.5 spikes/s; a value approximately two times higher than the mean spontaneous rate. The mean normalized current-evoked spike rate began to decline at minute six, one minute after the start of salicylate treatment and reached a minimum of 69 ± 10% of baseline one-minute into the washout period (Figure 3B). The mean evoked rate began to recover 2-3 minutes after the start of washout and recovered to 90 ± 7% of control values 6 minutes after the start of washout. Depolarization evoked spike rates were significantly different over the pre- and post-salicylate recording interval (one-way repeated measure ANOVA, P <0.001). Evoked spike rates following salicylate treatment were significantly less than baseline from minutes 8-12 (post-hoc analysis, Mann-Whitney Rank Sum Test, P < 0.05).
Cartwheel cells
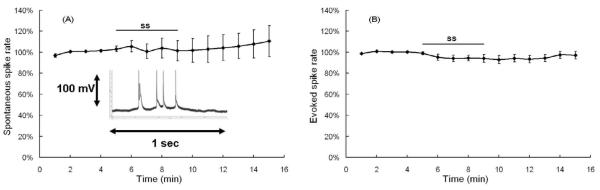
To evaluate the generality of these findings, recordings were also obtained from cartwheel cells. In addition to their shape and location within the DCN, cartwheel cells were easily identified by their complex pike shape (inset Figure 4A). Figure 4A shows the mean (n = 7) normalized spontaneous spike rates recorded from cartwheel cells before and after salicylate treatment. Unlike fusiform cells, the mean spontaneous spike rate of cartwheel cells did not change significantly over time (one-way repeated measure ANOVA, P > 0.05).
Figure 4.
Effect of 5 minutes of 1.4 mM salicylate (SS) on cartwheel cells. Typical ‘complex spikes’ used to physiologically classify cartwheel cells. (A) Mean spontaneous spike rates (N=7, +/− SE). None of the individual post-treatment points were significantly different from baseline. (B) Mean rate evoked by a +126 pA depolarization current (N=6, +/− SE). None of the individual post-treatment points were significantly different from baseline.
The electrically evoked spike rate was also evaluated in 6 cartwheel cells before and after salicylate treatment. The mean spike rate showed a slight decrease after salicylate treatment (Figure 4B). A one-way repeated measure ANOVA indicated that the evoked rates were significantly different (P < 0.05) over time; however, none of the individual time points were significantly different from the baseline control rate (post-hoc analysis, Mann-Whitney Rank Sum Test, P > 0.05).
Effect of short-duration salicylate treatment on fusiform cells
Since the spontaneous spike rate of fusiform cells only partially recovered after 5 minute treatment with 1.4 mM salicylate, another group of fusiform cells was evaluated with a 3 minute treatment to determine if they would fully recover (Figure 5A). The spontaneous rate began to decline at the end of the 3 minute treatment period and reached at minimum of 78±17% 3 minutes into the washout period. By the end of the washout period, the spontaneous spike rate had fully recovered (102±13%). Although there was a significant difference in spontaneous rate during the recording interval (one way repeated measures ANOVA, P < 0.05), none of the individual time points was significantly different from the control rate (post-hoc analysis, Mann-Whitney Rank Sum Test, P > 0.05).
Figure 5.
Effect of 3 minutes of 1.4 mM sodium salicylate (SS) on fusiform cells. (A) Mean spontaneous spike rate (N=5; +/− SE); none of the individual points were significantly different from baseline. (B) Mean spike rates evoked by +126 pA depolarization current (N= 8; +/− SE); none of the individual points were significantly different from baseline.
The current-evoked spike rate was evaluated in 8 fusiform cells treated for 3 minutes with salicylate (Figure 5B). The mean current-evoked spike rate declined during the salicylate treatment, reached a minimum of approximately 81±8% of baseline 3 minutes into the washout period and fully recovered (100±5%) by the end of the washout period. There was an overall significant difference in spike rate over the recording interval (one way repeated measure ANOVA, P< 0.05). A post-hoc analysis showed that the evoked spike rates at minute 9 and minute 10 were significantly different from control (post-hoc analysis, Mann-Whitney Rank Sum Test, P < 0.05). These results indicate that both the spontaneous and evoked spike rates of fusiform cells fully recover following a short, 3 minute treatment with 1.4 mM salicylate.
