Abstract
Infarct volume independently predicts cardiovascular events. Fragmented QRS complexes (fQRS) may complement Q-waves for identifying infarction; however, their utility in advanced coronary disease is unknown. We tested whether fQRS could improve the ECG prediction of infarct volume by PET in 138 patients with ischemic cardiomyopathy (EF 0.27±0.09). Indices of infarction (pathologic Q-waves, fQRS, and Selvester QRS Score) were analyzed by blinded observers. In patients with QRS duration <120ms, number of leads with pathologic Q-waves (mean 1.6±1.7) correlated weakly with infarct volume (r=0.30, p<0.05). Adding fQRS increased the number of affected leads (3.6±2.5), but the significant correlation with infarct volume was lost (r=0.02, p=0.10). Selvester Score was the most accurate (mean 5.9±4.9 points; r=0.49, p<0.001). fQRS was not predictive of infarct size in patients with QRS duration ≥120ms (r=0.02, p=0.19). Thus in ischemic cardiomyopathy, consideration of fQRS complexes does not improve Q-wave prediction of infarct volume, but Selvester Score was more accurate.
Keywords: Electrocardiography, Positron Emission Tomography, Infarct Volume, Ischemic Cardiomyopathy
BACKGROUND
In patients with left ventricular (LV) systolic dysfunction and heart failure, the extent of infarction has been shown to predict the progression of symptoms, survival, and response to therapies (i.e. biventricular pacing), and appears to be more useful than ejection fraction (EF) or LV volumes.(1,2) In addition, coronary revascularization offers greater benefit among those with limited infarction. (3,4) Although infarct volume can be accurately quantified by magnetic resonance imaging (MRI),(5,6) single photon emission computed tomography (SPECT)(7–9) or positron emission tomography (PET),(1,3,10) an accurate electrocardiographic (ECG) parameter would be desirable in view of its routine acquisition and affordability. The most specific ECG sign of a previous myocardial infarction (MI) is the presence of pathological Q-waves. However, Q-waves are relatively insensitive due to poor representation of certain myocardial regions (posterior segments), the increasing incidence of non Q-wave infarctions, and the eventual disappearance of Q-waves in approximately one-third of patients. (11) Despite these well acknowledged limitations, Q-waves are still useful in assessing infarct location and extent(6), and a greater “Q-wave burden” has been shown to predict larger infarct volume in patients with ischemic cardiomyopathy.(10)
Recent data has shown that in patients with suspected coronary artery disease, the additional consideration of fragmented QRS complexes (fQRS) can improve the sensitivity for identifying previous infarction(8) and can also predict greater stress perfusion abnormalities.(9) Furthermore, fQRS are present in a majority of patients with previous Q-wave infarction,(12) frequently persist even when Q-waves disappear,(12) and may be applicable to patients with wide QRS complexes (≥ 120 ms).(13) We therefore hypothesized that the consideration of fQRS complexes should improve the identification of myocardial infarction in patients with more extensive coronary artery disease and ischemic cardiomyopathy, and fQRS should complement Q-wave burden in the prediction of infarct volume. In order to gauge the accuracy with which the combined criteria of Q-wave or fQRS could predict infarct volume as quantified by PET, these correlations were also compared with the previously validated Selvester QRS scoring system for infarct volume estimation. (14,15)
METHODS
Patients for this investigation were drawn from the PAREPET (Prediction of ARrhythmic Events with Positron Emission Tomography) study which is an ongoing National Institutes of Health-sponsored observational trial evaluating PET imaging to predict sudden cardiac death (SCD) in patients with ischemic cardiomyopathy.(16) This study is enrolling patients with documented coronary artery disease, New York Heart Association (NYHA) functional class I-III heart failure symptoms, and an EF of ≤ 0.35 who are eligible for primary prevention of SCD. Patients with recent MI or revascularization were excluded, as were those who had indications for the secondary prevention of SCD (i.e. unexplained syncope, sustain ventricular arrhythmias). Consecutive patients (n = 138) with both a 12-lead ECG and PET-quantified infarct volume were included.
Electrocardiographic Indices of Infarction
Twelve lead ECGs were recorded using H12+ Holter recorders (V3.12, Mortara Instruments, Milwaukee, WI). To optimize signal quality, the patient’s skin was shaved (if necessary), rubbed with alcohol wipes until thoroughly clean, and briskly dried with gauze to stimulate capillary flow. Disposable pre-gelled silver chloride electrodes were applied in the Mason-Likar lead configuration, in which limb lead electrodes are placed on the torso rather than the distal extremities. All leads were simultaneously acquired at a high resolution (1,000 samples/second) resulting in high fidelity recordings with a frequency response of 0.05 to 60 Hz. Notably, the Selvester QRS score and fQRS analysis requires recording of frequencies up to 120 Hz for high-frequency features of the QRS complex, including notching and fractionation, that could be missed with a recording using the Holter standard of 60 Hz.(13) The first ECG of the monitoring period was selected in order to ensure a resting supine position, and with the ELI LINK program (Mortara Instruments) a single 12-lead ECG was exported into a portable document format (.pdf) with the standard filter setting at 0.05 – 150Hz for subsequent analysis.
