Abstract
Objective
To investigate the relative predictive value of CD4+ metrics for serious clinical endpoints.
Design
Observational
Methods
Patients (3012; 20317 person-years) from control arms of ESPRIT and SILCAAT trials were followed prospectively. We used Cox regression to identify CD4+ metrics (latest, baseline and nadir CD4+count, latest CD4+%, time spent with CD4+count below certain thresholds and CD4+ slopes) independently predictive of i)all-cause mortality; ii) non-AIDS deaths; iii) non-AIDS (cardiovascular, hepatic, renal and non-AIDS malignancy) and iv) AIDS events. Akaike Information Criteria (AIC) was calculated for each model. Significant metrics (p<0.05) were then additionally adjusted for latest CD4+ count.
Results
Non-AIDS deaths occurred at a higher rate than AIDS deaths (rate-ratio: 6.48, 95%CI: 5.1–8.1) and similarly, non-AIDS events (rate-ratio: 1.72, 95%CI: 1.65–1.79). Latest CD4+count was strongly predictive of lower risk of death (HR per log2 rise: 0.48, 95%CI: 0.43–0.54), with lowest AIC of all metrics. CD4+ slope over 7-visits, after additional adjustment for latest CD4+count, was the only metric to be independent predictor for all-cause (HR for slope<-10/mm3/month vs. 0±10: 3.04, 95%CI: 1.98–4.67) and non-AIDS deaths (HR for slope <-10/mm3/month vs. 0±10: 2.62, 95%CI: 1.62–4.22). Latest CD4+ count (per log2 rise) was the best predictor across all endpoints (i–iv) and predicted hepatic (HR: 0.46, 95%CI: 0.33–0.63) and renal events (HR: 0.39, 95%CI: 0.21–0.70), but not cardiovascular events (HR: 1.05, 95%CI: 0.77–1.43) or non-AIDS cancers (HR: 0.78, 95%CI: 0.59–1.03).
Conclusion
Latest CD4+count is the best predictor of serious endpoints. CD4+ slope independently predicts all-cause and non-AIDS deaths.
Keywords: CD4+, CD4+ counts, serious non-AIDS events, immunodeficiency, AIDS
Introduction
The disease burden in HIV-infected population with adequate access to combination anti-retroviral therapy (cART) is increasingly due to serious non-AIDS events, with a lesser proportion being contributed by AIDS-related events.[1–4] These non-AIDS events (including cardiovascular, renal, and hepatic events and non-AIDS cancers) [2–9] are most likely to be multi-factorial in origin, with aging[10], high-risk behavior[11], co-infections[12] and cART toxicity[7] being contributing factors.
Recently, the role of immunodeficiency in the development of non-AIDS events has been investigated. In the SMART study, these events were more common in the arm with less cumulative exposure to cART and consequently, more time spent with incremental levels of immunodeficiency.[13–14] Subsequently, observational studies have been used to investigate whether any association exists between CD4+ count, HIV RNA and non-AIDS events.[15–18] The findings from these studies suggest that higher recent CD4+ count is strongly associated with a lesser risk of non-AIDS events as an composite end-point,[19] although results for specific event categories, such as non-fatal cardiovascular and renal events, have been equivocal.[19] However, traditional latest or recent CD4+ count levels do not fully reflect cumulative time spent in immunodeficiency or the rate of changes in CD4+ counts and only provide a snapshot of immunological status at a single time point. Recent studies suggest other aspects of immunodeficiency, such as time spent with CD4+ count below particular thresholds could be of predictive value.[14, 20] It is not known if these CD4+ metrics provide any additional predictive value, especially for non-AIDS events, than to that provided by latest absolute CD4+ count.
In the present study, we first define the relationship between latest CD4+ count and various serious clinical end-points in our cohort. We then examine whether CD4+ metrics other than latest (most recent) CD4+ count (namely baseline and nadir CD4+ count, CD4+%, CD4+ slopes over short and long term, and time spent with CD4+ count below particular thresholds) provide any additional explanatory effect for major event categories, than that provided by latest CD4+ count. We use data from the control arms of ESPRIT and SILCAAT trials,[21] which constitute a large heterogeneous group of HIV-infected patients, on cART for nearly a decade, with 4-monthly assessments and low rates of loss to follow-up, and well documented and validated serious clinical endpoints.
Methods
Study population
We analysed the pooled follow-up data for patients enrolled in the control arms of ESPRIT and SILCAAT trials. Details of the study design and primary results of both trials have been published elsewhere.[21–22] Briefly, 4111 HIV infected adults with CD4+ count ≥300/mm3 and 1971 with CD4+ count of 50 to 299/mm3 were enrolled in ESPRIT and SILCAAT trials respectively, and randomised equally to the control arm or interleukin-2 treatment arm. Both arms of each trial only included patients on cART. All patients were followed-up every 4 months for clinical assessment and the measurement of CD4+ count/mm3 and HIV RNA copies/mL.
Study Endpoint
The study endpoints were defined as follows: i) all cause mortality; ii) non-AIDS deaths; iii) non-AIDS events, which include fatal or non-fatal serious clinical events in one of the four broad categories: a) cardiovascular (CVD) including stroke, myocardial infarction, coronary artery disease (CAD) requiring procedure, other fatal heart/vascular events and sudden death, b) hepatic, including cirrhosis or liver failure, c) renal including end-stage renal disease or kidney failure and d) non-AIDS malignancy (excluding skin cancers); and iv) AIDS events.
An Endpoint Review Committee validated the underlying cause of death using the Coding of Death in HIV (CoDE) system.[23] Non-AIDS events were validated by the endpoint committee for the ESPRIT trial and were reported as grade 4 adverse events for the SILCAAT trial. Grade 4 events were defined as potentially life-threatening events requiring medical intervention according to the toxicity table of the Division of AIDS of the NIAID, and were coded according to the Medical Dictionary for Regulatory Activities (version 12.0).
