Abstract
Calcium phosphate (Ca-P) minerals such as hydroxyapatite are able to bind a diverse range of biological molecules due to the presence of anions and cations in their crystal structure. The well-characterized ability of Ca-P minerals to bind and release plasmid DNA, coupled with the ability of biodegradable Ca-P coatings to form on the surface of common biomaterials, provides a potential mechanism for controlled release of plasmid DNA from various biomaterials. In this study we hypothesized that the release of plasmid DNA from Ca-P coatings formed on poly(lactide-co-glycolide) (PLG) substrates would be dependent on both the intrinsic properties of the Ca-P mineral coating and the surrounding solution conditions. Experiments were designed to consider two general parameters: i) the stability of various Ca-P mineral coatings in solution environments that are relevant to physiological conditions; and ii) the relationship between mineral stability and sustained plasmid DNA release. Our results corroborate previous studies that have demonstrated a direct relationship between intrinsic mineral composition and mineral stability. In addition, we further demonstrate that ion composition and pH of the surrounding solution environment can significantly influence mineral stability. In turn, mineral stability significantly influenced release of plasmid DNA from mineral coatings in vitro, and the DNA release efficiency could be tuned by controlling the mineral properties under various solution environments. These Ca-P mineral coatings may be a useful platform for plasmid DNA delivery applications using various biomaterial platforms.
Keywords: Calcium phosphate mineral, hydroxyapatite, simulated body fluid (SBF), dissolution, gene delivery
Introduction
Biomimetic fluids have been used for at least three decades to grow calcium phosphate (Ca-P)-based mineral coatings on natural and synthetic biomaterials, starting with the initial pioneering work in the 1970s by Kokubo and co-workers [1–3]. These biomimetic coatings provide unique interfaces for fundamental biological studies and clinical orthopedic applications, due in part to the ability to grow the coatings on a variety of biomaterials and their similarity to natural bone mineral. For example, investigators have grown “bone-like” Ca-P mineral coatings on polymers [4–6], ceramics [7, 8], and metals [9–11] using a simulated body fluid (SBF), which has a pH and ion concentrations similar to human blood plasma. Although the coatings formed in SBF are primarily composed of a hydroxyapatite (HA) mineral phase, differences in phase, morphology, nanostructure, and crystallinity have been achieved by modulating the pH, temperature, and ion composition in the SBF solutions. These differences significantly influence cell behavior on mineral coatings, including cell attachment, growth, proliferation, and morphology [12–16]. Indeed, a large body of previous studies has explored the relationship between the structure of mineral coatings and their function, which is typically defined by their ability to enhance osteoconductivity and promote differentiation of bone precursor cells into bone-forming osteoblasts.
In addition to their favorable interaction with bone-forming cells, another Ca-P mineral function is the ability to bind a diverse range of biological molecules due to the presence of anions and cations in their crystal structure. For example, HA has long been used as a resin for column purification of plasmid DNA (pDNA), since pDNA’s anionic phosphate backbone can bind to HA’s cationic Ca2+ ions [17, 18]. The well-characterized ability of Ca-P minerals to bind pDNA, coupled with the ability of biomimetic, biodegradable Ca-P coatings to form on the surface of several common biomaterials, provides a potentially useful mechanism for sustained release of pDNA. Furthermore, the dissolution rate of Ca-P coatings is typically influenced by mineral properties, and these intrinsic properties of the Ca-P coatings may provide a mechanism for controlled release of pDNA in response to changes in the surrounding environment. Toward that end, we hypothesized that the release of pDNA from Ca-P coatings formed on biodegradable polymer substrates would be dependent on both the intrinsic properties of the Ca-P mineral coating and the surrounding solution conditions.
It is well known that the composition and structure of Ca-P minerals formed from SBF solutions can be varied by manipulating the temperature, pH, ion concentrations, and ion content in SBF solutions. Multiple distinct Ca-P mineral phases have been reported during mineral growth in various SBF solutions, including HA, and less stable phases such as carbonate-substituted HA, octacalcium phosphate (OCP) and dicalcium phosphate dihydrate (DCPD). For example, previous studies reported that increased calcium concentrations in SBF solutions can lead to production and maintenance of OCP [19, 20]. Another set of recent studies has demonstrated that the pH of SBF influences the final structure of crystalline HA. For example, Chou et al. reported precursor apatite spheres grown in SBF solution at pH 5.8 and 6.5 transformed into plate-like structures, differing in the amount and size of crystals, in Mg2+ -free SBF solution [21]. Taken together, these differences in Ca-P mineral properties have direct implications for function of Ca-P coatings. In one previous example of this structure-function relationship, Shen et al. examined the effect of mineral properties on pDNA release from Ca-P mineral coatings [22]. Specifically, coatings were formed in SBF solutions in the presence of pDNA, and the ion content and concentration of the SBF were varied. The resulting co-precipitated pDNA-containing mineral coatings had different Ca/P ratios and mineral structures, and these differences resulted in varying pDNA release rates in simulated physiological conditions. This elegant study demonstrated that differences in structure and composition of Ca-P minerals could influence mineral stability and, in turn, the release rates of bound pDNA. In addition, the gene transfer efficiency from mineralized surface indicated that gene expression was dependent on pDNA amount. This dependence was also observed previously by Jang et al., in which PEI/DNA complexes immobilized on PLG disks transfected cells at significantly lower dosage when compared with transfection using bolus delivery [23]. Previous studies have also demonstrated that sustained release of pDNA can promote the prolonged gene expression, which can induce tissue formation [24, 25]. However, the release conditions in these previous studies were limited to solution conditions that mimicked physiological homeostasis. In contrast, in vivo environment surrounding biomaterials during new tissue formation often differs from homeostatic conditions. For example, the local pH within the ruffled border of osteoclasts during bone remodeling is between 4.0 and 5.0, and the pH during the inflammatory phase of wound healing is often acidic.
In view of the diverse range of solution environments present during tissue formation in vivo, our current study characterized pDNA release from multiple Ca-P mineral coatings in various simulated physiologic environments. The pDNA in our studies was simply bound to the calcium phosphate coatings via pDNA-mineral affinity rather than co-precipitated during mineral growth. Our guiding premise was that the stability of Ca-P mineral coatings depends not only on intrinsic mineral properties, but also on the characteristics of the surrounding solution environment, which could be tailored to promote mineral dissolution or mineral re-precipitation. Thus, experiments were designed to consider two general parameters: i) the stability of Ca-P mineral coatings in solutions that are physiologically significant; and ii) the release of bound pDNA from mineral coatings of varying stability. Compositionally and structurally different mineral coatings were grown on hydrolyzed PLG films in SBF using a general approach detailed previously [26, 27], but here modulating SBF ion concentrations and pH. Mineral coatings were then exposed to multiple solution conditions: (1) solutions with physiologic pH and varying Ca2+ and PO43− ion concentrations in the range from no Ca2+ or PO43−, to [Ca2+] and [PO43−] identical to physiologic conditions; (2) solutions with Ca2+ and PO43− ion concentrations identical to physiologic conditions with varying pH in the range from 4.0 to 7.4. These conditions were chosen based on the aforementioned variations that are common in vivo during new tissue formation. The results in this study indicate that mineral stability depends on both intrinsic mineral properties (e.g. morphology, composition, structure), and the surrounding solution environment during mineral dissolution. Importantly, these environment-dependent changes in mineral stability directly influenced release of bound pDNA. Therefore, these Ca-P mineral coatings may be a useful platform for pDNA delivery applications in future studies.