Computer-quantified QRS duration was averaged over one minute using all leads, and patients were divided into those with narrow (<120 ms) versus wide (≥ 120 ms) QRS complexes(8). Although computer-quantified intervals tend to overestimate manual measurements, automated measurements have less variability and are thus more reproducible(17). All ECGs (n = 138) were printed on paper at standard speed (25 mm/sec) and calibration (10 mm/mV) for evaluation of ECG indices of infarction (fQRS, Q-waves, and Selvester Score). Wide complex ECGs were only evaluated for fQRS complexes due to the poor specificity of Q waves in this setting.(18,19)
Pathologic Q waves and QRS Fragmentation
Two investigators, blinded to all clinical data, independently interpreted the ECG for the presence or absence of pathologic Q waves and fQRS complexes (Figure 1). For ECGs with a narrow QRS (n = 52), pathological Q waves were defined as ≥ 40 msec duration and greater than one fourth the voltage of the subsequent R wave.(8) fQRS was defined as: a) any RSR morphology, b) notching in the nadir of the S wave or c) more than one R wave (R’), with the exclusion of incomplete right bundle branch block.(8) In the presence of an intrinsic wide QRS rhythm (n = 45), fQRS was defined as various RSR’ patterns with or without Q-waves, with >2 R-waves (R’) or > 2 notches in the R-wave, or >2 notches in the S-wave.(13) In the presence of ventricular paced rhythms (n = 41), fQRS was defined as the presence of >2 R’ or >2 notches in the nadir of the S-wave.(13) Leads V1 and aVR were excluded from the analysis because of the frequent presence of non-specific Q-waves,(20) and to maintain consistency with previously published analyses.(13) In cases of disagreement, final characterization was determined by a third investigator who was also blinded to all clinical information. For the combined analysis of “Q waves or fQRS” a lead was considered to have evidence of infarction if there was either a Q wave or fQRS (for a maximum of 10 leads). In addition to a simple summation of the number of leads with evidence for infarction,(6,10) an analysis was performed requiring two contiguous leads within a major coronary territory (Anterior – leads V2, V3, V4 and V5; Inferior – leads II, III, and aVF; Lateral – leads I, aVL, and V6.(8,21) Subjects were subsequently divided into the following groups: ≥ 1 territory with evidence of infarction, ≥ 1 lead but no territory with evidence of infarction, and no leads with evidence of infarction.
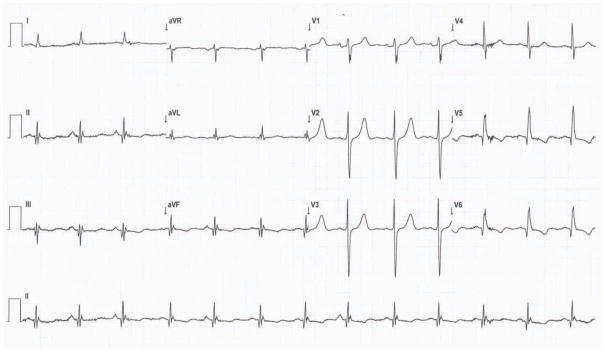
Figure 1. Representative Electrocardiographic Indices of Infarction.
This 12-lead ECG with the Mason-Likar lead configuration demonstrates pathological Q waves (II, III, aVF, V5 and V6) and fQRS complexes (II, III, aVF, aVL,V5, and V6) in the inferior and lateral lead. The Selvester QRS Score for this tracing was 5, which equates to an estimated infarct size of 15% of the LV. The infarct volume by PET was 22% of the LV.
Selvester QRS Score
The predictive accuracy of Q waves and fQRS complexes to estimate myocardial infarct volume was compared with the previously validated Selvester QRS Score,(14) by a reviewer blinded to all clinical data. This scoring system consists of 57 ECG criteria used to assign up to 32 points, each of which corresponds to infarction of 3% of the LV. Criteria are based on wave duration (Q or R), wave amplitude (R or S), and amplitude ratios (R/Q or R/S).
In order to determine the potential role of lead configuration on our results, ECGs performed with the standard lead configuration in a subset of subjects (n = 20) were reviewed, and compared to ECGs in the Mason-Likar lead configuration. The Selvester Scores derived using the two electrode configurations was strongly correlated (r = 0.72, p < 0.001). Among this subset of subjects the correlation between Selvester Score and infarct volume was not statistically significant for either electrode configuration. However, the correlation coefficient was actually higher for the Mason-Likar configuration (r = 0.39, p = 0.09) than the standard leads (r = 0.30, p = 0.20). Thus, it is unlikely that the Mason-Likar lead configuration confounded our Selvester Score results.
Quantification of Infarct Volume by Positron Emission Tomography
All patients (n = 138) underwent PET imaging with 13N-ammonia and insulin-stimulated 18F-2-flouro-2-deoxyglucose (FDG). The radiopharmaceutical synthesis and imaging protocol have been previously described. (16) Infarct volume was quantified using the PET image analysis program MyoPC® (University of Ottawa Heart Institute, Ontario, Canada)(3,10,16), by a reviewer blinded to all clinical data. Briefly, the heart was divided into 496 sectors corresponding to mid-myocardial voxels of ~5mm3. Regional perfusion was assessed with 13N-ammonia uptake, and regional tracer activity was determined as a percent of maximal activity for each patient. In order to control for potential heterogeneity in FDG uptake in viable myocardium, FDG deposition was normalized in those sectors with normal perfusion (≥ 80% of peak perfusion).(3,10,16) After normalization, FDG sectors with values >100% were reset to 100%. The volume of infarct in sectors with <80% perfusion was determined by the corresponding FDG uptake and expressed as a percentage of the total LV by the following equation: infarct volume (% LV) = ((Σ(100 − normalized FDG score)/49,600)) × 100 (Figure 2).(3,10,16) The presence of infarction in a single versus multiple coronary artery distribution was determined using the 17 segment model of the left ventricle and the standard assignment of coronary territories(22).
