Abstract
As a nanotechnology has been actively applied to the overall areas of scientific fields, it is necessary to understand the characteristic features, physical behaviors and the potential effects of exposure to nanomaterials and their toxicity. In this article we review the immunological influences induced by several nanomaterials and emphasize establishment of the animal models to estimate the impact of these nanomaterials on development of immunological diseases.
Keywords: Nanomaterials, Immune response, Cytokines, Immunological diseases
INTRODUCTION
A nanotechnology which has appeared since the mid-twenties century has provided the methods by which the limitations in the industrial application of each scientific technology could be overcome. It has also brought another revolutionary change to the overall areas in the scientific technology and the related industries. A nanotechnology has become a basis of the integrated development of scientific technology which has independently been developed. It has also been actively applied to the overall areas of scientific fields. The characteristics of nanomaterials are subject to their surface property, according to which the desirable physical property can be given deliberately to them.
As described here, products manufactured using nanomaterials are characterized to overcome the areas which cannot be resolved with the previous technology. It can therefore be stated that they have a higher degree of applicability. Synchronously, there is an increasing concern that they would be new hazardous factors for both humans and nature (1). In identifying and then clarifying risks due to exposure to nanomaterials, it is extremely important to clarify the physicochemical property and physical behavior of nanomaterials and to understand the biological and physiological actions of them in an in vivo setting (2). Based on this, a systematic approach should be made to examine the possible detrimental effects on the production, environment and health. In general, methods for assessing the detrimental effects of conventional types of chemicals are divided into the definition of detrimentalness, the assessment of dose-response, that of exposure and that of detrimental characteristics. These methods can also be applied to nanomaterials. In nanomaterials, however, as their surface area is increased, their responsiveness and toxicity to viable tissues are also increased (3). It is of prime importance to clarify new physicochemical property and to evaluate the resulting toxicity of them. Accordingly in the assessment of detrimentalness of nanomaterials, it is necessary to understand the characteristic features, physical behaviors, the potential effects of exposure to nanomaterials and their toxicity (4).
IMMUNOLOGICAL EFFECTS OF NANOMATERIALS
Nanomaterials have a structure that the size of one of three surfaces is 1~100 nm according to the definition made by ISO/TC 229. As described here, based on a smaller size of <100 nm, nanomaterials have physicochemical properties which are greatly different from general types of other substances. Due to a higher degree of surface reactivity and the cell membrane permeability, an in vivo influx of nanomaterials produces the stress on a cellular level.
Until the year of 2006, 69% of total manuscripts published on a peer-reviewed journal are related to the toxicity and the remaining 31% are related to the exposure. In particular, since 2008, research articles about the toxicity have been abruptly increased. These articles have reported the pulmonary toxicity, neurotoxicity and the permeability to blood-brain-barrier. Besides, peer-reviewed articles about the immunotoxicity have also been increasingly published.
Nanomaterials are crystalline, fine particles with a large surface area, and they have a higher degree of surface charge and proton exchangeability. Besides, it is also expected that they might have a positive effect on the environment because they facilitate the synthesis and transportation of contaminants in the environment. In addition, the potential problems of nanomaterials are not limited to the environmental pollution. By various routes such as an inhalation, they are absorbed into the body and then induce the biological toxicity (5,6). This makes nanomaterials further interesting. The possible routs by which nanomaterials migrate in the human body include the following:
Endocytosis: Nanomaterials enter the cells when they are surrounded by the cell membrane without passing it.
Cell membrane penetration occurring with the action of hydrophobic particles.
Transportation of nanoparticles with a size of <5 nm across the cell membrane channel.
As described here, it has been proposed that the contactable nanoparticles might stimulate the immune system in the body. By contrast, recent studies have been conducted to examine industrial nanoparticles. Industrial nanoparticles are mainly contacted in a working place by daily commodities or pharmaceutical products in customers. The total amount of exposure may be relatively lower or the actual one occurring in the local tissue might be relatively higher. Nanoparticles produce oxygen radicals (7,8). Besides, the mitochondrial perturbation and apoptosis (9) are induced, which are followed by the cytotoxicity. With the reactions with body proteins and other biological substances, various types of hazardous reactions might occur.
One of the advantages of nanoparticles, a biological permeability makes an intracellular and intranuclear influx of nanomaterials possible. Based on these findings, it has therefore been postulated that the oxidative stress and inflammatory responses induce the occurrence of immunotoxicity directly or indirectly. It is therefore imperative that the technology for assessing the exposure be established to examine the physicochemical properties of nanomaterials and to clarify the related immunotoxic mechanisms.