Statistical analysis
We used Cox regression with time-updated variables to analyse the relationship between various CD4+ metrics and development of endpoints defined above. CD4+ metrics were defined as follows: i) latest CD4+ count correspond to the CD4+ count measured closest to the event. This metric was time-updated and analysed as both, a) categorical (as >500, 350–500, 200–350, 50–200 and <50 cells/mm3) and b) continuous variable log2 transformed (i.e. doubling) and per 100 cell rise. ii) Time-updated CD4+ percent as categorical variable (as >25%, 14–25% and <14%). iii) Time-updated CD4+ slope over 3 consecutive visits, as change in CD4+ count per month determined by linear regression. The regression slope was determined from three consecutive CD4+ counts (current (at time t) and past two CD4+ counts (at time t-1 and t-2)). A slope less than zero was interpreted as decline in CD4+ count and vice versa. The median time between two-visits was 3.5 months (IQR: 2.4–4.2). iv) Time-updated CD4+ slope for every 7 consecutive visits (averaging a time-span of approximately two years) using linear regression. We defined CD4+ counts to be at plateau if CD4+ slope lay within the bounds of ±10 cells/mm3 change per month. It indicates the stability of CD4+ count over a prolonged period, as opposed to increasing (> 10 cells rise per month) or decreasing CD4+ counts (>10 cells decrease per month) over that period. v) Time spent (per year) with CD4+ count below 200, below 100, and below 50/mm3, as time-updated variables. vi) Nadir CD4+ count as known at randomization vii) Baseline CD4+ count, as measured at randomization.
We analysed each of the above CD4+ metrics as predictors of each of the above defined endpoints in adjusted Cox models, stratified by trial type (ESPRIT or SILCAAT). Models were fitted for each CD4+ metric, a priori adjusted for variables available for both the trials which are known to be associated with non-AIDS endpoints or death. These were: sex, age, prior AIDS at baseline, ART duration at baseline, current ART class, region, race, and time-updated HIV RNA load (categorized as <=500, 500–10,000, and >10,000 copies/mL). For all time-updated variables, missing data were imputed by carrying forward (but not backward) the last observation, till the last follow-up date. Akaike information criteria (AIC) were then calculated to assess the fit of the each adjusted model (lower AIC indicates better fit). [24]
Following this, those CD4+ metrics significant in the adjusted models, two-sided α <0.05, were additionally adjusted for latest CD4+ count (as log2 transformed) to see if they provide any additional explanatory effect as to that provided by latest CD4+ count. Sensitivity analysis was performed by lagging CD4+ count and HIV RNA by 6-months for analyzing mortality related endpoints. We also tested for any interaction between HIV RNA category (<500 or >500 copies/mL) and latest CD4+ count.
Follow-up data were censored at the first of: lost to follow-up, date of death or the closing date of study (15 November 2008). Patients who met multiple endpoints were counted for each endpoint when they were considered separately. Findings are summarised as hazard ratios (HR) and 95% confidence intervals (CI). All analyses were performed using STATA (StataCorp, USA) version 10.
Results
Patient characteristics
There were a total of 3024 patients randomised to the control arms of ESPRIT and SILCAAT (2040 and 984, respectively), of which 3012 patients were included in the analysis. No follow-up data were available for 12 patients. The population at baseline was characterised as: 2488 (82.3%) male; median age of 41 years; predominantly of white race (n= 2315; 76. 8%); mean CD4+ count 400 cells/mm3; mean nadir CD4+ count 167cells/mm3; mean CD4+% 21.5%; 2438 (80.8%) had HIV RNA below the detection limit of 500 copies/mL; 847 (28%) had history of AIDS; and the mean cumulative duration of ART was 57.4 months (Table-1). Except for baseline CD4+ count, nadir CD4+ count and baseline CD4+%, which were higher in the ESPRIT patients, there were no meaningful differences in baseline characteristics between the two trials (see Table-1). Hepatitis B and C co-infection status and likely mode of HIV infection were not collected for SILCAAT patients and therefore these covariates were not used in any further analysis. The median follow-up time was 7-years (IQR: 6–7.76), providing 20317 person-years of follow-up.
Table 1.
Baseline Characteristics
| Characteristics | ESPRIT | SILCAAT | Overall |
|---|---|---|---|
| No. of patients | 2040 | 984 | 3024 |
| Gender-Male (%) | 1659 (81.0) | 829(84.0) | 2488(82.3) |
| Mean age in years(SD) | 41(9.1) | 42(8.7) | 41 (9.0) |
| Race/Ethnicity (%) | |||
| Asian | 220 (10.8) | 12(1.2) | 232(7.7) |
| Black | 180(8.9) | 90(9.1) | 270(9.0) |
| White | 1541(75.8) | 774(78.6) | 2315(76.8) |
| Other/Unknown | 90(4.4) | 108(11.0) | 198(6.6) |
| Mean CD4 count (SD) | 495 (169.3) | 201.7(72.0) | 400 (200.0) |
| Mean nadir CD4 count (SD) | 211(152.0) | 73.45(58.2) | 166.63 (144.6) |
| Mean CD4% at baseline (SD) | 24.84 (8.5) | 15 (6.0) | 21.5(9.0) |
| HIV RNA copies/mL (%) | |||
| ≤500 | 1637(80.4) | 801(81.5) | 2438(80.8) |
| 500–10,000 | 278(13.6) | 164(16.6) | 442(14.6) |
| >10,000 | 121(5.9) | 18(1.83) | 139(4.6) |
| History of Prior AIDS at baseline (%) | 541(26.5) | 306(31.2) | 847(28.0) |
| Mean ART duration in months (SD) | 56.5(38.4) | 59.4(42.8) | 57.4 (39.8) |
| ARV categories at baseline (%): | |||
| NRTI | 2000(98.2) | 971(98.8) | 2971(98.4) |
| NNRTI | 977(48.0) | 455(46.3) | 1432(47.4) |
| PI | 968(47.5) | 630(64.0) | 1598 (53.0) |
| Other | 13(0.6) | 3(0.3) | 16(0.5) |
| Likely Infection mode$ (%) | |||
| IV drug abuse | 205(10.0) | ||
| MSM | 1136(55.7) | ||
| Heterosexual sex | 766(37.5) | ||
| Blood products | 36(1.8) | ||
| Other | 37(1.8) | ||
| Unknown | 73(3.6) | ||
| Hep B Positive$(%) | 127(7.2) | ||
| Hep C Positive$(%) | 268(15.6) | ||
These variables were not available for SILCAAT trial and were therefore not included as covariates in Cox regression analysis. SD= Standard deviation.
CD4= CD4+ T-cells/mm3, unless otherwise stated.