Materials and Methods
Preparation of mineral coatings on poly (lactide-co-glycolide) (PLG) films
Poly (lactide-co-glycolide) (PLG, lactide: glycolide = 85:15, average MW = 50,000–70,000) was purchased from Sigma-Aldrich (St. Louis, MO). Prior to mineral formation, PLG was dissolved in chloroform (Sigma-Aldrich, St Louis, MO) and dried to make PLG films (1 cm × 1 cm). Each PLG film was first incubated in 1 M NaOH solution for 1 h for hydrolysis to create a surface rich in COOH and OH groups, then rinsed with DI water (18 MΩ cm). Various mineral solutions were prepared by dissolving NaCl, KCl, MgSO4, MgCl2, NaHCO3, CaCl2, KH2PO4, and Tris-base (Fisher Scientific, Pittsburgh, PA) in DI water, and pH was adjusted by adding HCl and NaOH solution for supersaturated mineral solutions. The ion concentrations and pH of various mineral solutions are listed in Table 1. For mineral formation, PLG films were incubated in various mineral solutions at 37 °C for 10 d. The solutions were refreshed daily and resulting mineral-coated PLG films were rinsed in DI water and freeze dried for further analysis.
Table 1.
Ion concentrations of human blood plasma and SBF solutions
| mM | Na+ | K+ | Ca2+ | Mg2+ | Cl− | HCO3− | HPO42− | SO42− | pH |
|---|---|---|---|---|---|---|---|---|---|
| Blood plasma | 142.0 | 5.0 | 2.5 | 1.5 | 103.0 | 27.0 | 1.0 | 0.5 | 7.4 |
| 2 × SBF | 145.2 | 6.0 | 5.0 | 1.5 | 157 | 4.2 | 2.0 | 0.5 | 6.8 |
| 3.5 × SBF | 145.2 | 7.5 | 8.75 | 1.5 | 164.5 | 4.2 | 3.5 | 0.5 | 6.3 |
| 5 × SBF | 145.2 | 9.0 | 12.5 | 1.5 | 172 | 4.2 | 5.0 | 0.5 | 6.0 |
Characterization of mineral coatings on PLG films
The morphology of mineral coatings on PLG films was examined by scanning electron microscopy (SEM) (Carl Zeiss SMT, model LEO-1530) operating at 5 kV. The samples were coated with gold using a sputter coater (Denton Vacuum, model DESK II) under 50 mTorr pressure, 40 mA current, and 35 s coating time. To characterize mineral composition, energy dispersive X-ray spectroscopy (EDS) analysis was carried out in conjunction with SEM. A Fourier-transformed infrared (FT-IR) spectrometer (Bruker, model EQUINOX 55) was used for further compositional analysis. The minerals were scraped from PLG films, mixed with potassium bromide (KBr), pressed into a KBr pellet, and analyzed. All FT-IR spectra were recorded in the range of 400–2000 cm−1, and hydroxyapatite powders from Sigma-Aldrich were used as a reference material. The crystal phases of mineral coatings on PLG films were analyzed using X-ray diffractometry (XRD) (Bruker AXS, model HI-STAR). The minerals scraped from PLG films were mounted by glass number 50 capillary tubes (Hampton research, Aliso Viejo, CA) and analyzed under Cu Kα radiation. XRD spectra were taken for 10 min scanning in the range of 2θ = 10–50°.
Dissolution and re-precipitation of mineral coatings
To characterize the influence of Ca2+ and PO43− ion concentrations on mineral coating dissolution, coated films were incubated in DI water with 0.05M Pipes buffer (Boston BioProducts, Worcester, MA) at pH 7.4 with varying amounts of CaCl2 (0, 0.75, 1.5, or 2.5 mM) and KH2PO4 (0, 0.3, 0.6, or 1 mM KH2PO4). To characterize the influence of solution pH on mineral dissolution, coated films were incubated in DI water with 0.05M Pipes buffer with 2.5 mM CaCl2 and 1mM KH2PO4, and the pH was varied (pH 6.2, 6.6, 7, or 7.4). Mineral-coated films were incubated in 1 ml of each solution in 24-well plates and incubated at 37°C for 3 weeks. At specified times, each solution was assayed for soluble calcium and replaced by 1 ml of fresh solution. To assay for soluble calcium concentrations, a 5 µl aliquot of each solution was added to a 195 µl calcium assay working solution composed of 0.4 mM Arsenazo III (MP Biomedicals, Solon, OH) in 0.02 M Tris-base at pH 7.4. Absorbance at 650 nm was converted to Ca2+ concentration using standard curves relating absorbance intensity to Ca2+ concentration.
Plasmid DNA release from mineral coatings
pDNA release was characterized after binding of pDNA on mineral coatings. The process involved the following steps. pDsRed pDNA was amplified in competent TOF10F’ E. coli (Invitrogen, Carlsbad, CA) and purified using a Mega plasmid purification kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Mineral-coated PLG films were incubated in 1ml of preparation buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7) containing 20 µg of pDNA for 1 d to allow for binding. Samples were then rinsed with DI water and dried in air. The amount of bound pDNA was calculated by subtracting the amount of pDNA that remained in preparation buffer from the initially added amount of pDNA. The influence of Ca2+ and PO43− ion concentrations on pDNA release was characterized by incubating samples in 0.05M Pipes solutions (pH = 7.4) containing 0, 0.75, 1.5, or 2.5 mM CaCl2 and 0, 0.3, 0.6, or 1 mM KH2PO4, respectively. The influence of solution pH on pDNA release was characterized by incubating samples in solutions containing 2.5 mM CaCl2 and 1mM KH2PO4, and buffering these solutions with either 0.05 M sodium acetate trihydrate (EMD, San Diego, CA) (pH = 4 or pH = 5) or 0.05 M Pipes (pH = 6 or pH = 7.4). Each sample was incubated in 1 ml of solution in 24-well plates and incubated at 37°C for 3 weeks. At specified times, release solutions were removed for analysis and replaced with a 1 ml of fresh solution. A 50 µl aliquot of the release solution was added to a 150 µl of working solution prepared using the Quant-iT Picogreen dsDNA assay kit (Invitrogen, Carlsbad, CA). The fluorescence measured at 520 nm was converted to the amount of released pDNA using standard curves prepared with known concentration of pDNA in solutions used in each pDNA release experiment (Supp. Figure 5).