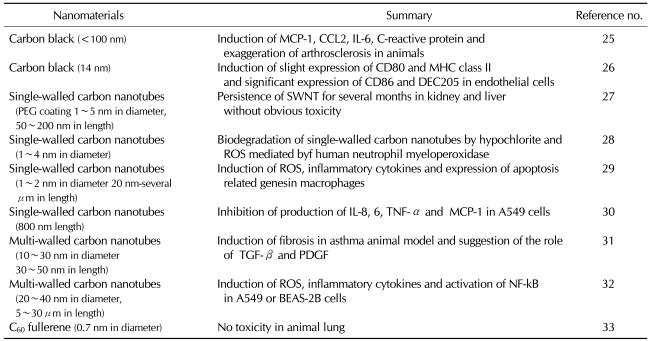
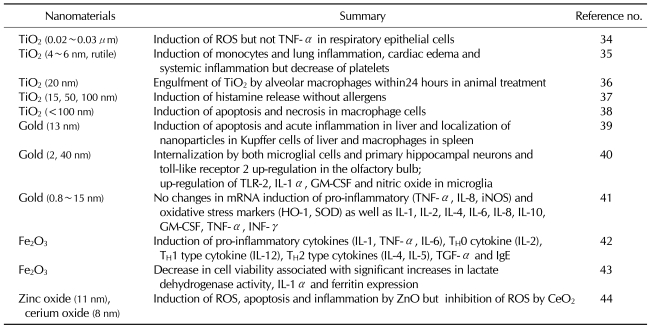
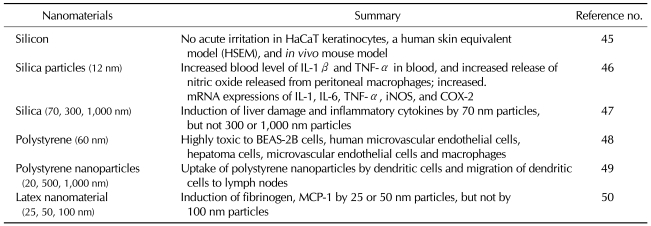
Peer-reviewed articles about the toxicity of nanomaterials have been summarized based on the chemical constituents. Based on the classification system into carbon nanomaterials, metals, metal-oxide nanomaterials and other silicas, a review of the above peer-reviewed journals was made. Results were represented in Tables I, II and III. For example, of carbon nanomaterials, carbon black induces the immune responses. In particular, multi-walled carbon nanotubes (MWCNT) rather than single-walled carbon nanotubes (SWCNT) induce the activation of immune responses to a greater extent (Table I). Of metals and metal oxides, iron oxide nanoparticles strongly induce the immune responses. Besides, gold nanoparticles induce a moderate degree of the immune responses. Furthermore, TiO2 induces a lower degree of the immune responses (Table II). In addition, it has also been reported that such substances as silica, polystyrene and latex nanomaterials have an immunotoxicity (Table III).
Table I.
Immunotoxicity of carbon-based nanomaterials
Table II.
Immunotoxicity of metal-based nanomaterials
Table III.
Immunotoxicity related of silica or polystyrene-based nanomaterials
THE NECESSITY FOR CONDUCTING STUDIES ABOUT THE RELATEDNESS TO DISEASE THROUGH AN ANIMAL EXPERIMENTAL MODEL OF PULMONARY DISEASES IN AN IN VIVO SETTING
Relatedness of nanomaterials to diseases can be presumed based on studies about the particulate matter (PM) associated with air pollution and the diseases (10-12). A long-term exposure to particulate matter (PM) increases the incidence of complications associated with pulmonary (13,14) and cardiovascular diseases (15) and the resulting mortality. The occurrence of malignant mesothelioma due to an inhalation of asbestos fiber (16,17), which has become a socially issue in recent years, well illustrates the hazardousness of microparticles. Particulate matters (PM10) of <10 µm in size are associated with respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), acute/chronic bronchitis and lower respiratory tract diseases and cardiovascular diseases such as myocardial infarction, arrhythmias and arteriosclerosis. Besides, particulate matters (PM2.5) of <2.5 µm in size have a poorer effect on the respiratory system and cardiovascular one as compared with PM10 and they also raise the mortality. An analysis was performed for the correlation between the concentration of PM2.5 and the mortality in the USA, according to which 100,000 people are found to die directly or indirectly due to PM2.5 every year.
