Abstract
Background
Asthma exacerbations, most often due to respiratory tract infections, are the leading causes of asthma morbidity and comprise a significant proportion of asthma-related costs. Vitamin D status may play a role in preventing asthma exacerbations.
Objectives
To assess the relationship between serum vitamin D levels and subsequent severe asthma exacerbations.
Methods
We measured 25-hydroxyvitamin D (25(OH)D) levels in serum collected from 1,024 mild to moderate persistent asthmatic children at the time of enrollment in a multi-center clinical trial of children randomized to receiving budesonide, nedocromil, or placebo (as-needed beta-agonists), the Childhood Asthma Management Program. Using multivariable modeling we examined the relationship between baseline vitamin D level and the odds of any hospitalization or emergency department (ED) visit over the 4 years of the trial.
Results
35% of all subjects were vitamin D insufficient, as defined by a level ≤ 30 ng/ml 25(OH)D. Mean vitamin D levels were lowest in African-American subjects, and highest in whites. After adjusting for age, sex, BMI, income, and treatment group, insufficient vitamin D status was associated with a higher odds of any hospitalization or ED visit (odds ratio [OR] 1.5 [95% confidence interval [CI]: 1.1 – 1.9] P =0.01).
Conclusion
Vitamin D insufficiency is common in this population of North American children with mild to moderate persistent asthma, and is associated with higher odds of severe exacerbation over a four year period.
Keywords: Asthma, Vitamin D, inhaled corticosteroids, asthma exacerbations
INTRODUCTION
Asthma is a major public health problem affecting an estimated 22 million people in the United States 1 and 300 million people worldwide.2 Much of the burden of the disease is a consequence of asthma exacerbations, which results in missed time from work, increased absence from school, and increased health-care expenditures. Asthma is the third leading cause of hospitalization among children under 15 yrs of age, with 26.2 discharges per 10,000 population.3 In the United States, asthma healthcare costs are estimated to be $19.7 billion each year4, and hospitalizations from exacerbations are estimated to be responsible for a third of these costs.5
Vitamin D has been shown to have several effects on the innate and adaptive immune systems that may modulate the severity of asthma exacerbations. Airway epithelia contain high levels of the enzyme that converts circulating 25-OH-vitamin D3 to its active form, 1,25-OH-vitamin D3. This active form of vitamin D has local effects in response to infections6 and may dampen the inflammation that is the consequence of these infections.7 Vitamin D also has potentially beneficial effects on the adaptive immune system through its effects on Th1, Th2, and T-regulatory cells.8 Through these mechanisms, vitamin D may also have a therapeutic role in reducing asthma exacerbations.
Our group recently published the first epidemiological study demonstrating an association between low vitamin D levels and increased markers of asthma severity, including serum IgE, eosinophil count, hospitalizations in the previous year, and inhaled steroid use.9 However, this study was cross-sectional and retrospective, and limited to a single geographic location and age group (children 7.5 – 10.5 years of age) in Costa Rica. We now examine the association between vitamin D levels and risk of hospitalization or Emergency Department (ED) visit in a prospective manner in the Childhood Asthma Management Program (CAMP) study, a diverse population of North American asthmatic children.
METHODS
Study Population
CAMP was a multicenter, randomized, double-blind, placebo-controlled trial established to investigate the long-term effects of commonly prescribed asthma treatment regimens. In total, 1,041 children were randomized to receive inhaled budesonide, inhaled nedocromil, or placebo. Participants were subsequently followed for a mean of 4.3 years with lung function studies and questionnaires at regular intervals. Serum IgE and eosinophil count were measured at the time of enrollment in the study, and other outcomes such as lung function, response to methacholine, symptom scores as calculated from diary data were recorded at baseline and at regular intervals over the 4-year follow-up period. At regularly scheduled follow-up visits (approximately every 4 months) children and caretakers were asked about hospitalizations or ED visits specifically due to an asthma exacerbation. At the screening visit, children and caretakers were also asked about any ED or hospitalizations that occurred in the year prior to enrollment in the study. Trial design, methodology, and the primary outcomes analysis of the CAMP study have been previously published.10, 11
At enrollment, CAMP participants had mild-to-moderate persistent asthma based on the presence of symptoms, the use of inhaled bronchodilators at least twice weekly, or the use of daily asthma medication for at least 6 months in the year before screening. Patients with severe persistent and mild-intermittent asthma were excluded from the study. Spirometry was performed at least 4 hours after short-acting bronchodilator use and 24 hours after long-acting bronchodilator use. Spirometry was required to meet American Thoracic Society criteria for acceptability and reproducibility. At least 3 spirometric maneuvers were performed, with at least 2 reproducible maneuvers required for each test. Bronchodilator response to albuterol was assessed at randomization and at all subsequent visits except when a methacholine challenge was performed. Post-bronchodilator spirometric values were obtained at least 15 minutes after the administration of 2 puffs of albuterol (90 mcg per puff).
Approval was obtained from the institutional review boards at each of the CAMP-participating institutions before initiation of the trial. Informed consent was obtained from the parent or guardian of the participant, and the child’s assent was obtained before study enrollment.
Serum 25–Hydroxyvitamin D3 (25(OH)D)
Serum levels of 25–hydroxyvitamin D3 (hereafter referred to as vitamin D) are considered as the best circulating biomarker of vitamin D metabolic status and reflect contributions from all sources of vitamin D (i.e. diet and sun exposure). 12, 13 A single measurement of vitamin D was obtained on 1,024 subjects (98% of enrolled subjects) using a radioimmunoassay method in Dr. Bruce Hollis’ laboratory at the Medical University of South Carolina.14, 15 Vitamin D levels have been shown to be relatively stable when specimens have been properly stored.16 We categorized vitamin D levels into insufficient (≤ 30 ng/ml), and sufficient (>30 ng/ml) based on previous recommendations.17–19
Statistical Analysis
A descriptive analysis of univariate predictors and outcomes was performed using a dichotomous vitamin D variable with cut-off of less than or equal to 30 ng/ml. For the purposes of this analysis, severe exacerbation was defined as any hospitalization or ED visit specifically due to an asthma exacerbation over the four year course of the study. P values were calculated using two-sided t-tests for continuous predictors with equal variance, and Wilcoxon rank sum test for continuous predictor with unequal variances. Chi-square tests were performed to obtain p values for binary variables.