The rate per 1000 person-years for each endpoint was: 9.89 for all-cause mortality, 1.32 for AIDS-related deaths, 8.56 for non-AIDS deaths, 6.70 for AIDS events and 11.53 for non-AIDS events. The rates were higher for non-AIDS deaths (as compared to AIDS deaths; overall rate-ratio: 6.48, 95%CI 5.1–8.1) and for non-AIDS events (as compared to AIDS events; overall rate-ratio: 1.72, 95%CI 1.65–1.79) in both the trials and overall.
Association of CD4+ metrics with all-cause mortality
There were a total of 201 deaths overall. Adjusted for key covariates, latest CD4+ count was significantly predictive, with the risk halving per doubling of latest CD4+ count (HR per log2 rise: 0.48, 95%CI 0.43–0.54) (Table-2). Baseline CD4+ count (HR per 100 cells/mm3 rise: 0.77, 95%CI 0.68–0.89); nadir CD4+ count (HR per 100 cells/mm3 rise:0.84, 95%CI 0.73–0.97); latest CD4+% <14% (HR 2.42, 95%CI 1.55–3.78 vs. CD4+% >25%); time (per year) spent with CD4+ count below 200 (HR 1.29, 95%CI 1.18–1.41); below 100 (HR 1.35, 95%CI 1.20–1.51); and below 50 cells/mm3 (HR 1.45, 95%CI 1.12–1.87) were all significant individual predictors (adjusted for other covariates) (Table-2). Also, time-updated CD4+ slope over 7-consecutive visits was significantly associated with risk of death (HR for slope<-10/mm3/month: 3.32, 95%CI: 2.14–5.15 vs. CD4+ slope= 0±10/mm3, i.e. plateau).
Table-2.
All cause death
| Adjusted analysis * | AIC | ||||
|---|---|---|---|---|---|
| Predictors | HR | p | p for trend | 95% CI | |
| Latest CD4 per log2 rise(i.e. doubling) | 0.48 | <0.001 | 0.43–0.54 | 2512.65 | |
| Latest CD4>500 | ref | ||||
| 350–500 | 2.17 | 0.002 | <0.001 | 1.33–3.54 | 2510.90 |
| 200–350 | 3.19 | <0.001 | 1.93–5.29 | ||
| 50–200 | 13.38 | <0.001 | 7.94–22.52 | ||
| <50 | 37.26 | <0.001 | 18.44–75.30 | ||
| Latest CD4 per 100 cells rise | 0.58 | <0.001 | 0.52–0.64 | 2517.07 | |
| Baseline CD4 per 100 cell rise | 0.77 | <0.001 | 0.68–0.89 | 2623.46 | |
| Latest CD4% >25 | ref | ||||
| CD4% 14–25 | 1.14 | 0.494 | <0.001 | 0.78–1.66 | 2622.98 |
| CD4% <14 | 2.42 | <0.001 | 1.55–3.78 | ||
| Nadir CD4 count per 100 cell rise | 0.84 | 0.020 | 0.73–0.97 | 2632.66 | |
| CD4 slope over 3 consecutive visits | |||||
| >0 | ref | ||||
| <=0 | 1.33 | 0.058 | 0.016 | 0.016 | 2634.31 |
| CD4 slope over 7 consecutive visits | |||||
| ±10 (i.e. plateau) | ref | ||||
| <-10 cells/mm3 per month | 3.32 | <0.001 | 0.004 | 2.14–5.15 | 2614.92 |
| >10 cells/mm3 per month | 0.91 | 0.789 | 0.46–1.80 | ||
| Time spent (per year) with CD4<200 | 1.29 | <0.001 | 1.18–1.41 | 2607.44 | |
| Time spent (per year) with CD4<100 | 1.35 | <0.001 | 1.20–1.51 | 2619.71 | |
| Time spent (per year) with CD4<50 | 1.45 | 0.004 | 1.12–1.87 | 2633.41 | |
| Effect of addition of significant CD4+ metrics** | |||||
| Model1 | |||||
| Baseline CD4 per 100 cell rise | 0.96 | 0.573 | 0.84–1.09 | ||
| Model2 | |||||
| Latest CD4% >25 | ref | ||||
| CD4% 14–25 | 0.83 | 0.361 | 0.438 | 0.57–1.22 | |
| CD% <14 | 0.75 | 0.296 | 0.44–1.27 | ||
| Model3 | |||||
| CD4 slope over 7 consecutive visits | |||||
| ±10 (i.e. plateau) | ref | ||||
| <-10 cells/mm3 per month | 3.04 | <0.001 | 0.004 | 1.98–4.67 | |
| >10 cells/mm3 per month | 1.55 | 0.210 | 0.77–3.10 | ||
| Model4 | |||||
| Nadir CD4 count per 100 cell rise | 0.97 | 0.693 | 0.84–1.11 | ||
| Model5 | |||||
| Time spent (per year) with CD4<200 | 1.03 | 0.529 | 0.93–1.14 | ||
| Model6 | |||||
| Time spent (per year) with CD4<100 | 1.01 | 0.881 | 0.87–1.16 | ||
| Model7 | |||||
| Time spent (per year) with CD4<50 | 0.89 | 0.617 | 0.58–1.37 | ||
CD4= CD4+ T-cells/mm3, unless otherwise stated.
Stratified by trial and adjusted for gender, age, prior aids at baseline, ART duration at baseline, current ART class, region, race, HIV RNA (as time-updated variable). AIC= Akaike information criteria.
Each model is the adjusted model for log2 rise in CD4+ (highlighted above) after addition of each of the significant CD4+ metrics above. Log2 rise in CD4+ remained statistically significant in all models (1–7) at p<0.001.
When CD4+ metrics significant in the adjusted models were additionally adjusted for latest CD4+ count, only CD4+ slope over 7-consecutive visits remained statistically significant (HR for slope<-10/mm3/month vs. plateau: 3.04, 95%CI 1.98–4.67) (Models 1–7 in Table-2). The results did not change appreciably when latest CD4+ count and HIV RNA were lagged by 6-months (not shown).