The number of particulate matters in the atmosphere has been reported to be associated with the occurrence of respiratory diseases. Since then, it has been proposed that nanoparticles inhaled through a mathematical model of the expansion of human lungs might be deposited in an area ranging from the trachea to terminal bronchioles. The surface area of particles rather the amount of particles are associated with the occurrence of inflammatory responses. At the present, no studies have provided the possibility that carbon nanotubes, nano-fibers and nano-wire products might induce the detrimentalness to such an equivalent extent to asbestos. Nevertheless, to rule out this possibility, it is imperative that the safety following a long-term exposure to above materials be audited. An experimental animal model of a long-term exposure would therefore be mandatory.
Nanoparticles contained in particulate matter are known as a strong inducer of oxidative stress in macrophages and epithelial cells lining the airway tract. They increase the activity such molecules as MAP kinase, NF-κB and AP-1 and also promotes the synthesis of oxygen radicals (18,19). This phenomenon is explained as one of the key pathophysiologic mechanisms by which COPD occurs, one of the representative chronic inflammatory respiratory tract diseases. It has been hypothesized that COPD might be a type of dust-induced diseases. In this regard, the use of particulate matters and biomass fuel which has recently been of increasing interest has been considered as a pathogenesis of COPD. Nanoparticles contained in the atmosphere have a possibility for aggravating the respiratory diseases.
In a murine model of asthma, one of the respiratory diseases, particulate matter of 0.2~10 µm in size was experimentally administered. According to this experiment, as the size of particles was relatively smaller, the deposition to the lower respiratory tracts was increased. Following the administration of microparticles into the airway tract in the above experimental model, the concentration of protein in the bronchoalveolar lavage is increased (20) and this is also accompanied by the increased occurrence of eosinophilic and neutrophilic inflammations occurring in the airway tract and the elevated levels of TH2 cytokines (IL-4, IL-5 and eotaxin) (21,22) as well as TH1 cytokines (IFN-γ, IL-6 and TNF-α) (23). Besides, following an intranasal administration of oil flash ash, a key substance for pathological phenomena due to particulate matters in a murine model of asthma, the occurrence of respiratory hypersensitivity and eosinophilic inflammation was further increased (24).
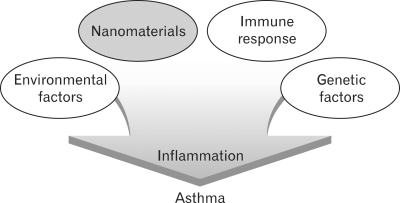
In regard to the effects of nanoparticles on the asthma, a murine model of albumin-induced asthma has shown that the inflammatory responses and the production of immunoglobluins were increased by nanoparticles in the respiratory tract (Fig. 1). These inflammatory phenomena are explained by the expression of IL-5 and eotaxin which was induced in the early stage of inflammation. Subsequently, with the induced expression of such cytokines as IL-13, RANTES and MCP-1, the degree of inflammation was further increased. Moreover, based on the reports that TiO2 which has frequently been used for cosmetic products aggravates the atopic dermatitis, it has been proposed that nanoparticles might aggravate the immune diseases.
Figure 1.
Potential contribution of nanomaterials on asthma pathophysiology.
In conclusion, with the development of nanotechnology, a great deal of nanoproducts and the related materials have been produced. Due to a lack of the clarification of the potential hazard of technical development, however, demand and supply of the products have not been activated up to present. At the present, therefore, if guidelines or landmarks should be provided for safety assessment or data about the safety range including the immune toxicity of nanomaterials, this would greatly contribute to the activation of nanoindustry.
ACKNOWLEDGEMENTS
This work was supported by grants from the Korea Food and Drug Administration in 2007 (07142KFDA764), 2009 (09152 KFDA 695), the NCRC (R15-2004-024-00000-0) and by Nano R&D program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2009-0082417).
Footnotes
The author have no financial conflict of interest.