Multivariable models were constructed using linear and logistic regression, and proportional hazards models for time-to-event analysis. We initially conducted a retrospective analysis relating baseline vitamin D with the subjects’ (or parents’) report of an ED visit or hospitalization for asthma in the previous year, obtained at the initial screening visit. The analysis is retrospective because the events had occurred prior to measurement of vitamin D levels, and recapitulates the analysis we conducted in the Costa Rica study.9 We then conducted the main prospective analyses relating baseline vitamin D with subsequent reports of ED visits or hospitalizations for asthma over the four years of the trial. Potential confounders were included in the multivariable models if they were associated with vitamin D insufficiency on a univariate basis at p ≤.05, or if they were plausibly related to both vitamin D levels and asthma severity. The baseline covariates are age, sex, income, and body mass index (BMI). Prospective models for asthma hospitalizations or ED visits were additionally controlled for treatment group. Additional confounders that are possibly causally related to the relationship between vitamin D and the outcome of interest were then added in a stepwise fashion, and include season of vitamin D level draw, baseline asthma severity as determined by a physician, and race. To assess for effect modification by inhaled steroid use, all models were additionally stratified by treatment group (budesonide vs. either placebo or nedocromil). In the stratified analysis, the term for treatment group was removed from the models. All analyses were performed using SAS version 9.1 and JMP 7 (both from SAS Institute, Cary, NC).
RESULTS
Characteristics of the study population
The baseline characteristics for the study population stratified by vitamin D sufficiency are shown in Table I. Thirty-five percent of the children were vitamin D insufficient as defined by a level ≤ 30 ng/ml. In a univariate analysis, vitamin D insufficiency was significantly associated with older age, higher BMI, and African-American race. A higher proportion of the vitamin D sufficient group was randomized to the budesonide treatment group, although the blood samples were drawn before randomization. Vitamin D insufficiency was not more common among individuals with low income (<$30,000 per year) in a univariate analysis.
Table I.
Baseline Characteristics of Study Participants
| Vitamin D | ||||
|---|---|---|---|---|
| Characteristics1 | All children N = 1,024 |
Insufficient (≤ 30) N = 361 (35%) |
Sufficient (> 30) N = 663 (65%) |
p value2,3 |
| Age (years) | 8.9 (7.2 – 10.6) | 9.3 (7.6–11.0) |
8.7 (7.0–10.4) |
.0002 |
| Female gender | 413 (40%) | 155 (43%) |
258 (39%) |
.21 |
| BMI | 17.2 (15.7 – 19.8) | 17.7 (16.2–20.8) |
17.0 (15.5–19.2) |
<.0001 |
| African-American race | 134 (13%) | 88 (24%) | 46 (7%) | <.0001 |
| Income < $30,000 | 239 (24%) | 87 (25%) | 152 (24%) | .61 |
| Budesonide group | 305 (30%) | 89 (25%) | 216 (33%) | .008 |
| Moderate severity4 | 538 (53%) | 195 (54%) |
343 (52%) |
.49 |
| Skin test positivity to any allergen |
898 (88%) | 326 (90%) | 572 (86%) | .06 |
| Total IgE, IU/mL | 437 (174 – 1216) | 468 (178–1380) |
427 (170–1175) |
.15 |
| Eosinophil count, cells/mm3 Post-bronchodilator lung function |
398 (200 – 646) | 389 (200–617) |
398 (200–676) |
.44 |
| FEV1, L5 | 1.85 (1.83 – 1.87) | 1.82 (1.78 – 1.85) |
1.87 (1.84 – 1.89) |
.009 |
| FEV1/FVC ratio | 86 (82 – 90) | 86 (81–90) |
86 (82–90) |
.79 |
| Bronchodilator response (% of FEV1) |
8% (4 – 15) | 9% (4–15) |
8% (4–15) |
.66 |
| Health–care utilization | ||||
| Hospitalizations for asthma in the year prior to randomization |
328 (32%) |
142 (39%) |
186 (28%) |
.0002 |
| Any hospitalization or ED visit over 4 years |
346 (34%) |
136 (38%) |
210 (32%) |
.05 |
Results expressed as median (interquartile range) for continuous variables, and number of subjects (percent of group) for categorical variables.
Information missing on some subjects for eosinophil count (n=14), spirometry and bronchodilator response (n=2), and BMI (n=10).
p values are 2-sided t-test or Wilcoxon rank sum test (depending on equality of variances) for continuous variables, and Chi square test for binary variables.
Only subjects with mild persistent or moderate persistent asthma were enrolled in the study.
FEV1 is the predicted value from regression model including age, sex, and vitamin D status (for stratified columns), for subject of mean age 9 and male sex.
Distribution and predictors of serum vitamin D levels in the study population
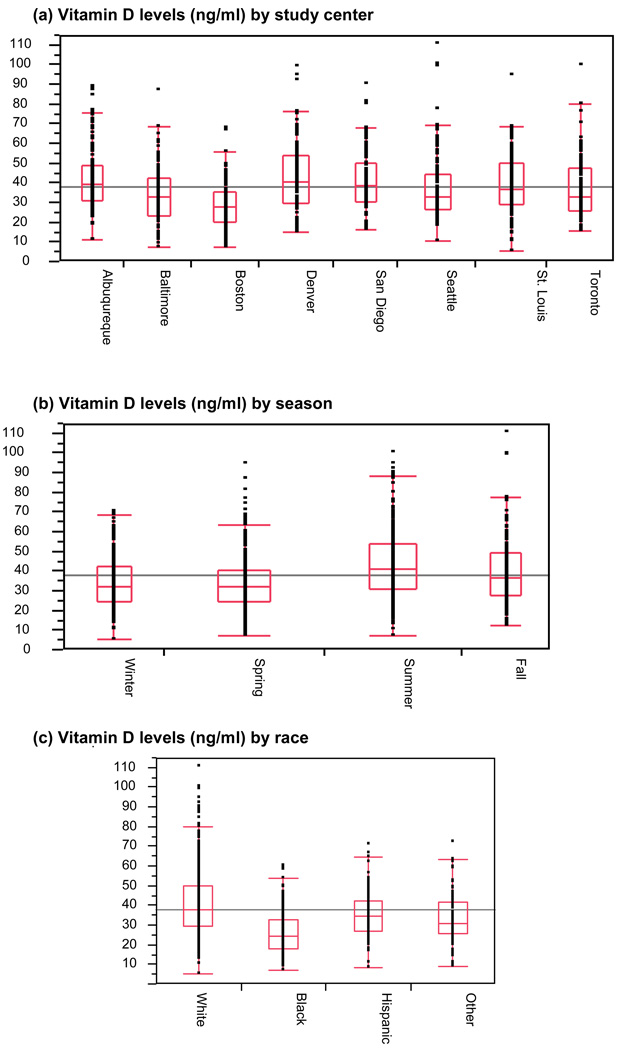
The distribution of vitamin D levels by study center, season, and subjects’ self-reported race are illustrated in Figure 1. The highest average levels were in Denver, Colorado, and Albuquerque, New Mexico, and the lowest were in Boston, Massachusetts. Vitamin D levels also varied by season, with summer having the highest mean level, while winter and spring had the lowest. African-American subjects had the lowest average vitamin D levels, and whites had the highest, although there was a wide range of levels among the white subjects.