Results and Discussion
Characterization of mineral coatings grown in various SBF solutions
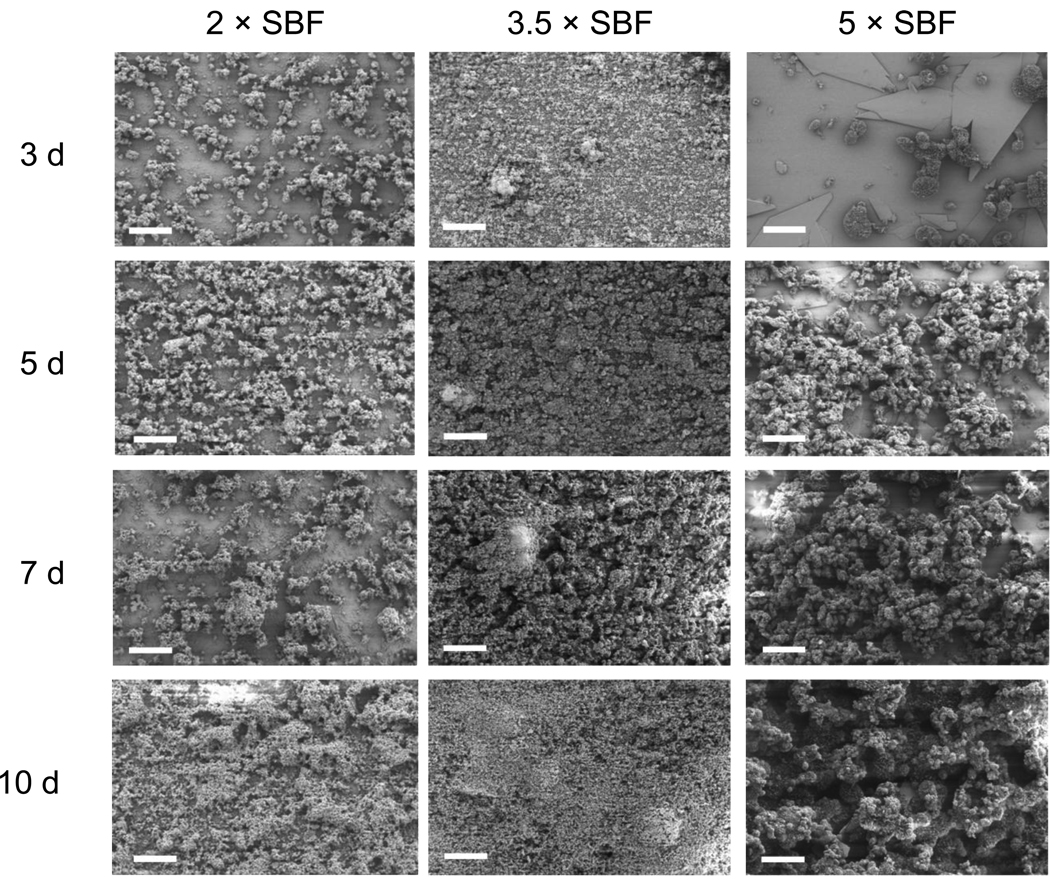
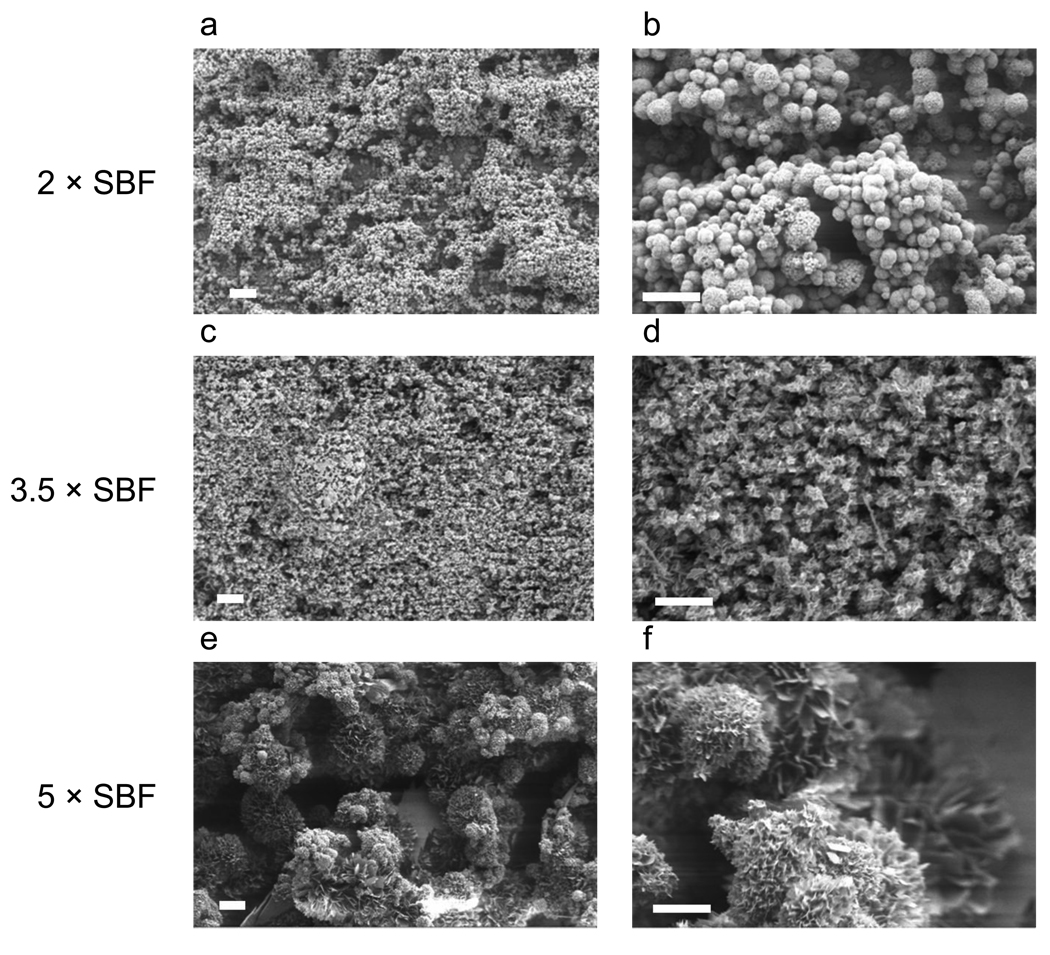
The morphologies of mineral coatings grown on PLG films depended on the Ca2+ and PO43− ion concentrations and pH in SBF solutions. The mineral coatings were continuous on the PLG surface after 10 days of incubation in all SBF solutions studied (Figure 1). SEM observations showed spherulitic microstructure and plate-like nanostructure in all cases. The average diameters of the mineral spherulites grown in 2 × SBF (3.1 ± 0.92 µm) and 3.5 × SBF (3.5 ± 0.90 µm) were significantly smaller than those grown in 5 × SBF (28.9 ± 12.37 µm) (Figure 2).
Figure 1.
SEM micrographs of Ca-P mineral coatings grown on pre-hydrolyzed PLG films in 2 ×, 3.5 ×, or 5 × SBF solutions at 37 °C for 3, 5, 7, or 10 days. Continuous layers were formed after 10 days incubation and the layers consist of spherulitic microstructure. The scale bars indicate 100 µm.
Figure 2.
SEM micrographs of Ca-P mineral coatings grown on pre-hydrolyzed PLG films in 2 ×, 3.5 ×, or 5 × SBF solution at 37 °C for 10 days. The spherulitic microstructure formed from 2 ×, 3.5 ×, or 5 × SBF solutions has different average spherulite diameter (3.1 ± 0.92 µm, 3.5 ± 0.90 µm and 28.9 ± 12.37 µm, respectively). The scale bars indicate 20 µm in a, c, and e and 10 µm in b, d, and f.
