Abstract
Endogenous neurosteroids and their synthetic analogs (neuroactive steroids) are potent modulators of GABAA receptors. Thus, they are of physiological and clinical relevance for their ability to modulate inhibitory function in the central nervous system. Despite their importance, fundamental issues of neurosteroid actions remain unresolved. Recent evidence suggests that glutamatergic principal neurons, rather than glia, are major sources of neurosteroid synthesis. Other recent studies have identified putative neurosteroid binding sites on GABAA receptors. In this Opinion, we argue that neurosteroids require a membranous route of access to transmembrane domain binding sites within GABAA receptors. This has implications for the design of future neuroactive steroids, since the lipid solubility and related accessibility properties of the ligand may be key determinants of receptor modulation.
Overview
Endogenous neurosteroids and their synthetic analogues (neuroactive steroids) are among the most potent and effective modulators of γ-aminobutyric acid-A (GABAA) receptors known [1]. Although a role for neurosteroids as endogenous modulators has been postulated for nearly thirty years, fundamental issues of neurosteroid actions on circuits, cells, and receptors remain unresolved. For instance, which cells in the CNS synthesize neurosteroids? Once synthesized, how, where, and at what concentrations do these modulators reach their target receptors? Recent evidence suggests that principal neurons, rather than glia, may dominate CNS neurosteroid synthesis. Other studies have identified putative neurosteroid binding sites on GABAA receptors. Parallel pharmacological approaches have implied a non-classical interaction between neurosteroid ligands and GABAA receptors, involving a membrane route of access and a low-affinity receptor interaction for neurosteroids. Here, we discuss our opinion that local synthesis and a non-classical transmembrane binding site for neurosteroids suggest very local actions for neurosteroids that differ from the diffuse, hormonal effects usually associated with steroid actions. We also argue that the atypical nature of the neurosteroid interaction with GABAA receptors implies that future drug design should consider both specific (drug pharmacophore) and non-specific (membranous access) properties of the ligand.
Neurosteroid synthesis and CNS effects
Neurosteroids augment GABAergic inhibition by two principal effects. At low nanomolar aqueous concentrations, they potentiate the actions of GABA, increasing receptor sensitivity and GABA’s effectiveness, through specific changes in the kinetics of GABA-activated channels [1–3]. At higher concentrations, neurosteroids directly open GABAA channels in the absence of GABA. Although both actions enhance inhibition, potentiation probably dominates both endogenous modulation and exogenous drug actions. Although neurosteroid effects do not strictly depend on specific GABAA receptor subunits, effects are greater at certain receptor subtypes and depend on GABA concentration, the receptor’s affinity for GABA, and the efficacy with which GABA gates its channel. The threshold for potentiation by (3α,5α)-3-hydroxypregnan-20-one (allopregnanolone or 3α5αP) is 1–10 nM, with maximal enhancement of up to 20-fold at low GABA concentration. Interestingly, enhancement by low neurosteroid concentrations develops more slowly during exogenous administration than expected from simple aqueous diffusion to a receptor site [3–5].
At synapses, where GABA achieves brief, high concentrations, neurosteroids enhance postsynaptic charge transfer by prolonging the decay of inhibitory postsynaptic currents (IPSCs) [6]. Neurosteroids also augment tonic inhibition at extrasynaptic GABAA receptors [7], particularly those containing delta (δ) subunits. GABA gates δ-containing channels with low efficacy, and neurosteroids potentiate partly by increasing GABA efficacy [8, 9]. The large effects on δ-containing receptors have led to the proposal that they may be preferred sites of action of neurosteroids.
Neurosteroids also target non-GABAA receptors and channels [10–14]. For example, sulfated neurosteroids affect N-methyl-D-aspartate (NMDA) receptors at supra-physiological concentrations and σ1 receptors at low, physiological concentrations. Modulation of these receptors, like modulation of GABAA receptors, influences behavior and may be important for the etiology and treatment of neuropsychiatric disorders [13, 14]. Neurosteroid actions on NMDA and sigma (σ1) receptors are complex. Sulfated neurosteroids can either positively or negatively modulate NMDA receptors, depending on neurosteroid structure and receptor subunit combination [12, 13]. Likewise, sulfated neurosteroids and dehydroepiandrosterone (DHEA) exhibit complex modulation of σ1 receptors [14]. Direct neurosteroid binding of both NMDA and σ1 receptors is likely, although crosstalk between these two systems may also occur [14].
