Abstract
It is well established that the neuropeptide oxytocin (OT) is involved in regulating social behavior, anxiety, and hypothalamic-pituitary-adrenal (HPA) axis physiology in mammals. Because individuals with major depression often exhibit functional irregularities in these measures, we test in this pilot study whether depressed subjects (n=11) exhibit dysregulated OT biology compared to healthy control subjects (n=19). Subjects were hospitalized overnight and blood samples were collected hourly between 1800 and 0900 h. Plasma levels of OT, the closely related neuropeptide argine-vasopressin (AVP), and cortisol were quantified. Results indicated that depressed subjects exhibit increased OT levels compared to healthy control subjects, and this difference is most apparent during the nocturnal peak. No depression-related differences in AVP or cortisol levels were discerned. This depression-related elevation in plasma OT levels is consistent with reports of increased hypothalamic OT-expressing neurons and OT mRNA in depressed patients. This present finding is likewise consistent with the hypothesis that dysregulated OT biology may be a biomarker of the emotional distress and impaired social relationships which characterize major depression. Additional research is required to elucidate the role of OT in the pathophysiology of this psychiatric disorder.
Keywords: cortisol, HPA axis, stress, vasopressin
1. Introduction
The neuropeptide oxytocin (OT) is synthesized in the hypothalamus and released into systemic circulation via the posterior pituitary. OT is also released into the brain via widely distributed oxytocinergic pathways, and OT receptors are found in a variety of socially-relevant and stress-sensitive brain regions (Gimpl and Fahrenholz, 2001). Central OT facilitates social contact between conspecifics, maternal-infant attachment, and pair-bond formation in a variety of mammals (Pedersen et al., 1992; Witt et al., 1992; Lim and Young, 2006). Centrally administered OT also possesses anxiolytic properties (Landgraf and Neumann, 2004), whereas OT gene deletion enhances anxiety (Amico et al., 2004) in rodents.
In addition to regulating social behavior and anxiety, OT is released into the brain and periphery in response to acute psychogenic stressors in mammals (Landgraf and Neumann, 2004; Onaka, 2004). Although the role of endogenous OT in stress biology is poorly understood, particularly under chronically stressful conditions, exogenously administered OT attenuates acute activation of the hypothalamic-pituitary-adrenal (HPA) axis in primates and rodents (Windle et al., 2004; Parker et al., 2005), the primary neuroendocrine mediator of the stress response.
Social abnormalities, enhanced anxiety, and dysregulated HPA axis physiology are frequently observed in major depression (Meyer et al., 2001; Parker et al., 2003). This evidence and preliminary findings reviewed below suggest that functional irregularities in OT biology may be involved in the pathophysiology of depressive disorders. Indeed, significant correlations between OT levels and depressive symptoms have been reported in patient populations with obsessive compulsive disorder (Swedo et al., 1992), fibromyalgia (Anderberg and Uvnas-Moberg, 2000), and major depression (Scantamburlo et al., 2007; Cyranowski et al., 2008). Greater numbers of OT-immunoreactive expressing neurons and higher levels of OT mRNA in the hypothalamus also have been found in patients with depressive disorders (Purba et al., 1996; Meynen et al., 2007).
Although promising, it is difficult to draw conclusions about the role of OT in major depression from these studies. This is because most of the prior research was conducted within a disordered population with no control group (Swedo et al., 1992; Scantamburlo et al., 2007), or comparisons were made between patients and control subjects, but patient groups were comprised of mixed populations (e.g., patients had either bipolar or major depression; patients had concomitant fibromyalgia and major depression) (Purba et al., 1996; Anderberg and Uvnas-Moberg, 2000). Another limitation of most of the neuroendocrine studies in humans is that they relied on collection and analysis of single biological samples, which precluded detailed analyses of OT levels across multiple time points.
