Abstract
Objectives
The goal of this work was to reduce the scan time of contrast-enhanced whole-heart coronary magnetic resonance angiography (MRA) by using a gradient echo interleaved echo planar imaging (GRE-EPI) sequence at 3T field strength.
Materials and Methods
A GRE-EPI sequence was optimized to acquire contrast-enhanced whole-heart coronary MRA at 3T. First order phase correction was used for alignment of the odd and even echoes in the GRE-EPI echo train. Single and dual reference scan techniques for estimation of the linear phase correction parameters were evaluated using both phantom and volunteer studies. The GRE-EPI readout was combined with parallel imaging for a further reduction in scan time. To avoid image distortions, calibration signals for coil sensitivity estimation were acquired in a separate low resolution GRE scan prior to the whole-heart GRE-EPI scan. 8 healthy volunteers were scanned with the optimized contrast-enhanced GRE-EPI sequence. GRE-EPI images were acquired during slow infusion (0.3 ml/sec) of 0.1 mmol/kg body weight of Gd-BOPTA. For comparison purposes, the same 8 volunteers were scanned again in a separate scan session using a traditional GRE sequence with double the dose (0.2 mmol/kg body weight) of the same contrast agent with the same injection rate. The contrast-enhanced GRE-EPI and contrast-enhanced GRE techniques were compared in terms of relative SNR and CNR, image quality scores and visualized vessel lengths.
Results
Both, phantom and volunteer studies demonstrated that the dual reference scan phase correction technique was a key step for obtaining satisfactory image quality using GRE-EPI at 3T. Whole-heart coronary MRA with a spatial resolution of 1.0 × 1.0 × 2.0 mm3 was acquired with the GRE-EPI sequence in an average scan time of 2.5 ± 0.6 minutes, compared with 8.6 ± 2.7 minutes for the GRE technique. The GRE-EPI technique had lower relative CNR compared with the GRE sequence. The image quality and coronary artery visualization with the GRE-EPI technique were adequate and there was no statistically significant difference in the image quality scores, relative SNR and visualized coronary artery lengths between the GRE-EPI and GRE techniques.
Conclusions
Contrast-enhanced whole-heart coronary MRA using the GRE-EPI technique resulted in excellent delineation of all the major coronary arteries and compared with current GRE techniques demonstrated a factor of two reduction in contrast agent dose and a factor of three reduction in scan time.
Introduction
Whole-heart coronary MRA (1) acquires a thick axial slab covering the entire heart and allows the coronary arteries to be imaged in a single volume. The main advantage of this technique is that it eliminates the time-consuming process of localization of the coronary arteries and substantially improves the workflow of coronary MRA. Furthermore, it is possible to retrospectively reconstruct arbitrary views for optimal visualization of each vessel. Whole-heart coronary MRA has previously been applied at both 1.5T (2) and 3T (3) and has shown promising results for detecting coronary artery disease. However, the major drawback of this technique is the relatively long data acquisition time on the order of 10-15 minutes despite an acceleration factor of 2 with the use of parallel imaging methods (1-7). Further increase in the acceleration factor is limited by the available SNR and coil array geometry. Drifts in diaphragm position, patient motion and heart rate variations during these long imaging times may compromise the robustness of whole-heart coronary MRA and result in imaging artifacts (8).
Echo planar imaging (EPI) has the potential to reduce the imaging time of whole-heart coronary MRA, but this technique is sensitive to flow and off-resonance artifacts. Gradient echo interleaved EPI (GRE-EPI) (9) is a hybrid technique that provides a trade-off between scanning efficiency and artifacts and has previously been successfully applied to coronary MRA (10-14). All of these studies were performed at 1.5T or lower and had limited slab coverage and spatial resolution. Recently, a GRE-EPI based method using slow infusion of contrast agent (15) was proposed for contrast-enhanced whole-heart coronary MRA at 1.5T. This study achieved whole-heart coverage in a scan time of approximately 5 minutes.
The use of 3T field strength could lead to a further reduction in scan time by combining GRE-EPI with parallel imaging methods because of the increased SNR at 3T. However, the increased B0 field inhomogeneity at 3T can compromise the image quality of GRE-EPI. The goal of this work was to optimize a GRE-EPI acquisition scheme at 3T field strength for further reducing the imaging time of contrast-enhanced whole-heart coronary MRA. Phantom studies were used to optimize the GRE-EPI sequence. Healthy volunteers were scanned with the proposed sequence. Slow infusion of an extravascular, paramagnetic contrast agent, gadobenate dimeglumine (Gd-BOPTA) was used to enhance the SNR of the whole-heart GRE-EPI acquisition.