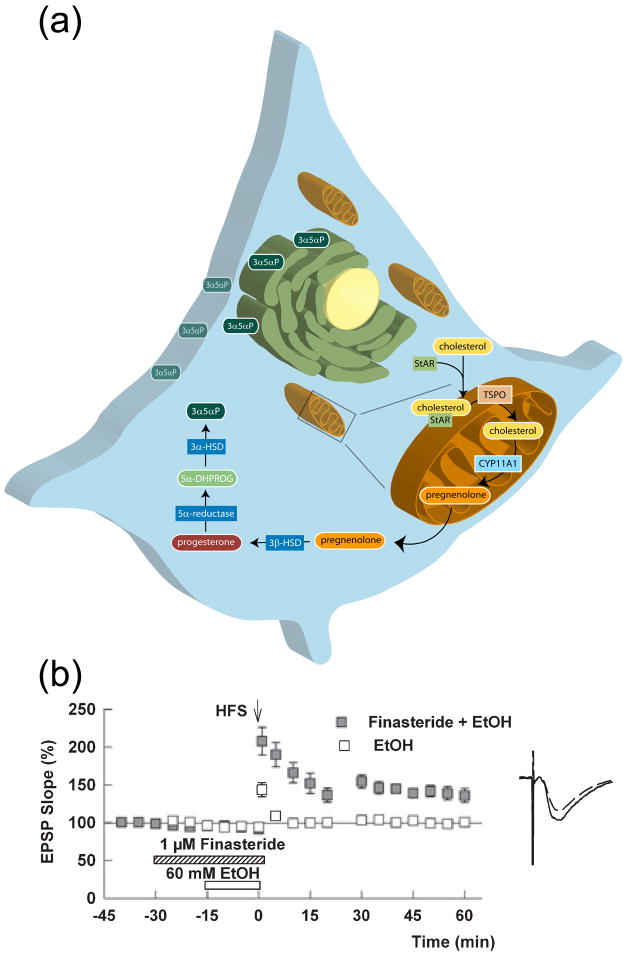
Endogenous neurosteroids are synthesized from cholesterol [15] (Figure 1a). Steroidogenic acute regulatory protein (StAR) is an inducible chaperone that delivers cytoplasmic cholesterol to the outer mitochondrial membrane, where translocator protein 18 kDa (TSPO) triggers movement to the inner mitochondrial membrane [16], the rate limiting step in neurosteroid biosynthesis. TSPO is part of a multi-protein complex, together with the voltage-dependent anion channel and adenine nucleotide transporter. TSPO was previously known as the mitochondrial (peripheral) benzodiazepine receptor based on its ability to bind anxiolytic drugs [17]. At the inner mitochondrial membrane, CYP11A1, a cytochrome P450-side chain cleavage enzyme, converts cholesterol to pregnenolone. After exiting mitochondria, pregnenolone undergoes reactions catalyzed by 3β-hydroxysteroid dehydrogenase/isomerase, 5α-reductase and 3α-hydroxysteroid dehydrogenase to generate 3α5αP, the major GABA-modulating product of this pathway [7].
Figure 1.
Biosynthesis of neurosteroids and endogenous steroid effects. (a) Protein and enzymes involved in steroid synthesis are: StAR, which shuttles cholesterol from the cytoplasm to the outer mitochondrial membrane; TSPO, which moves cholesterol from the outer to the inner mitochondrial membrane; CYP11A1, which converts cholesterol to pregnenolone. After exiting mitochondria, pregnenolone is catalyzed by 3β-hydroxysteroid dehydrogenase/isomerase, 5α-reductase and 3α-hydroxysteroid dehydrogenase to generate 3α5αP, the major product of this pathway, which likely accumulates in the endoplasmic reticulum and plasma membrane. (b) Finasteride (1 μm, hatched bar), an inhibitor of 5α-reduced steroid synthesis, blocks the ability of 60 mM ethanol (EtOH, white bar) to inhibit long-term potentiation (LTP). Panel b is reprinted by permission from reference [32].
Other neurosteroids, including sulfates that inhibit GABAA receptors and interact with NMDA and σ1 receptors, are also synthesized from cholesterol and pregnenolone via offshoots of this scheme. Cytosolic sulfotransferases catalyze the addition of sulfate groups onto pregnenolone and DHEA [12]. DHEA is synthesized from pregnenolone by P450-17α-hydroxylase, but this enzyme has not been demonstrated in brain [18]. Neurosteroid synthesis can be inhibited at multiple points along the pathway, and drugs like finasteride that target 5α-reductase are particularly effective at diminishing 3α5αP formation.
A number of types of brain cells synthesize neurosteroids. Early studies described astroglial synthesis [19, 20]. Recent studies, however, suggest the importance of neurons and demonstrate that synthetic machinery for neurosteroids, including StAR [21, 22], CYP11A1 [21] and 5α-reductase [23] is highly, if not exclusively, expressed in principal (excitatory) neurons in many brain regions, including hippocampal and neocortical pyramidal neurons. Additionally, 5α-reduced neurosteroid immunoreactivity was demonstrated in excitatory neurons, but not in interneurons or glia [24]. Thus, GABA-enhancing neurosteroids have potentially important paracrine and perhaps autocrine effects on excitatory output neurons.
How are neuronal neurosteroid synthesis and release regulated? Behavioral stressors acutely increase 3α5αP production in specific brain regions [25, 26]. How these stressors increase neurosteroid levels is not well understood. Intriguingly, activation of NMDA receptors and calcium influx into pyramidal neurons may contribute [21]. While speculative, this implies that increased excitatory activity or activation of extrasynaptic glutamate receptors may trigger neurosteroid synthesis to regulate overexcitation and excitotoxicity.
Certain abused and clinical drugs also enhance neurosteroid production. These include ethanol, etifoxine, gamma-hydroxybutyrate, and clinically-used psychotropic medications, including the antidepressant fluoxetine and the antipsychotics clozapine and olanzepine [27–29]. It remains unclear whether neurosteroids contribute to actions of the latter agents, although sedating and anxiolytic effects could result from this mechanism. Some behavioral [30] and electrophysiological [31] effects of ethanol involve neurosteroids, and ethanol’s inhibition of hippocampal long-term potentiation (LTP) may partly involve local production of a 5α-reduced neurosteroid and enhanced GABAergic inhibition of pyramidal neuron spiking [32] (Figure 1b). These effects could contribute to acute ethanol-induced memory impairment. However, effects on GABAergic inhibition are complex, and neurosteroids cannot account for all of ethanol’s actions. In particular, ethanol interacts directly with GABAA receptors; δ–containing receptors may be particularly sensitive to ethanol [33, 34], although other studies contradict this [35].
This discussion highlights the powerful effects of neurosteroids and their potential roles as local modulators of principal neurons. A second key piece of the puzzle of neurosteroid modulation involves the pharmacology of neurosteroid actions. Once neurosteroids are synthesized, how do they access and interact with their targets (e.g. the GABAA receptor)?
Possible models for neurosteroid actions
There are three general possibilities for the actions of neurosteroids at GABAA (and other) receptor targets. First, the direct target of the neurosteroid could be different than the receptor itself. Second, the neurosteroid could target the receptor through a conventional “lock-and-key” site on an aqueous domain of the receptor (similar to benzodiazepines and GABA), or through aqueous access to a transmembrane domain. Third, neurosteroids might target the receptor through a site that is accessible through lateral membrane diffusion rather than aqueous access. The identification of neurosteroid binding sites within the transmembrane domain of GABAA receptors, together with our own findings, have shifted our perspective toward the latter view. In the following sections, we will briefly discuss the first two options and then focus our discussion on the evidence supporting the latter view.