The primary goal of this study is to begin to bridge the gaps in our understanding of the role of OT in major depression. Here we test whether depressed compared to healthy control subjects exhibit dysregulated OT biology by examining depression-related differences in plasma OT levels over a 16-h period. A secondary goal of this research is to examine whether depression-related changes in OT biology co-occur with changes in other stress-related hormones. These hormones include argine-vasopressin (AVP), a neuropeptide closely related to OT, and cortisol.
2. Methods
2.1 Subjects
Adult subjects were recruited through Stanford University Medical Center, as well as through online and print advertisements, as part of a larger study examining cortisol levels in depressive disorders (Keller et al., 2006). The present pilot study was initiated after the beginning of the larger parent study, and resulted in recruitment of 11 outpatient subjects (n=7 women; n=4 men) with major depression and 19 healthy control subjects (n=9 women; n=10 men). Subjects from these two groups differed on clinical, but not demographic, characteristics (Table 1). This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Stanford University. Informed written consent was obtained from all participants.
Table 1.
Demographic and clinical characteristic of depressed and healthy control subjects.
| Demographics | Depressed Subjects (N = 11) | Healthy Subjects (N = 19) | Analyses |
|---|---|---|---|
| Gender | |||
| Female | N = 7 | N = 9 | |
| Male | N = 4 | N = 10 | |
| Age (years) | 40.64 (± 14.71) | 34.26 (± 15.24) | t (28) = 1.12; p = 0.27 |
| Education (years) | 14.73 (± 2.10) | 15.45 (± 2.24) | t (28) = 0.72; p = 0.39 |
| Clinical Diagnosis | |||
| HDRS (21 Item) | 25.64 (± 3.26) | 0.26 (± 0.45) | t (28) = 33.75; p < 0.0001 |
| CES | 9.64 (± 1.63) | 0.05(± 0.23) | t (28) = 25.53; p < 0.0001 |
Mean ± S.D. presented for continuous variables
Depressed subjects were required to meet the following inclusion criteria: a score of 21 or higher on the 21-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960); and a score of at least 7 on the Thase Core Endogenomorphic Scale (Thase et al., 1983). Control subjects were required to score less than a 6 on the HDRS and have no history of Axis I disorders as determined by the Structured Clinical Interview for DSM-IV-TR (First et al., 1997). Depressed subjects were allowed to remain on psychiatric medications provided the dose had not been adjusted in the last week. Psychiatric medications included: antidepressants (n=6), concomitant anxiolytics and antidepressants (n=1), and no medication (n=4). Control subjects were free of psychiatric medications. Exclusion criteria were as follows: electroconvulsive shock therapy or substance abuse problems in the last six months, major medical illness, history of seizures, major head trauma, abnormal clinical laboratory tests, unstable or untreated hypertension, cardiovascular disease, endocrine disorders, pregnancy, lactation, or use of steroids.
2.2 Procedures
Subjects were admitted to the Stanford University Hospital General Clinical Research Center at 1500 h. At 1600 h, an intravenous line kept patent with saline infusion was started in one arm and 8 cc of blood were drawn into heparinized vacutainer tubes every hour from 1800 to 0900 h. Subjects were required to be supine in bed 15 min prior to each blood sample collection. No food or sleep restrictions were imposed. Blood samples were subsequently centrifuged and the plasma fraction was stored at −70° C prior to hormone quantification.
2.3 Plasma hormone quantification
Plasma OT and AVP were measured using established radioimmunoassays (Amico et al., 1981; Amico et al., 1985). The sensitivity of the OT and AVP assays in extracted plasma is 0.5 pg/ml. Cortisol was quantified using the Access Immunoassay System (Beckman Coulter, Chaska, MN). The sensitivity of this assay is 0.4 ug/dl (11 nmol/L). The intra- and inter-assay coefficients of variation for all assays are typically below 10%.