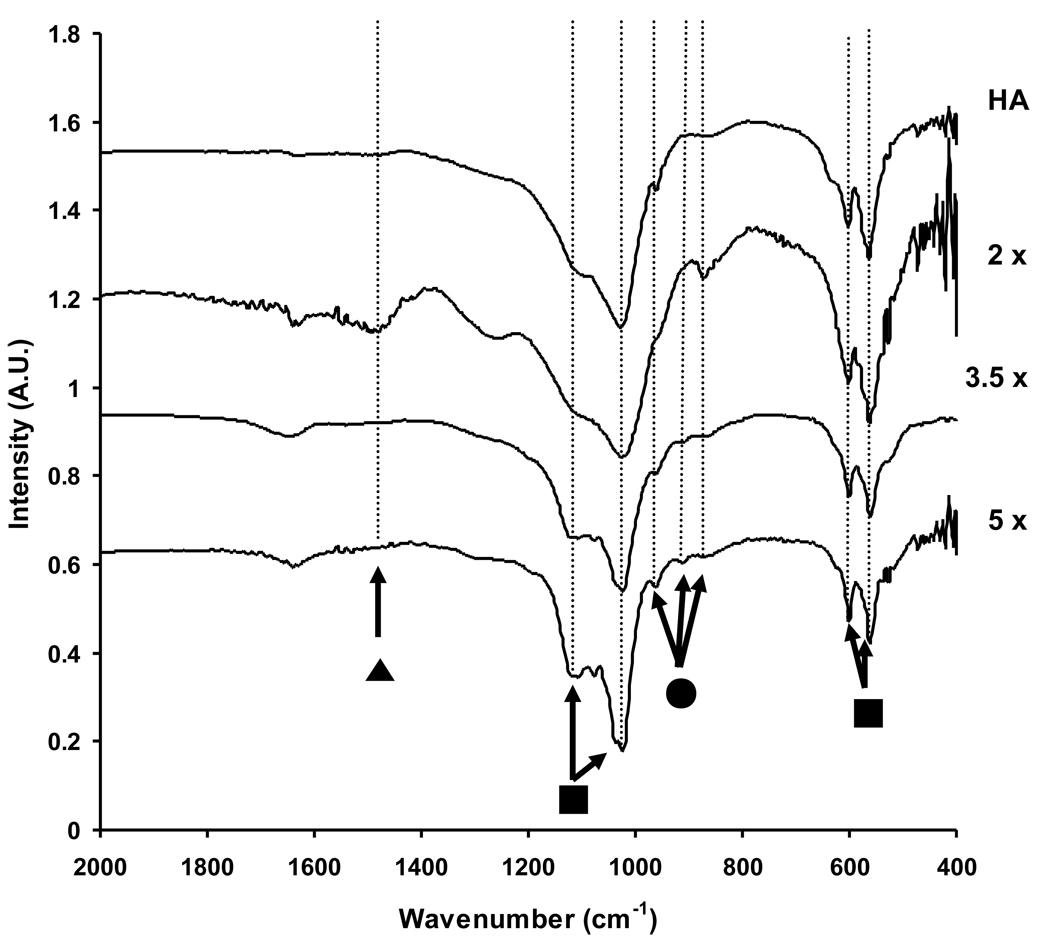
The Ca2+ concentration, PO43− concentration, and pH in SBF solutions influenced both phase (Figure 3) and composition (Figure 4) of mineral coatings. The appearance of two main X-ray diffraction peaks at 26° and 31° indicated that all mineral coatings are primarily composed of a HA mineral phase (Figure 3). The first main peak at 26° corresponds to the (002) plane of HA and the second main peak at 31° corresponds to the (211), (112), and (202) planes. Carbonate-substituted HA also has been previously shown to display peaks at 26° and 31°, which correspond to the (002) plane and the (112) and (300) planes of carbonate-substituted HA, respectively. Therefore, the XRD spectra were consistent with the possibility of carbonate substitution in the HA mineral. We also performed FT-IR analysis to gain more detailed insight into the extent of carbonate incorporation into the HA coatings (Figure 4). FT-IR spectra showed two dominant peaks that can be attributed to O–P–O bending and asymmetric P–O stretch from the PO43− group of hydroxyapatite (450–600 cm−1 and 900–1200 cm−1, respectively). The vibration peaks at 876, 1427, and 1483 cm−1, which can be assigned to the carbonate (CO32−) group, were more strongly detected in mineral coatings formed in 2 × SBF when compared with those in 3.5 × and 5 × SBF. Therefore, carbonate incorporation into mineral coatings was enhanced in the 2 × SBF condition. Although Müller et al. previously showed enhanced CO32− substitution for PO43− and OH− by increasing the [CO32−] in the SBF [28], our results here indicated that CO32− substitution can be varied without changing the [CO32−].
Figure 3.
XRD spectra of commercially available HA and Ca-P mineral coatings grown on PLG films incubated in 2 ×, 3.5 ×, or 5 × SBF solutions for 10 d at 37 °C. The first main peak at 26° corresponds to (002) plane of HA and the second main peak at 31° corresponds to (211), (112), and (202) planes of HA (●). The peaks at 16° and 24° correspond to (−101) and (−211) plane of octacalcium phosphate respectively and the peak at 33° corresponds to (260), (2–41), (−1–42) and (331) planes of OCP (▲).
Figure 4.
FT-IR spectra of commercially available HA and Ca-P mineral coatings grown on PLG films incubated in 2 ×, 3.5 ×, or 5 × SBF solutions for 10 d at 37 °C. All sample showed peaks associated with O–P–O bending and asymmetric P–O stretch from the PO43− group (■). The peak for HPO42− was increased with the increase of [Ca2+] and [PO43−] in SBF solutions (●). The CO32− peak was more strongly detected in mineral coatings formed in 2 × SBF when compared with those formed in 3.5 × and 5 × SBF (▲).
High Ca2+ and PO43− ion concentrations and low pH in the SBF solutions provided favorable conditions for growth of the octacalcium phosphate (OCP) mineral phase. XRD spectra showed peaks at 16°, 24°, and 33°, which can be attributed to the OCP phase (Figure 3). These OCP peaks became more distinct with increasing Ca2+ and PO43− ion concentrations in SBF solutions. In a previous study, Lu et al. analyzed the effect of high Ca2+ and PO43− ions in a SBF solution on the free energy change (Δ G) and nucleation rate (J) of HA and OCP [29]. Their analysis indicated that Δ G of HA was lower than that of OCP, and J of OCP was higher than that of HA in the range from 1 × to 5 × of Ca2+ and PO43− ion concentrations in three different SBF solutions (pH 5, 7.4, or 10). However, they also showed that J of OCP became much higher than that of HA as the pH of SBF solution was lowered to pH 6. Therefore, in our study the lower initial pH of 3.5 × SBF (pH = 6.3) and 5 × SBF (pH = 6.0) may have provided a more favorable environment for OCP nucleation, resulting in enhanced OCP content in the mineral coatings. These results are significant, since OCP is a less stable phase than HA in physiologic conditions.
The FT-IR peak at 1075 cm−1 provided additional evidence that the OCP mineral phase was present, and that its abundance was dependent on SBF conditions (Figure 4). In particular, the peak at 1075 cm−1 was more distinct with increasing Ca2+ and PO43− ion concentrations in SBF solutions. Similar behavior was reported previously by Horváthová et al., in which OCP structure was transformed into HA in SBF solution and resulted a less substantial peak at 1075 cm−1 [20]. In addition, the intensities of peaks at 856, 914 and 962 cm−1, which indicate the presence of HPO42−, were increased with increasing Ca2+ and PO43− ion concentrations during mineral formation. This increased abundance of HPO42− may be caused by the differences in the pH of the different SBF solutions during mineral formation. In 5 × SBF (pH = 6.0), the molar ratio of protonated PO43− ions is higher than those in 2 × SBF (pH = 6.8) or 3.5 × SBF (pH = 6.3), which may result in greater incorporation of HPO42− ions into the mineral structure. In general, the presence of protonated PO43− ions results in more acidic Ca-P phases, which have increased solubility (decreased stability) in physiologic conditions.
The Ca/P ratios measured via EDS analysis were in good agreement with the presence of CO32− and HPO42− ions measured via FT-IR. EDS analysis indicated that the Ca/P ratio decreased with increasing Ca2+ and PO43− ion concentrations in SBF solutions. Specifically, the Ca/P ratios of minerals produced from 2 × SBF (1.67 ± 0.03) were higher than minerals produced from 3.5 × SBF (1.58 ± 0.05), and 5 × SBF (1.43 ± 0.02). We can therefore hypothesize that the Ca/P ratio decreased with the increase of Ca2+ and PO43− ion concentrations due to a decrease in CO32− substitution for PO43− and increased incorporation of HPO42− at low pH.