Association of CD4+ metrics with non-AIDS deaths
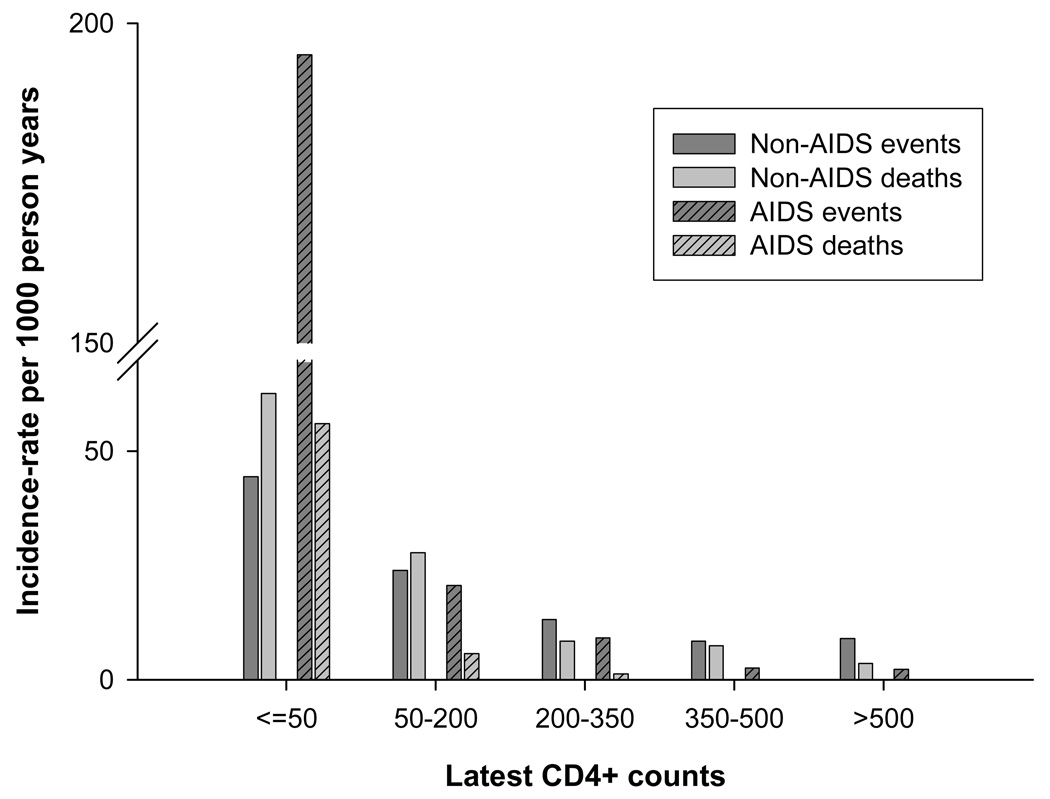
There were a total of 174 deaths due to non-AIDS causes (30 due to cardiovascular causes, 19 due to hepatic causes, 39 due to non-AIDS cancers, 14 due to non-AIDS infections, 16 due to suicides or accidents and 56 due to other/unknown causes) and 27 deaths due to AIDS. Besides latest CD4+ count (HR per log2 rise: 0.53, 95%CI 0.46–0.60), baseline CD4+ count, latest CD4%, CD4+ slope over 7-consecutive visit, and time spent (per year) with CD4+ count below 200 and 100/mm3 were other significant predictors, with lowest AIC for latest CD4+ count (data not shown). However, only CD4+ slope over 7-consecutive visits retained significance (HR for slope <-10/mm3/month vs. plateau: 2.62, 95% CI 1.62–4.22) when they were additionally adjusted for latest CD4+ count. When rates of AIDS and non-AIDS deaths were compared across different CD4+ categories, non-AIDS deaths occurred at higher rates in all CD4+ categories, with the difference more marked in higher CD4+ categories (Figure-1).
Figure-1. Rates by latest CD4+ counts.
Rates of non-AIDS events and deaths and AIDS events and deaths by CD4+ category. Non-AIDS events include fatal or non-fatal events only in one of the four categories: cardiovascular, renal, hepatic and non-aids malignancy.
Association of CD4+ metrics with non-AIDS events
There were a total of 226 non-AIDS events (95 cardiovascular events, 95 non-AIDS cancers, 28 hepatic and 8 renal events). In the adjusted models, latest CD4+ count was strongly associated with non-AIDS events as an endpoint (HR per log2 rise: 0.73, 95%CI 0.62–0.86). Time (per year) spent with CD4+ count below 200 (HR 1.14, 95%CI 1.02–1.27), below 100 (HR 1.30, 95% CI 1.10–1.53) and below 50 cells/mm3 (HR 1.80, 95%CI 1.05–3.08) were other significant predictors in the adjusted model (Table 3). However, they were no longer significant when the models were additionally adjusted for latest CD4+ count (Models 1–3 in Table 3). Other CD4+ metrics were not found to be significant predictors of non-AIDS events (Table 3).
Table-3.
Non-AIDS events
| Adjusted Analysis* | AIC | ||||
|---|---|---|---|---|---|
| Predictors | HR | p | p for trend | 95%CI | |
| Latest CD4 per log2 rise(i.e. doubling) | 0.73 | <0.001 | 0.62–.86 | 3000.46 | |
| Latest CD4>500 | ref | ||||
| 350–500 | 0.79 | 0.246 | 0.001 | 0.53–1.17 | 2997.93 |
| 200–350 | 1.20 | 0.371 | 0.80–1.79 | ||
| 50–200 | 2.11 | 0.003 | 1.29–3.45 | ||
| <50 | 4.81 | 0.001 | 1.89–12.25 | ||
| Latest CD4 per 100 cells rise | 0.93 | 0.073 | 0.86–1.00 | 3009.88 | |
| Baseline CD4 per 100cell rise | 0.97 | 0.573 | 0.87–1.07 | 3012.93 | |
| Latest CD% >25 | ref | ||||
| CD4% 14–25 | 1.26 | 0.162 | 0.161 | 0.90–1.76 | 3013.33 |
| CD% <14 | 1.49 | 0.072 | 0.96–2.32 | ||
| Nadir CD4 count per 100 rise | 0.93 | 0.286 | 0.81–1.06 | 3011.62 | |
| CD4 slope over 3 consecutive visits | |||||
| >0 | ref | ||||
| <=0 | 0.95 | 0.745 | 0.413 | 0.72–1.25 | 3008.02 |
| CD4 slope over 7 consecutive visits | |||||
| ±10 (i.e. Plateau) | ref | ||||
| <-10 cells/mm3 per month | 1.25 | 0.500 | 0.345 | 0.64–2.43 | 3016.04 |
| >10 cells/mm3 per month | 1.08 | 0.784 | 0.62–1.88 | ||
| Time spent (per year) with CD4<200 | 1.14 | 0.015 | 1.02–1.27 | 3007.72 | |
| Time spent (per year) with CD4<100 | 1.30 | 0.002 | 1.10–1.53 | 3005.93 | |
| Time spent (per year) with CD4<50 | 1.80 | 0.030 | 1.05–3.08 | 3010.03 | |
| Effect of addition of significant CD4+ metrics** | |||||
| Model1 | |||||
| Time spent (per year) with CD4<200 | 1.04 | 0.453 | 0.92–1.18 | ||
| Model2 | |||||
| Time spent (per year) with CD4<100 | 1.16 | 0.129 | 0.95–1.41 | ||
| Model3 | |||||
| Time spent (per year) with CD4<50 | 1.29 | 0.416 | 0.69–2.38 | ||
CD4= CD4+ T-cells/mm3, unless otherwise stated.