Effect of high dose salicylate on fusiform cells
Spontaneous spike rates were recorded from 5 fusiform cells (only 5 of 10 fusiform cells were spontaneously active) during 5 minute treatment with 5 mM salicylate. The mean normalized spontaneous rate showed a noticeable increase after the start of salicylate application (Figure 6A). The mean spike rate reached a peak of 148 ± 49% at minute six and then declined moderately during the remainder of the treatment. During the washout period, the mean spike rate decreased to a minimum of 65 ±18 % of baseline at minute 13. Afterwards, the spontaneous rates started to recover and reached 81 ± 18% of baseline at the end of the wash out period. However, statistical analysis showed that these changes were not significant (one way repeated measures ANOVA; P > 0.05) presumably due to the large variance.
Figure 6.
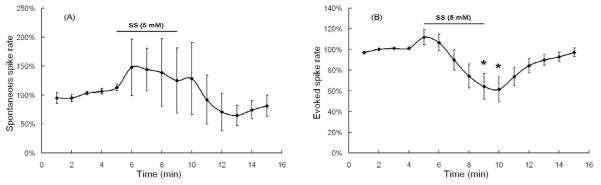
Effect of 5 minutes of 5 mM sodium salicylate (SS) on fusiform cells. (A) Mean spontaneous spike rate (N= 5; +/− SE); none of the individual points were significantly different from baseline. (B) Mean fusiform spike rate evoked by +126 pA depolarization current (N= 10; +/− SE); * indicate points significantly difference from pre-SS baseline.
Spike rates evoked by current injection were recorded from 10 fusiform cells during 5 minute treatment with 5 mM salicylate (figure 6B). During the first 2 minutes of treatment, the average evoked spike rate increased slightly to 112 ± 7%. Afterwards, the evoked spike rate fell below the baseline reaching a minimum value of 61 ± 12% at minute 10 (1 minute into the washout period). Afterwards, the evoked spike rate gradually recovered to 97±4% of baseline by the end of the washout period. There was a significant difference in evoked rate over the recording period (one way repeated measures ANOVA; P < 0.05). The evoked spike rates at minutes 9 and 10 were significantly different from baseline control values (post-hoc analysis, Mann-Whitney Rank Sum Test, P < 0.05).
Fusiform cell IPSC
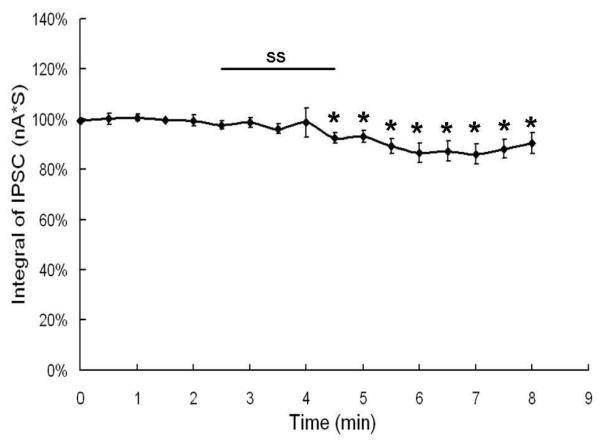
To determine if the decrease in spontaneous and evoked spike rate seen in fusiform cells was the result of increased inhibition, we measured the spontaneous IPSC before and after salicylate treatment. Figure 7 shows the normalized integral of the IPSC from 8 fusiform cells over the recording period. The integral of the IPSC showed little change during salicylate treatment and a moderate decrease (maximum decrease ~15%) during salicylate washout. There was a significant difference in the integral of the IPSC over the recording period (one way repeated measures ANOVA; P < 0.05). The integral of the IPSC during the entire post-treatment period (minutes 4.5-8) was significantly less than baseline control values (post-hoc analysis, Mann-Whitney Rank Sum Test, P < 0.05).