Neurosteroids may indirectly target receptor function
Neurosteroids might modulate channel activity through an indirect target. Because steroids alter transcription, gene regulation is one possible indirect mechanism of altering receptor function. In the case of GABAA and NMDA receptors, this possibility is excluded most directly by the persistence of neurosteroid effects on channel function in excised membrane patches. It should be noted, however, that neurosteroids do regulate transcription of GABAA receptor subunits and can thus alter inhibition over more prolonged time courses than we are considering here [36].
Steroids could also interact with protein kinases or phosphatases, whose post-translational regulation of GABAA receptors alters receptor function [1]. Although excised patch experiments exclude most cytoplasmic regulators, patch excision would not necessarily alter membrane bound modulators. Furthermore, in some systems there is a direct and important role for receptor phosphorylation in neurosteroid sensitivity of GABAA receptors (reviewed in [37]). In these cases, phosphorylation appears to modulate sensitivity of the receptor for neurosteroids, so a direct neurosteroid target on the receptor is implied.
Neurosteroids may directly interact with receptor targets
A subset of neurosteroids (including 3α5αP) are known to exhibit general anesthetic actions when given exogenously at high concentrations. Important components of neurosteroid structure necessary for such anesthetic actions include a 3α–hydrogen-bond donor (A-ring substitutent) and a 17β-hydrogen-bond acceptor (D-ring substituent) [38], whereas 3β or 17α diastereomers do not potentiate GABAA receptors or induce general anesthesia. Neurosteroid effects on GABAA receptor function are strongly enantioselective [5, 39–43]. Enantioselectivity is widely interpreted to imply a mechanism of action that involves a proteinaceous, chiral binding site. Because proteins consist entirely of L-amino acids, the absolute configuration of a ligand influences its interactions with protein binding sites [44, 45]. Importantly, enantioselectivity argues against the hypothesis that nonspecific drug/lipid interactions alone are responsible for receptor modulation [46]. Enantiomeric steroid analogues behave indistinguishably in model membranes [47], and fluorescently-labeled enantiomeric steroids accumulate in neurons with identical time courses and indistinguishable cellular partitioning [5], suggesting that their effects on lipid bilayers are non-specific and do not correlate with their mechanism of action.
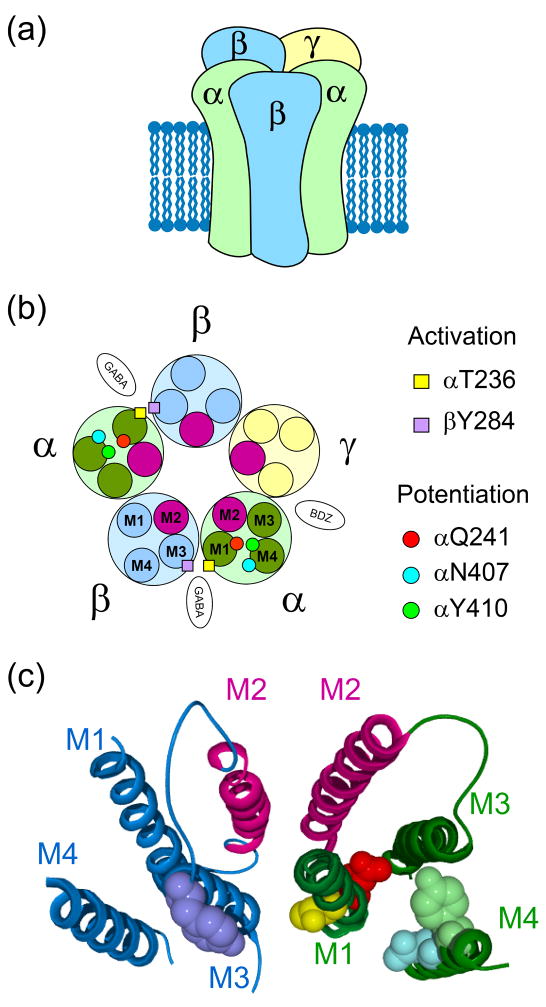
Neurosteroids likely interact directly with a site on GABAA receptors. GABAA receptors belong to the cys-loop family of ligand-gated ion channels and are heteropentameric combinations of 2 α, 2 β and usually a γ or δ subunit [48] (Figure 2a). Residues on α and β subunits are critical for neurosteroid actions [49, 50] (Figure 2b and 2c). Especially important residues lie within M1 and M4 transmembrane domains of α subunits. These residues may form hydrophobic binding pockets for neurosteroids that lead to potentiated receptor activity in the presence of agonist. A realistic model is important for interpreting the effect of mutations because it is often difficult to exclude the hypothesis that mutated residues are unimportant for binding but rather are important for transducing binding into a conformational change associated with “potentiation.” The neurosteroid 3α-OH group was hypothesized to pair with the Q241 residue, while the C17 hydrogen bond acceptor interacts with the M4 residues 407/410 with the GABAA receptor α subunit [49, 50] In homology models, the M4 transmembrane domain of cys-loop receptors has the most contact with the surrounding lipid environment (Figure 2b, c)[51]. As discussed below, this may have significance for the access of lipophilic neurosteroids to these putative binding sites.
Figure 2.
Transmembrane neurosteroid sites on GABAA receptors. (a) Heteropentameric GABAA receptor representation and (b) schematic view showing residues involved in potentiation (Q241, N407 and Y410 residues within α subunits) and activation (residues T236 and Y284 within the α and β subunits, respectively) by neurosteroids. GABA and benzodiazepine (BDZ) binding sites are also indicated. (c) Neurosteroid binding residues in ribbon structures of α1 and β2 subunits. Residues are color coded as in (b). Panels b and c are reprinted with permission of Macmillian Publishers Ltd from reference [49].