References
- 1.Dreher KL. Health and environmental impact of nanotechnology: toxicological assessment of manufactured nanoparticles. Toxicol Sci. 2004;77:3–5. doi: 10.1093/toxsci/kfh041. [DOI] [PubMed] [Google Scholar]
- 2.Borm PJ, Klaessig FC, Landry TD, Moudgil B, Pauluhn J, Thomas K, Trottier R, Wood S. Research strategies for safety evaluation of nanomaterials, Part V: role of dissolution in biological fate and effects of nanoscale particles. Toxicol Sci. 2006;90:23–32. doi: 10.1093/toxsci/kfj084. [DOI] [PubMed] [Google Scholar]
- 3.Rushton EK, Jiang J, Leonard SS, Eberly S, Castranova V, Biswas P, Elder A, Han X, Gelein R, Finkelstein J, Oberdörster G. Concept of assessing nanoparticle hazards considering nanoparticle dosemetric and chemical/biological response metrics. J Toxicol Environ Health A. 2010;73:445–461. doi: 10.1080/15287390903489422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivière G. European and international standardisation progress in the field of engineered nanoparticles. Inhal Toxicol. 2009;21(Suppl 1):2–7. doi: 10.1080/08958370902942590. [DOI] [PubMed] [Google Scholar]
- 5.Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Targeting. 2004;12:635–641. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- 6.Bergamaschi E, Bussolati O, Magrini A, Bottini M, Migliore L, Bellucci S, Iavicoli I, Bergamaschi A. Nanomaterials and lung toxicity: interactions with airways cells and relevance for occupational health risk assessment. Int J Immunopathol Pharmacol. 2006;19(4 Suppl):3–10. [PubMed] [Google Scholar]
- 7.Jou MJ. Pathophysiological and pharmacological implications of mitochondria-targeted reactive oxygen species generation in astrocytes. Adv Drug Deliv Rev. 2008;60:1512–1526. doi: 10.1016/j.addr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Xia T, Li N, Nel AE. Potential health impact of nanoparticles. Annu Rev Public Health. 2009;30:137–150. doi: 10.1146/annurev.publhealth.031308.100155. [DOI] [PubMed] [Google Scholar]
- 9.Møller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, Vesterdal LK, Forchhammer L, Wallin H, Loft S. Role of oxidative damage in toxicity of particulates. Free Radic Res. 2010;44:1–46. doi: 10.3109/10715760903300691. [DOI] [PubMed] [Google Scholar]
- 10.Girod CE, King TE., Jr COPD: a dust-induced disease? Chest. 2005;128:3055–3064. doi: 10.1378/chest.128.4.3055. [DOI] [PubMed] [Google Scholar]
- 11.Biswas P, Wu CY. Nanoparticles and the environment. J Ai Waste Manage Assoc. 2005;55:708–746. doi: 10.1080/10473289.2005.10464656. [DOI] [PubMed] [Google Scholar]
- 12.Duffin R, Mills NL, Donaldson K. Nanoparticles-a thoracic toxicology perspective. Yonsei Med J. 2007;48:561–572. doi: 10.3349/ymj.2007.48.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alley D, Langley-Turnbaugh S, Gordon N, Wise J, Van Epps G, Jalbert A. The effect of PM10 on human lung fibroblasts. Toxicol Ind Health. 2009;25:111–120. doi: 10.1177/0748233709103185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, Brauer M. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010;118:284–290. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogueira JB. Air pollution and cardiovascular disease. Rev Port Cardiol. 2009;28:715–733. [PubMed] [Google Scholar]
- 16.Heintz NH, Janssen-Heininger YM, Mossman BT. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol. 2010;42:133–139. doi: 10.1165/rcmb.2009-0206TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rinaudo C, Croce A, Musa M, Fornero E, Allegrina M, Trivero P, Bellis D, Sferch D, Toffalorio F, Veronesi G, Pelosi G. Study of inorganic particles, fibers, and asbestos bodies by variable pressure scanning electron microscopy with annexed energy dispersive spectroscopy and micro-Raman spectroscopy in thin sections of lung and pleural plaque. Appl Spectrosc. 2010;64:571–577. doi: 10.1366/000370210791414380. [DOI] [PubMed] [Google Scholar]