Figure 7.
Effect of 1.4 mM sodium salicylate (SS) on mean (n=8; +/− SE) of the integral of IPSC recorded from fusiform cells; * indicate points significantly difference from pre-SS baseline.
Discussion
High doses of salicylate have previously been reported to alter the spontaneous activity of cochlear nucleus neurons in vitro; spontaneous activity increased in roughly a third of the units, decreased in another third and was unaffected in the remainder. However, it was unclear what regions of the cochlear nucleus were assessed and what cell types were affected by salicylate treatment (Basta et al., 2008). The present study, carried out with physiologically relevant salicylate concentrations (Boettcher et al., 1990; Jastreboff et al., 1986b; Jastreboff et al., 1997), identifies for the first time the specific effects salicylate has on the two major cell types in the DCN, namely fusiform cells and cartwheel cells. Five minute application of 1.4 mM salicylate suppressed both spontaneous and depolarization evoked spike rates in fusiform cells; the suppressive effects of salicylate partially recovered during the 6 minute washout period. The same general trends were observed with a shorter duration treatment (3 minutes) or a higher concentration (5 mM) of salicylate. In contrast, the same 1.4 mM dose of salicylate had no effect on the spontaneous and depolarization evoked spike rates of cartwheel cells. The first two findings indicate that the effects of salicylate are specific to fusiform cells and are not the result of a global (e.g., toxicity, pH, osmolarity) effect of salicylate on the DCN.
Spontaneous hyperactivity in the DCN is believed to arise from a shift in the balance of excitation and inhibition (Brozoski et al., 2002; Brozoski et al., 2007; Kaltenbach, 2007; Kaltenbach and Zhang, 2007). Fusiform cells receive strong glycinergic inhibitory inputs from cartwheel cells (Backoff et al., 1997; Juiz et al., 1996; Kaltenbach et al., 2005; Kaltenbach et al., 2002; Moore et al., 1996; Mugnaini, 1985; Rubio and Juiz, 2004), and recent evidence indicates that salicylate can inhibit glycine receptors containing alpha1-subunits (Lu et al., 2009). Therefore, we speculated that salicylate might increase the firing rate of fusiform cells by suppressing IPSC. However, 5 minute application of 1.4 mM salicylate unexpectedly reduced the spike rates of fusiform cells. Considering the facts that the spontaneous rates of cartwheel cells were unaffected by salicylate and IPSC in fusiform cells were slightly decreased, the salicylate-induced reduction of fusiform cell spontaneous activity cannot be due to an increase of inhibition.
High doses of salicylate have been reported to increase spontaneous activity in the guinea pig inferior colliculus (Jastreboff and Sasaki, 1986a). This raises the possibility that the changes seen in the inferior colliculus are initiated by hyperactivity originating in the DCN. However, our results conflict with this hypothesis since salicylate either suppressed or had no effect on the spontaneous rates of fusiform and cartwheel cells respectively. Similarly, suppression of activity in putative fusiform cells has been reported after intense noise exposure (Chang et al., 2002). The down regulation of spontaneous activity seen in fusiform cells after noise and salicylate could perturb activity either within local or remote neural networks and lead to tinnitus. Our salicylate data, however, are compatible with more recent in vitro data showing that salicylate suppresses spontaneous activity in the mouse inferior colliculus (Ma et al., 2006) and other more rostral sites in the auditory pathway (Yang et al., 2007). Collectively, these data suggest salicylate-induced tinnitus does not arise from hyperactivity in fusiform or cartwheel cells in the DCN, but from some other mechanism or from some other site in the central auditory pathway.