Effects of solution ion concentrations and pH on dissolution and re-precipitation of mineral coatings
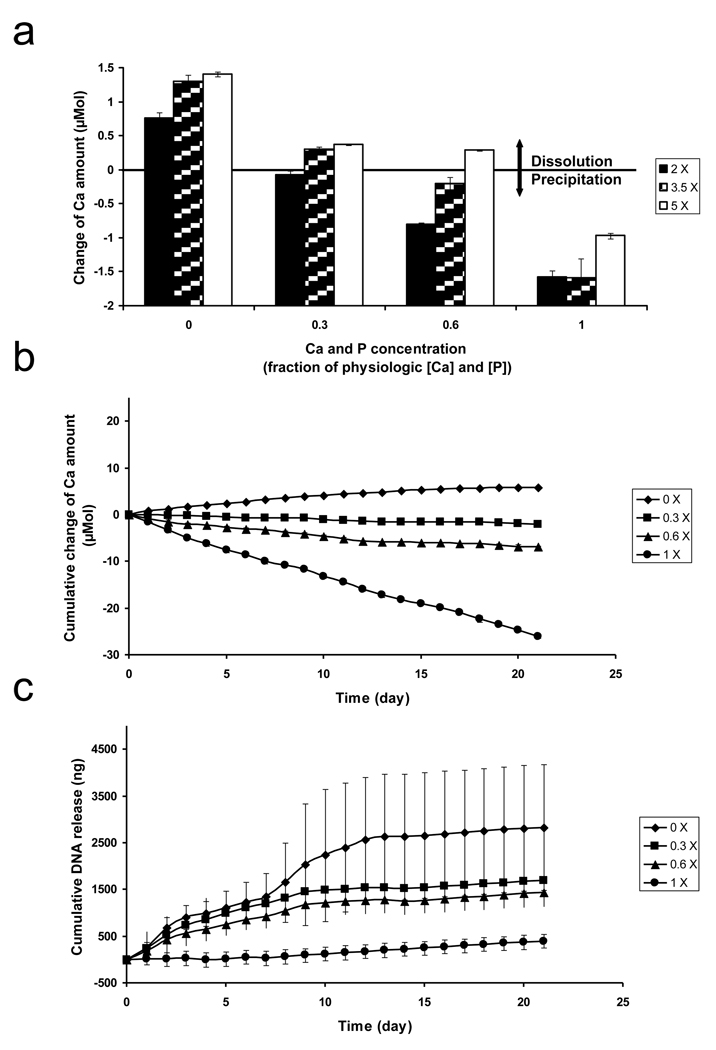
The stability of mineral coatings formed in SBF solutions depended on the saturation level of dissolution environments, which was dictated by Ca2+ and PO43− ion concentrations and pH (Figure 5(a) and Figure 6(a)). Here we characterized mineral dissolution by measuring Ca2+ release into solution. Mineral dissolution was increased in solution environments with decreasing Ca2+ and PO43− ion concentrations at pH 7.4 (Figure 5(a)). The dissolution rate was highest when the mineral coatings were immersed in a solution environment without Ca2+ and PO43− ions. Mineral dissolution was decreased with increasing Ca2+ and PO43− ion concentrations in the dissolution environment, indicating that solution Ca2+ ions were incorporating into the mineral coating during a mineral re-precipitation process. The amount of Ca2+ ions released from mineral coatings formed in 2 × SBF was less than Ca2+ release from mineral coatings formed in 3.5 × and 5 × SBF solutions in all dissolution environments studied. These differences in dissolution rates may correspond not only to the differences in mineral morphology and composition, which were demonstrated by the SEM and EDS data, but also the increased presence of the OCP phase, which was demonstrated by XRD and FT-IR analysis. Driessens et al. previously reported that incorporation of CO32− into HA has a profound influence on the dissolution rate [30]. In addition, a previous study by Johnsson et al. demonstrated that different phases of Ca-P minerals show different stability in contact with aqueous solutions, with OCP releasing Ca2+ and PO43− ions at a higher rate than HA [31]. Therefore, our mineral stability data generally corroborate previous evidence.
Figure 5.
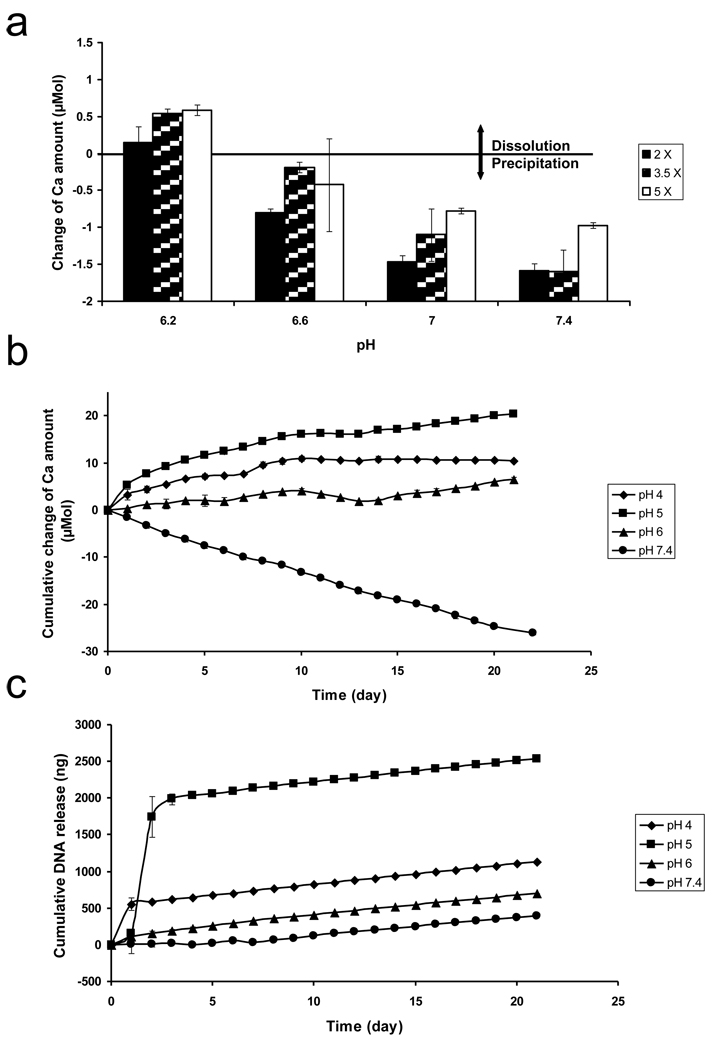
(a) The change of Ca2+ amount after Ca-P mineral coatings grown on pre-hydrolyzed PLG films in 2 ×, 3.5 ×, or 5 × SBF solutions were immersed for 24 h in 0.05 M Pipes-buffered solutions containing 0 ×, 0.3 ×, 0.6 ×, or 1 × of physiologic [Ca2+] and [PO43−] at pH 7.4. The change of Ca2+ amount calculated by subtracting initial Ca2+ amount in different solutions from Ca2+ amount after 24 h incubation, (b) Cumulative change of Ca2+ and (c) Cumulative plasmid DNA amounts from Ca-P mineral coatings grown on pre-hydrolyzed PLG films in 2 × SBF solution after plasmid DNA binding. Ca-P mineral coatings with bound plasmid DNA were immersed for 3 weeks in 0.05 M Pipes-buffered solutions containing 0 ×, 0.3 ×, 0.6 ×, or 1 × of physiologic [Ca2+] and [PO43−] at pH 7.4. The amount of released Ca2+ and plasmid DNA were increased with decreasing [Ca2+] and [PO43−] in the solutions.
Figure 6.