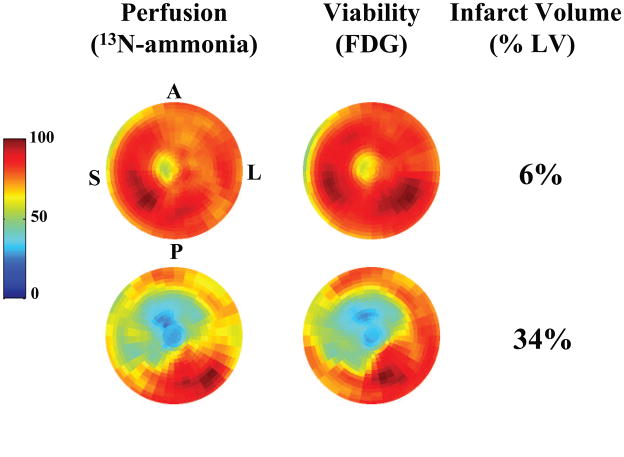
Figure 2. Representative PET Images in Patients with Ischemic Cardiomyopathy.
In these polar tomograms the apex of the LV is in the middle of each image, with the basal segments at the periphery. Normal tracer deposition (perfusion with 13N-ammonia on the left and viability with FDG on the right) is shown in dark red and dark blue denotes no tracer activity. The upper pair of tomograms is from a patient with a small infarct primarily involving the apex (6% of LV). The lower pair of tomograms shows extensive infarction involving the septal, anterior, anterolateral and apical regions (34% of the LV). (A – anterior, P – posterior, L – lateral, S – septal)
Statistical Analysis
All values are mean ± standard deviation. Agreement between investigators for the presence of pathological Q-waves and fQRS in individual leads was evaluated by a κ coefficient. Categorical values were compared by χ2 test, t-tests and ANOVA were used for continuous data. The degree of association between scar volume and various electrocardiographic parameters was determined using the Pearson’s correlation coefficient (r). With a sample size of 52 patients with QRS duration of <120 ms, there was 97% power to detect a correlation that would account for at least 25% of the variability in infarct size (with α = 0.05). A Bland-Altman analysis was also used to compare infarct volume determinations by PET and Selvester QRS Score. All statistical analyses were performed with SPSS (Version 17). P < 0.05 was considered statistically significant.
RESULTS
Patient Demographics
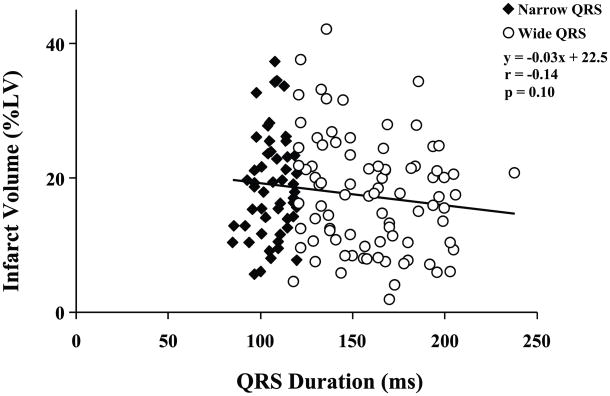
The demographic characteristics of the study sample are shown in Table 1. The average age was 68 ± 12 yrs, with an average EF of 0.27 ± 0.09. Subjects were mostly men. All patients had stable heart failure and angina symptoms, and were appropriately managed with beta-blockers (96%) and angiotensin converting enzyme inhibitors or angiotensin II receptor blockers (88%). As expected in a sample of patients with ischemic cardiomyopathy, all patients had evidence of myocardial infarction by PET imaging, averaging 17.9 ± 8.1% of the LV. As illustrated in Figures 3, and consistent with previous reports,(10) there was a wide variability in infarct volume among patients (range, 2 – 42%). Not surprisingly, patients with wide QRS complexes and or paced rhythms (n = 86) had worse prognostic features, including older age, worse NYHA functional class, lower EF, and greater LV end-diastolic volumes.(23) Nevertheless, there was no significant difference in infarct volume between those with narrow versus wide QRS complexes, nor was there any correlation between QRS duration and infarct volume (r = −0.14, p = 0.10; n = 138, Figure 3).
Table 1.
Baseline Characteristics n=138
| n | Age | Class HF | Ejection Fraction | End Diastolic Volume (EDV) | Infarct Size (% LV) | |
|---|---|---|---|---|---|---|
| Consecutive Patients | 138 | 68.2±11.7 | 2.1±0.67 | 0.27±0.09 | 180±56 | 17.9±8.1 |
| Narrow QRS (<120 ms) | 52 | 65.2±12.3 | 1.8±0.61 | 0.30±0.10 | 165±52 | 18.7±7.7 |
| Intrinsic Wide QRS (≥ 120 ms) | 45 | 65.9±10.9 | 2.0±0.64* | 0.26±0.08* | 208±60* | 18.4±9.2 |
| Paced Complexes | 41 | 74.5±9.6*† | 2.4±0.63*† | 0.24±0.09* | 169±46† | 16.3±7.2 |
Values are mean ± SD;
p<0.05 vs. Narrow QRS;
p<0.05 vs. Intrinsic Wide QRS, per ANOVA with post hoc Bonferroni correction
Figure 3. QRS Duration and Infarct Volume.
Among all 138 patients QRS duration ranged from 85 to 238 msec (mean 140 ± 34 msec). Data from patients with QRS duration of < 120 ms (Narrow QRS) are represented by black diamonds, and those with QRS duration ≥ 120 ms (Wide QRS) are shown as white circles. There was no significant correlation between QRS duration and infarct volume (r = −0.14, p = 0.10).