2.4 Statistical analyses
OT and AVP were analyzed with separate regression models, using first-order autoregressive structures to model the relationship among measurements over time with diagnostic group as a fixed effect (SAS Institute Inc., Cary, NC USA). Given the known diurnal rhythm of cortisol and the potential confounds of using a regression model (high variability in individual cortisol data points due to its pulsatile production), we instead fit the cortisol data to a single harmonic sine wave, with a fixed period of 24 hours, using a nonlinear least squares fitting analysis based upon the Levenberg-Marquardt method (Microcal Origin v.6.1, Microcal Software, Northampton, MA). The three coefficients in the equation represent the amplitude of the daily cortisol oscillation (½ peak-trough), the mesor, and the time of the mesor-crossing. Depression-related differences in cortisol parameters were then compared. Test statistics were evaluated with two-tail probabilities (α < 0.05) and descriptive statistics are presented below as mean ± S.D.
3. Results
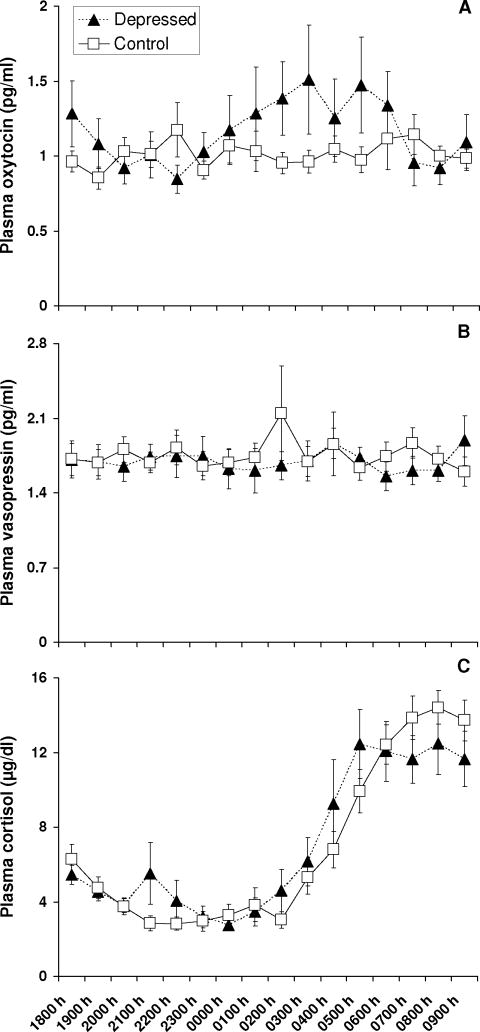
Depressed subjects exhibited significantly higher plasma OT concentrations compared to healthy control subjects (F1,102=4.46, P=0.037) (Figure 1). No depression-related differences in AVP levels were discerned (F1,102=0.07, P =0.788). There was no difference in the amplitude of cortisol between depressed and control subjects, as described by the fitted mesor (8.31 ± 1.58 μg/dl vs. 8.89 ± 2.22 μg/dl; t28=−0.76, P=0.456) or fitted amplitude (6.01 ± 1.41 μg/dl vs. 7.21 ± 1.74 μg/dl; t28=−1.95, P=0.062). The timing of the fitted peak of cortisol was also not different between depressed and control subjects (9:21 ± 4:53 vs. 10:39 ± 1:49; t12=−2.18, P=0.411).
Figure 1.
Plasma OT levels are elevated in individuals with major depression. Mean hourly plasma concentrations of oxytocin (panel A), vasopressin (panel B), and cortisol (panel C) collected between 1800 and 0900 h are presented for depressed (n=11) and healthy control (n=19) subjects.
4. Discussion
This experiment examined depression-related differences in plasma OT levels over a 16-h period. Results indicate that plasma OT levels are increased in depressed compared to healthy control subjects. Hyperoxytocinergic activity in the present study is similar to previous reports demonstrating that the number of OT-expressing neurons and amount of OT mRNA in the hypothalamus are increased in depressed compared to control individuals (Purba et al., 1996; Meynen et al., 2007). Although the relationship between peripheral and central oxytocin activity remains poorly understood in mammals, these systems exhibit functional coordination during certain states (Landgraf and Neumann, 2004), and the aforementioned neuroendocrine and neuroanatomical data suggest the intriguing possibility that major depression may be one of them.