Figure 1.
Vitamin D levels and distributions by (a) study center, (b) season, and (c) race.
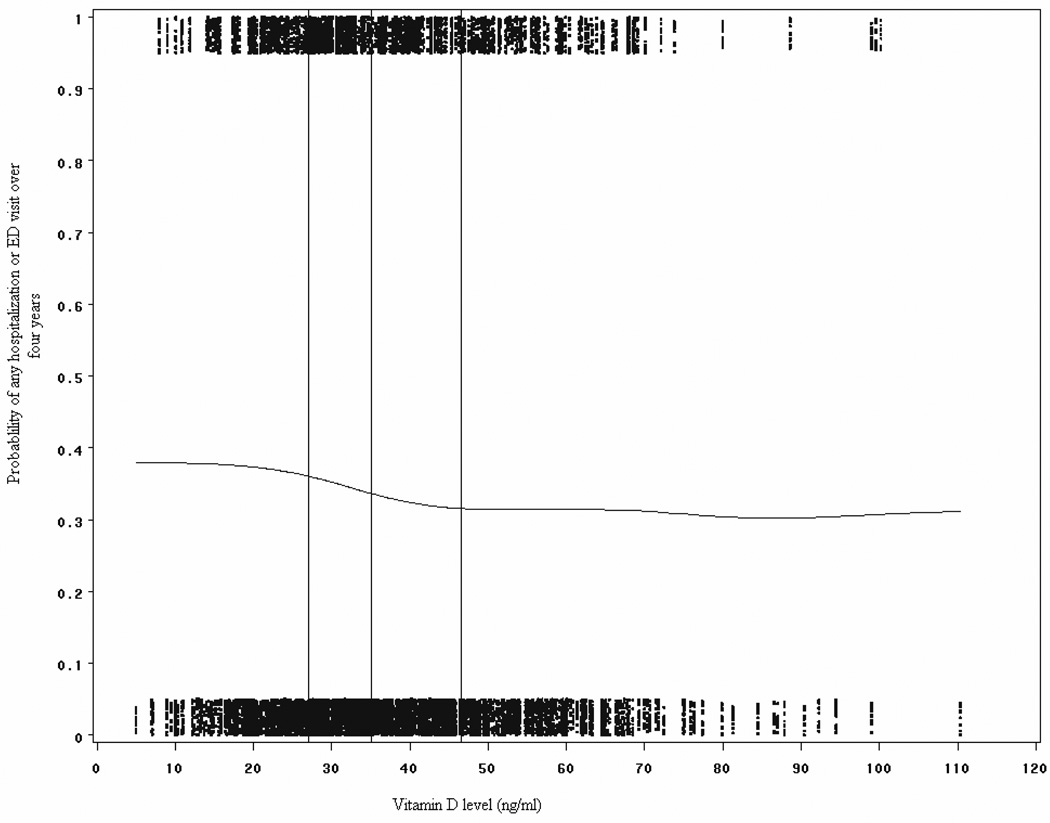
Among the entire cohort of children with asthma the distribution of vitamin D levels were right skewed, with a few individuals having levels greater than 90 ng/ml, but 95% of subjects had levels between 14 and 72 ng/ml (see Figure E1 in the Online Repository). In addition, a plot of the probability of having an ED visit or hospitalization over the four years of the trial versus serum vitamin D level is demonstrated in Figure 2. A smoothing function was used to fit a line between these points to assess for non-linear trends over the range of vitamin D levels in the study. At around 30 ng/ml, the probability of having an asthma exacerbation decreases. Given this finding, and in keeping with prior recommendations for “sufficient” levels, all subsequent analyses were performed with a dichotomous variable for vitamin D levels.
Figure 2.
Sensitivity analysis of risk of hospitalization or ED visit by vitamin D level. The probabilities of severe exacerbations were plotted for the range of vitamin D levels in the study, and a smoothing function was used to draw a line through the values. Vertical lines represent quartiles of vitamin D levels. The risk of hospitalization start to decline around vitamin D levels of 30 ng/ml.
Severe asthma exacerbations
For the purposes of this analysis, any asthma exacerbation requiring hospitalization or emergency room visit was defined as severe, according to GINA guidelines.20 Over the four years of the study, 106 children were hospitalized for asthma, and 352 children had either a hospitalization or a visit to the emergency room for an asthma exacerbation. Univariate and multivariable analyses of the relation between vitamin D and odds of ED visit or hospitalization prior to and during the CAMP study are shown in Table II. The odds of any ED visit or hospitalization in the year prior to enrolling in the trial (retrospective analysis) was significantly elevated in the vitamin D insufficient group, even after controlling for age, sex, BMI, and physician severity at baseline. We additionally adjusted for race and season of blood draw in a stepwise fashion, although these covariates are strong determinants of vitamin D levels and therefore may be an overadjustment. The effect remained significant after additionally adjusting for these variables, although the effect size was attenuated.
Table II.