ECG Parameters of Infarction versus Infarct Volume in the Setting of Narrow QRS Complexes (n=52)
Q-Waves
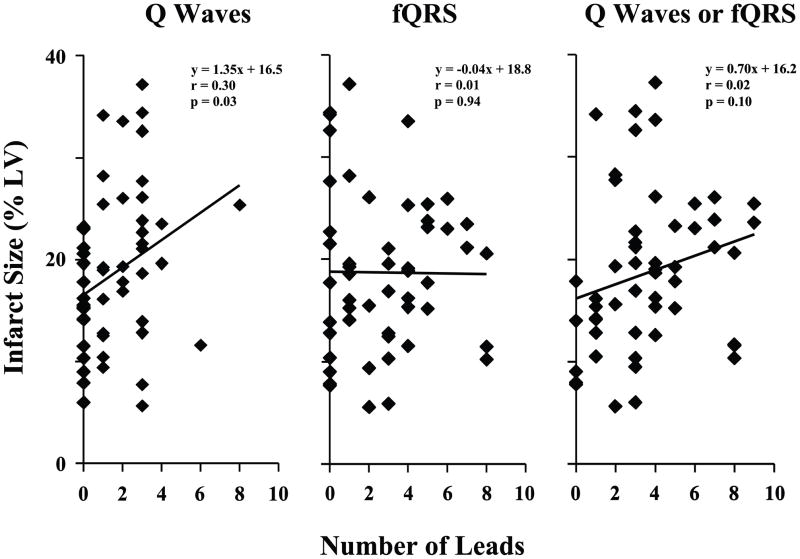
Overall there was excellent agreement between the two investigators for the presence of pathological Q-waves (κ = 0.94) and fQRS complexes (κ = 0.90) on individual leads. At least one pathologic Q-wave (in the 10 leads after the exclusion of leads V1 and aVR) was present in 33 of these 52 patients with narrow QRS complexes (63%), with an average of 1.6 ± 1.7 per patient (range, 0 to 8). Although there was a statistically significant correlation between number of Q-waves and infarct volume, the correlation was weak, accounting for only 9% of the variability in infarct volume (r = 0.30, p = 0.03; Figure 4, left graph). Limited specificity of isolated Q-waves may have influenced this poor correlation since infarct volume was significantly greater in the 15 patients (27%) with two or more leads with pathologic Q-waves in a coronary artery territory (ANOVA p = 0.03).
Figure 4. Infarct Volume as Predicted by Q Waves Alone, fQRS Alone, or Q Waves Combined with fQRS.
The left graph shows the correlation between infarct volume and the number of leads with pathologic Q waves (with leads V1 and aVR excluded) in patients with narrow QRS complexes (n = 52). Although the correlation was statistically significant, the relationship was weak and Q waves only accounted for 9% of the variability in infarct volume. The middle graph shows that although fQRS complexes are common in patients with ischemic cardiomyopathy, the number of leads with fQRS did not correlate with infarct volume. By combining Q waves and fQRS as indices of infarction (right graph), the number of affected leads significantly increased (to 3.6 ± 2.5, p < 0.001 vs. either Q waves or fQRS alone), but there was no improvement in the correlation with infarct volume than with Q waves alone.
fQRS Complexes
fQRS complexes were common in patients with ischemic cardiomyopathy, occurring in 39 of 52 patients with narrow QRS complexes (75%). There was an average of 2.7 ± 2.4 fQRS per patient (range 0 to 8). Despite the previous associations of fQRS with infarction in patients with less prevalent coronary artery disease(8,21), there was no correlation between number of leads with fQRS and infarct volume in patients with ischemic cardiomyopathy (Figure 4, middle graph). This result was not improved by requiring two or more leads with fQRS in a coronary artery territory (ANOVA, p = 0.86).
Q-waves or fQRS
The consideration of fQRS in addition to Q-waves increased the sensitivity to identify patients with ischemic cardiomyopathy, with at least one lead suggesting infarction in 47 of 52 patients with narrow QRS complexes (90%). There was an average of 3.6 ± 2.5 leads with ‘Q-waves or fQRS’ per patient (range 0 to 9, p < 0.001 vs. both Q-waves alone and fQRS alone). Nevertheless, the number of leads with either ‘Q-waves or fQRS’ was not significantly correlated with infarct volume (r = 0.023, p = 0.10; Figure 4, right graph). By considering ‘Q-waves or fQRS’, there were 38 patients (73%) with two or more leads indicative of infarction within a coronary territory. Nevertheless, there was no difference in infarct volume among groups determined by coronary territories (ANOVA, p = 0.09).