Stratified by trial and adjusted for gender, age, prior aids at baseline, ART duration at baseline, current ART class, region, race, HIV RNA (as time-updated variable). AIC= Akaike information criteria.
Each model is the adjusted model for log2 rise in CD4+ (highlighted above) after addition of each of the significant CD4+ metrics above. Log2 rise in CD4+ remained statistically significant in all models (1–3) at p<0.004.
In comparison, for AIDS events (n=132), latest CD4+ count, baseline CD4+ count, nadir CD4+ count, CD4+%, CD4+ slope over 3-visits and time (per year) spent with CD4+ count below 200,below 100 and below 50/mm3 were all found to be significant predictors in the adjusted model, with lowest AIC for latest CD4+ count (data not shown). Only CD4+% <14% retained significance when additionally adjusted for latest CD4+ count. When rates of AIDS and non-AIDS events were compared across different CD4+ categories, non-AIDS events and AIDS events occurred nearly at equal rates in higher CD4+ categories (Figure-1).
Association of latest CD4+ count with specific non-AIDS sub-categories
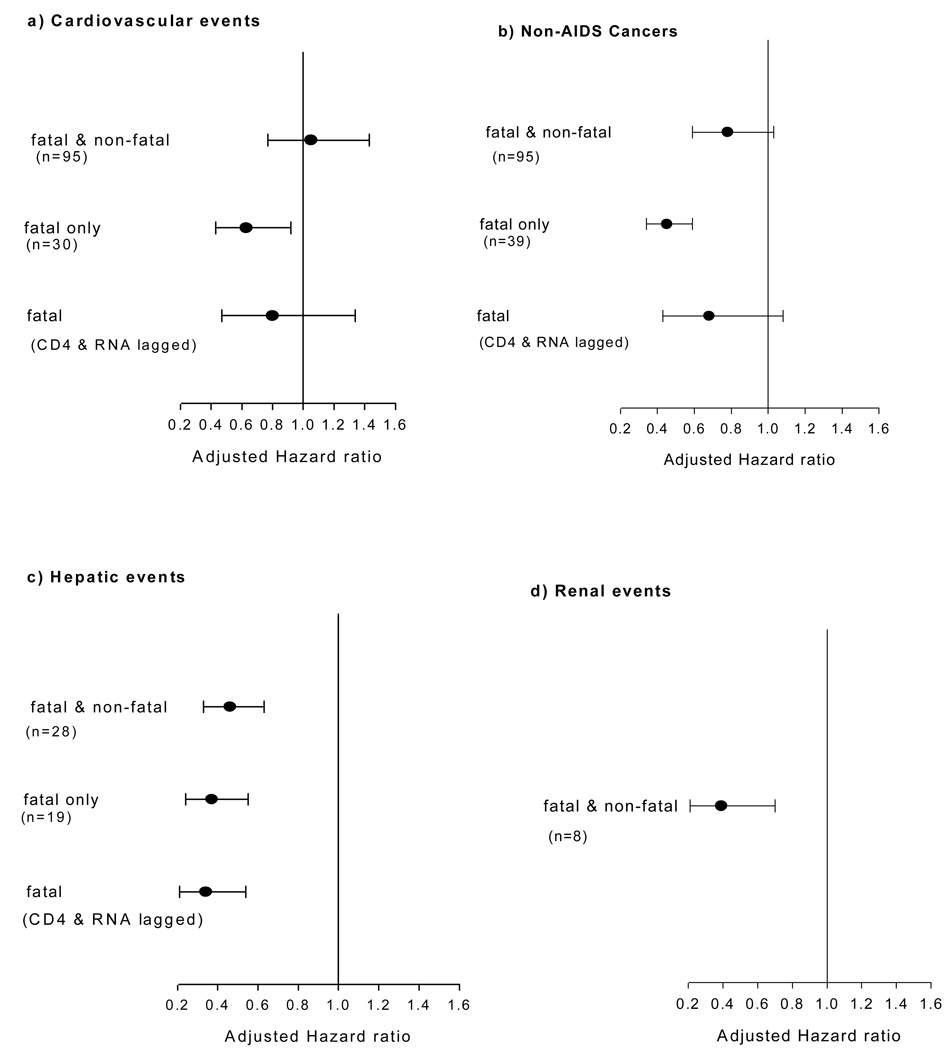
The risk of fatal or non-fatal cardiovascular events was not significantly associated with latest CD4+ count (adjusted HR per log2 rise: 1.05, 95%CI 0.77–1.43) (Figure-2a). When only fatal cardiovascular events were considered, risk was 37% lower (adjusted HR per log2 rise in CD4+: 0.63, 95%CI 0.43–0.92). However, the association was no longer significant when CD4+ count and HIV RNA were lagged by 6-months (Figure-2a).