Hospitalizations or ED visits: Values are odds ratio (95% CI) (p value) for severe exacerbations in vitamin D insufficient group (≤30 ng/ml) vs. vitamin D sufficient group (>30ng/ml)
| Unadjusted odds ratio | Adjusted for age, sex, BMI, income, treatment group1 |
Additionally adjusted for season of vitamin D draw |
Additionally adjusted for baseline asthma severity |
Additionally adjusted for race |
|
|---|---|---|---|---|---|
| Retrospective analysis2 | |||||
| ED or hospitalization in year prior to study3 |
1.7 (1.3 – 2.2) (.0002) | 1.9 (1.4 – 2.5) (<.0001) |
2.0 (1.5 – 2.7) (<.0001) |
1.9 (1.4 – 2.6) (<.0001) |
1.7 (1.2 – 2.7) (.001) |
| Prospective analysis | |||||
| ED/Hospitalization over 4 years of study |
1.3 (1.0 – 1.7) (.05) | 1.5 (1.1 – 1.9) (.01) | 1.5 (1.2 – 2.1) (.004) | 1.5 (1.1 – 2.0) (.006) | 1.4 (1.0 – 1.9) (.03) |
| Vitamin D insufficiency at baseline (placebo or nedocromil) |
1.2 (0.9 – 1.6) (.36) | 1.3 (1.0 – 1.9) (.08) | 1.4 (1.0 – 2.0) (.03) | 1.4 (1.0 – 2.0) (.06) | 1.3 (.9 – 1.8) (.15) |
| Vitamin D insufficiency at baseline(budesonide) |
1.7 (1.0 – 2.8) (.06) | 1.8 (1.0 – 3.2) (.05) | 1.8 (1.0 – 3.3) (.06) | 1.8 (1.0 – 3.4) (.05) | 1.7 (.9 – 3.2) (.11) |
Models for hospitalization prior to randomization and stratified models are not adjusted for treatment group.
The terms retrospective and prospective refer to the timing of the outcomes relative to the timing of phlebotomy from which vitamin D levels were measured.
The odds of any hospitalization or ED visit over the four years of the CAMP study (prospective analysis) in the vitamin D insufficient group were also elevated after adjustment for age, sex, BMI, and baseline physician severity. We also analyzed the association stratified by treatment group. Subjects who were randomized to inhaled budesonide and were vitamin D insufficient tended to have higher odds of hospitalizations than subjects who were randomized to nedocromil or placebo and were vitamin D insufficient. This effect was largest in subjects randomized to budesonide, and non-significant in subjects randomized to placebo or nedocromil.
Since inhaled corticosteroids had a large protective effect on severe exacerbations,11 we calculated the risks of hospitalization or ED visit over 4 years for all combinations of vitamin D status and inhaled corticosteroid use (see Table III). Compared with the reference group of children on inhaled steroids and with sufficient vitamin D levels, all other combinations had increased odds of severe exacerbation, with the highest risk occurring in children who were not on inhaled corticosteroids and who were vitamin D insufficient. Since children who were on inhaled corticosteroids and who were vitamin D insufficient also had increased risks for severe exacerbations, these results also suggest that having sufficient vitamin D levels conferred additional benefit to inhaled corticosteroids. Children not on inhaled steroids and with insufficient vitamin D levels had the highest risk of hospitalization compared with reference group.
Table III.
Risk of severe exacerbation over four years by combination of inhaled corticosteroid use and vitamin D status
| No | Yes | % | Odds ratio1 | |
|---|---|---|---|---|
| On inhaled steroids, sufficient vitamin D |
162 | 52 | 24% | 1.0 (reference) |
| On inhaled steroids, insufficient vitamin D |
58 | 31 | 35% | 1.7 (1.0–2.9) |
| Not on steroids, sufficient vitamin D |
289 | 158 | 35% | 1.7 (1.2 – 2.5) |
| Not on steroids, insufficient vitamin D |
166 | 105 | 39% | 2.0 (1.3 – 2.9) |
Odds ratios are for each stratum in reference to a subject on inhaled corticosteroids with sufficient vitamin D. The Cochran-Armitage two-sided P-value for trend over the four strata is .0009.
Since some children had more than 1 severe exacerbation in the 4 years of the trial, we also conducted a time-to-first event analysis. Using a proportional hazards model, vitamin D insufficiency conferred a higher hazard ratio for hospitalization or ED visit (see Table E1 in the Online Repository). As in the logistic regression analysis, the effect was strongest in the budesonide treatment arm, and remained significant after controlling for season of vitamin D draw, baseline asthma severity, and race.
Measures of atopy and asthma symptoms
After adjustment for potential confounders there was no significant difference in skin test reactivity, total serum IgE levels, or eosinophil count between the vitamin D sufficient and insufficient subject (see Table E2 in the Online Repository). Subjects with insufficient vitamin D at randomization also had a significantly lower percentage of visits with reports of moderate symptoms (defined as at least two days of symptoms in each of the four weeks prior to the visit). We found no difference in the odds of receiving a prednisone burst over the four years of the study in the vitamin D insufficient children.
Spirometric measures of lung function and airway hyperreactivity
Table E2 also shows results of multivariate models for prediction of post-bronchodilator FEV1 and FEV1/FVC, and natural log dose of methacholine required to cause a 20% drop in FEV1. After controlling for confounders, insufficient vitamin D status predicts a slightly lower post-bronchodilator FEV1, and a trend towards slightly higher FEV1/FVC ratio. There was no difference in airway hyperreactivity as measured by log-transformed PC20 dose.
DISCUSSION
Our group previously reported an inverse relationship with vitamin D levels and several markers of asthma and allergy severity in Costa Rican children.9 The present data from the CAMP cohort of 1,024 children confirms the finding in the Costa Rican study that low vitamin D levels are associated with increased odds of asthma-related ED visits or hospitalization in the previous year. In this study, we further demonstrate that after adjustment for age, sex, BMI, and baseline asthma severity, vitamin D insufficiency at baseline is associated prospectively with increased odds of severe asthma exacerbations, defined as hospitalizations and/or ED visits, over the four year course of the study. In addition, children with insufficient vitamin D levels had a slightly lower mean FEV1 compared with children with sufficient levels. However, unlike the Costa Rica study, we did not find any association between vitamin D levels and allergy markers.