Selvester QRS Score
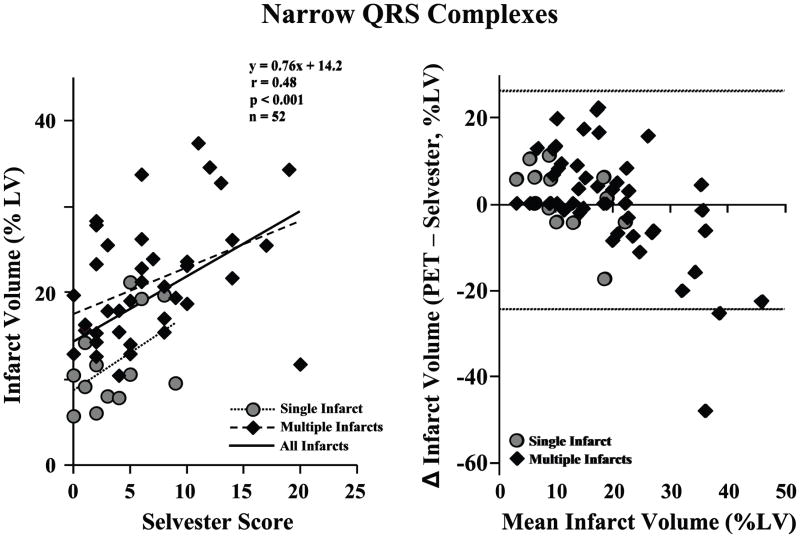
The ability to predict infarct volume by the frequency of pathologic Q-waves or fQRS complexes was compared to the previously validated Selvester QRS Score.(14,15) The average Selvester QRS Score was 5.9 ± 4.9 points (range 0 to 20), and this was moderately correlated to infarct volume (r = 0.49, p < 0.001; Figure 5, left graph). Because correlation does not necessarily imply good agreement, a Bland Altman plot (Figure 5, right graph) was generated to determine the degree of agreement between infarct volume using the PET imaging versus the Selvester QRS Score. There was no significant difference between the average infarct volumes determined by PET versus Selvester QRS Score, although the 95% confidence intervals include a range of 50% of the LV.
Figure 5. Selvester QRS Score and Infarct Volume by PET Imaging with Narrow QRS Complexes.
The left graph shows the correlation between infarct volume and the Selvester QRS Score. Among all patients with narrow QRS complexes (n = 52), the Selvester Score was moderately correlated with infarct size (black line, r = 0.48, p < 0.001). The correlation between Selvester QRS score and infarct volume among subjects with single infarcts (gray circles and dotted line; n = 13, r = 0.49, p = 0.09) was similar to that of subjects with multiple infarcts (black diamonds and dashed line; n = 39, r = 0.41, p=0.01). The Bland-Altman plot (right graph) shows the degree of agreement between infarct volumes using the PET and the Selvester QRS Score. The average infarct size by PET was similar to that estimated by the Selvester QRS Scores, with a mean difference of < 1% of the LV. There was no significant difference in predictive accuracy between subjects with single versus multiple infarcts. The horizontal dotted lines denote the 95% confidence limits.
Coronary Distribution of Infarction
As expected in a population of patients with ischemic cardiomyopathy, a majority of subjects (n = 39, 75%) had evidence of infarction in multiple coronary territories. Among subjects with a single infarct territory, anterior infarction was most common (10 of 13, 77%). Not surprisingly, infarct volume by PET was larger in those with multiple infarct territories (21.0 ± 7.0% vs. 11.7 ± 5.2%, p < 0.001; Figure 5). Although infarction in multiple coronary infarction territories has been noted to be particularly challenging for ECG scoring systems(24), there was a statistically significant correlation between Selvester Score and infarct volume in those subjects with multiple infarcts (r = 0.41, p = 0.01; Figure 5, left graph). Although the correlation between Selvester Score and infarct volume by PET was not statistically significant in the single infarct subgroup (r = 0.49, p = 0.09), the correlation coefficient was somewhat higher than among those with multiple infarcts.
fQRS Complexes to Predict Infarct Volume in the Setting of Wide QRS Complexes (n=86)
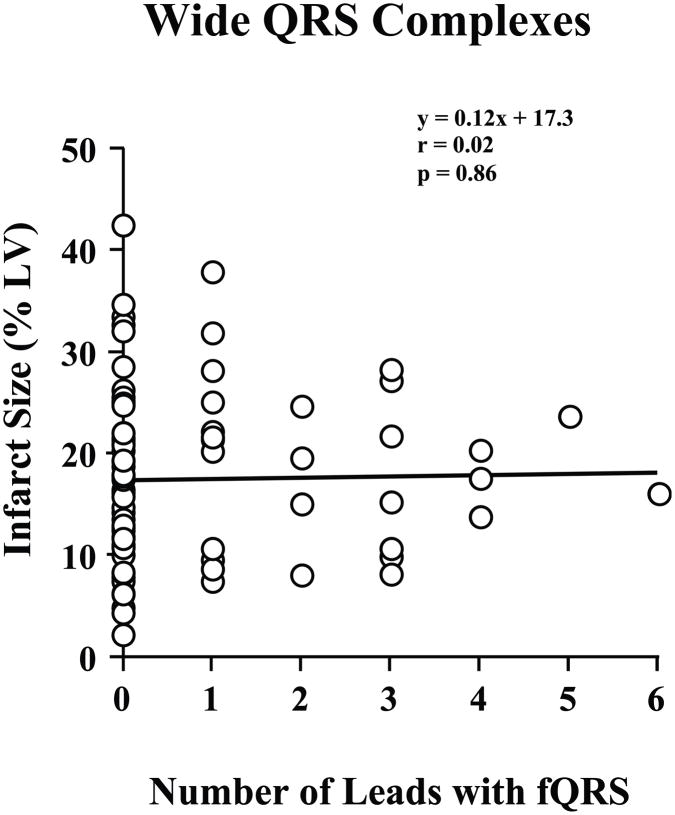
As compared to those with narrow QRS complexes, fQRS complexes were less frequent among those with wide QRS complexes (29 of 86 patients, 34%, χ2 p = 0.01 vs. narrow QRS complexes). There was an average of 0.8 ± 1.3 leads with fQRS per patient (range 0 to 6), and no significant correlation between number of leads with fQRS and infarct volume (r = 0.02, p = 0.86; Figure 6).