A separate site involving the M1 domain of the α1 subunit and the M3 domain of the β subunit of GABAA receptors may mediate neurosteroid gating of the channel [49, 50] (Figure 2b and 2c). Although each native receptor contains two neurosteroid binding sites arising from two α subunits, studies with mutated and wildtype concatemeric receptors (receptors constructed from two or more subunits tethered to each other by linking amino acids) show that a single functional neurosteroid site can yield nearly full potentiation [52, 53]. Taken together with the enantiomeric specificity properties of neurosteroids, these studies demonstrate that neurosteroids likely augment GABAA receptor function by selective interactions with specific sites within the receptor.
Neurosteroids may access an aqueous accessible domain on receptor targets
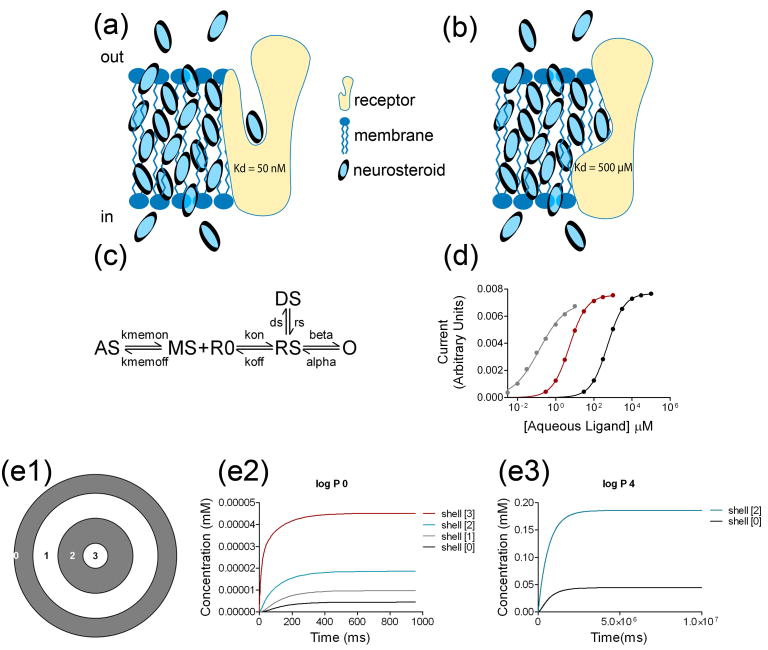
One possibility for explaining how neurosteroids interact with GABAA receptors is that they could target an aqueous accessible domain of the receptor (Figure 3a). Although this is a conventional model for a ligand-receptor interaction, we know of no direct evidence that it pertains to neurosteroid interactions with GABAA receptors. If neurosteroids indeed bind within transmembrane domains [49], it is difficult to envision how aqueous ligands access these sites. Nevertheless, some receptors, such as adrenergic receptors (ARs), have ligand binding sites within water-filled transmembrane domains [54].
Figure 3.
Implications of membranous, low affinity binding site(s) for neurosteroids on GABAA receptors. (a) A simplified cartoon showing how ligand might access a transmembrane domain via aqueous access. In this case aqueous concentration is directly relevant to receptor Kd. Although neurosteroid may accumulate in the membrane at much higher concentrations than in the aqueous phase, the membrane concentration is irrelevant to binding. (b) An alternative view is that the transmembrane binding site is accessed by lateral membrane diffusion. The high membrane concentration is directly relevant to ligand binding at transmembrane interaction sites. (c) Kinetic scheme for simulations of conventional aqueous ligand access versus membrane access to a receptor. AS: aqueous steroid ligand; MS: membrane steroid; R0: unbound receptor; RS: ligand bound receptor; O: open channel; DS: a liganded desensitized state. Only the open state is conducting. (d) Concentration-response curves for peak responses from data generated from the model. Simulated agonist log P values of 0 (black), 2 (red), and 4 (gray) are shown. EC50 values: 519.4 μM, 5.26 μM and 0.14 μM, respectively; Hill coefficient of 0.99, 0.97 and 0.64, respectively. The lower Hill coefficient for the gray trace may result from the slower delivery of steroid to the receptor transmembrane compartment, combined with receptor desensitization. All parameters except Kmemon and Kmemoff were held constant in all simulations, including Kon and Koff. For aqueous ligand, Kmemon and Kmemoff were set to equal values, more rapid than the other rate constants in the scheme. For ligand of log P of 2 and 4, Kmemoff was set to be 100-fold and 10000-fold slower respectively than Kmemon. See reference [5] for additional details. Panels c and d are reprinted by permission from [5] (e) Simulations of intracellular steroid accumulation. (e1) Cross section of a model cell, 30 μm in diameter and length. For simulations in e2, all the concentric shells represent aqueous compartments. For simulations in e3, the gray shells represent membranous compartments, whereas the white shells represent aqueous compartments. The inner shell has a radius of 2.5 μm and the distance from the outer edge of the shell to the inner edge of the next shell is 5 μm. The numbers in the shells represent the locations of calculated concentrations. (e2) A uniform aqueous environment (no membrane accumulation). Synthesis occurs in Shell[3] at an arbitrary rate of 50 nmoles μm−1 ms−1. Concentration decreases monotonically from this site in the more distal shells. (e3) Same simulation, except that Shell[0] represents the plasma membrane and Shell[2] represents intracellular membrane. A log P of 4 is assumed. The approximately 10,000-fold difference in time scales between e2 and e3 is a direct result of the change in rates used to simulate a ligand of log P = 4. In panel e3, concentration in the aqueous compartments is not discernible on this scale. Modeling was performed in the NEURON simulation environment (http://www.neuron.yale.edu/neuron/).