Materials and Methods
8 healthy volunteers (6 male and 2 female, average age 36 ± 13.8 years) were scanned on a clinical 3T scanner (MAGNETOM Trio, A Tim System, Siemens AG Healthcare Sector, Erlangen, Germany). Gd-BOPTA (Multihance, Bracco Imaging SpA, Milan, Italy) was used as the contrast agent. Written consent was obtained from volunteers in compliance with the Institutional Review Board of our institution.
Sequence Design Considerations
An ECG-triggered, fat saturated 3D GRE-EPI sequence similar to that proposed in Ref. (15) was used for contrast-enhanced coronary MRA at 3T. To achieve blood/myocardium contrast during contrast-enhanced imaging, inversion recovery (IR) preparation with an adiabatic inversion pulse was used. This has the additional side-effect of nulling the fat signal, leading to excellent fat suppression in the presence of increased B0 and B1 inhomogeneities at 3T. All acquisitions were performed during free breathing with navigator pulses placed on the dome of the right hemidiaphragm. A ± 3 mm acceptance window with a correlation factor of 0.6 (which describes the scaling in the measured motion of the diaphragm before application to the heart in the superior-inferior direction) was used for prospective real-time adaptive motion correction (16). An echo train length of 6 was used and k-space was filled in an interleaved fashion (9). Echo shifting (17) was used to minimize the phase discontinuities in the central k-space region. Asymmetric k-space sampling was used in the phase encoding direction in order to achieve a shorter TE to minimize off-resonance and flow artifacts. Partial Fourier reconstruction was used to synthesize the unacquired k-space region (18). Further details about the GRE-EPI sequence, reordering scheme and selection of inversion time (TI) and flip angle can be found in Ref. (15).
A bipolar readout gradient was used to maximize scan efficiency. First order phase correction was used to align the even (acquired with positive readout gradient) and odd (acquired with negative readout gradient) echoes. The first order phase correction parameters were estimated using a reference scan acquired without any phase encoding gradients. The nth even echo in the reference scan, after one-dimensional Fourier transformation is given by (19),
| [1] |
where, ρ(x,y) and Δω0(x,y) are the spin density and off-resonance frequency at location (x,y) respectively, TEn is the echo time of the nth echo, Mn(x) and An(x) are the magnitude and phase of the integral in Eq. [1]. In similar fashion, the nth odd echo, after one-dimensional Fourier transformation is given as,
| [2] |
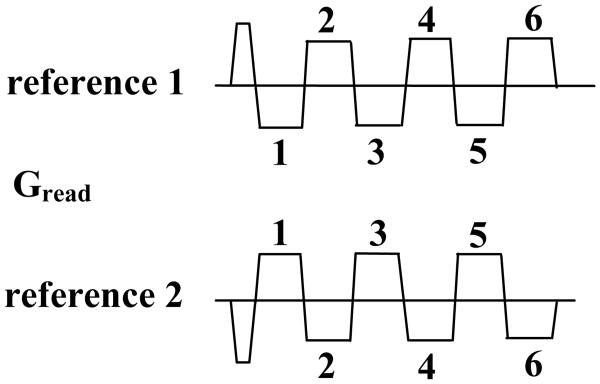
The linear phase term i(αx + β) is due to various factors including system imperfections, gradient delays and eddy currents (20) and has opposite polarity in the even and odd echoes due to the opposite readout gradient polarities. This linear phase term needs to be estimated using reference scans, and subsequently removed from the corresponding echoes during image reconstruction. Traditionally, a single reference scan is acquired for phase correction (20). In this situation, the even and odd echoes have different TE's and the phase difference between them, in addition to the linear phase term contains the term [An(x)−An+1(x)] due to off-resonance effects. At 3T, this off-resonance phase term interferes with accurate estimation of the first order phase correction parameters. A “dual reference scan” method similar to that proposed in references (19,21) was used to overcome this problem. The acquisition for this scan involves acquiring 2 sets of reference lines, with opposite readout gradient polarities (Fig. 1). With the dual reference scans the even and odd echoes are acquired with the same TE and the phase difference between them is given by
Figure 1.
Dual reference scan phase correction method acquiring 2 sets of reference lines with opposite readout gradient polarities.
| [3] |
This phase difference is independent of the off-resonance phase and contains only the linear phase term which can easily estimated. In this work the autocorrelation method (22) was used for estimation of the phase correction parameters.