Significant increases in spontaneous activity typically appear in the DCN after acoustic overstimulation or cisplatin treatment (Brozoski et al., 2002; Kaltenbach et al., 2005; Melamed et al., 2000; Rachel et al., 2002). These physiological results along with relevant behavioral metrics suggest that tinnitus may be related to spontaneous hyperactivity in the DCN and that the axons of fusiform cells relay this information to the inferior colliculus via the dorsal acoustic stria (Brozoski et al., 2002; Cant and Benson, 2003; Imig and Durham, 2005; Kaltenbach et al., 2008; Melamed et al., 2000; Shore et al., 2007). However, recent study showed that selective ablation of DCN did not abolish the psychophysical evidence of tinnitus or actually increase it in some cases (Brozoski and Bauer, 2005). Our results also do not support a local mechanism of action for salicylate in the DCN hyperactivity model of tinnitus since we found salicylate quickly suppressed spontaneous rates in fusiform cells and had no effect on cartwheel cells. More complex models of salicylate-induced tinnitus may be required in which salicylate disrupts neuronal function at multiple sites along the auditory pathway perhaps by increasing the gain of the central auditory system (Lu et al., 2009; Sun et al., 2009; Wang et al., 2008).
There are several possible explanations why we may not have observed hyperactivity in the DCN after salicylate treatment. Our in vitro brain slice preparation eliminates many of the local and descending inputs to the DCN as well as inputs from the cochlea; the physiological effects of salicylate on DCN spontaneous activity could conceivably be fundamentally different. In addition, we only recorded from fusiform cells, whose axons project out of the cochlear nucleus, and cartwheel cells, that inhibit fusiform cells. The salicylate-induced hyperactivity observed in the cochlear nucleus by others could arise from other cell types we did not record from (Basta et al., 2008). High doses of salicylate also affect the cochlea; several studies report that salicylate increases the spontaneous rates of auditory nerve fibers (Evans and Borerwe, 1982; Ruel et al., 2008) while others have reported the exact opposite (Muller et al., 2003). Since the spiral ganglion neurons are absent from our brain slice preparation, the peripheral effects of salicylate were eliminated from our recordings. Despite these limitation, the local effects of salicylate observed in our DCN preparation provide important new information on the effect that salicylate has on fusiform and cartwheel cells, IPSC and the role the DCN plays in salicylate-induced tinnitus. The local effects of salicylate seem pertinent to models of tinnitus involving the DCN and other models that posit a central origin since DCN hyperactivity and tinnitus persist even after cochlea ablation (Coad et al., 2001; Zacharek et al., 2002).
Salicylate selectively suppressed spontaneous and evoked spike rate in fusiform cells, but not cartwheel cells. This cell-specific effect is presumably related to some unique, but unidentified properties of fusiform cells. Salicylate has been reported to affect many different types of ion channels including L-type calcium channels, potassium channels and sodium channels (Liu and Li, 2004; Liu et al., 2005a; Liu et al., 2005b). In addition, salicylate also influences numerous types of receptors such as NMDA, GABAergic, serotonergic and glycinergic receptors (Bauer et al., 1999; Finlayson and Kaltenbach, 2009; Milton et al., 2009; Peng et al., 2003; Ruel et al., 2008; Wang et al., 2006). Therefore, the salicylate-induced suppression of fusiform firing rates could be caused by the combined effect of one or more these ion channels or receptors as well as other unknown factors.