Neurosteroids may target a membrane-accessible domain of receptors
We believe that at least six direct and indirect lines of evidence support our view that neurosteroids access their binding sites through membrane access (Figure 3b) [3–5, 55]. First, fluorescently-labelled neurosteroid analogues that retain activity at GABAA receptors accumulate and departition into cells and membranes, and this non-specific lipid accumulation and departure temporally coincide with biological effects [3–5]. Second, cell-attached patch recordings show that channels in a patch of membrane isolated from direct aqueous exposure to neurosteroid are modulated by bath-applied neurosteroid [3]. Third, potentiation in excised membrane patches persists following removal of aqueous neurosteroid [3]. Fourth, a membrane-impermeant neuroactive steroid potentiates only when applied to the intracellular side of a membrane patch [3]. Fifth, neurosteroid directly applied intracellularly through a whole-cell patch pipette can modulate GABAA receptors [3]. Finally, and most directly, offset kinetics of neurosteroid potentiation, after removal of free aqueous ligand, are dramatically speeded by an extracellular scavenger of free neurosteroid [3, 5, 56]. This suggests that neurosteroids do not bind tightly to the receptor, where the neurosteroid would be inaccessible to the scavenger. Rather, this observation is more consistent with the idea that ligand rapidly associates with and dissociates from the receptor into the lipid phase, where it is accessible to the scavenger. Taken together, these results suggest that the partitioning of neurosteroids into the plasma membrane is an important step for effective neurosteroid modulation of GABAA receptors, although additional approaches towards testing this hypothesis must be undertaken.
These concepts of neurosteroid access have precedent with other ligands and receptors. Although there is evidence for an extracellular binding site for pregnenolone sulfate on NMDA receptors [57], other work suggests pregnenolone sulfate and also ketamine access receptors through a membrane route [58, 59]. Ethanol may interact with protein targets through transmembrane access [60]. Lipophilic cannabinoids partition into plasma membranes before accessing their receptor sites. Cannabinoid hydroxyl groups face the membrane surface and interact with polar head groups of phospholipids, while the tricyclic ring structure or the unsaturated carbon tail of anandamide is accommodated by the hydrophobic tails of phospholipids [61]. Other documented examples of modulators that use membranes to access a transmembrane target protein include toxins and neuroactive peptides that act on potassium channels and mechanosensitive channels, respectively [62, 63]. Thus, membrane access is likely to be more relevant than is currently widely realized.
Conceptualizing lipid versus receptor binding contributions to neurosteroid actions
One implication of membrane access might be that for more lipophilic neurosteroid analogues, lower aqueous concentrations are needed to achieve the same steady-state membrane concentration compared to less lipophilic ligands. Therefore, while the EC50 for two analogues may differ dramatically, their affinity may be similar (Box 2 and Figure 3) [5]. To conceptualize how both steady-state and kinetic parameters are affected by lipophilicity and to demonstrate how high sensitivity (low EC50) can arise from a low-affinity transmembrane ligand interaction site, we produced a simple model of a ligand-receptor interaction (Figure 3c). In this model, rather than a typical aqueous diffusion-limited reaction, neurosteroid-receptor binding occurs from an intermediate compartment, meant to represent the lipid bilayer. Rate constants out of this membrane compartment were adjusted to simulate neurosteroids of different log P (=log{[neurosteroid]octanol/[neurosteroid]water}) values (ie. the higher a neurosteroid’s lipophilicity, the higher the log P value). Parameters for the receptor were chosen to simulate a low-affinity neurosteroid-receptor interaction. As expected, simulating a dose-response curve using rate constants that allow for rapid equilibration of neurosteroid between aqueous and membrane compartments without accumulation in the membrane (log P = 0) confirms a low affinity interaction (Figure 3d, black curve). Changing only the rate constants for neurosteroid accumulation in the membrane compartment such that log P = 2 results in a simulated dose-response curve that is shifted to the left (more potent) compared to the baseline dose-response curve (Figure 3d, red curve). Changing only the rate constants for neurosteroid accumulation such that log P = 4 results in a further left-shift of the dose-response curve (Figure 3d4, gray curve). Note that the rates of neurosteroid-receptor binding and unbinding (the Kd) were not changed in any of these simulations. Thus, increasing neurosteroid lipophilicity in the simulations results in higher neurosteroid apparent affinity (EC50, Figure 3d). Although these data are only correlative, kinetics and steady-state EC50 values in this simple model match experimental data obtained from neurosteroids of different lipophilicity [5].
Box 2. EC50 vs. affinity.
EC50 is an empirical term that relates free (aqueous) concentration of a ligand to a measured effect. The EC50 is the free ligand concentration required to achieve a half maximum response (maximum binding, maximum electrical current, maximum heart rate, etc.).
By contrast to EC50, affinity is a parameter associated with a receptor’s microscopic association and dissociation rate constants for a ligand. Typically, affinity is measured as Kd, the ratio of the dissociation rate constant to the ligand’s association rate constant (koff/kon in the parlance used in Figure 3). As for EC50, units are in molarity.
Although the temptation is to equate EC50 with affinity (Kd), there are several well documented examples of ligand-receptor interactions in which EC50 is distinct from affinity (Kd) [64, 71, 72]. For instance, mutations can affect a ligand-gated receptor’s EC50 for agonist by affecting the rates of entry into or out of the channel open state, which define the agonist’s efficacy, but do not affect the microscopic binding constants (Kd) [64]. In these examples, EC50 changes but affinity does not.
We raise another example of potentially discrepant EC50 and affinity. In this case the incongruity arises from using the wrong independent variable to define EC50. The free aqueous concentration of a steroid is usually used to define receptor sensitivity and EC50; typical values for neurosteroid EC50 are ~100 nM. This is usually interpreted as a relatively high affinity interaction. However, for a lipophilic ligand, which may interact with a receptor from a lipid route of access rather than an aqueous route of access, the free membrane concentration would be more relevant for steady-state binding than the free aqueous concentration. From calculated log P values, the free steroid membrane concentration may be 4 orders of magnitude higher than the free aqueous concentration. Therefore, the true EC50 is probably closer to 1 mM, suggesting a weak interaction between steroid and receptor.