To further reduce the scan time, GRAPPA (23) was used in the phase-encoding direction with an acceleration factor of 2. The use of auto-calibration for GRAPPA requires the central k-space views to be fully sampled and the outer k-space views to be undersampled by the desired acceleration factor. This variable density k-space sampling combined with the GRE-EPI readout results in different k-space traversal velocities in the central and outer k-space regions. This results in more off-resonance phase accumulation in the central k-space region and lesser phase accumulation in the outer k-space region. As a result, the low frequency parts of the image (corresponding to the central k-space region), are more distorted compared with the high frequency parts of the image (corresponding to the outer k-space region) (24). To avoid such image distortions, auto-calibration was not used and the reference lines were acquired using a separate low resolution GRE scan prior to the whole-heart GRE-EPI scan (25). The reference lines were acquired during free-breathing without any ECG or navigator gating in approximately 4-5 seconds with a spatial resolution of 4 × 4 × 4 mm3.
Phantom Studies
The goal of the phantom studies was to evaluate the effectiveness of the dual reference scan phase correction technique in the presence of off-resonance effects. The phantom setup consisted of two water and fat bottles. To simulate off-resonance effects images were acquired with the scanner frequency set to the water and fat resonance frequencies respectively. Images were reconstructed with the dual reference scan method. For comparison purposes, images were also reconstructed with a traditional “single reference scan” method in which only one reference scan (20) was used to obtain the phase correction parameters. The GRE-EPI sequence parameters used for the phantom studies were the same as those used for volunteer imaging (described in the next section) with the only difference being that the fat suppression pulse was removed in order to visualize both the water and fat components of the phantom.
Volunteer Studies
An inversion recovery prepared GRE-EPI sequence was used to acquire contrast-enhanced whole-heart coronary MRA during free breathing. Parameters for the GRE-EPI sequence were: TR = 10.6 ms, TE = 3.5 ms, 6 echoes after each RF pulse, flip angle = 25°, readout bandwidth = 1221 Hz/pixel, nonselective inversion pulse with a TI of 350 ms, 60 to 78 lines per heartbeat with 10 to 13 RF excitations in a data acquisition window of 106 to 138 ms, FOV = 265 × 174-232 × 112-128 mm3 (readout × phase encoding × partition encoding), matrix size = 256 × 168-224 × 56-64, voxel size = 1.0 × 1.0 × 2.0 mm3 interpolated to 0.5 × 0.5 × 1.0 mm3. The estimated imaging time for the GRE-EPI whole-heart scan would be 1 minute, assuming a heart-rate of 60 bpm and navigator gating efficiency of 100%. 0.1 mmol/kg body weight of Gd-BOPTA was injected at a rate of 0.3 ml/sec followed by a flush of saline using the same amount and rate. The amount of contrast agent was half of that used in previous studies (15,26). This was possible due to the reduced imaging time of the GRE-EPI acquisition. For maximal SNR, a previously described method was used to automatically trigger the scan during peak contrast enhancement (27). The partitions were acquired in a centrically reordered scheme to acquire the central k-space lines during peak signal enhancement.
For comparison purposes, all the volunteers were scanned again in a separate scan session using a traditional GRE sequence (26) with double the dose (0.2 mmol/kg body weight) of the same contrast agent with the same injection rate. The increased contrast dose was similar to previous studies (26) and was required due to the longer scan time of the GRE acquisition. The matrix size and spatial resolution were the same as the GRE-EPI acquisition. The scan parameters for the GRE sequence were: TR = 3.1 ms, TE = 1.5 ms, flip angle = 20°, non selective inversion pulse with TI = 200 ms, 34 to 44 lines per heartbeat in a data acquisition window of 106 to 138 ms, readout bandwidth = 700 Hz/pixel, GRAPPA acceleration factor of 2 in the phase encoding direction. For all the scans, a four chamber cine scan was used to determine the quiescent period (in mid-diastole) for coronary artery imaging.