Hourly plasma collection occurred in this study between 1800-0900 h as a means by which to repeatedly assess OT levels within the same individuals. Although there is doubt as to whether plasma OT exhibits circadian or diurnal rhythmicity (Amico et al., 1983), it is interesting to note that depression-related increases in plasma OT were most pronounced during the nocturnal period in which OT has been reported to peak (Forsling et al., 1998). Individual differences in depressed subjects’ plasma OT levels were also most apparent during this period. Whether or not disrupted sleep patterns in the depressed subjects contributed to these individual or group differences is unknown, and merits investigation. Additional research is also required to determine whether group differences in OT levels continue to be evident between 1000–1700 h.
Exactly why plasma OT levels are elevated in depressed individuals is unknown. One post hoc explanation, however, is that dysregulated OT biology is a biomarker of the emotional distress and impaired social relationships which characterize major depression. This hypothesis is supported by several studies: higher plasma OT levels are associated with impairments in social functioning in healthy (Taylor et al., 2006), anxious (Hoge et al., 2008), and depressed (Cyranowski et al., 2008) women, and greater hypothalamic OT cell numbers are associated with social isolation-induced anhedonia in prairie voles (Grippo et al., 2007).
Several limitations of this research should be considered. It is possible that variables such as gender, age, and medication status (e.g., oral contraceptives; antidepressants) may have affected endogenous hormone levels measured in this study (Amico et al., 1981; Forsling et al., 1998; Uvnas-Moberg et al., 1999; Kramer et al., 2004). However, this pilot study consisted of a small sample size that was not powered to examine the interaction effects of these variables with psychiatric status on plasma hormone levels. This study was also not powered to examine the relationships between hormone levels and both depressive symptoms and social functioning. Finally, because the depressed subjects in this study were outpatients and therefore less likely to exhibit hypercortisolemia (Peeters et al., 2004), we were unable to examine OT levels in the context of dysregulated HPA axis physiology. Research with more subjects, including hypercortisolemic patients, is required to test these important questions.
In conclusion this pilot study determined that individuals with major depression exhibit elevated OT levels compared to healthy control subjects. Elevated OT levels occurred in the absence of depression-related changes in cortisol and AVP levels, suggesting an effect independent of the neuroendocrine stress axis. Results from this study are consistent with the hypothesis that dysregulated OT biology may serve as a biomarker of social distress in major depression. The role of OT in the pathophysiology of major depression remains unknown and merits further investigation.
Acknowledgments
This research was supported by grants MH50604, MH66537, and RR-00070 from the National Institutes of Health, Bethesda, MD and The Pritzker Foundation, New York, NY. We gratefully acknowledge Hou Ming Cai for her technical assistance with the oxytocin and vasopressin assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amico JA, Seif SM, Robinson AG. Oxytocin in human plasma: correlation with neurophysin and stimulation with estrogen. Journal of Clinical Endocrinology and Metabolism. 1981;52:988–993. doi: 10.1210/jcem-52-5-988. [DOI] [PubMed] [Google Scholar]
- Amico JA, Tenicela R, Johnston J, Robinson AG. A time-dependent peak of oxytocin exists in cerebrospinal fluid but not in plasma of humans. Journal of Clinical Endocrinology and Metabolism. 1983;57:947–951. doi: 10.1210/jcem-57-5-947. [DOI] [PubMed] [Google Scholar]
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. Journal of Neuroendocrinology. 2004;16:319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Amico JA, Ervin MG, Leake RD, Fisher DA, Finn FM, Robinson AG. A novel oxytocin-like and vasotocin-like peptide in human plasma after administration of estrogen. Journal of Clinical Endocrinology and Metabolism. 1985;60:5–12. doi: 10.1210/jcem-60-1-5. [DOI] [PubMed] [Google Scholar]