Figure 6. fQRS and Infarct Volume by PET Imaging with Wide QRS Complexes.
Among patients with a QRS duration ≥ 120 ms, there was no correlation between infarct volume and the number of ECG leads with an fQRS complex (n = 86).
DISCUSSION
There are several novel findings from the present study that improve our knowledge regarding the potential utility of fQRS complexes in the identification of prior infarction, as well as the more general theme of the ECG estimation of infarct volume. First, in patients with ischemic cardiomyopathy the evaluation of fQRS does increase the sensitivity of the ECG to identify evidence of previous infarction. However, in patients with narrow QRS complexes, infarct volume was weakly correlated to the number of leads with Q-waves, but not to the number of leads with fQRS (Figure 4). Furthermore, when either Q-waves or fQRS were considered evidence of infarction, the estimation of infarct volume was not improved over Q-waves alone (Figure 4). Finally, our data validates the Selvester QRS Score in patients with ischemic cardiomyopathy and multiple infarcts (Figure 5), and indicates that in this population it is superior in predicting infarct volume as compared to Q-waves (with or without fQRS, Figure 4).
Fragmented QRS Complexes in Patients with Coronary Artery Disease
In ischemic heart disease, the course notching of the fQRS complex suggests the irregularity of the border zone of infarction causing the waveform direction to reverse, or notch as it encounters the margin.(25) Several recent studies have highlighted the potential clinical utility of fQRS, in which prior myocardial infarction can result in altered ventricular depolarization without Q-waves.(8) For example, in patients undergoing stress testing for known or suspected coronary disease, Das and colleagues have shown that fQRS complexes can predict the presence of SPECT diagnosed myocardial infarction with significantly higher sensitivity and negative predictive value than pathologic Q-waves.(8) This group subsequently showed that fQRS in patients without Q-waves were associated with greater stress perfusion defects and wall motion abnormalities.(9) In a higher risk cohort with prior Q-wave myocardial infarction, fQRS were found to be common (53% of patients), but did not portend a poorer prognosis over ~2 years.(12) Nevertheless, when Q-waves resolved on the ECG the presence of fQRS was associated with a 2-fold increase in cardiac events as compared to patients with neither Q-waves or fQRS, as well as those with persistent Q-waves.(12) Das et al. have confirmed the prognostic significance of fQRS in patients referred for stress testing.(21) Over an average follow-up of ~4.5 years, the presence of fQRS was shown to predict cardiac events independent of multiple risk factors (including Q-waves and the results of perfusion imaging).(21)
In our study, we have extended this previous research by evaluating fQRS in patients with more severe coronary artery disease and ischemic cardiomyopathy. Consistent with the previous findings in patients with a lower likelihood of coronary artery disease(8,9), the additional consideration of fQRS did improve the sensitivity to identify myocardial infarction over Q waves alone in this population. However, the consideration of fQRS did not improve the prediction of infarct volume (either on an individual lead or a territory basis) over Q-waves alone. This was surprising in view of the fact that infarct volume was better correlated with Selvester QRS Score than Q-waves, and the Selvester Score considers R-wave notching in the evaluation of some ECG leads.(14,15)
Electrocardiographic Estimation of Infarct Volume
The diagnosis of myocardial infarction remains a cornerstone function of the 12-lead ECG; and Q-waves, despite their limitations, can be used to predict infarct volume. For example, investigators at the Ottawa Heart Institute determined the ability of Q-waves to estimate infarct volume in patients with ischemic cardiomyopathy (ejection fraction 0.27 ± 0.07) using the same PET algorithm to quantify infarction volume as the present study.(10) Although the number of leads with Q-waves were not considered as a continuous variable, patients with 0 to 4 Q-waves were shown to have had smaller infarcts than patients with 5 or more Q-waves (13.9 ± 7.3 vs. 20.6 ± 8.1%, p = 0.001). More recently, Rovai et al. used magnetic resonance imaging to show that Q-waves can not only predict infarct volume, but also the transmural extent of first myocardial infarction.(6)
Other ECG parameters in addition to Q-waves have been shown to improve the predictive accuracy of the 12-lead ECG to estimate infarct volume. The most successful of these algorithms is the quantitative Selvester QRS Score. This was initially developed as a simulation based on normal human ventricular activation,(26) and subsequently refined using the extent of akinesis by biplane contrast ventriculography in patients with proximal coronary occlusions.(14,26) In previous studies that compared infarct volume with the Selvester QRS Score, the vast majority of enrolled patients had a single, relatively recent myocardial infarction. This included the initial validation studies against postmortem examination(14) as well as more recent studies that used 99mTc-sestamibi SPECT(7) or magnetic resonance imaging.(5) The Selvester QRS score has also been shown to outperform other infarct scoring methods (Minnesota Score, Novacode, and Cardiac Infarction Injury Score) in estimating inferior and posterolateral infarcts, but all methods were poorly predictive of infarct size in the presence of multiple infarcts.(24)
Our results are consistent with these previous studies, and extend them in several ways. We have confirmed that the number of ECG leads with pathologic Q-waves (as a continuous variable) correlates with infarct volume even in patients with ischemic cardiomyopathy, although the correlation is weak and unlikely to be clinically useful. Consistent with previous interpretations, this likely reflects the poor sensitivity of Q-waves, not only due to non Q-wave myocardial infarctions and the eventual resolution of many Q-waves,(27) but also to regional differences in Q-wave pathogenesis.(14) This later point is underscored by the recent magnetic resonance imaging data showing that Q-waves are much more accurate at predicting infarct volume and transmural extent of infarctions involving the anterior wall.(6) Importantly, the limitations of Q-wave quantification cannot be overcome by the relatively simple additional consideration of fQRS complexes in patients with ischemic cardiomyopathy. Thus, at present, the Selvester QRS Score provides the most useful electrocardiographic approximation of infarct volume, even when infarctions are multiple(28) and temporally remote as in the present population.