Implications of neurosteroid membrane access
We speculate that these considerations supporting membranous receptor access may help reconcile some of the anomalies that are starting to emerge with regard to amino acid residues implicated in the binding of neurosteroids to receptor targets (see Box 1). The high sensitivity of GABAA receptors for neurosteroids (low EC50) is observed only with respect to aqueous concentrations. The plasma membrane concentration at steady-state is likely >10,000 times that of the aqueous compartment (this calculation is based on a log P value of 4.2, which is the estimated log P of 3α5αP). Thus, although aqueous EC50 concentrations for neurosteroid potentiation of GABAA receptors may be 3–100 nM [4, 55], the membrane concentration (i.e., at the binding site) is likely 50 μM - 1.7 mM. This range may better reflect the site’s affinity (although even these estimates do not directly reflect affinity because of transduction steps between binding and receptor modulation [64]). Thus, neurosteroid binding to transmembrane GABAA receptors likely differs from classical steroid binding to non-membrane receptors, where direct binding studies on soluble receptors confirm a genuine high-affinity ligand-receptor interaction in the sub-nanomolar range (e.g., [65]). Thus, for membranous ligands like neurosteroids, EC50 values in the nanomolar range may be misleading.
Box 1. Caveats of the classical receptor binding site view.
Anomalies inconsistent with a simple lock-and-key binding site for neurosteroids are emerging. For instance, recent evidence supports a homology model of GABAA receptors in which putative ends of the binding cavity for neurosteroids are not sufficiently close to each other to accommodate neurosteroid binding [67]. In addition, the neurosteroid direct gating site is predicted to overlap with the binding of the general anesthetic etomidate, a modulator whose site has been established through photoaffinity labeling [68]. Neurosteroids, however, do not occlude etomidate binding [69], questioning the validity of homology models on which the putative neurosteroid binding pockets are based. Other results, including steroid analogues whose actions at GABAA receptors are unaffected by mutation of the specific residues outlined above [42] and non-steroid modulators whose actions are affected by these mutations [70], are difficult to reconcile with a simple “lock-and-key” site contributed by these residues. Perhaps surrounding residues can also contribute to the binding site in the case of non-steroidal ligands, or perhaps the interaction site is not as structurally-selective as a traditional “lock-and-key” site suggests.
We propose that there are at least three implications of the idea that neurosteroids follow a membranous route of access to a low-affinity receptor target. First, some drug lipophilicity must be maintained when designing new steroid molecules, in order to achieve a reasonable drug ED50. If the compound is too hydrophilic, it will not reach the receptor target at reasonable aqueous concentrations. Second, different lipid compositions of cell membranes may result in kinetic and/or steady-state sensitivity differences of neurosteroid actions [66]. Finally, for endogenous neurosteroids synthesized within a cell, retention in cell membranes will likely dramatically alter the time course and concentration that reaches the target (Figure 3e). Induced synthesis would need to proceed for minutes rather than seconds to produce relevant membrane concentrations (Figure 3e2 vs. Figure 3e3). Whether the binding site for neurosteroids is aqueous or membranous, retention of lipophilic neurosteroids by intracellular and plasma membranes will hinder the departure of newly synthesized neurosteroids to free circulation. It thus seems likely that neurosteroid actions, like those of lipophilic endocannabinoids, are limited to local spheres of autocrine and paracrine influence near sites of synthesis. Neurosteroid log P values are lower than those of cannabinoids, and cannabinoids are well documented to diffuse short intercellular distances (e.g., across synaptic clefts). Thus, some neurosteroid likely escapes beyond the cell of origin. Because GABAA receptors are confined mostly to postsynaptic compartments, neurosteroids synthesized by glutamatergic neurons may mainly influence somatodendritic regions of other nearby principal neurons. Finally, because most measurements of endogenous neurosteroid concentrations do not distinguish intracellular, membrane, and aqueous concentrations, it is difficult to predict the strength of receptor modulation by endogenous neurosteroids.
Concluding remarks and future directions
Neurosteroids remain of interest because of their strong potentiating effects on GABAA receptor function and their potential clinical relevance. Recent work demonstrates that excitatory principal neurons produce neurosteroids. Non-specific cellular retention and membrane accumulation may be important to the time course and steady-state effects of neurosteroids in the context of transmembrane domain interaction sites. Neurosteroids may be an example of a growing list of ligands that access their receptor by a membranous, rather than aqueous, route. A low-affinity transmembrane site would promote local influences among the principal cells that synthesize neurosteroids, possibly effectively fostering dampened local circuit excitation.
Future work, using complementary and novel experimental approaches to those described here, will be valuable in testing and refining these ideas. For example, to test the idea of local synthesis, additional immunostaining and in situ hybridization studies for steroidogenic enzymes will confirm cell types where synthesis occurs and hopefully clarify conditions under which synthesis is stimulated in various cell types. Ideally, future techniques should be developed for distinguishing endogenous steroid concentration in membrane compartments versus soluble fractions in an effort to elucidate the actual concentration localized near receptors. To test how far neurosteroids diffuse from their source of synthesis, fluorescently-tagged neuroactive steroids may be helpful [3]. In addition, artificial bilayer experiments, manipulations of the plasma membrane composition of cells, and molecular dynamics simulations may all be helpful to further test the idea that neurosteroid-plasma membrane interactions are important in the kinetic and steady-state properties of neurosteroid-receptor interactions. Improved understanding of neurosteroid access to, and interactions with, receptors may also improve future drug design and increase the prospects for a clinically useful neuroactive steroid.