Higher vitamin D levels are likely associated with decreased risk of severe exacerbations through multiple mechanisms. One mechanism may be through improved response to respiratory infections, since vitamin D has been shown to induce the production of antimicrobial proteins (AMPs), such as cathelicidin (which has both antimicrobial and antiviral properties) 6, 21 and defensin beta 4.22 Induction of AMPs has been shown to occur at the airway epithelium. In addition to the induction of AMPs, vitamin D may modulate the inflammatory response to viral infections. Airway epithelial cells exposed to vitamin D produce less inflammatory cytokines (without adversely affecting viral clearance) than cells not exposed to vitamin D when infected with viruses.6, 7 These findings suggest that, although higher vitamin D levels may not prevent the occurrence of infections, higher levels may allow improved handling of these infections and decreased inflammatory responses, resulting in less severe disease and sequelae of these viral infections. This is supported by our data, which shows no difference in the odds of receiving a prednisone burst in the vitamin D insufficient group, but a higher odds of hospitalization or ED visit, suggesting that the number of exacerbations are the same, but the severity of exacerbations are worse in the vitamin D insufficient children. However, we are unable to directly test the hypothesis that vitamin D improved the host response to infections since we did not have specific information regarding infections.
In addition to the response to infections, another mechanism of vitamin D in asthma may be through enhancement of response to both exogenous and endogenous steroids. This may explain why we saw a stronger beneficial vitamin D effect among the children randomized to the budesonide group. While inhaled steroids were effective in significantly decreasing exacerbations in this trial,11 children who continued to have severe exacerbations despite inhaled corticosteroids may be a subgroup that is not responding fully to inhaled corticosteroids. Our results suggest that having sufficient levels prevents some of these exacerbations while on inhaled corticosteroids. These results, in particular, are consistent with a proposed mechanism, demonstrated in vitro by Xystrakis et. al., that vitamin D restores the ability of steroid-treated T regulatory cells from steroid-resistant asthmatics to secrete IL-10, a potent anti-inflammatory cytokine in airway epithelial cells thus enhancing steroid uptake and effectiveness.23
In contrast to our findings on severe exacerbations, vitamin D insufficiency was significantly associated with the report of fewer symptoms. A possible explanation is that daily symptoms and exacerbations may be related to vitamin D through different mechanisms. Since vitamin D likely reduces the severity of viral infections rather than eliminate them, another explanation is that vitamin D reduces severe exacerbations by preventing the severe sequelae of viral infections without affecting the incidence of such infections. Further investigation will be required to resolve these inconsistencies.
While this study confirms and extends the results of the Costa Rica study with regard to asthma exacerbations, we did not see similar results for markers of allergy, such as serum IgE level and eosinophil count. The CAMP and Costa Rica cohorts have important similarities and differences which may explain some of these discrepant findings. Since Costa Rica was designed as a genetic epidemiology study, the study population is homogenous by design; all subjects were required to have 6 out of 8 grandparents from the Central Valley of Costa Rica, and all subjects live in or near the Central Valley. In contrast, CAMP has a diverse outbred population composed of predominantly of whites, blacks, and Hispanics. Subjects were recruited from eight different centers across the country, each at different latitudes and altitudes. Atopy is highly prevalent in both populations with 88% of CAMP subjects having skin test positivity to at least one allergen compared with 85% for Costa Rica. Unlike equatorial Costa Rica, which does not have much variability in UV exposure throughout the year, the vitamin D levels of CAMP subjects vary by season. So while the overall median values for vitamin D levels in CAMP and Costa Rica are similar (35.1 vs. 35.7 ng/ml respectively), there is likely much more variability in vitamin D level over the course of a year in the CAMP population. To the extent to which this is true it would represent a null bias in our results. Finally, it is possible that vitamin D influences atopy only in certain environmental backgrounds. As a multi-center trial, the participants in CAMP would be expected to have quite different quantitative and qualitative seasonal allergen exposures when compared with subjects in Costa Rica. An important area of future research is to clarify the interaction between vitamin D and environmental exposures on allergic outcomes as well as asthma severity.
Since Costa Rica is a cross-sectional study it was difficult to establish causality for the association between vitamin D levels and asthma severity. It is possible that children with more severe disease are more likely to spend time indoors, which can lead to an association that is spurious. A major advantage of the current study is that we were able to measure vitamin D levels on blood samples collected at the time of enrollment and perform an analysis where the hospitalizations or ED visits occurred after the time of phlebotomy, which is a stronger study design than a retrospective analysis. This prospective design reveals more modest effects of vitamin D than that seen in the retrospective design, but these effects remain statistically significant after adjustment for age, sex, BMI, treatment group, and physician severity. Since vitamin D production in the skin is dependent on skin pigmentation and amount of UVB exposure, 18 covariates such as race and season of vitamin D measurement are strongly related to vitamin D levels, so adjusting for them in a multivariate model likely represents over-adjustment for vitamin D. However, since both race and season likely affect asthma severity through pathways independent from vitamin D, they are also likely to confound the association between vitamin D and severe asthma exacerbations. Accordingly, we have presented models with race and season added in stepwise fashion. Despite this concern for over-adjustment, the full models including race and season predict a significantly higher odds ratio for ED/hospitalization in the vitamin D insufficient group. At this point, a randomized, placebo-controlled trial of vitamin D supplementation to prevent asthma exacerbations is needed to confirm the findings from our observational studies.
Another potential confounder of our results is vitamin D supplementation. While significant vitamin D supplementation will be reflected in higher vitamin D levels, this may also reflect a “healthy user” effect, or act as a proxy for intake of other vitamins which may influence asthma severity. For this type of confounding to significantly affect our results, vitamin D supplementation would have to substantially change serum levels, and one would have to postulate that those who were insufficient at the start of the study were supplemented with relatively large doses. As Heaney et. al. demonstrate,24 standard recommended replacement doses of vitamin D (400 IU/d) do not have any substantial impact of serum levels, so we believe this is unlikely to be a significant source of error. At the time the CAMP study was conducted, vitamin D supplementation was not as publicized as it is today; thus, it is likely that children who were insufficient remained in the insufficient range throughout most of the study.