Image Reformatting and Data Analysis
Whole-heart coronary artery images were reformatted using the CoronaViz software (Siemens Corporate Research, Inc., Princeton, NJ, USA) to project multiple vessel branches onto a single image (28). Blood SNR and blood-myocardium CNR were evaluated in the contrast-enhanced GRE and GRE-EPI images. Blood signal was measured in the ascending aorta at the level of the left main (LM) or right coronary artery (RCA). Myocardial signal was measured in the left ventricle adjacent to left anterior descending coronary artery (LAD). Image noise was estimated as the standard deviation in background air. SNR of blood was calculated as the ratio of blood signal to noise standard deviation. CNR between blood and myocardium was calculated as the difference between blood and myocardium signals divided by the noise standard deviation (29). To avoid problems associated with spatially varying noise profile in images reconstructed with parallel imaging, the SNR and CNR measurements were made from similar ROI's in the GRE-EPI and GRE images. Since these two images have similar coil position, imaging volume, and reconstruction method, a relative comparison between SNR and CNR is possible as suggested in Ref. (26). The SNR and CNR measurements made with this approach are referred to as relative SNR (rSNR) and relative CNR (rCNR) respectively. The GRE-EPI and GRE images were also compared in terms of image quality scores and visualized vessel lengths. Image quality was evaluated based on the raw images by two observers blinded to the technique. Image quality was graded as 1, poor (non-assessable); 2, fair (mild to moderate artifacts); 3, good (minimum to mild artifacts); 4, excellent (minimum or no artifacts). The visualized vessel lengths were measured on curved multi planar reconstructions of each artery. Quantitative comparison between the rSNR, rCNR, image quality scores and visualized coronary artery lengths was performed using a paired t-test.
Results
Phase Correction
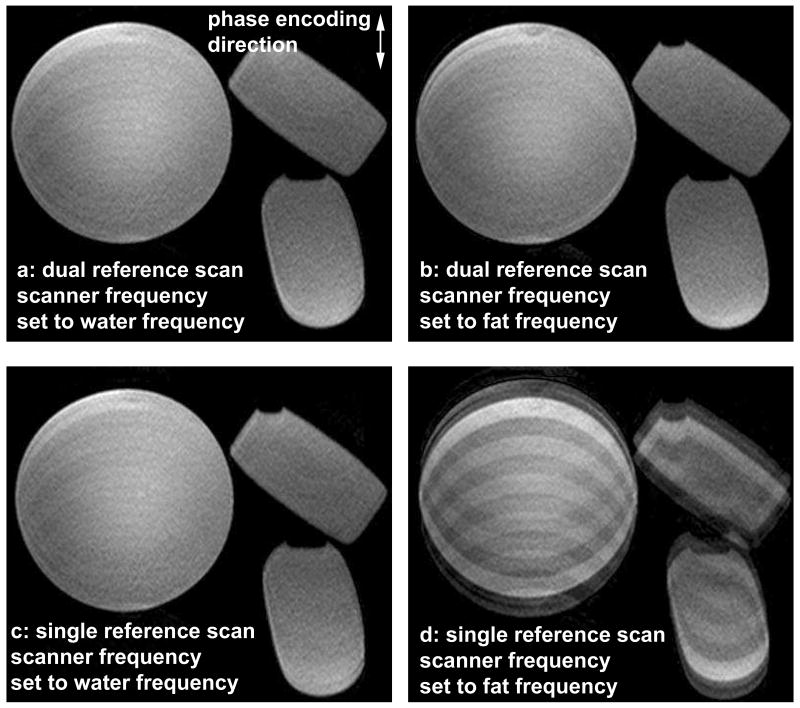
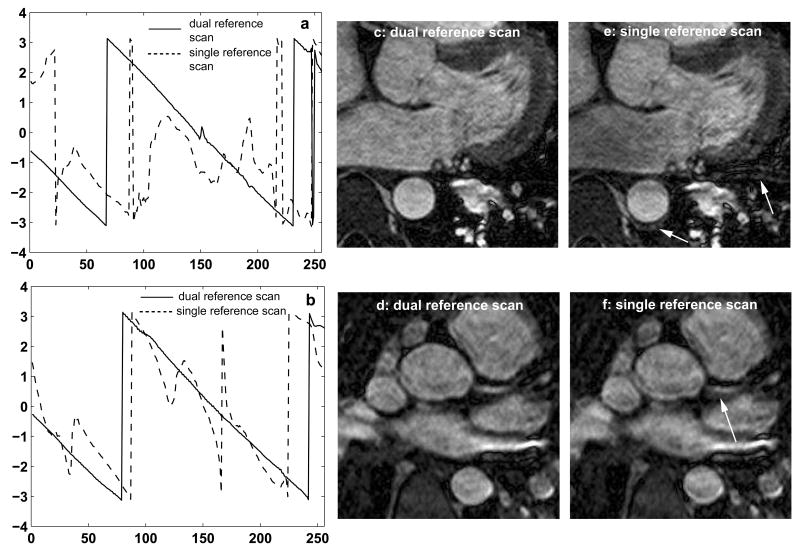
Fig. 2 shows phantom images using the dual (2a and b) and single (2c and d) reference scan techniques. 2a and c have the scanner frequency set to the water resonance frequency and 2b and d have the scanner frequency set to the fat resonance frequency. The dual reference scan technique results in images without ghosting artifacts at both frequencies (2a, b), demonstrating the robustness of this approach in the presence of a wide range of off-resonance effects. The single reference scan method gives results similar to the dual reference scan method when the scanner frequency is set to the water frequency (2c) but results in ghosting artifacts when the scanner frequency is set to the fat frequency (2d). Fig. 3 shows a comparison between the dual and single reference scan techniques in 2 healthy volunteers. Figs. 3a and b show the phase difference between the even and odd echoes using the dual (solid line) and single (dotted line) reference scan techniques. With the dual reference scan technique, the linear phase difference between the even and odd echoes is obvious and can be accurately estimated. For the single reference scan technique, this phase difference is influenced by the off-resonance phase effects and deviates significantly from the expected linear approximation, making the estimation inaccurate. The effect of phase correction on the images is seen in Figs. 3c-f. 3c and d were obtained with the dual reference scan technique, and 3e and f were obtained with the single reference scan technique. Residual ghosting and blurring artifacts shown by the white arrows in the images obtained with the single reference scan technique are eliminated by using the dual reference scan technique.