(a) The change of Ca2+ amount after Ca-P mineral coatings grown on pre-hydrolyzed PLG films in 2 × 3.5 ×, or 5 × SBF solutions were immersed for 24 h in 0.05 M Pipes-buffered solutions containing 1 × of physiologic [Ca2+] and [PO43−] at various pH. The change of Ca2+ amount calculated by subtracting initial Ca2+ amount in different solutions from Ca2+.amount after 24 h incubation, (b) Cumulative change of Ca2+ and (c) Cumulative plasmid DNA amounts from Ca-P mineral coatings grown on pre-hydrolyzed PLG films in 2 × SBF solution after plasmid DNA binding. Ca-P mineral coatings with bound plasmid DNA were immersed for 3 weeks in 0.05 M acetate-buffered solution at pH 4 and 5 or in 0.05 M Pipes-buffered solution at pH 6 and 7.4 with 1 × of physiologic [Ca2+] and [PO43−]. The amount of released Ca2+ and plasmid DNA were increased with decreasing pH in the solutions.
The total extent of released Ca2+ after a 24 h incubation increased linearly with decreasing pH in the dissolution environments (Figure 6(a)). Ca2+ release from mineral coatings formed in 2 ×, 3.5 × and 5 × SBF solutions reached maxima after 24 h of incubation at pH 6.2 (0.150 ± 0.214 µmol, 0.546 ± 0.056 µmol, and 0.587 ± 0.068 µmol, respectively). The released Ca2+ amount into the surrounding environment gradually decreased with increasing pH, indicating that Ca2+ was being consumed during mineral re-precipitation at higher pH. The dissolution rate of mineral coatings formed in 2 × SBF was lower than mineral coatings formed in 3.5 × SBF or 5 × SBF in the pH range from 6.2 to 7.4. This result may be attributed to the increased presence of the OCP phase in the minerals formed in 3.5 × and 5 × SBF, as it has been reported previously that the solubility product of HA (5.5 × 10−118 (mol/L)18) is lower than that of OCP (2.5 × 10−99 (mol/L)16) [32].
Taken together, our results corroborate previous studies that have demonstrated a direct relationship between intrinsic mineral properties and mineral stability [22, 30, 31]. Our results here further demonstrate that ion composition and pH of the surrounding solution also significantly influence mineral stability. It is noteworthy that other factors not systematically examined here may also influence the dissolution rate of mineral coatings. LeGeros et al. previously indicated that biodegradation of minerals is influenced by mineral porosity, surface area, and crystallinity [33]. Therefore, there may be a broad range of additional variables that can be explored in future studies as a mechanism to modulate mineral coating dissolution, and associated mineral coating functions.
Effects of solution ion concentrations and pH on pDNA release from mineral coatings
The binding efficiency of pDNA on Ca-P mineral surfaces was significantly influenced by mineral properties. The amount of bound pDNA on the surface (surface area = 1 cm2) of mineral coatings formed in 2 × SBF (15.8 ± 1.5 µg) was significantly higher than that on coatings formed in 3.5 × SBF (6.1 ± 1.3 µg) or 5 × SBF (3.0 ± 0.6 µg). A previous study indicated that protein binding capacity strongly correlated with the specific surface area of HA particles [34]. Similarly, in our study the apparent differences in mineral morphology demonstrated by SEM results may result in differences in specific surface area and, in turn, significant differences in pDNA binding capacity.
The release rates of pDNA from mineral coatings correlated with the mineral dissolution rates (Figure 5(c) and Figure 6(c)). The release rates of pDNA were increased with decreasing concentrations of Ca2+ and PO43− ions in the solution environment, and the largest amount of total pDNA released into the solution devoid of Ca2+ and PO43− ions. As noted above, the [Ca2+] and [PO43−] in the solution environment had a similar influence on the mineral dissolution rate (Figure 5(b)). Therefore, these results indicated that the pDNA release rates were strongly influenced by changes in mineral dissolution rate. Interestingly, pDNA was still released from mineral coatings in some experimental conditions in which Ca2+ ions were consumed during the incubation. For example, solution environments containing 0.3 × of physiologic Ca2+ and PO43− ion concentrations led to decreases in [Ca2+] consistent with re-precipitation, but also led to sustained release of pDNA. This result may be explained by the weak saturation level of the solution environment when compared to the solution containing [Ca2+] and [PO43−] equivalent to physiologic conditions, in which pDNA release from the mineral surface was completely hindered by re-precipitation (Figure 5(c)).
After 3 weeks of pDNA release, the amount of pDNA released from coatings formed in 2 × SBF (0.4 – 2.8 µg) remained below the initially bound pDNA amount (15.8 ± 1.5 µg) in all solution environments. Previous studies have demonstrated the presence of Ca2+-rich-layer at the mineral-solution interface during HA dissolution [35–37]. Therefore, the release of pDNA at the mineral-solution interface during Ca-P mineral dissolution may be limited by a Ca2+-rich layer, which could interact with anionic phosphate backbone of pDNA and lead to slower pDNA release. It is also possible that pDNA/Ca-P mineral co-precipitates formed in solution environments that became locally saturated in the near-surface region during mineral dissolution. Jordan et al. previously demonstrated that the formation of DNA/Ca-P co-precipitates in aqueous solution is dependent on Ca2+ and PO43− ion concentrations [38]. Although the range of Ca2+ and PO43− ion concentrations shown to promote formation of DNA/Ca-P mineral co-precipitates in previous studies is higher than the conditions explored this study, it is possible that the interface between mineral surfaces and surrounding solutions may have high enough [Ca2+] and [PO43−] near the interface to form pDNA/Ca-P mineral co-precipitates. Regardless of the operative mechanism, these results indicated that the saturation level of the surrounding solution environment directly influences dissolution of mineral coatings, which in turn influences the release rate of bound pDNA.
Plasmid DNA release from dissolving mineral coatings was also significantly influenced by the pH of the solution environments, which also contained physiologic Ca2+ (2.5 mM) and PO43− (1.0 mM) concentrations (Figure 6(c)). The release of pDNA was hindered in solution environments at near physiological pH. However, the total amount of released pDNA significantly increased as the pH was decreased. This result is consistent with a previous study by Jongpaiboonkit et al., in which mineral-coated PLG was used as a carrier for protein, and protein release was influenced by pH-dependent mineral dissolution [39]. Similarly, Matsumoto et al. demonstrated that protein release from Ca-P particles is enhanced by increased mineral dissolution at low pH [34]. Importantly, pH-dependent pDNA release was directly related to pH-dependent dissolution of mineral coatings (Figures 6(b) and (c)). Specifically, acidic solutions destabilized the mineral structure more quickly than near-neutral solutions, resulting in more total pDNA release at low pH. It is interesting to note one exception to this trend – mineral dissolution and pDNA release at pH 4 were lower than those at pH 5. Based on this result we hypothesize that high dissolution rates of mineral coatings at pH 4 contribute to high local [Ca2+] and [PO43−] at the mineral-solution interface, leading to local supersaturation and rapid re-precipitation of minerals. This phenomenon was observed previously in a study by Zhang et al., in which minerals with high dissolution rates showed high re-precipitation rates due to enhanced ion concentrations in solution and increased nucleation sites on the mineral surface [40].
Taken together, our results indicated that pDNA was retarded in near physiological conditions (2.5 mM of Ca2+ and 1 mM of PO43− ions and pH 7.4), while pDNA release was increased in solutions with lower [Ca2+] and [PO43−] or lower pH in the surrounding environment. These results suggest the intriguing possibility that mineral coatings like those generated in this study could ultimately be designed to release pDNA selectively in response to changes in physiologic parameters that vary during new tissue formation in vivo, such as [Ca2+], [PO43−], and pH.