Acknowledgements
Research supported in part by grants from NIH (R01DC009091; R01DC009219) and Mark Diamond Research Fund from University at Buffalo
Abbreviations
- DCN
dorsal cochlear nucleus
- IPSC
inhibitory postsynaptic current
- HG-ACSF
high glucose artificial cerebral spinal fluid
- SS
sodium salicylate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Axelsson A, Ringdahl A. Tinnitus--a study of its prevalence and characteristics. Br. J. Audiol. 1989;23:53–62. doi: 10.3109/03005368909077819. [DOI] [PubMed] [Google Scholar]
- Backoff PM, Palombi PS, Caspary DM. Glycinergic and GABAergic inputs affect short-term suppression in the cochlear nucleus. Hear. Res. 1997;110:155–63. doi: 10.1016/s0378-5955(97)00081-6. [DOI] [PubMed] [Google Scholar]
- Basta D, Ernst A. Effects of salicylate on spontaneous activity in inferior colliculus brain slices. Neurosci. Res. 2004;50:237–43. doi: 10.1016/j.neures.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Basta D, Goetze R, Ernst A. Effects of salicylate application on the spontaneous activity in brain slices of the mouse cochlear nucleus, medial geniculate body and primary auditory cortex. Hear. Res. 2008;240:42–51. doi: 10.1016/j.heares.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M. Behavioral model of chronic tinnitus in rats. Otolaryngol. Head Neck Surg. 1999;121:457–62. doi: 10.1016/S0194-5998(99)70237-8. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Bancroft BR, Salvi RJ. Concentration of salicylate in serum and perilymph of the chinchilla. Arch. Otolaryngol. Head Neck Surg. 1990;116:681–4. doi: 10.1001/archotol.1990.01870060039005. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear. Res. 2005;206:227–36. doi: 10.1016/j.heares.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J. Neurosci. 2002;22:2383–90. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Spires TJ, Bauer CA. Vigabatrin, a GABA transaminase inhibitor, reversibly eliminates tinnitus in an animal model. J Assoc Res Otolaryngol. 2007;8:105–18. doi: 10.1007/s10162-006-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res. Bull. 2003;60:457–74. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Chang H, Chen K, Kaltenbach JA, Zhang J, Godfrey DA. Effects of acoustic trauma on dorsal cochlear nucleus neuron activity in slices. Hear. Res. 2002;164:59–68. doi: 10.1016/s0378-5955(01)00410-5. [DOI] [PubMed] [Google Scholar]
- Chen GD, Jastreboff PJ. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear. Res. 1995;82:158–78. doi: 10.1016/0378-5955(94)00174-o. [DOI] [PubMed] [Google Scholar]
- Coad ML, Lockwood A, Salvi R, Burkard R. Characteristics of patients with gaze-evoked tinnitus. Otol. Neurotol. 2001;22:650–4. doi: 10.1097/00129492-200109000-00016. [DOI] [PubMed] [Google Scholar]
- Coles RR. Epidemiology of tinnitus: (1) prevalence. J. Laryngol. Otol. Suppl. 1984;9:7–15. doi: 10.1017/s1755146300090041. [DOI] [PubMed] [Google Scholar]
- Davis A, Refaie AE. Epidemiology of tinnitus. In: Tyler R, editor. Tinnitus Handbook. Singular; San Diego, CA: 2000. pp. 1–23. [Google Scholar]
- Dobie RA. Clinical Trials and Drug Therapy for Tinnitus. In: Snow JBJ, editor. Tinnitus: Theory and Management. BC Decker, Inc.; Hamilton: 2004. pp. 266–277. [Google Scholar]
- Eggermont JJ, Kenmochi M. Salicylate and quinine selectively increase spontaneous firing rates in secondary auditory cortex. Hear. Res. 1998;117:149–60. doi: 10.1016/s0378-5955(98)00008-2. [DOI] [PubMed] [Google Scholar]
- Evans EF, Borerwe TA. Ototoxic effects of salicylates on the responses of single cochlear nerve fibres and on cochlear potentials. Br. J. Audiol. 1982;16:101–8. doi: 10.3109/03005368209081454. [DOI] [PubMed] [Google Scholar]
- Finlayson PG, Kaltenbach JA. Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear. Res. 2009;256:104–17. doi: 10.1016/j.heares.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Oertel D. Physiological identification of the targets of cartwheel cells in the dorsal cochlear nucleus. J. Neurophysiol. 1997;78:248–60. doi: 10.1152/jn.1997.78.1.248. [DOI] [PubMed] [Google Scholar]