Acknowledgments
We thank lab members for support and Drs. Joe Henry Steinbach, Alex Evers, Gustav Akk, and Dave Reichert for discussion and their contributions to the work described here. We would like to dedicate this article to the memories of Drs. Erminio Costa and Alastair Hosie, both of whom made pivotal contributions to the field. This work was supported by NIGMS, NIMH, NIAAA, NINDS and the Bantly Foundation.
Footnotes
Disclosure statement. DFC and CFZ hold patents on several novel neurosteroid analogues.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 2.Akk G, et al. Neuroactive steroids have multiple actions to potentiate GABAA receptors. J Physiol (Lond) 2004;558:59–74. doi: 10.1113/jphysiol.2004.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akk G, et al. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li P, et al. Neurosteroid migration to intracellular compartments reduces steroid concentration in the membrane and diminishes GABAA receptor potentiation. J Physiol (Lond) 2007;584:789–800. doi: 10.1113/jphysiol.2007.142794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chisari M, et al. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low-affinity interaction. J Neurophysiol. 2009;102:1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zorumski CF, et al. Enantioselective modulation of GABAergic synaptic transmission by steroids and benz[e]indenes in hippocampal microcultures. Synapse. 1998;29:162–171. doi: 10.1002/(SICI)1098-2396(199806)29:2<162::AID-SYN7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Herd MB, et al. Neurosteroid modulation of synaptic and extrasynaptic GABAA receptors. Pharmacol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheleznova N, et al. α1β2δ, a silent GABAA receptor: recruitment by tracazolate and neurosteroids. Br J Pharmacol. 2008;153:1062–1071. doi: 10.1038/sj.bjp.0707665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todorovic SM, Jevtovic-Todorovic V. Regulation of T-type calcium channels in the peripheral pain pathway. Channels (Austin) 2007;1:238–245. doi: 10.4161/chan.4953. [DOI] [PubMed] [Google Scholar]
- 11.Mensah-Nyagan AG, et al. Evidence for a key role of steroids in the modulation of pain. Psychoneuroendocrinology. 2009;34:S169–S177. doi: 10.1016/j.psyneuen.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Schumacher M, et al. Pregnenolone sulfate in the brain: a controversial neurosteroid. Neurochem Int. 2008;52:522–540. doi: 10.1016/j.neuint.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Mony L, et al. Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol. 2009;157:1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurice T, et al. Neuro(active)steroids actions at the neuromodulatory sigma1 (σ1) receptor: biochemical and physiological evidences, consequences in neuroprotection. Pharmacol Biochem Behav. 2006;84:581–597. doi: 10.1016/j.pbb.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- 16.Rone MB, et al. Targeting and insertion of the cholesterol-binding translocator protein into the outer mitochondrial membrane. Biochemistry. 2009;48:6909–6920. doi: 10.1021/bi900854z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadopoulos V, et al. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006;138:749–756. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 18.Maayan R, et al. Is brain dehydroepiandrosterone synthesis modulated by free radicals in mice? Neurosci Lett. 2005;377:130–135. doi: 10.1016/j.neulet.2004.11.086. [DOI] [PubMed] [Google Scholar]
- 19.Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- 20.Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]
- 21.Kimoto T, et al. Neurosteroid synthesis by cytochrome P450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142:3578–3589. doi: 10.1210/endo.142.8.8327. [DOI] [PubMed] [Google Scholar]
- 22.King SR, et al. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J Neurosci. 2002;22:10613–10620. doi: 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agis-Balboa RC, et al. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saalmann YB, et al. Cellular distribution of the GABAA receptor-modulating 3α-hydroxy, 5α-reduced pregnane steroids in the adult rat brain. J Neuroendocrinol. 2007;19:272–284. doi: 10.1111/j.1365-2826.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- 25.Dong E, et al. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biggio G, et al. Stress, ethanol, and neuroactive steroids. Pharmacol Ther. 2007;116:140–171. doi: 10.1016/j.pharmthera.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verleye M, et al. The anxiolytic etifoxine activates the peripheral benzodiazepine receptor and increases the neurosteroid levels in rat brain. Pharmacol Biochem Behav. 2005;82:712–720. doi: 10.1016/j.pbb.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci USA. 1999;96:13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danovich L, et al. The influence of clozapine treatment and other antipsychotics on the 18 kDa translocator protein, formerly named the peripheral-type benzodiazepine receptor, and steroid production. Eur Neuropsychopharmacol. 2008;18:24–33. doi: 10.1016/j.euroneuro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 30.VanDoren MJ, et al. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanna E, et al. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izumi Y, et al. GABAergic neurosteroids mediate the effects of ethanol on long-term potentiation in rat hippocampal slices. Eur J Neurosci. 2007;26:1881–1888. doi: 10.1111/j.1460-9568.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- 33.Wallner M, et al. Ethanol enhances α4 β3 δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundstrom-Poromaa I, et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borghese CM, et al. The δ subunit of γ-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- 36.Maguire J, Mody I. Steroid hormone fluctuations and GABAAR plasticity. Psychoneuroendocrinology. 2009;34S1:S84–S90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belelli D, et al. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience. 2006;138:821–829. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Phillipps GH. Structure-activity relationships in steroidal anaesthetics. J Steroid Biochem. 1975;6:607–613. doi: 10.1016/0022-4731(75)90041-2. [DOI] [PubMed] [Google Scholar]