Effects of intrinsic mineral properties on mineral dissolution and pDNA release
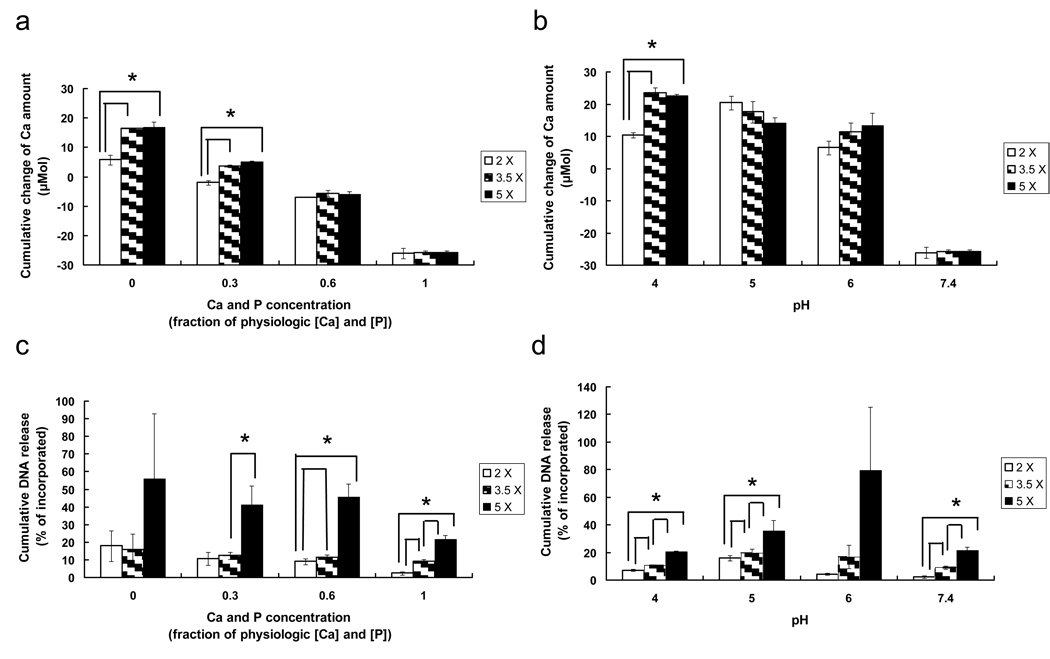
Importantly, intrinsic mineral properties also correlated with mineral dissolution and pDNA release efficiency after 3 weeks of incubation in various simulated physiologic environments (Figure 7). The cumulative amount of Ca2+ ions released after 3 weeks incubation was increased in solution environments with decreasing Ca2+ and PO43− ion concentrations or pH (Figures 7(a) and (b)). In addition, the cumulative amount of Ca2+ ions from mineral coatings formed in 2 × SBF was lower than that from mineral coatings formed in 3.5 × SBF or 5 × SBF in multiple solution conditions. This result indicated that the dissolution behavior of mineral coatings after 24 h incubation (Figure 5(a) and Figure 6(a)) was prolonged until 3 weeks incubation. Importantly, these differences in intrinsic mineral stability also influenced pDNA release from mineral coatings. Mineral coatings formed in 5 × SBF showed a higher pDNA release efficiency when compared to coatings formed in 2 × SBF or 3.5 × SBF in all dissolution environments studied (Figures 7(c) and (d)). Here pDNA release efficiency is a measure of the fraction of initially bound pDNA that was released during the 3 week incubation. These results indicate that pDNA release was strongly influenced by differences in intrinsic mineral properties, which include morphology, composition, and pDNA binding efficiency.
Figure 7.
(a and b) Cumulative change of Ca2+ amount and (c and d) plasmid DNA release efficiency released from Ca-P mineral coatings grown on pre-hydrolyzed PLG films in 2 ×, 3.5 ×, or 5 × SBF solution after 3 weeks of incubation in 0.05 M Pipes-buffered solutions containing 0 ×, 0.3 ×, 0.6 ×, or 1 × of physiologic [Ca2+] and [PO43−] at pH 7.4 (a and c) and in 0.05 M acetate-buffered solution at pH 4 and 5 or in 0.05 M Pipes-buffered solution at pH 6 and 7.4 with 1 × of physiologic [Ca2+] and [PO43−] (b and d). plasmid DNA release efficiency is defined by the percentage of cumulative plasmid DNA amount released after 3 weeks of incubation relative to the amount of plasmid DNA initially bound. The cumulative change of Ca2+ amount and the plasmid DNA release efficiency were each dependent on intrinsic mineral properties. * indicates statistical significance (p < 0.05).
It is noteworthy that the choice of analysis time points can affect the values for kinetics of Ca2+ and pDNA release. A Ca2+ assay within the span of 24 h indicated that the rate of Ca2+ release is reduced with increasing incubation time and no significant change in the rate of Ca2+ release was detected after 10 h (Supp. Figure 2). This result suggests that daily replacement of fresh buffer allows the solution-substrate system to reach equilibrium. Therefore, the choice of time points may lead to change of cumulative amount of released Ca2+ and pDNA during the experiment period, particularly if the timepoint is less than 10 h.
Conclusion
The results in this study indicate that mineral coating stability depends on both intrinsic mineral properties (e.g. morphology, composition, structure), and the surrounding solution environment during mineral dissolution. Specifically, SBF solutions with varying ion concentrations and pH were used to form coatings with different mineral structure, morphology, and composition. Dissolution of mineral coatings was strongly dependent on these intrinsic mineral properties, but also dependent on the pH and ion content of the surrounding solution environment. Importantly, environment-dependent changes in mineral dissolution directly influenced pDNA release. In particular, pDNA release was hindered in solutions containing physiologic [Ca2+], [PO43−], and pH, but pDNA release increased as each of these parameters was decreased. These results indicated that the release rate of pDNA could be tailored by varying the properties of the solution environment, and that mineral coatings may be designed to release pDNA in response to changes in the surrounding solution environment. Therefore, these Ca-P mineral coatings may be a useful platform for pDNA delivery applications in future studies.