Figure 2.
Phantom images using the dual (a, b) and single (c, d) reference scan phase correction techniques with the scanner frequency set to the water (a, c) and fat (b, d) frequencies. The dual reference scan technique results in images without ghosting artifacts at both frequencies. In comparison, the single reference scan method results in ghosting artifacts when the scanner frequency is set to the fat frequency.
Figure 3.
Comparison between the dual and single reference scan phase correction techniques in 2 healthy volunteers (a, c, e: volunteer 1 and b, d, f: volunteer 2). a, b: Phase difference between the even and odd echoes. With the dual reference scan technique (solid line) the phase difference is linear, whereas with the single reference scan technique (dotted line) it deviates from the expected linear approximation. c-f: effect of dual (c, d) and single (e, f) reference scan phase correction techniques on the images. Ghosting and blurring artifacts shown by the white arrows in the images obtained with the single reference scan technique are eliminated by using the dual reference scan technique.
Volunteer Studies
All the volunteer studies were successfully completed. Quantitative comparison between the contrast-enhanced GRE-EPI and GRE acquisitions is shown in Table 1. The average scan time for the GRE-EPI sequence was 2.5 ± 0.6 minutes with an average navigator efficiency of 43.1 ± 10.9%. In comparison, the average scan time for the GRE technique was 8.6 ± 2.7 minutes with an average navigator efficiency of 41.3 ± 13.1%. Compared to the GRE technique the GRE-EPI technique had: i) rSNR decreased by 15% (37.3 ± 15.4 to 31.5 ± 11.9, the difference between them was not statistically significant p value > 0.05), and, ii) rCNR decreased by 35% (30.0 ± 10.9 to 19.8 ± 8.9, the difference between them was statistically significant p value < 0.05). The average image quality score for the contrast-enhanced GRE-EPI acquisition was 2.6 ± 0.5, compared to 2.9 ± 0.8 for the contrast-enhanced GRE acquisition. The difference between them was not statistically significant (p value > 0.05). The average visualized vessel lengths of the RCA and LAD were similar for both the techniques (p value > 0.05 for both).
Table 1.
Quantitative comparison between the contrast-enhanced GRE-EPI and GRE techniques.