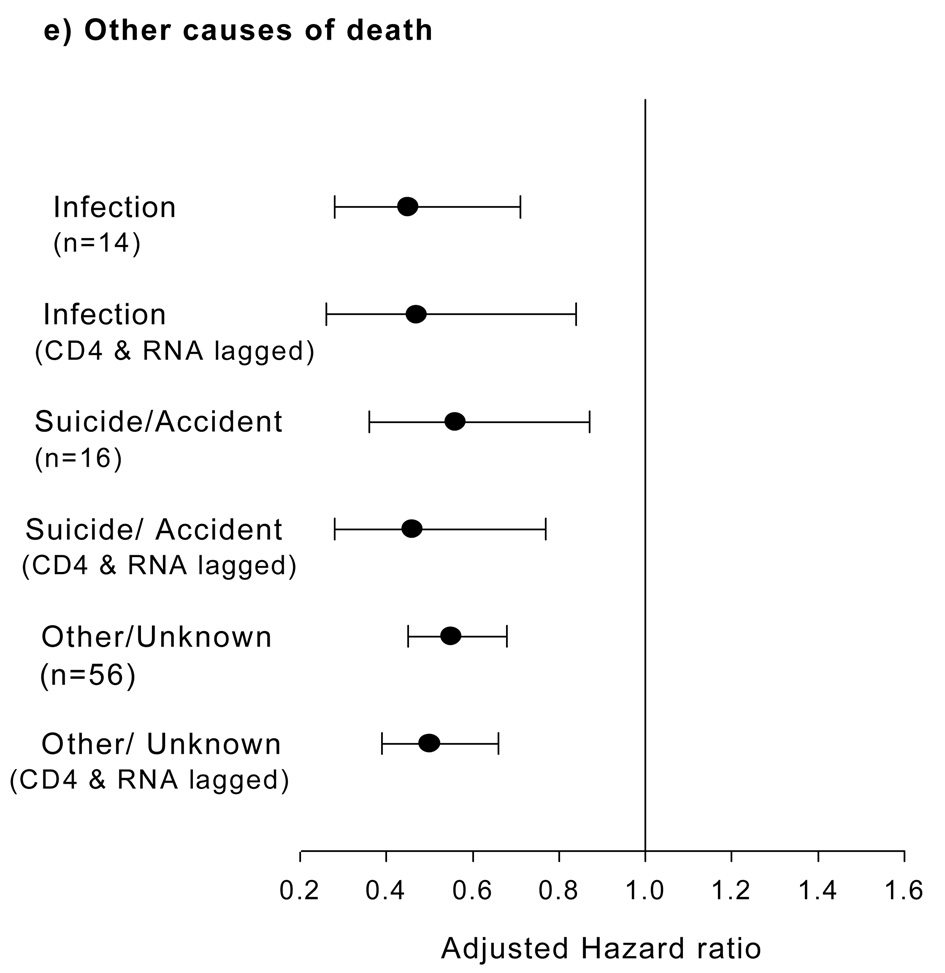
Figure-2. Latest CD4+ counts and specific non-AIDS events categories.
(a to e): Adjusted Hazard ratios (per log2 rise in CD4+ counts, with 95% CI) for the association between latest CD4 count and categories of serious non-AIDS diseases. For fatal events, CD4+ and RNA were lagged by 6-months in the model and adjusted hazard per log2 rise in CD4+ counts plotted.
There were a total of 95 non-AIDS cancers. These included respiratory tract (n=13), anal (n=10), other gastrointestinal (n=9), prostatic (n=8), lip and oropharyngeal (n=7), laryngeal (n=5), hepatic (n=4) and breast (n=4) neoplasms and 13 were of unknown/unspecified type. The risk of fatal or non-fatal non-AIDS cancers tended to be lower per log2 rise in CD4+ count, but was not significant (adjusted HR per log2 rise: 0. 78, 95%CI 0.59–1.03). Also, association between CD4+ count and fatal non-AIDS cancers lost its significance when CD4+ count and HIV RNA were lagged by 6 months (Figure-2b).
Risk of serious hepatic events was significantly associated with latest CD4+ count (adjusted HR per log2 rise: 0.46, 95%CI 0.33–0.63) and this significance did not diminish when CD4+ count and HIV RNA were lagged by 6 months while analyzing fatal hepatic events (Figure-2c). Although there were only 8 renal events, their risk was significantly associated with latest CD4+ count (adjusted HR per log2 rise: 0.39, 95%CI: 0.21–0.70) (Figure-2d).
Latest CD4+ count was significantly associated with risk of all remaining causes of non-AIDS death, even when CD4+ count and HIV RNA were lagged by 6-months (Figure-2e).
Among the other covariates included in the model adjusted for latest CD4+ count, age was an independent predictor of all-cause mortality, non-AIDS deaths and non-AIDS events, and HIV RNA was an independent predictor of AIDS events (data not shown). We did not find significant interaction between HIV RNA and latest CD4+ count for all the endpoints (p=0.41 for all-cause mortality); due possibly to the fact that more than 80% patients had undetectable HIV RNA at baseline and for most of the follow-up time. Results were therefore not stratified by HIV RNA category.
Discussion
In assessing the relationship between various CD4+ metrics and the risk of serious clinical end-points, including all-cause mortality, non-AIDS deaths, fatal or non-fatal non-AIDS and AIDS events; we found that latest absolute CD4+ count was the best predictor across all the endpoints. Negative CD4+ slope over approximately 2-years was also independently associated with higher risk of all-cause and non-AIDS deaths, even after additional adjustment for latest CD4+ count.
Our findings confirm that in the cART era, non-AIDS events dominate the disease burden in HIV-infected population and their risk is related to the degree of immunodeficiency, even in the cohort which had undetectable HIV RNA levels for most of the follow-up time. In our cohort, lower latest CD4+ count was significantly associated with higher risk of non-AIDS events or deaths. Also, the risk of non-AIDS events tended to increase with more time spent in lower CD4+ categories. These findings are consistent with similar studies conducted in other cohorts.[15, 18–19] A recent analysis which pooled the ESPRIT and SMART cohorts and focused on risk of death after AIDS and non-AIDS events, did not find a significant association between latest CD4+ count and non-AIDS events, due possibly to the greater number of CVD events and non-AIDS malignancies in those studies, as well as the higher CD4+ counts (>300/mm3) at baseline.[25] In our study, patients with latest CD4+ count 350–500/mm3 were not found to be at higher risk of non-AIDS events, as compared to those with latest CD4+ count>500/mm3.