The current study is limited by only having one measure of serum vitamin D at enrollment. An ideal analysis would be to perform repeated measures over time for testing in a proportional hazards model, which would be able to account for seasonal variations in vitamin D level. Our inability to capture this variability does not invalidate these results, rather a low correlation between repeated measures would reduce power to detect a true association, possibly resulting in a false negative result25. Furthermore, work by Hilgenfeld et. al. suggests that while seasonal variation in vitamin D levels in school-age children occurs, over the course of the year there was no variation in urinary calcium excretion, suggesting that these fluctuations in serum levels may not be physiologically relevant.26
Because asthma exacerbations account for a large proportion of overall asthma-related healthcare costs, and because vitamin D deficiency is so common, we calculated the population attributable risk (PAR) for vitamin D insufficiency on asthma exacerbations. Given the prevalence of insufficiency in our study in both the budesonide group and in the total group of children in CAMP, the PAR% is at least 11%. While this may appear small, decreasing asthma health care costs by this proportion translates into over $2 billion in savings per year if circulating vitamin D levels could be brought up to at least 30 ng/ml in all asthmatics. Furthermore, in a recent analysis of the National Health and Nutrition Examination Survey (NHANES) data collected between 2001 and 2006, 48% of children had levels below 30 ng/ml27, so our PAR% estimate is likely conservative. Although this prevalence of vitamin D insufficiency is higher than in the CAMP cohort, we point out that CAMP subjects were recruited from 1993 through 1995, and vitamin D insufficiency has become more prevalent in the years since28.
The current recommended level of sufficiency (≥30 ng/ml) is thought to be the minimum necessary level for overall health, since beneficial effects on musculoskeletal function are seen beginning at this level.17 However, the circulating level of vitamin D for optimal immune function is unknown but there are suggestions that this level may very well be above 40 ng/ml.27, 28 Beneficial effects are likely to be greatest in blacks and in those already on inhaled steroids. Given the potential public health benefits, a randomized controlled trial is indicated to confirm the observational results presented here.
In summary, we demonstrate that vitamin D insufficiency is common in childhood asthmatics in the US and it predicts increased odds of severe exacerbations over a four year period, even after adjusting for a range of potential confounders. Even in those already on inhaled steroids, vitamin D insufficiency increased this risk. These results confirm and extend the findings from the Costa Rica cohort in regards to severe exacerbations. A randomized controlled trial should be done to confirm these results and to generalize to other subgroups of asthmatics.
Key Messages
Vitamin D insufficiency is common in a North American population of mild to moderate childhood asthmatics.
Vitamin D insufficiency is associated with higher odds of severe asthma exacerbation over a four year period.
Having sufficient vitamin D levels conferred protection against severe exacerbations that was in addition to the effect of being on inhaled corticosteroids.
Acknowledgments
Funding/Support: We acknowledge the CAMP investigators and research team, supported by NHLBI, for collection of CAMP Genetic Ancillary Study data. All work on data collected from the CAMP Genetic Ancillary Study was conducted at the Channing Laboratory of the Brigham and Women’s Hospital under appropriate CAMP policies and human subject’s protections. The CAMP Genetics Ancillary Study is supported by U01 HL075419, U01 HL65899, P01 HL083069, R01 HL086601, and T32 HL07427 from the National Heart, Lung and Blood Institute, National Institutes of Health. We also acknowledge the Asthma Clinical Research Network (ACRN) investigators and research teams supported by U01 HL51510, U01 HL51834, U01 HL51831, U01 HL51845, U01 HL 51843, M01 RR00079, M01 RR03186, from the NHLBI. This work was also supported by National Institutes of Health: R21HL089842.
Abbreviations
- 25(OH)D
25-hydroxy-vitamin D
- ED
Emergency department
- CAMP
Childhood Asthma Management Program
- BMI
Body mass index
- GINA
The Global Initiative for Asthma
- AMP
Anti-microbial proteins
- UV
Ultraviolet
- PAR
Population attributable risk
* Members of the CAMP Research Group
Source of funding
The Childhood Asthma Management Program is supported by contracts NO1-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052 with the National Heart, Lung, and Blood Institute and General Clinical Research Center grants M01RR00051, M01RR0099718-24, M01RR02719-14, and RR00036 from the National Center for Research Resources.
Members of the CAMP Research Group:
Clinical centers
ASTHMA, Inc, Seattle, WA: Gail G. Shapiro, MD (Director); Thomas R. DuHamel, PhD (Co-Director); Mary V. Lasley, MD (Co-Director); Tamara Chinn, MSN, ARNP (Coordinator). Michele Hinatsu, MSN, ARNP; Clifton T. Furukawa, MD; Leonard C. Altman, MD; Frank S. Virant, MD; Paul V. Williams, MD; Michael S. Kennedy, MD; Jonathan W. Becker, MD; Grace White. C. Warren Bierman, MD (1992–1997); Dan Crawford, RN (1996–2002); Heather Eliassen, BA (1996–1999); Babi Hammond (1996–1999); Dominick A. Minotti, MD (1992–2003); Chris Reagan (1992–2003); Marian Sharpe, RN (1992–1994); Timothy G. Wighton, PhD (1994–1998).
Brigham & Women’s Hospital, Boston, MA: Scott Weiss, MD, MS (Director); Anne Fuhlbrigge, MD (Principal Investigator); Anne Plunkett, NP, MS (Coordinator). Nancy Madden, RN, BSN; Peter Barrant, MD; Christine Darcy; Kelly Thompson, MD. Walter Torda, MD (Co-Investigator Director, 1993–2003); Martha Tata, RN (1993–2002); Sally Babigian, RN (1997–1999); Linda Benson (1998–2004); Jose Caicedo (1998–1999); Tatum Calder (1998–2001); Anthony DeFilippo (1994–2000); Cindy Dorsainvil (1998–2001); Julie Erickson (1998–1999); Phoebe Fulton (1997); Mary Grace, RN (1994–1996); Jennifer Gilbert (1997–1998); Dirk Greineder, MD (1993–2000); Stephanie Haynes (1993–1998); Margaret Higham, MD (1996–1998); Deborah Jakubowski (1999); Susan Kelleher (1993–1997); Jay Koslof, PhD (1993–1995); Dana Mandel (1996–1998); Patricia Martin (2001–2003); Agnes Martinez (1994–1997); Jean McAuliffe (1994–1995); Erika Nakamoto (2002–2004); Paola Pacella (1993–1998); Paula Parks (1993–1995); Johanna Sagarin (1998–1999); Kay Seligsohn, PhD (1995–2004); Susan Swords (2003–2005); Meghan Syring (1998–2001); June Traylor, MSN, RN (1996–1998); Melissa Van Horn, PhD (1996–1999); Carolyn Wells, RN (1993–1995); Ann Whitman, RN (1994–1996).