| Imaging sequence (n = 8) |
Imaging time (minutes) |
Navigator efficiency (%) |
rSNR | rCNR | Image quality score | Vessel length (cm) | |
|---|---|---|---|---|---|---|---|
| LM + LAD | RCA | ||||||
| contrast-enhanced GRE-EPI | 2.5 ± 0.6 | 43.1 ±10.9 | 31.5 ± 11.9 | 19.8 ± 8.9 | 2.6 ± 0.5 | 10.3 ± 3.5 | 10.7 ± 1.6 |
| contrast-enhanced GRE | 8.6 ± 2.7* | 41.3 ±13.1 | 37.3 ± 15.4 | 30.0±10.9* | 2.9 ± 0.8 | 10.2 ± 2.9 | 10.9 ± 1.7 |
indicates statistically significant
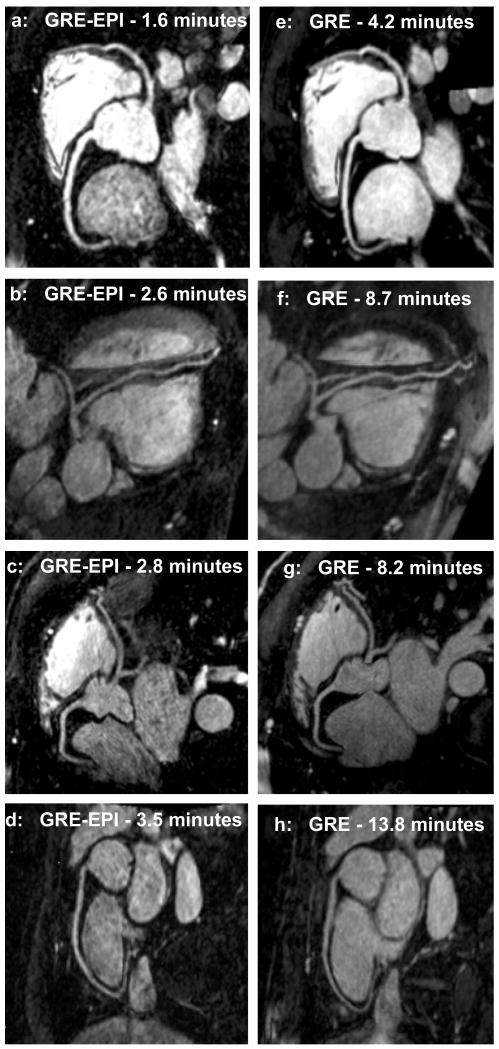
Fig. 4 shows reformatted coronary artery images from 4 healthy volunteers using the contrast-enhanced GRE-EPI acquisition (4a-d) and contrast-enhanced GRE acquisition (4e-h), acquired in separate scan sessions. The coronary artery delineation between the two sequences is similar, however, compared with the GRE sequence, the GRE-EPI sequence has, i) half the dose of contrast agent (0.1 mmol/kg as opposed to 0.2 mmol/kg) and, ii) imaging time reduced approximately by a factor of 3.
Figure 4.
Reformatted coronary artery images from 4 volunteers using a contrast-enhanced GRE-EPI sequence (4a-d) and a contrast-enhanced GRE sequence (4e-h), acquired in separate scan sessions. The GRE-EPI sequence has contrast agent dose reduced by a factor of 2 and scan time reduced by a factor of 3.
Discussion
In this study, contrast-enhanced whole-heart coronary artery images were acquired at 3T in an average scan time of 2.6 minutes using the GRE-EPI sequence. This is approximately a factor of 3 reduction compared with previous protocols reported at both 1.5T and 3T (1-5,7). This short scan time minimizes respiratory and cardiac motion artifacts due to changes in breathing pattern, patient motion and heart rate variations. It should also lead to increased patient comfort.
GRE-EPI was previously proposed for contrast-enhanced whole-heart coronary MRA at 1.5T (15). In the current study, we used the GRE-EPI technique at 3T field strength. In our previous work at 1.5T, a single reference scan phase correction technique was found to be adequate for alignment of the even and odd echoes. At 3T, we found that the single reference scan technique did not work well due to the increased off-resonance effects, and so we used a dual-reference scan technique (19) for linear phase correction of the GRE-EPI data. Both phantom and volunteer studies showed that this dual reference scan technique is critical for obtaining satisfactory image quality using GRE-EPI at 3T.
A number of different phase correction techniques have been proposed for echo alignment in GRE-EPI. One of these (30) is an image based phase correction method which was initially proposed for single-shot EPI and later extended for multishot EPI (31-33). This method calculates the phase correction as a one dimensional linear or non linear function and has the advantage that no reference scan is required. However, since it requires manual selection of ROI's in the image (from which the phase correction parameters are estimated), it was not used in this study. Another method is based on acquiring fully phase encoded reference scan data (21) with the gradient polarities reversed between image and reference scan acquisition, and results in a full 2D phase correction. The dual-reference scan technique used in this work is somewhat similar to this method since it acquires two reference scans with opposite readout gradient polarities. However, the difference is that it does not acquire any phase-encoded reference scans due to the unrealistic scan time requirements of a 3D phase encoded reference scan. Based on our experiments, a simple one dimensional linear phase correction based on the dual reference scan technique was adequate for echo alignment over a wide range of off-resonance frequencies which are present at 3T field strength.