Supplementary Material
Acknowledgment
The authors acknowledge financial support from the National Institutes of Health (R21HL084547) and the AO Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kokubo T. Formation of biologically active bone-like apatite on metals and polymers by a biomimetic process. Thermochim Acta. 1996;280/281:479–490. [Google Scholar]
- 2.Koutsopoulos S, Paschalakis PC, Dalas E. The calcification of Elastin in vitro. Langmuir. 1994;10:2423–2428. [Google Scholar]
- 3.Koutsopoulos S, Dalas E. The calcification of fibrin in vitro. J Cryst Growth. 2000;216:450–458. [Google Scholar]
- 4.Tanahashi M, Yao T, Kokubo T, Minoda M, Miyamoto T, Nakamura T, Yamamuro T. Apatite coating on organic polymers by a biomimetic process. J Am Ceram Soc. 1994;77:2805–2808. [Google Scholar]
- 5.Kretlow JD, Mikos AG. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Engineering. 2007;13:927–938. doi: 10.1089/ten.2006.0394. [DOI] [PubMed] [Google Scholar]
- 6.Kang S, Yang HS, Seo S, Han DK, Kim B. Apatite-coated poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for bone tissue engineering. J Biomed Mater Res Part A. 2007;85A:747–756. doi: 10.1002/jbm.a.31572. [DOI] [PubMed] [Google Scholar]
- 7.Kokubo T, Ito S, Huang ZT, Hayashi T, Sakka S, Kitsugi T, Yamamuro T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J Biomed Mater Res. 1990;24:331–343. doi: 10.1002/jbm.820240306. [DOI] [PubMed] [Google Scholar]
- 8.Fujibayashi S, Neo M, Kim HM, Kokubo T, Nakamura T. A comparative study between in vivo bone ingrowth and in vitro apatite formation on Na2O-CaO-SiO2 glasses. Biomaterials. 2003;24:1349–1356. doi: 10.1016/s0142-9612(02)00511-2. [DOI] [PubMed] [Google Scholar]
- 9.Takadama H, Kim HM, Kokubo T, Nakamura T. TEM-EDX study of mechanism of bonelike apatite formation on bioactive titanium metal in simulated body fluid. J Biomed Mater Res. 2001;57:441–448. doi: 10.1002/1097-4636(20011205)57:3<441::aid-jbm1187>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Barrère F, Layrolle P, van Blitterswijk CA, de Groot K. Biomimetic coatings on titanium: a crystal growth study of calcium phosphate. J Mater Sci:Mater Med. 2001;12:529–534. doi: 10.1023/a:1011271713758. [DOI] [PubMed] [Google Scholar]
- 11.Barrère F, van Blitterswijk CA, de Groot K, Layrolle P. Nucleation of biomimetic Ca-P coatings on Ti6AAl4V from a SBF × 5 solution: influence of magnesium. Biomaterials. 2002;23:2211–2220. doi: 10.1016/s0142-9612(01)00354-4. [DOI] [PubMed] [Google Scholar]
- 12.Melville AJ, Harrison J, Gross KA, Forsythe JS, Trounson AO, Mollard R. Mouse embryonic stem cell colonisation of carbonated apatite surfaces. Biomaterials. 2006;27:615–622. doi: 10.1016/j.biomaterials.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Chou L, Marek B, Wagner WR. Effects of hydroxylapatite coating crystallinity on biosolubility, cell attachment efficiency and proliferation in vitro. Biomaterials. 1999;20:977–985. doi: 10.1016/s0142-9612(98)00254-3. [DOI] [PubMed] [Google Scholar]
- 14.Xin R, Leng Y, Chen J, Zhang Q. A comparative study of calcium phosphate formation on bioceramics in vitro and in vivo. Biomaterials. 2005;26:6477–6486. doi: 10.1016/j.biomaterials.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Chou YF, Huang W, Dunn JC, Miller TA, Wu BM. The effect of biomimetic apatite structure on osteoblast viability, proliferation, and gene expression. Biomaterials. 2005;26:285–295. doi: 10.1016/j.biomaterials.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Barrère F, van Blitterswijk CA, de Groot K. Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics. Int J Nanomedicine. 2006;1:317–332. [PMC free article] [PubMed] [Google Scholar]
- 17.Colman A, Byers MJ, Primrose SB, Lyons A. Rapid purification of plasmid DNA by hydroxyapatite chromatography. Eur J Biochem. 1978;91:303–310. doi: 10.1111/j.1432-1033.1978.tb20966.x. [DOI] [PubMed] [Google Scholar]
- 18.Giovannini R, Freitag R. Comparison of different types of ceramic hydroxyapatite for the chromatographic separation of plasmid DNA and a recombinant anti-Rhesus D antibody. Bioseparation. 2000;9:359–368. doi: 10.1023/a:1011174905511. [DOI] [PubMed] [Google Scholar]
- 19.Iijima M, Kamemizu H, Wakamatsu N, Goto T, Doi Y, Moriwaki Y. Effects of Ca addition on the formation of octacalcium phosphate and apatite in solution at pH 7.4 and at 37°C. J Cryst Growth. 1998;193:182–188. [Google Scholar]
- 20.Horváthová R, Müller L, Helebrant A, Greil P, Müller FA. In vitro transformation of OCP into carbonated HA under physiological conditions. Mater Sci Eng C. 2008;28:1414–1419. [Google Scholar]
- 21.Chou YF, Chiou WA, Xu Y, Dunn JC, Wu BM. The effect of pH on the structural evolution of accelerated biomimetic apatite. Biomaterials. 2004;25:5323–5331. doi: 10.1016/j.biomaterials.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 22.Shen H, Tan J, Saltzman WM. Surface-mediated gene transfer from nanocomposites of controlled texture. Nature Materials. 2004;3:569–574. doi: 10.1038/nmat1179. [DOI] [PubMed] [Google Scholar]
- 23.Jang J, Bengali Z, Houchin TL, Shea LD. Surface adsorption of DNA to tissue engineering scaffolds for efficient gene delivery. J Biomed Mater Res A. 2006;77:50–58. doi: 10.1002/jbm.a.30643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polyemr matrices for tissue engineering. Nature Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 25.Storrie H, Mooney DJ. Sustained delivery of plasmid DNA from polymeric scaffolds for tissue engineering. Adv Drug Deliv Rev. 2006;58:500–514. doi: 10.1016/j.addr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Murphy WL, Gilhool KA, Kohn DH, Mooney DJ. Effect of growth factor presence on mineralization of porous poly(lactide-co-glycolide) scaffolds in vitro. Mat. Res. Soc. Symp. Proc. 2000;599:347–352. [Google Scholar]
- 27.Murphy WL, Kohn DH, Mooney DJ. Growth of continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro. J Biomed Mater Res Part A. 1999;50:50–58. doi: 10.1002/(sici)1097-4636(200004)50:1<50::aid-jbm8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 28.Müller L, Müller FA. Preparation of SBF with different HCO3− content and its influence on the composition of biomimetic apatites. Acta Biomaterialia. 2006;2:181–189. doi: 10.1016/j.actbio.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Lu X, Leng Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials. 2005;26:1097–1108. doi: 10.1016/j.biomaterials.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 30.Driessens FC, van Dijk JW, Borggreven JM. Biological calcium phosphates and their role in the physiology of bone and dental tissues I. composition and solubility of calcium phosphate. Calcif Tissue Res. 1978;26:127–137. doi: 10.1007/BF02013247. [DOI] [PubMed] [Google Scholar]
- 31.Johnsson MSA, Nancollas GH. The role of brushite and octacalcium phosphate in apatite formation. Crit Rev Oral Biol Med. 1992;3:61–82. doi: 10.1177/10454411920030010601. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Nancollas GH. Calcium orthophosphates: crystallization and dissolution. Chem Rev. 2008;108:4628–4669. doi: 10.1021/cr0782574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeGeros R, Parsons JR, Daculsi G, Driessens F, Lee D, Liu ST, Metsger S, Peterson D, Walker M. Significance of the porosity and physical chemistry of calcium phosphate ceramics. Biodegradation-bioresorption. Ann N Y Acad Sci. 1988;523:268–271. doi: 10.1111/j.1749-6632.1988.tb38519.x. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto T, Okazaki M, Yamaguchi S, Kusunose T, Toyonaga T, Hamada Y, Takahashi J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials. 2003;25:3807–3812. doi: 10.1016/j.biomaterials.2003.10.081. [DOI] [PubMed] [Google Scholar]
- 35.Thomann JM, Voegel JC, Gramain P. Kinetics of dissolution of calcium hydroxyapatite powder lV. Interfacial calcium diffusion controlled process. Coll Surf. 1991;54:145–159. [Google Scholar]
- 36.Mafé S, Manzanares JA, Reiss H, Thomann JM, Gramain P. Model for the dissolution of calcium hydroxyapatite powder. J Phys Chem. 1992;96:861–866. [Google Scholar]
- 37.Thomann JM, Voegel JC, Gramain P. Quantitative model for the dissolution of calcium hydroxyapatite with a permselective ionic interface. J Colloid Interface Sci. 1993;157:369–374. [Google Scholar]
- 38.Jordan M, Schallhorn A, Wurm FM. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucl Acids Res. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jongpaiboonkit L, Franklin-Ford T, Murphy WL. Mineral-coated polymer microspheres for controlled protein binding and release. Adv Mater. 2009;21:1–4. [Google Scholar]
- 40.Zhang Q, Chen J, Feng J, Cao Y, Deng C, Zhang X. Dissolution and mineralization behaviors of HA coatings. Biomaterials. 2003;24:4741–4748. doi: 10.1016/s0142-9612(03)00371-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.