- 18.Eom HJ, Choi J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2B. Toxicol In Vitro. 2009;23:1326–1332. doi: 10.1016/j.tiv.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Eom HJ, Choi J. Oxidative stress of CeO2 nanoparticles via p38-Nrf-2 signaling pathway in human bronchial epithelial cell, Beas-2B. Toxicol Lett. 2009;187:77–83. doi: 10.1016/j.toxlet.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Inoue K, Takano H, Yanagisawa R, Sakurai M, Ichinose T, Sadakane K, Yoshikawa T. Effects of nano particles on antigen-related airway inflammation in mice. Respir Res. 2005;6:106. doi: 10.1186/1465-9921-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberg T, Cassee FR, Groeng EC, Dybing E, Løvik M. Fine ambient particles from various sites in europe exerted a greater IgE adjuvant effect than coarse ambient particles in a mouse model. J Toxicol Environ Health A. 2009;72:1–13. doi: 10.1080/15287390802414471. [DOI] [PubMed] [Google Scholar]
- 22.Park EJ, Yoon J, Choi K, Yi J, Park K. Induction of chronic inflammation in mice treated with titanium dioxide nanoparticles by intratracheal instillation. Toxicology. 2009;260:37–46. doi: 10.1016/j.tox.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Jiao F, Qiu Y, Li W, Lao F, Zhou G, Sun B, Xing G, Dong J, Zhao Y, Chai Z, Chen C. The effect of Gd@C82(OH)22 nanoparticles on the release of Th1/Th2 cytokines and induction of TNF-alpha mediated cellular immunity. Biomaterials. 2009;30:3934–3945. doi: 10.1016/j.biomaterials.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Arantes-Costa FM, Lopes FD, Toledo AC, Magliarelli-Filho PA, Moriya HT, Carvalho-Oliveira R, Mauad T, Saldiva PH, Martins MA. Effects of residual oil fly ash (ROFA) in mice with chronic allergic pulmonary inflammation. Toxicol Pathol. 2008;36:680–686. doi: 10.1177/0192623308317427. [DOI] [PubMed] [Google Scholar]
- 25.Niwa Y, Hiura Y, Sawamura H, Iwai N. Inhalation exposure to carbon black induces inflammatory response in rats. Circ J. 2008;72:144–149. doi: 10.1253/circj.72.144. [DOI] [PubMed] [Google Scholar]
- 26.Koike E, Takano H, Inoue KI, Yanagisawa R, Sakurai M, Aoyagi H, Shinohara R, Kobayashi T. Pulmonary exposure to carbon black nanoparticles increases the number of antigen-presenting cells in murine lung. Int J Immunopathol Pharmacol. 2008;21:35–42. doi: 10.1177/039463200802100105. [DOI] [PubMed] [Google Scholar]
- 27.Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NW, Chu P, Liu Z, Sun X, Dai H, Gambhir SS. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 28.Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, Vlasova II, Belikova NA, Yanamala N, Kapralov A, Tyurina YY, Shi J, Kisin ER, Murray AR, Franks J, Stolz D, Gou P, Klein-Seetharaman J, Fadeel B, Star A, Shvedova AA. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotechnol. 2010;5:354–359. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou CC, Hsiao HY, Hong QS, Chen CH, Peng YW, Chen HW, Yang PC. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008;8:437–445. doi: 10.1021/nl0723634. [DOI] [PubMed] [Google Scholar]
- 30.Herzog E, Byrne HJ, Casey A, Davoren M, Lenz AG, Maier KL, Duschl A, Oostingh GJ. SWCNT suppress inflammatory mediator responses in human lung epithelium in vitro. Toxicol Appl Pharmacol. 2009;234:378–390. doi: 10.1016/j.taap.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, Bonner JC. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009;4:747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye SF, Wu YH, Hou ZQ, Zhang QQ. ROS and NF-kappaB are involved in upregulation of IL-8 in A549 cells exposed to multi-walled carbon nanotubes. Biochem Biophys Res Commun. 2009;379:643–648. doi: 10.1016/j.bbrc.2008.12.137. [DOI] [PubMed] [Google Scholar]