- 39.Zorumski CF, et al. Effects of neurosteroid and benz[e]indene enantiomers on GABAA receptors in cultured hippocampal neurons and transfected HEK-293 cells. Neuropharmacology. 1996;35:1161–1168. doi: 10.1016/s0028-3908(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 40.Wittmer LL, et al. Enantioselectivity of steroid-induced γ-aminobutyric acidA receptor modulation and anesthesia. Mol Pharmacol. 1996;50:1581–1586. [PubMed] [Google Scholar]
- 41.Li W, et al. Enantiomers of neuroactive steroids support a specific interaction with the GABAC receptor as the mechanism of steroid action. Mol Pharmacol. 2006;69:1779–1782. doi: 10.1124/mol.106.022863. [DOI] [PubMed] [Google Scholar]
- 42.Li P, et al. Natural and enantiomeric etiocholanolone interact with distinct sites on the rat α1β2γ2L GABAA receptor. Mol Pharmacol. 2007;71:1582–1590. doi: 10.1124/mol.106.033407. [DOI] [PubMed] [Google Scholar]
- 43.Hu Y, et al. Neurosteroid analogues. Part 5 Enantiomers of neuroactive steroids and benz[e]indenes: total synthesis, electrophysiological effects on GABAA receptor function and anesthetic actions in tadpoles. J Chem Soc, Perkin Trans. 1997;1:3665–3671. [Google Scholar]
- 44.Westover EJ, Covey DF. The enantiomer of cholesterol. J Membr Biol. 2004;202:61–72. doi: 10.1007/s00232-004-0714-7. [DOI] [PubMed] [Google Scholar]
- 45.Covey DF. ent-Steroids: novel tools for studies of signaling pathways. Steroids. 2009;74:577–585. doi: 10.1016/j.steroids.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cantor RS. The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Toxicol Lett. 1998;00–101:451–458. doi: 10.1016/s0378-4274(98)00220-3. [DOI] [PubMed] [Google Scholar]
- 47.Alakoskela JM, et al. Lack of enantiomeric specificity in the effects of anesthetic steroids on lipid bilayers. Biochim Biophys Acta. 2007;1768:131–145. doi: 10.1016/j.bbamem.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 49.Hosie AM, et al. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 50.Hosie AM, et al. Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 51.Unwin N. Structure and action of the nicotinic acetylcholine receptor explored by electron microscopy. FEBS Lett. 2003;555:91–95. doi: 10.1016/s0014-5793(03)01084-6. [DOI] [PubMed] [Google Scholar]
- 52.Akk G, et al. Activation and modulation of concatemeric GABAA receptors expressed in human embryonic kidney cells. Mol Pharmacol. 2009;75:1400–1411. doi: 10.1124/mol.108.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bracamontes JR, Steinbach JH. Steroid interaction with a single potentiating site is sufficient to modulate GABAA receptor function. Mol Pharmacol. 2009;75:973–981. doi: 10.1124/mol.108.053629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huber T, et al. Structural basis for ligand binding and specificity in adrenergic receptors: implications for GPCR-targeted drug discovery. Biochemistry. 2008;47:11013–11023. doi: 10.1021/bi800891r. [DOI] [PubMed] [Google Scholar]
- 55.Shu HJ, et al. Slow actions of neuroactive steroids at GABAA receptors. J Neurosci. 2004;24:6667–6675. doi: 10.1523/JNEUROSCI.1399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shu HJ, et al. Cyclodextrins sequester neuroactive steroids and differentiate mechanisms that rate limit steroid actions. Br J Pharmacol. 2007;150:164–175. doi: 10.1038/sj.bjp.0706973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jang MK, et al. A steroid modulatory domain on NR2B controls N-methyl-D-aspartate receptor proton sensitivity. Proc Natl Acad Sci USA. 2004;101:8198–8203. doi: 10.1073/pnas.0401838101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bowlby MR. Pregnenolone sulfate potentiation of N-methyl-D-aspartate receptor channels in hippocampal neurons. Mol Pharmacol. 1993;43:813–819. [PubMed] [Google Scholar]
- 59.Orser BA, et al. Multiple mechanisms of ketamine blockade of N-methyl-D-aspartate receptors. Anesthesiology. 1997;86:903–917. doi: 10.1097/00000542-199704000-00021. [DOI] [PubMed] [Google Scholar]
- 60.Treistman SN, Martin GE. BK Channels: mediators and models for alcohol tolerance. Trends Neurosci. 2009;32:629–637. doi: 10.1016/j.tins.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Makriyannis A, et al. How lipophilic cannabinergic ligands reach their receptor sites. Prostaglandins & Other Lipid Mediators. 2005;77:210–218. doi: 10.1016/j.prostaglandins.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 62.Lee SY, MacKinnon R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature. 2004;430:232–235. doi: 10.1038/nature02632. [DOI] [PubMed] [Google Scholar]
- 63.Suchyna TM, et al. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–240. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- 64.Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure- activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hosie AM, et al. Neurosteroid binding sites on GABAA receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 66.Sooksawate T, Simmonds MA. Increased membrane cholesterol reduces the potentiation of GABAA currents by neurosteroids in dissociated hippocampal neurones. Neuropharmacology. 1998;37:1103–1110. doi: 10.1016/s0028-3908(98)00113-0. [DOI] [PubMed] [Google Scholar]
- 67.Bali M, et al. GABA-induced intersubunit conformational movement in the GABAA receptor α1M1-β2M3 transmembrane subunit interface: experimental basis for homology modeling of an intravenous anesthetic binding site. J Neurosci. 2009;29:3083–3092. doi: 10.1523/JNEUROSCI.6090-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li GD, et al. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26:11599–11605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li GD, et al. Neurosteroids allosterically modulate binding of the anesthetic etomidate to gamma-aminobutyric acid type A receptors. J Biol Chem. 2009;284:11771–11775. doi: 10.1074/jbc.C900016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li P, et al. Mechanisms of potentiation of the mammalian GABAA receptor by the marine cembranoid eupalmerin acetate. Br J Pharmacol. 2008;153:598–608. doi: 10.1038/sj.bjp.0707597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang Y, Weiss DS. Channel opening locks agonist onto the GABAC receptor. Nat Neurosci. 1999;2:219–225. doi: 10.1038/6313. [DOI] [PubMed] [Google Scholar]
- 72.Colquhoun D. The quantitative analysis of drug-receptor interactions: a short history. Trends Pharmacol Sci. 2006;27:149–157. doi: 10.1016/j.tips.2006.01.008. [DOI] [PubMed] [Google Scholar]