Compared to our previous study at 1.5T, in this work the higher SNR at 3T field strength was used to combine the GRE-EPI readout with parallel imaging for a further reduction in scan time by a factor of 2. GRAPPA was used in this work as opposed to techniques like SENSE due to its insensitivity to motion (24). To avoid artifacts due to different k-space traversal velocities in different k-space regions (24), the reference scan for GRAPPA coefficient estimation was acquired using a separate low resolution GRE scan prior to the high resolution GRE-EPI scan. Based on our initial testing we observed that the reference scan could be acquired during free-breathing, without ECG or navigator gating. This resulted in a short scan time of 4 to 5 seconds for the reference scan.
The reduced scan time in this study allowed the contrast agent dose to be reduced from 0.2 mmol/kg (used in previous studies (15,26)) to 0.1 mmol/kg. One of the disadvantages of using 0.2 mmol/kg contrast agent for whole-heart coronary MRA is the increased safety concern and the inability to acquire first pass perfusion scans in the same imaging session. Since the current study acquires whole-heart coronary MRA with a contrast dose of 0.1 mmol/kg, it can potentially be combined with first pass perfusion scans using an additional 0.1 mmol/kg of contrast agent, resulting in a total dose of 0.2 mmol/kg. In addition, the coronary MRA and perfusion scans could be followed by a delayed enhancement scan, making a comprehensive cardiac MRI examination at 3T viable.
In this study the GRE-EPI sequence was compared with a traditional GRE sequence. Compared with the GRE sequence, the GRE-EPI sequence had contrast agent dose reduced by a factor of 2, and imaging time reduced by a factor of 3. The GRE-EPI technique had lower rCNR compared with the GRE sequence. The reduction in rCNR is probably due to the longer TI (350 ms) in the GRE-EPI sequence compared with the GRE sequence (200 ms). This TI for GRE-EPI was selected to avoid image artifacts due to signal decay within each heart-beat (15), and leads to increased myocardium signal, resulting in lower CNR. Inspite of this reduction in rCNR, which is one of the disadvantages of using GRE-EPI, this technique still resulted in comparable rSNR and adequate coronary artery delineation.
Another disadvantage of GRE-EPI is its sensitivity to flow and cardiac motion artifacts. In this study, these artifacts were minimized by using relatively small TE (3.5 ms) and TR (10.6 ms) values. With these values, we did not observe any apparent flow or motion artifacts in the volunteer studies. Flow compensation mechanisms (34) could potentially be incorporated into the GRE-EPI technique to make it more robust to flow and motion artifacts; however, this would be at the expense of reduced efficiency. Another drawback of GRE-EPI is that the T2* decay is expected to introduce some blurring in the images. This is a trade-off for using GRE-EPI and is partially offset by the fact that the shorter imaging time makes breathing pattern drifts and heart rate changes less likely, resulting in fewer motion artifacts. Another generic limitation of contrast-enhanced studies is that it is difficult to repeat the scan if the results are not adequate. Also, with the recent concerns of nephrogenic systemic fibrosis (35) it might not be applicable in all patients.
In conclusion, a GRE-EPI sequence was optimized to acquire contrast-enhanced whole-heart coronary MRA at 3T in an average scan time of 2.5 minutes, using 0.1 mmol/kg of contrast agent. Compared with current techniques, this represents a factor of two reduction in contrast agent dose and a factor of three reduction in scan time. Clinical utility of the technique needs to be tested on a patient population.
Acknowledgments
Himanshu Bhat would like to acknowledge support from the Captain and Mrs. Roberts Fellowship.
Grant Sponsor: National Institute of Health grants nos. NIBIB EB002623 and NHLBI HL38698; Siemens Medical Solutions USA, Inc., Malvern, PA; National Natural Science Foundation of China grant nos. 30828009 and 30900355; Captain and Mrs. Roberts Fellowship.