The Hospital for Sick Children, Toronto, Ontario, Canada: Ian MacLusky, MD, FRCP(C) (Director); Joe Reisman, MD, FRCP(C), MBA (Director, 1996–1999); Henry Levison, MD, FRCP(C) (Director, 1992–1996); Anita Hall, RN (Coordinator). Jennifer Chay; Melody Miki, RN, BScN; Renée Sananes, PhD. Yola Benedet (1994–1999); Susan Carpenter, RN (1998–2001); Michelle Collinson, RN (1994–1998); Jane Finlayson-Kulchin, RN (1994–1998); Kenneth Gore, MA (1993–1999); Noreen Holmes, RRT (1998–1999); Sharon Klassen, MA(1999–2000); Joseé Quenneville, MSc (1993–1995); Christine Wasson, PhD (1999).
Johns Hopkins Asthma & Allergy Center, Baltimore, MD: N. Franklin Adkinson, Jr, MD (Director); Peyton Eggleston, MD (Co-Director); Elizabeth H. Aylward, PhD; Karen Huss, DNSc (Co-Investigator); Leslie Plotnick, MD (Co-Investigator); Margaret Pulsifer, PhD (Co-Investigator); Cynthia Rand, PhD (Co-Investigator); Nancy Bollers, RN (Coordinator). Deborah Bull, LPN; Robert Hamilton, PhD; Kimberly Hyatt; Susan Limb, MD; Mildred Pessaro; Stephanie Philips, RN; Barbara Wheeler, RN, BSN.
National Jewish Medical and Research Center, Denver, CO: Stanley Szefler, MD (Director); Harold S. Nelson, MD (Co-Director); Bruce Bender, PhD (Co-Investigator); Ronina Covar, MD (Co-Investigator); Andrew Liu, MD (Co-Investigator); Joseph Spahn, MD (Co-Investigator); D Sundström (Coordinator). Melanie Phillips; Michael P. White. Kristin Brelsford (1997–1999); Jessyca Bridges (1995–1997); Jody Ciacco (1993–1996); Michael Eltz (1994–1995); Jeryl Feeley, MA (Coordinator, 1992–1995); Michael Flynn (1995–1996); Melanie Gleason, PA-C (1992–1999); Tara Junk-Blanchard (1997–2000); Joseph Hassell (1992–1998); Marcia Hefner (1992–1994); Caroline Hendrickson, RN (1995–1998; Coordinator, 1995–1997); Daniel Hettleman, MA (1995–1996); Charles G. Irvin, PhD (1992–1998); Jeffrey Jacobs, MD (1996–1997); Alan Kamada, PharmD (1994–1997); Sai Nimmagadda, MD (1993–1996); Kendra Sandoval (1995–1997); Jessica Sheridan (1994–1995); Trella Washington (1993–1997); Eric Willcutt, MA (1996–1997). We also thank the pediatric allergy and immunology fellows for their participation (Kirstin Carel, MD; Neal Jain, MD; Harvey Leo, MD; Beth Macomber, MD; Chris Mjaanes, MD; Lora Stewart, MD; Ben Song, MD).
University of California, San Diego and Kaiser Permanente Southern California Region, San Diego, CA: Robert S. Zeiger, MD, PhD (Director); Noah Friedman, MD (Co-Investigator); Michael H. Mellon, MD (Co-Investigator); Michael Schatz, MD (Co-Investigator); Kathleen Harden, RN (Coordinator). Elaine M. Jenson; Serena Panzlau; Eva Rodriguez, RRT. James G. Easton, MD (Co-Director, 1993–1994); M. Feinberg (1997–1998); Linda L. Galbreath (1991–2002); Jennifer Gulczynski (1998–1999); Ellen Hansen (1995–1997); Al Jalowayski, PhD (Co-Investigator, 1991–2005); Alan Lincoln, PhD (Co-Investigator, 1991–2003); Jennie Kaufman (1994); Shirley King, MSW (1992–1999); Brian Lopez (1997–1998); Michaela Magiari-Ene, MA (1994–1998); Kathleen Mostafa, RN (1994–1995); Avraham Moscona (1994–1996); Catherine A. Nelle, RN (1991–2005); Jennifer Powers (2001–2003); Karen Sandoval (1995–1996); Nevin W. Wilson, MD (Co-Director, 1991–1993).
University of New Mexico, Albuquerque, NM: H. William Kelly, PharmD (Director); Aaron Jacobs (Co-Investigator); Mary Spicher, RN (Coordinator). Hengameh H. Raissy. Robert Annett, PhD (Co-Investigator, 1993–2004); Teresa Archibeque (1994–1999); Naim Bashir, MD (Co-Investigator, 1998–2005); H. Selda Bereket (1995–1998); Marisa Braun (1996–1999); Shannon Bush (2002–2006); Michael Clayton, MD (Co-Investigator, 1999–2001); Angel Colon-Semidey, MD (Co-Investigator, 1997–2000); Sara Devault (1993–1997); Roni Grad, MD (Co-Investigator, 1993–1995); David Hunt, RRT (1995–2004); Jeanne Larsson, RN (1995–1996); Sandra McClelland, RN (Coordinator, 1993–1995); Bennie McWilliams, MD (Co-Investigator, Director, 1992–1998); Elisha Montoya (1997–2000); Margaret Moreshead (1996–1999); Shirley Murphy, MD (Co-Investigator, 1992–1994); Barbara Ortega, RRT (1993–1999); David Weers (1997–1998); Jose Zayas (1995–1996).
Washington University, St. Louis, MO: Robert C. Strunk, MD (Director); Leonard Bacharier, MD (Co-Investigator); Gordon R. Bloomberg, MD (Co-Investigator); James M. Corry, MD (Co-Investigator); Denise Rodgers, RFPT (Coordinator). Lila Kertz, MSN, RN, CPNP; Valerie Morgan, RRT; Tina Oliver-Welker, CRTT; Deborah K. White, RPFT, RRT.