- Henry JA, Dennis KC, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. J. Speech Lang. Hear. Res. 2005;48:1204–35. doi: 10.1044/1092-4388(2005/084). [DOI] [PubMed] [Google Scholar]
- Hoffman HJ, Reed GW. Epidemiology of tinnitus. In: Snow JB, editor. Tinnitus: Theory and management. BC Decker; Lewiston, NY: 2004. pp. 16–41. [Google Scholar]
- Imig TJ, Durham D. Effect of unilateral noise exposure on the tonotopic distribution of spontaneous activity in the cochlear nucleus and inferior colliculus in the cortically intact and decorticate rat. J. Comp. Neurol. 2005;490:391–413. doi: 10.1002/cne.20674. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Sasaki CT. Salicylate-induced changes in spontaneous activity of single units in the inferior colliculus of the guinea pig. J. Acoust. Soc. Am. 1986a;80:1384–91. doi: 10.1121/1.394391. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Hansen R, Sasaki PG, Sasaki CT. Differential uptake of salicylate in serum, cerebrospinal fluid, and perilymph. Arch. Otolaryngol. Head Neck Surg. 1986b;112:1050–3. doi: 10.1001/archotol.1986.03780100038004. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Zhou S, Jastreboff MM, Kwapisz U, Gryczynska U. Attenuation of salicylate-induced tinnitus by Ginkgo biloba extract in rats. Audiol. Neurootol. 1997;2:197–212. doi: 10.1159/000259244. [DOI] [PubMed] [Google Scholar]
- Juiz JM, Helfert RH, Bonneau JM, Campos ML, Altschuler RA. Distribution of glycine and GABA immunoreactivities in the cochlear nucleus: quantitative patterns of putative inhibitory inputs on three cell types. J. Hirnforsch. 1996;37:561–74. [PubMed] [Google Scholar]
- Kaltenbach JA. The dorsal cochlear nucleus as a contributor to tinnitus: mechanisms underlying the induction of hyperactivity. Prog. Brain Res. 2007;166:89–106. doi: 10.1016/S0079-6123(07)66009-9. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, McCaslin D. Increases in spontaneous activity in the dorsal cochlear nucleus following exposure to high intensity sound: a possible neural correlate of tinnitus. Aud. Neurosci. 1996;3:57–78. [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J. Intense sound-induced plasticity in the dorsal cochlear nucleus of rats: evidence for cholinergic receptor upregulation. Hear. Res. 2007;226:232–43. doi: 10.1016/j.heares.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA. Dorsal cochlear nucleus hyperactivity and tinnitus: are they related? Am. J. Audiol. 2008;17:S148–61. doi: 10.1044/1059-0889(2008/08-0004). [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear. Res. 2005;206:200–26. doi: 10.1016/j.heares.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci. Lett. 2004;355:121–5. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA, Neumann JB, McCaslin DL, Afman CE, Zhang J. Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear. Res. 1998;124:78–84. doi: 10.1016/s0378-5955(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Rachel JD, Mathog TA, Zhang J, Falzarano PR, Lewandowski M. Cisplatin-induced hyperactivity in the dorsal cochlear nucleus and its relation to outer hair cell loss: relevance to tinnitus. J. Neurophysiol. 2002;88:699–714. doi: 10.1152/jn.2002.88.2.699. [DOI] [PubMed] [Google Scholar]
- Kenmochi M, Eggermont JJ. Salicylate and quinine affect the central nervous system. Hear. Res. 1997;113:110–6. doi: 10.1016/s0378-5955(97)00137-8. [DOI] [PubMed] [Google Scholar]