Our data did not reveal any significant correlation between QRS duration and infarct volume, which is in contrast to the finding in patients with non-ischemic cardiomyopathy.(29) Although both studies used PET imaging for the quantification of infarct volume, the previous study determined the presence or absence of infarction in each of 24 segments.(29) This resulted in larger infarct volumes (mean 25 ± 18% of the LV, range 0 – 63%) than in the present study (p < 0.001). Furthermore, there are differences in the mechanism of scar formation in ischemic and non-ischemic cardiomyopathy, with variable effects on ventricular depolarization and electrical conduction. For example, in patients with ischemic heart disease prolonged ventricular depolarization can result from a small infarct involving the His-Purkinje system, whereas a large infarct outside of the conduction system might have a normal QRS duration. In contrast, fibrosis in non-ischemic cardiomyopathy is more likely to be the result of ventricular dilation and myocardial remodeling, which would also contribute to a prolongation of ventricular depolarization. This argument is supported by the worse heart failure class of the patients in the previous study (limited to NYHA Class III and IV), and the greater QRS duration in those with non-ischemic cardiomyopathy (excluding ventricular pacing, 144 ± 38 vs. 127 ± 27 msec in the present study, p < 0.01).
Methodological Limitations
The 12-lead ECGs used for the primary analyses in the present study were performed using the Mason-Likar lead configuration in which limb lead electrodes are placed on the torso. Although this improves signal quality and patient comfort during continuous ambulatory monitoring, it is different than the lead configuration used for previous validation of the Selvester QRS score.(15) As compared to ECGs performed with electrodes on the limbs, with the Mason-Likar configuration the R-wave amplitude in the lateral leads is reduced while inferior R-wave amplitude may increase and Q-wave amplitudes are underestimated.(30) To address this potential for confounding, ECGs using the standard electrode configuration from a subset of subjects were also reviewed and compared to the Mason-Likar configuration. As expected, the Selvester Scores using the two different lead configurations were strongly correlated (r = 0.72, p < 0.001). Furthermore, among these patients the Mason-Likar lead configuration actually resulted in a greater correlation with PET infarct volume than the standard configuration. Thus, our data support the utility of the Selvester QRS Score for estimating infarct size using the Mason-Likar lead configuration, and reinforce the recently published contention that the standard 12-lead ECG could be acquired with limb leads placed on the body torso as many criteria have been developed from tracings acquired during invasive cardiac electrophysiology studies, exercise stress tests, and ambulatory monitoring; all of which use torso positioned limb leads.(31)
Clinical Implications
Although fQRS complexes were not shown to improve the prediction of infarct volume in the present study, this does not exclude their usefulness in predicting prognosis, as has been shown in previous investigations (12,21). The PAREPET trial was designed to test various non-invasive predictors of cause-specific mortality (16), and long-term follow-up of this population will directly test the role for fQRS to predict events in patients with ischemic cardiomyopathy.
Acknowledgments
Supported by Grants from the National Institutes of Health (K23 NR-009716, MGC) and (RO1 HL-076252, JMC and JAF)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feola M, Biggi A, Chauvie S, et al. Myocardial scar and insulin resistance predict cardiovascular events in severe ischaemic myocardial dysfunction: a perfusion-metabolism positron emission tomography study. Nucl Med Commun. 2008;29:448–54. doi: 10.1097/MNM.0b013e3282f5d2bc. [DOI] [PubMed] [Google Scholar]
- 2.Yokota H, Heidary S, Katikireddy CK, et al. Quantitative characterization of myocardial infarction by cardiovascular magnetic resonance predicts future cardiovascular events in patients with ischemic cardiomyopathy. J Cardiovasc Magn Reson. 2008;10:17. doi: 10.1186/1532-429X-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beanlands RS, Ruddy TD, deKemp RA, et al. Positron emission tomography and recovery following revascularization (PARR-1): the importance of scar and the development of a prediction rule for the degree of recovery of left ventricular function. J Am Coll Cardiol. 2002;40:1735–1743. doi: 10.1016/s0735-1097(02)02489-0. [DOI] [PubMed] [Google Scholar]
- 4.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151–1158. doi: 10.1016/s0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 5.Engblom H, Hedstrom E, Heiberg E, et al. Size and transmural extent of first-time reperfused myocardial infarction assessed by cardiac magnetic resonance can be estimated by 12-lead electrocardiogram. Am Heart J. 2005;150:920. doi: 10.1016/j.ahj.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Rovai D, Di Bella G, Rossi G, et al. Q-wave prediction of myocardial infarct location, size and transmural extent at magnetic resonance imaging. Coron Artery Dis. 2007;18:381–9. doi: 10.1097/MCA.0b013e32820588c2. [DOI] [PubMed] [Google Scholar]