Resource centers
Chair's Office, National Jewish Medical and Research Center, Denver, CO: Reuben Cherniack, MD (Study Chair).
Coordinating Center, The Johns Hopkins University, Baltimore, MD: James Tonascia, PhD (Director); Curtis Meinert, PhD (Co-Director). Patricia Belt; Karen Collins; Betty Collison; Ryan Colvin, MPH; John Dodge; Michele Donithan, MHS; Judith Harle; Rosetta Jackson; Hope Livingston; Jill Meinert; Kapreena Owens; Michael Smith; Alice Sternberg, ScM; Mark Van Natta, MHS; Margaret Wild; Laura Wilson, ScM; Robert Wise, MD; Katherine Yates, ScM.
Project Office, National Heart, Lung, and Blood Institute, Bethesda, MD: Virginia Taggart, MPH (Project Officer); Lois Eggers; James Kiley, PhD; Gang Zheng, PhD. Paul Albert, PhD (1991–1999); Suzanne Hurd, PhD (1991–1999); Sydney Parker, PhD (1991–1994); Pamela Randall (1992–2003); Margaret Wu, PhD (1991–2001).
Committees
Data and Safety Monitoring Board: Howard Eigen, MD (Chair); Michelle Cloutier, MD; John Connett, PhD; Leona Cuttler, MD; David Evans, PhD; Meyer Kattan, MD; Rogelio Menendez, MD; F. Estelle R. Simons, MD. Clarence E. Davis, PhD (1993–2003); Sanford Leikin, MD (1993–1999).
Executive Committee: Reuben Cherniack, MD (Chair);Robert Strunk, MD; Stanley Szefler, MD; Virginia Taggart, MPH; James Tonascia, PhD. Curtis Meinert, PhD (1992–2003).
Steering Committee: Reuben Cherniack, MD (Chair); Robert Strunk, MD (Vice-Chair); N. Franklin Adkinson, MD; Robert Annett, PhD (1992–1995, 1997–1999); Bruce Bender, PhD (1992–1994, 1997–1999); Mary Caesar, MHS (1994–1996); Thomas R. DuHamel, PhD (1992–1994, 1996–1999); H. William Kelly, PharmD; Henry Levison, MD (1992–1996); Alan Lincoln, PhD (1994–1995); Ian MacLusky, MD; Bennie McWilliams, MD (1992–1998); Curtis L. Meinert, PhD; Sydney Parker, PhD (1991–1994); Joe Reisman, MD, FRCP(C), MBA (1991–1999); Denise Rodgers (2003–2005); Kay Seligsohn, PhD (1996–1997); Gail G. Shapiro, MD; Marian Sharpe (1993–1994); D Sundström (1998–1999); Stanley Szefler, MD; Virginia Taggart, MPH; Martha Tata, RN (1996–1998); James Tonascia, PhD; Scott Weiss, MD, MS; Barbara Wheeler, RN, BSN (1993–1994); Robert Wise, MD; Robert Zeiger, MD, PhD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.CDC National Center for Health Statistics. Asthma prevalence, health care use and mortality: United States. 2003–05 [cited] [Google Scholar]
- 2.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 3.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2007;13:1–209. [PubMed] [Google Scholar]
- 4.American Lung Association. Asthma. American Lung Association Lung Disease Data. 2008 2008. [Google Scholar]
- 5.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009;39:193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory Epithelial Cells Convert Inactive Vitamin D to Its Active Form: Potential Effects on Host Defense. The Journal of Immunology. 2008;181:7090. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010;184:965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Curr Opin Allergy Clin Immunol. 2009 doi: 10.1097/ACI.0b013e32832b36cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in costa rica. Am J Respir Crit Care Med. 2009;179:765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Childhood Asthma Management Program Research Group. Control Clin Trials. 1999;20:91–120. [PubMed] [Google Scholar]
- 11.Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 12.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 13.Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352:515–516. doi: 10.1056/NEJM200502033520521. author reply −6. [DOI] [PubMed] [Google Scholar]
- 14.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I–labeled tracer. Clin Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- 15.Hollis BW, Napoli JL. Improved radioimmunoassay for vitamin D and its use in assessing vitamin D status. Clin Chem. 1985;31:1815–1819. [PubMed] [Google Scholar]
- 16.Agborsangaya C, Toriola AT, Grankvist K, Surcel HM, Holl K, Parkkila S, et al. The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione. Nutr Cancer. 2010;62:51–57. doi: 10.1080/01635580903191460. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 19.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 20.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 21.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. American Association for the Advancement of Science. 2006:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 22.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, et al. Convergence of IL-1 and VDR Activation Pathways in Human TLR2/1-Induced Antimicrobial Responses. PloS one. 2009:4. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 25.Brunekreef B, Noy D, Clausing P. Variability of exposure measurements in environmental epidemiology. Am J Epidemiol. 1987;125:892–898. doi: 10.1093/oxfordjournals.aje.a114606. [DOI] [PubMed] [Google Scholar]
- 26.Hilgenfeld MS, Simon S, Blowey D, Richmond W, Alon US. Lack of seasonal variations in urinary calcium/creatinine ratio in school-age children. Pediatr Nephrol. 2004;19:1153–1155. doi: 10.1007/s00467-004-1568-z. [DOI] [PubMed] [Google Scholar]
- 27.Saintonge S, Bang H, Gerber LM. Implications of a New Definition of Vitamin D Deficiency in a Multiracial US Adolescent Population: The National Health and Nutrition Examination Survey III. Pediatrics. 2009;123:797. doi: 10.1542/peds.2008-1195. [DOI] [PubMed] [Google Scholar]
- 28.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollis BW, Wagner CL, Drezner MK, Binkley NC. Circulating vitamin D(3) and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J Steroid Biochem Mol Biol. 2007 doi: 10.1016/j.jsbmb.2006.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taback SP, Simons FE. Anaphylaxis and vitamin D: A role for the sunshine hormone? J Allergy Clin Immunol. 2007;120:128–130. doi: 10.1016/j.jaci.2007.05.020. [DOI] [PubMed] [Google Scholar]