Nadir CD4+ count was not associated with the risk of non-AIDS events or deaths. This finding is consistent with DAD study which reported no association between nadir CD4+ count and the risk of non-AIDS malignancies[26]; and with study that pooled the ESPRIT and SMART cohorts, which reported only borderline association with non-AIDS events.[25] This suggests that the risk of these events may not be related to severity of past immunodeficiency. CD4+ plateau was considered as some patients are known to attain stability in their CD4+ count after approximately 3.5 years of cART.[27] It was found that negative CD4+ slope (an overall ongoing decline in CD4+ count) over approximately two years was independently associated with higher risk of all-cause and non-AIDS deaths, as compared to CD4+ plateau. This finding further suggests that relatively recent immunodeficiency may have a role in non-AIDS deaths. However, CD4+ slope was not found to be a better predictor of endpoints, than latest CD4+ counts. This is consistent with a recent CASCADE analysis of therapy-naïve individuals.[28] Other CD4+ metrics did not provide any additional explanatory effect than to that provided by latest CD4+ count.
Latest CD4+ count was significantly associated with the risk of hepatic and renal events, and this finding is consistent with that of other observational studies.[18–19, 29–30] This association remained even after we lagged the CD4+ count and HIV RNA by 6 months for fatal events, thereby confirming the role of immunodeficiency in these events, besides other factors such as co-infections. The risk of fatal or non-fatal non-AIDS cancers was not found to be associated with latest CD4+ count level. Further, any significant association between fatal non-AIDS cancers and CD4+ count was lost when CD4+ count and HIV RNA levels were lagged by 6 months. This is in contrast to findings from other observational studies, which reported a significant association between recent CD4+ count and non-AIDS malignancies.[15, 18, 26] This discrepancy could be due to lack of sufficient number of events (and therefore the power) in our analysis. Alternatively, our cohort may have experienced those non-AIDS cancers less associated with immunodeficiency. Non-AIDS cancers related to the infectious cause are most likely related to immunodeficiency.[31] However, we did not have enough events in specific subtypes to formally evaluate this hypothesis.
We did not find significant association between CD4+ count and cardiovascular events, which is consistent with other studies[18–19]. Although some association existed for fatal cardiovascular events, this was lost when we lagged the CD4+ count and HIV RNA by 6 months, thereby suggesting that CD4+ count may have decreased due to the illness, rather than vice versa. Increased rate of cardiovascular and possibly other non-AIDS events, in HIV-infected population could be due to subtle ongoing inflammatory process stimulated by residual viral replication[32–33] or the treatment.[7] The subclinical inflammation may not be best reflected by latest CD4+ count. More specific inflammation and coagulation biomarkers, such as IL-6, D-dimer, and C-reactive protein, may prove to be better predictors of these events.[33–34] Further research in this area should focus on elucidating the role of these, and possibly newer, biomarkers in predicting various non-AIDS events in HIV infected population. This would not only provide new clinical tools for predicting these events, but also provide better insight into their pathogenesis.
The strengths of our analysis include a large heterogeneous group of patients with wide range of baseline CD4+ counts, long term follow-up (7 years), low rates of loss to follow-up,[21] prospective validation of even non-fatal non-AIDS events (especially for ESPRIT trial, which was in majority) and a significant number of nonfatal non-AIDS events. The three main limitations to this analysis include the following. Firstly, some risk factors for non-AIDS events, including some behavioral risk factors, were not collected on all patients and hepatitis B and C status, and likely mode of HIV infection were not documented for the SILCAAT patients. Our inability to adjust for co-infection status is especially important, since co-infection is known to have a detrimental effect on CD4+ count[35] and also, may in part, be responsible for liver related events. Secondly, we could not compare the predictive value of various CD4+ metrics for specific non-AIDS sub-categories, due mainly to the smaller number of events and therefore, the lack of power. Lastly, these findings are based on control arms of two clinical trials, and therefore may not be as representative of participants in some observational cohorts.
In summary, we confirm the association between latest CD4+ count and non-AIDS events in HIV infected population on stable cART. We also showed that among various CD4+ metrics, latest CD4+ count is the best predictor of non-AIDS deaths and events. Inducing CD4+ proliferation by immune-based therapies, such as IL-2, has not provided any clinical benefit.[21] Whether or not other strategies that focus on CD4+ T cells separately from impacting viral load would be of benefit remains to be determined.
Also, there is currently considerable debate regarding the best time to initiate antiretroviral therapy for HIV infection. Since the differences in absolute risk of non-AIDS events between higher CD4 strata are rather low; even a small risk from antiretroviral treatment (especially for CVD events) could offset the absolute gain. The only definitive way to asses this is via randomized trial. The results from the START trial, which is an ongoing randomised study investigating whether starting cART at CD4+ count above 500/mm3 provide any additional benefit as compared to starting cART at CD4+ count ≤350/mm3, are likely to provide important answers to these questions.
Acknowledgements
The writing group acknowledges the efforts of thousands of patients, the many ESPRIT and SILCAAT investigators who collected these data, the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) Executive Committee (JD Neaton, D Abrams, A Babiker, J Baxter, DA Cooper, CJ Cohen, D Cohn, JH Darbyshire, W El-Sadr, S Emery, F Gordin, HC Lane, G Larson, MH Losso, JD Lundgren, J Nadler, AN Phillips) for their oversight of the ESPRIT study, and the SILCAAT Scientific Committee (Y Lévy, D Abrams, A Babiker, P Cahn, B Clotet, N Clumeck, DA Cooper, JH Darbyshire, S Emery, U Hengge, HC Lane, J Lange, G Levi, JD Lundgren, R Mitsuyasu, JD Neaton, JP Routy, G Tambussi) for their oversight of the SILCAAT study.
ESPRIT was supported by grants U01 AI46957 and U01 AI068641 from the National Institute of Allergy and Infectious Diseases (NIAID). SILCAAT was supported by Chiron and Novartis.
Funding source: ESPRIT was supported by grants U01 AI46957 and U01 AI068641 from the National Institute of Allergy and Infectious Diseases (NIAID). SILCAAT was supported by Chiron and Novartis. None of the funding bodies had any role in the proposal, analysis, and interpretation of the data or preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution
Amit C Achhra performed statistical analysis and drafted the first manuscript; Janaki Amin, Matthew G Law, Sean Emery and David A Cooper contributed to design of the study, review of the results and critical review of the manuscript; and James D Neaton, Fred M Gordin, Michael J Vjecha and Jan Gerstoft each contributed review of the results and provided critical revision to the manuscript.