- 33.Fujita K, Morimoto Y, Ogami A, Myojyo T, Tanaka I, Shimada M, Wang WN, Endoh S, Uchida K, Nakazato T, Yamamoto K, Fukui H, Horie M, Yoshida Y, Iwahashi H, Nakanishi J. Gene expression profiles in rat lung after inhalation exposure to C60 fullerene particles. Toxicology. 2009;258:47–55. doi: 10.1016/j.tox.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Muller L, Riediker M, Wick P, Mohr M, Gehr P, Rothen-Rutishauser B. Oxidative stress and inflammation response after nanoparticle exposure: differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways. J R Soc Interface. 2010;7(Suppl 1):S27–S40. doi: 10.1098/rsif.2009.0161.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nemmar A, Melghit K, Ali BH. The acute proinflammatory and prothrombotic effects of pulmonary exposure to rutile TiO2 nanorods in rats. Exp Biol Med (Maywood) 2008;233:610–619. doi: 10.3181/0706-RM-165. [DOI] [PubMed] [Google Scholar]
- 36.Geiser M, Casaulta M, Kupferschmid B, Schulz H, Semmler-Behnke M, Kreyling W. The role of macrophages in the clearance of inhaled ultrafine titanium dioxide particles. Am J Respir Cell Mol Biol. 2008;38:371–376. doi: 10.1165/rcmb.2007-0138OC. [DOI] [PubMed] [Google Scholar]
- 37.Yanagisawa R, Takano H, Inoue K, Koike E, Kamachi T, Sadakane K, Ichinose T. Titanium dioxide nanoparticles aggravate atopic dermatitis-like skin lesions in NC/Nga mice. Exp Biol Med (Maywood) 2009;234:314–322. doi: 10.3181/0810-RM-304. [DOI] [PubMed] [Google Scholar]
- 38.Morishige T, Yoshioka Y, Tanabe A, Yao X, Tsunoda S, Tsutsumi Y, Mukai Y, Okada N, Nakagawa S. Titanium dioxide induces different levels of IL-1beta production dependent on its particle characteristics through caspase-1 activation mediated by reactive oxygen species and cathepsin B. Biochem Biophys Res Commun. 2010;392:160–165. doi: 10.1016/j.bbrc.2009.12.178. [DOI] [PubMed] [Google Scholar]
- 39.Cho WS, Kim S, Han BS, Son WC, Jeong J. Comparison of gene expression profiles in mice liver following intravenous injection of 4 and 100 nm-sized PEG-coated gold nanoparticles. Toxicol Lett. 2009;191:96–102. doi: 10.1016/j.toxlet.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Hutter E, Boridy S, Labrecque S, Lalancette-Hébert M, Kriz J, Winnik FM, Maysinger D. Microglial response to gold nanoparticles. ACS Nano. 2010;4:2595–2606. doi: 10.1021/nn901869f. [DOI] [PubMed] [Google Scholar]
- 41.Brandenberger C, Rothen-Rutishauser B, Mühlfeld C, Schmid O, Ferron GA, Maier KL, Gehr P, Lenz AG. Effects and uptake of gold nanoparticles deposited at the air-liquid interface of a human epithelial airway model. Toxicol Appl Pharmacol. 2010;242:56–65. doi: 10.1016/j.taap.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Park EJ, Kim H, Kim Y, Yi J, Choi K, Park K. Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology. 2010 doi: 10.1016/j.tox.2010.06.002. PMID: 20540983. [DOI] [PubMed] [Google Scholar]
- 43.Zhong CY, Zhou YM, Smith KR, Kennedy IM, Chen CY, Aust AE, Pinkerton KE. Oxidative injury in the lungs of neonatal rats following short-term exposure to ultrafine iron and soot particles. J Toxicol Environ Health A. 2010;73:837–847. doi: 10.1080/15287391003689366. [DOI] [PubMed] [Google Scholar]
- 44.Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2:2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park YH, Kim JN, Jeong SH, Choi JE, Lee SH, Choi BH, Lee JP, Sohn KH, Park KL, Kim MK, Son SW. Assessment of dermal toxicity of nanosilica using cultured keratinocytes, a human skin equivalent model and an in vivo model. Toxicology. 2010;267:178–181. doi: 10.1016/j.tox.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Hu Y, Jin Z, Jiang H, Wen J. Silica-induced TNF-alpha and TGF-beta1 expression in RAW264.7 cells are dependent on Src-ERK/AP-1 pathways. Toxicol Mech Methos. 2009;19:51–58. doi: 10.1080/15376510802354201. [DOI] [PubMed] [Google Scholar]
- 47.Nishimori H, Kondoh M, Isoda K, Tsunoda S, Tsutsumi Y, Yagi K. Silica nanoparticles as hepatotoxicants. Eur J Pharm Biopharm. 2009;72:496–501. doi: 10.1016/j.ejpb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2008;2:85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- 49.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 50.Inoue K, Takano H, Yanagisawa R, Koike E, Shimada A. Size effects of latex nanomaterials on lung inflammation in mice. Toxicol Appl Pharmacol. 2009;234:68–76. doi: 10.1016/j.taap.2008.09.012. [DOI] [PubMed] [Google Scholar]