- Leske MC. Prevalence estimates of communicative disorders in the U.S. Language, hearing and vestibular disorders. ASHA. 1981;23:229–37. [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear. Res. 1984;16:55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li X. Effects of salicylate on voltage-gated sodium channels in rat inferior colliculus neurons. Hear. Res. 2004;193:68–74. doi: 10.1016/j.heares.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li X, Ma C, Liu J, Lu H. Salicylate blocks L-type calcium channels in rat inferior colliculus neurons. Hear. Res. 2005a;205:271–6. doi: 10.1016/j.heares.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Liu YX, Li XP, Liu JX, Shi GM, Lu H, Ma CS. Inhibition of salicylate on potassium channels in rat inferior colliculus neurons. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2005b;40:835–9. [PubMed] [Google Scholar]
- Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. U. S. A. 1999;96:15222–7. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC) Hear Res. 2004;190:109–14. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Yang G, Sun W, Ding D, Mirza N, Dalby-Brown W, Hilczmayer E, Fitzgerald S, Zhang L, Salvi R. Salicylate- and quinine-induced tinnitus and effects of memantine. Acta Otolaryngol. Suppl. 2006:13–9. doi: 10.1080/03655230600895408. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50:114–20. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- Lu YG, Tang ZQ, Ye ZY, Wang HT, Huang YN, Zhou KQ, Zhang M, Xu TL, Chen L. Salicylate, an aspirin metabolite, specifically inhibits the current mediated by glycine receptors containing alpha1-subunits. Br. J. Pharmacol. 2009;157:1514–22. doi: 10.1111/j.1476-5381.2009.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WL, Hidaka H, May BJ. Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hear. Res. 2006;212:9–21. doi: 10.1016/j.heares.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Mahlke C, Wallhausser-Franke E. Evidence for tinnitus-related plasticity in the auditory and limbic system, demonstrated by arg3.1 and c-fos immunocytochemistry. Hear. Res. 2004;195:17–34. doi: 10.1016/j.heares.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Manis PB, Spirou GA, Wright DD, Paydar S, Ryugo DK. Physiology and morphology of complex spiking neurons in the guinea pig dorsal cochlear nucleus. J. Comp. Neurol. 1994;348:261–76. doi: 10.1002/cne.903480208. [DOI] [PubMed] [Google Scholar]
- Melamed SB, Kaltenbach JA, Church MW, Burgio DL, Afman CE. Cisplatin-induced increases in spontaneous neural activity in the dorsal cochlear nucleus and associated outer hair cell loss. Audiology. 2000;39:24–9. doi: 10.3109/00206090009073051. [DOI] [PubMed] [Google Scholar]
- Milton S, Lobarinas S, West N, Mawer P, Butler A, Salvi R, Barnes N. Quantitative PCR analysis of 5-HT receptor expression in tissue from the inferior colliculus and cochlear nucleus of an animal model of tinnitus. Proc.Br. Pharmacol. Soc. 2009:139. [Google Scholar]
- Moore JK, Osen KK, Storm-Mathisen J, Ottersen OP. gamma-Aminobutyric acid and glycine in the baboon cochlear nuclei: an immunocytochemical colocalization study with reference to interspecies differences in inhibitory systems. J. Comp. Neurol. 1996;369:497–519. doi: 10.1002/(SICI)1096-9861(19960610)369:4<497::AID-CNE2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Mugnaini E. GABA neurons in the superficial layers of the rat dorsal cochlear nucleus: light and electron microscopic immunocytochemistry. J. Comp. Neurol. 1985;235:61–81. doi: 10.1002/cne.902350106. [DOI] [PubMed] [Google Scholar]
- Muller M, Klinke R, Arnold W, Oestreicher E. Auditory nerve fibre responses to salicylate revisited. Hear. Res. 2003;183:37–43. doi: 10.1016/s0378-5955(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Myers EN, Bernstein JM. Salicylate ototoxicity; a clinical and experimental study. Arch Otolaryngol. 1965;82:483–93. doi: 10.1001/archotol.1965.00760010485006. [DOI] [PubMed] [Google Scholar]
- Nondahl DM, Cruickshanks KJ, Wiley TL, Klein R, Klein BE, Tweed TS. Prevalence and 5-year incidence of tinnitus among older adults: the epidemiology of hearing loss study. J. Am. Acad. Audiol. 2002;13:323–31. [PubMed] [Google Scholar]