- 7.Barbagelata A, Di Carli MF, Califf RM, et al. Electrocardiographic infarct size assessment after thrombolysis: insights from the Acute Myocardial Infarction STudy ADenosine (AMISTAD) trial. Am Heart J. 2005;150:659–65. doi: 10.1016/j.ahj.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 9.Mahenthiran J, Khan BR, Sawada SG, Das MK. Fragmented QRS complexes not typical of a bundle branch block: a marker of greater myocardial perfusion tomography abnormalities in coronary artery disease. J Nucl Cardiol. 2007;14:347–53. doi: 10.1016/j.nuclcard.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Ananthasubramaniam K, Chow BJ, Ruddy TD, et al. Does electrocardiographic Q wave burden predict the extent of scarring or hibernating myocardium as quantified by positron emission tomography? Can J Cardiol. 2005;21:51–6. [PubMed] [Google Scholar]
- 11.Wu E, Judd RM, Vargas JD, et al. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet. 2001;357:21–8. doi: 10.1016/S0140-6736(00)03567-4. [DOI] [PubMed] [Google Scholar]
- 12.Pietrasik G, Goldenberg I, Zdzienicka J, Moss AJ, Zareba W. Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q-wave myocardial infarction. Am J Cardiol. 2007;100:583–6. doi: 10.1016/j.amjcard.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 13.Das MK, Suradi H, Maskoun W, et al. Fragmented wide QRS on a 12-lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008;1:258–68. doi: 10.1161/CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- 14.Selvester RH, Wagner GS, Hindman NB. The Selvester QRS scoring system for estimating myocardial infarct size. The development and application of the system. Arch Intern Med. 1985;145:1877–81. [PubMed] [Google Scholar]
- 15.Strauss DG, Selvester RH. The QRS complex--a biomarker that “images” the heart: QRS scores to quantify myocardial scar in the presence of normal and abnormal ventricular conduction. J Electrocardiol. 2009;42:85–96. doi: 10.1016/j.jelectrocard.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Fallavollita JA, Luisi JAJ, Michalek SM, et al. Prediction of ARrhythmic Events with Positron Emission Tomography: PAREPET study design and methods. Contemp Clin Trials. 2006;27:374–88. doi: 10.1016/j.cct.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Langley P, Smith FE, King ST, et al. Fully automated computer measurement of QT interval from the 12-lead electrocardiogram. Computers Cardiol. 2006;33:345–348. [Google Scholar]
- 18.Gussak I, Wright RS, Bjerregaard P, et al. False-negative and false-positive ECG diagnoses of Q wave myocardial infarction in the presence of right bundle-branch block. Cardiology. 2000;94:165–72. doi: 10.1159/000047312. [DOI] [PubMed] [Google Scholar]
- 19.Bayes-Genis A, Lopez L, Vinolas X, et al. Distinct left bundle branch block pattern in ischemic and non-ischemic dilated cardiomyopathy. Eur J Heart Fail. 2003;5:165–70. doi: 10.1016/s1388-9842(02)00203-9. [DOI] [PubMed] [Google Scholar]
- 20.Haisty WK, Jr, Pahlm O, Wagner NB, Pope JE, Wagner GS. Performance of the automated complete Selvester QRS scoring system in normal subjects and patients with single and multiple myocardial infarctions. J Am Coll Cardiol. 1992;19:341–6. doi: 10.1016/0735-1097(92)90489-a. [DOI] [PubMed] [Google Scholar]
- 21.Das MK, Saha C, El Masry H, et al. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4:1385–92. doi: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 23.Carey MG. Electrocardiographic predictors of sudden cardiac death. J Cardiovasc Nurs. 2008;23:175–182. doi: 10.1097/01.JCN.0000305069.64860.51. [DOI] [PubMed] [Google Scholar]
- 24.Pahlm US, Chaitman BR, Rautaharju PM, Selvester RH, Wagner GS. Comparison of the various electrocardiographic scoring codes for estimating anatomically documented sizes of single and multiple infarcts of the left ventricle. Am J Cardiol. 1998;81:809–15. doi: 10.1016/s0002-9149(98)00016-2. [DOI] [PubMed] [Google Scholar]
- 25.Boineau JP. New Criteria for Diagnosis of Concealed MI. St. Louis, MO: CardioRhythms, Inc; 2004. The ECG in Multiple Myocardial Infarction and the Progression of Ischemic Heart Disease. [Google Scholar]
- 26.Wagner GS, Freye CJ, Palmeri ST, et al. Evaluation of a QRS scoring system for estimating myocardial infarct size. I. Specificity and observer agreement. Circulation. 1982;65:342–7. doi: 10.1161/01.cir.65.2.342. [DOI] [PubMed] [Google Scholar]
- 27.Bauer WR, Hiller KH, Galuppo P, et al. Fast high-resolution magnetic resonance imaging demonstrates fractality of myocardial perfusion in microscopic dimensions. Circ Res. 2001;88:340–346. doi: 10.1161/01.res.88.3.340. [DOI] [PubMed] [Google Scholar]
- 28.Sevilla DC, Wagner NB, Pegues R, et al. Correlation of the complete version of the Selvester QRS scoring system with quantitative anatomic findings for multiple left ventricular myocardial infarcts. Am J Cardiol. 1992;69:465–9. doi: 10.1016/0002-9149(92)90987-a. [DOI] [PubMed] [Google Scholar]
- 29.O’Neill JO, McCarthy PM, Brunken RC, et al. PET abnormalities in patients with nonischemic cardiomyopathy. J Card Fail. 2004;10:244–9. doi: 10.1016/j.cardfail.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Papouchado M, Walker PR, James MA, Clarke LM. Fundamental differences between the standard 12-lead electrocardiograph and the modified (Mason-Likar) exercise lead system. Eur Heart J. 1987;8:725–33. doi: 10.1093/eurheartj/8.7.725. [DOI] [PubMed] [Google Scholar]
- 31.Drew BJ. The standard 12-lead electrocardiogram: Is the stardard wrong? J Electrocardiol. 2007;40:380–381. [Google Scholar]