References
- 1.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. EuroSIDA Study Group. Changing patterns of mortality across Europe in patients infected with HIV-1. Lancet. 1998;352:1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 2.Pacheco AG, Tuboi SH, Faulhaber JC, Harrison LH, Schechter M. Increase in non-AIDS related conditions as causes of death among HIV-infected individuals in the HAART era in Brazil. PLoS One. 2008;3:e1531. doi: 10.1371/journal.pone.0001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 4.Smit C, Geskus R, Walker S, Sabin C, Coutinho R, Porter K, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20:741–749. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 5.Burgi A, Brodine S, Wegner S, Milazzo M, Wallace MR, Spooner K, et al. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer. 2005;104:1505–1511. doi: 10.1002/cncr.21334. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi AK, Pfeiffer RM, Chang L, Goedert JJ, Biggar RJ, Engels EA. Elevated risk of lung cancer among people with AIDS. AIDS. 2007;21:207–213. doi: 10.1097/QAD.0b013e3280118fca. [DOI] [PubMed] [Google Scholar]
- 7.Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 8.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz EJ, Szczech LA, Ross MJ, Klotman ME, Winston JA, Klotman PE. Highly active antiretroviral therapy and the epidemic of HIV+ end-stage renal disease. J Am Soc Nephrol. 2005;16:2412–2420. doi: 10.1681/ASN.2005040340. [DOI] [PubMed] [Google Scholar]
- 10.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 11.Nahvi S, Cooperman NA. Review: the need for smoking cessation among HIV-positive smokers. AIDS Educ Prev. 2009;21:14–27. doi: 10.1521/aeap.2009.21.3_supp.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torti C, Lapadula G, Uccelli MC, Quiros-Roldan E, Regazzi M, Ladisa N, et al. Influence of viral chronic hepatitis co-infection on plasma drug concentrations and liver transaminase elevations upon therapy switch in HIV-positive patients. Int J Antimicrob Agents. 2007;29:185–190. doi: 10.1016/j.ijantimicag.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 13.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 14.Inferior Clinical Outcome of the CD4+ Cell Count-Guided Antiretroviral Treatment Interruption Strategy in the SMART Study: Role of CD4+ Cell Counts and HIV RNA Levels during Follow-up. Journal of Infectious Diseases. 2008;197:1145–1155. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 15.Baker JV, Peng G, Rapkin J, Abrams DI, Silverberg MJ, MacArthur RD, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruyand M, Thiebaut R, Lawson-Ayayi S, Joly P, Sasco AJ, Mercie P, et al. Role of uncontrolled HIV RNA level and immunodeficiency in the occurrence of malignancy in HIV-infected patients during the combination antiretroviral therapy era: Agence Nationale de Recherche sur le Sida (ANRS) CO3 Aquitaine Cohort. Clin Infect Dis. 2009;49:1109–1116. doi: 10.1086/605594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan RC, Kingsley LA, Gange SJ, Benning L, Jacobson LP, Lazar J, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith C the D:A:D Study Group. Association between Modifiable and Non-modifiable Risk Factors and Specific Causes of Death in the HAART Era: The Data Collection on Adverse Events of Anti-HIV Drugs Study. 16th Conference on Retroviruses and Opportunistic infections; Montreal. 2009. Abstract 145. [Google Scholar]
- 19.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22:2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kesselring A, Gras L, Smit C, Wolf Fd, Reiss P, Wit F. Longer duration of exposure to immunodeficiency and detectable viremia both are risk factors for non-AIDS defining malignancies in HIV-1 infected patients on combination antiretroviral therapy [abstract WEAB104]. International AIDS society (IAS) Conference; Cape Town, SA. 2009. [Google Scholar]
- 21.Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, et al. Interleukin-2 Therapy in Patients with HIV Infection. N Engl J Med. 2009;361:1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emery S, Abrams DI, Cooper DA, Darbyshire JH, Lane HC, Lundgren JD, et al. The evaluation of subcutaneous proleukin (interleukin-2) in a randomized international trial: rationale, design, and methods of ESPRIT. Control Clin Trials. 2002;23:198–220. doi: 10.1016/s0197-2456(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 23.Lifson AR, Belloso WH, Carey C, Davey RT, Duprez D, El-Sadr WM, et al. Determination of the underlying cause of death in three multicenter international HIV clinical trials. HIV Clin Trials. 2008;9:177–185. doi: 10.1310/hct0903-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akaike H. A new look at the statistical model identification. IEEE Transaction on Automatic Control. 1974;19:716–723. [Google Scholar]
- 25.Neuhaus J, Angus B, Kowalska JD, La Rosa A, Sampson J, Wentworth D, et al. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV. AIDS. 2010;24:697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monforte A, Abrams D, Pradier C, Weber R, Reiss P, Bonnet F, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS. 2008;22:2143–2153. doi: 10.1097/QAD.0b013e3283112b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarwater PM, Margolick JB, Jin J, Phair JP, Detels R, Rinaldo C, et al. Increase and plateau of CD4 T-cell counts in the 3(1/2) years after initiation of potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:168–175. doi: 10.1097/00126334-200106010-00012. [DOI] [PubMed] [Google Scholar]
- 28.Wolbers M, Babiker A, Sabin C, Young J, Dorrucci M, Chene G, et al. Pretreatment CD4 cell slope and progression to AIDS or death in HIV-infected patients initiating antiretroviral therapy--the CASCADE collaboration: a collaboration of 23 cohort studies. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000239. e1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 30.O Kirk AM, d'Arminio Monforte A, Eg Hansen A-B, Gatell JM, Caplinskas S, Fätkenheuer G, Reiss Peter, Vinogradova E, Lundgren JD the EuroSIDA Study Group. Deterioration of Renal Function Associated with Current Level of Immunodeficiency. 15th Conference on Retroviruses and Opportunistic Infections; Boston, USA. 2008. Abstract 971. [Google Scholar]
- 31.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 32.Aberg JA. Cardiovascular complications in HIV management: past, present, and future. J Acquir Immune Defic Syndr. 2009;50:54–64. doi: 10.1097/QAI.0b013e31818ceaa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundgren JDea. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS. 2008;22:7. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller MF, Haley C, Koziel MJ, Rowley CF. Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: a meta-analysis. Clin Infect Dis. 2005;41:713–720. doi: 10.1086/432618. [DOI] [PubMed] [Google Scholar]