- Anderberg UM, Uvnas-Moberg K. Plasma oxytocin levels in female fibromyalgia syndrome patients. Zeitschrift für Rheumatologie. 2000;59:373–379. doi: 10.1007/s003930070045. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosomatic Medicine. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Washington, DC: American Psychiatric Press; 1997. Patient Edition (SCID-I/P, 2/2001 revision) Edition. [Google Scholar]
- Forsling ML, Montgomery H, Halpin D, Windle RJ, Treacher DF. Daily patterns of secretion of neurohypophysial hormones in man: effect of age. Experimental Physiology. 1998;83:409–418. doi: 10.1113/expphysiol.1998.sp004124. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological Reviews. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic Medicine. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Pollack MH, Kaufman RE, Zak PJ, Simon NM. Oxytocin levels in social anxiety disorder. CNS Neuroscience and Therapeutics. 2008;14:165–170. doi: 10.1111/j.1755-5949.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Flores B, Gomez RG, Solvason HB, Kenna H, Williams GH, Schatzberg AF. Cortisol circadian rhythm alterations in psychotic major depression. Biological Psychiatry. 2006;60:275–281. doi: 10.1016/j.biopsych.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Cushing BS, Carter CS, Wu J, Ottinger M. Sex and species differences in plasma oxytocin using an enzyme immunoassay. Canadian Journal of Zoology. 2004;82:1194–1200. [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and Behavior. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Meyer SE, Chrousos GP, Gold PW. Major depression and the stress system: a life span perspective. Development and Psychopathology. 2001;13:565–580. doi: 10.1017/s095457940100308x. [DOI] [PubMed] [Google Scholar]
- Meynen G, Unmehopa UA, Hofman MA, Swaab DF, Hoogendijk WJ. Hypothalamic oxytocin mRNA expression and melancholic depression. Molecular Psychiatry. 2007;12:118–119. doi: 10.1038/sj.mp.4001911. [DOI] [PubMed] [Google Scholar]
- Onaka T. Neural pathways controlling central and peripheral oxytocin release during stress. Journal of Neuroendocrinology. 2004;16:308–312. doi: 10.1111/j.0953-8194.2004.01186.x. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Hormones and Behavior. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30:924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Peterson G, Walker CH, Mason GA. Oxytocin activation of maternal behavior in the rat. Annals of the New York Academy of Sciences. 1992;652:58–69. doi: 10.1111/j.1749-6632.1992.tb34346.x. [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J. Levels and variability of daily life cortisol secretion in major depression. Psychiatry Research. 2004;126:1–13. doi: 10.1016/j.psychres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Archives of General Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, Ansseau M, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Kruesi MJ, Rettew DC, Listwak SJ, Berrettini W, Stipetic M, Hamburger S, Gold PW, Potter WZ, Rapoport JL. Cerebrospinal fluid neurochemistry in children and adolescents with obsessive-compulsive disorder. Archives of General Psychiatry. 1992;49:29–36. doi: 10.1001/archpsyc.1992.01820010029004. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosomatic Medicine. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Thase ME, Hersen M, Bellack AS, Himmelhoch JM, Kupfer DJ. Validation of a Hamilton subscale for endogenomorphic depression. Journal of Affective Disorders. 1983;5:267–278. doi: 10.1016/0165-0327(83)90050-2. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Bjokstrand E, Hillegaart V, Ahlenius S. Oxytocin as a possible mediator of SSRI-induced antidepressant effects. Psychopharmacology (Berl) 1999;142:95–101. doi: 10.1007/s002130050867. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. Journal of Neuroscience. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt DM, Winslow JT, Insel TR. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacology Biochemistry and Behavior. 1992;43:855–861. doi: 10.1016/0091-3057(92)90418-f. [DOI] [PubMed] [Google Scholar]