- Oertel D, Young ED. What’s a cerebellar circuit doing in the auditory system? Trends Neurosci. 2004;27:104–10. doi: 10.1016/j.tins.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Peng BG, Chen S, Lin X. Aspirin selectively augmented N-methyl-D-aspartate types of glutamate responses in cultured spiral ganglion neurons of mice. Neurosci. Lett. 2003;343:21–4. doi: 10.1016/s0304-3940(03)00296-9. [DOI] [PubMed] [Google Scholar]
- Rachel JD, Kaltenbach JA, Janisse J. Increases in spontaneous neural activity in the hamster dorsal cochlear nucleus following cisplatin treatment: a possible basis for cisplatin-induced tinnitus. Hear. Res. 2002;164:206–14. doi: 10.1016/s0378-5955(02)00287-3. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Juiz JM. Differential distribution of synaptic endings containing glutamate, glycine, and GABA in the rat dorsal cochlear nucleus. J. Comp. Neurol. 2004;477:253–72. doi: 10.1002/cne.20248. [DOI] [PubMed] [Google Scholar]
- Ruel J, Chabbert C, Nouvian R, Bendris R, Eybalin M, Leger CL, Bourien J, Mersel M, Puel JL. Salicylate enables cochlear arachidonic-acid-sensitive NMDA receptor responses. J. Neurosci. 2008;28:7313–23. doi: 10.1523/JNEUROSCI.5335-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi R, Lobarinas E, Sun W. Pharmacological treatments for tinnitus: New and old. Drugs Future. 2009;34:381–400. doi: 10.1358/dof.2009.034.05.1362442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S, Zhou J, Koehler S. Neural mechanisms underlying somatic tinnitus. Prog. Brain Res. 2007;166:107–23. doi: 10.1016/S0079-6123(07)66010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JB. Report from the National Institute of Deafness and Other Communication Disorders. In: Reich GE, Vernon JA, editors. Proceedings of the Fifth International Tinnitus Seminar; Portland: American Tinnitus Association; 1995. pp. 11–15. [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ. Salicylate increases the gain of the central auditory system. Neurosci. 2009;159:325–34. doi: 10.1016/j.neuroscience.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveys O.o.P.C.a. General household survey: The prevalenc of tinnitus. In: O.M.R., editor. GHS83/1) Office of Population Statistics; London: 1983. [Google Scholar]
- Wang HT, Luo B, Zhou KQ, Xu TL, Chen L. Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear. Res. 2006;215:77–83. doi: 10.1016/j.heares.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Wang HT, Luo B, Huang YN, Zhou KQ, Chen L. Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear Res. 2008;236:42–51. doi: 10.1016/j.heares.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Weisz N, Muller S, Schlee W, Dohrmann K, Hartmann T, Elbert T. The neural code of auditory phantom perception. J. Neurosci. 2007;27:1479–84. doi: 10.1523/JNEUROSCI.3711-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–53. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Zacharek MA, Kaltenbach JA, Mathog TA, Zhang J. Effects of cochlear ablation on noise induced hyperactivity in the hamster dorsal cochlear nucleus: implications for the origin of noise induced tinnitus. Hear. Res. 2002;172:137–43. doi: 10.1016/s0378-5955(02)00575-0. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Kaltenbach JA, Wang J, Kim SA. Fos-like immunoreactivity in auditory and nonauditory brain structures of hamsters previously exposed to intense sound. Exp. Brain Res. 2003;153:655–60. doi: 10.1007/s00221-003-1612-4. [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Cartwheel and superficial stellate cells of the dorsal cochlear nucleus of mice: intracellular recordings in slices. J. Neurophysiol. 1993;69:1384–97. doi: 10.1152/jn.1993.69.5.1384. [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Neuronal circuits associated with the output of the dorsal cochlear nucleus through fusiform cells. J. Neurophysiol. 1994;71:914–30. doi: 10.1152/jn.1994.71.3.914. [DOI] [PubMed] [Google Scholar]