References
- 1.Weber OM, Martin AJ, Higgins CB. Whole-heart steady-state free precession coronary artery magnetic resonance angiography. Magn Reson Med. 2003;50(6):1223–1228. doi: 10.1002/mrm.10653. [DOI] [PubMed] [Google Scholar]
- 2.Sakuma H, Ichikawa Y, Chino S, et al. Detection of coronary artery stenosis with whole-heart coronary magnetic resonance angiography. J Am Coll Cardiol. 2006;48(10):1946–1950. doi: 10.1016/j.jacc.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Yang Q, Li K, Liu X, et al. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3.0-T a comparative study with x-ray angiography in a single center. J Am Coll Cardiol. 2009;54(1):69–76. doi: 10.1016/j.jacc.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gharib AM, Ho VB, Rosing DR, et al. Coronary artery anomalies and variants: technical feasibility of assessment with coronary MR angiography at 3 T. Radiology. 2008;247(1):220–227. doi: 10.1148/radiol.2471070274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuber M, Weiss RG. Coronary magnetic resonance angiography. J Magn Reson Imaging. 2007;26(2):219–234. doi: 10.1002/jmri.20949. [DOI] [PubMed] [Google Scholar]
- 6.Stehning C, Boernert P, Nehrke K. Advances in coronary MRA from vessel wall to whole heart imaging. Magn Reson Med Sci. 2007;6(3):157–170. doi: 10.2463/mrms.6.157. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Bi X, Huang J, et al. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3.0 T: comparison with steady-state free precession technique at 1.5 T. Invest Radiol. 2008;43(9):663–668. doi: 10.1097/RLI.0b013e31817ed1ff. [DOI] [PubMed] [Google Scholar]
- 8.Chang S, Cham MD, Hu S, et al. 3-T navigator parallel-imaging coronary MR angiography: targeted-volume versus whole-heart acquisition. AJR Am J Roentgenol. 2008;191(1):38–42. doi: 10.2214/AJR.07.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKinnon GC. Ultrafast interleaved gradient-echo-planar imaging on a standard scanner. Magn Reson Med. 1993;30(5):609–616. doi: 10.1002/mrm.1910300512. [DOI] [PubMed] [Google Scholar]
- 10.Bornert P, Jensen D. Coronary artery imaging at 0.5 T using segmented 3D echo planar imaging. Magn Reson Med. 1995;34(6):779–785. doi: 10.1002/mrm.1910340602. [DOI] [PubMed] [Google Scholar]
- 11.Botnar RM, Stuber M, Danias PG, et al. A fast 3D approach for coronary MRA. J Magn Reson Imaging. 1999;10(5):821–825. doi: 10.1002/(sici)1522-2586(199911)10:5<821::aid-jmri29>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Deshpande VS, Wielopolski PA, Shea SM, et al. Coronary artery imaging using contrast-enhanced 3D segmented EPI. J Magn Reson Imaging. 2001;13(5):676–681. doi: 10.1002/jmri.1095. [DOI] [PubMed] [Google Scholar]
- 13.Slavin GS, Riederer SJ, Ehman RL. Two-dimensional multishot echo-planar coronary MR angiography. Magn Reson Med. 1998;40(6):883–889. doi: 10.1002/mrm.1910400614. [DOI] [PubMed] [Google Scholar]
- 14.Wielopolski PA, Manning WJ, Edelman RR. Single breath-hold volumetric imaging of the heart using magnetization-prepared 3-dimensional segmented echo planar imaging. J Magn Reson Imaging. 1995;5(4):403–409. doi: 10.1002/jmri.1880050406. [DOI] [PubMed] [Google Scholar]
- 15.Bhat H, Zuehlsdorff S, Bi X, et al. Whole-heart contrast-enhanced coronary magnetic resonance angiography using gradient echo interleaved EPI. Magn Reson Med. 2009;61(6):1388–1395. doi: 10.1002/mrm.21963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Ehman RL. Retrospective adaptive motion correction for navigator-gated 3D coronary MR angiography. J Magn Reson Imaging. 2000;11(2):208–214. doi: 10.1002/(sici)1522-2586(200002)11:2<208::aid-jmri20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Feinberg DA, Oshio K. Phase errors in multi-shot echo planar imaging. Magn Reson Med. 1994;32(4):535–539. doi: 10.1002/mrm.1910320418. [DOI] [PubMed] [Google Scholar]
- 18.Noll DC, Nishimura DG, Macovski A. Homodyne detection in magnetic resonance imaging. IEEE Trans Med Imaging. 1991;10(2):154–163. doi: 10.1109/42.79473. [DOI] [PubMed] [Google Scholar]
- 19.Reeder SB, Faranesh AZ, Atalar E, et al. A novel object-independent “balanced” reference scan for echo-planar imaging. J Magn Reson Imaging. 1999;9(6):847–852. doi: 10.1002/(sici)1522-2586(199906)9:6<847::aid-jmri13>3.0.co;2-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruder H, Fischer H, Reinfelder HE, et al. Image reconstruction for echo planar imaging with nonequidistant k-space sampling. Magn Reson Med. 1992;23(2):311–323. doi: 10.1002/mrm.1910230211. [DOI] [PubMed] [Google Scholar]
- 21.Hu X, Le TH. Artifact reduction in EPI with phase-encoded reference scan. Magn Reson Med. 1996;36(1):166–171. doi: 10.1002/mrm.1910360126. [DOI] [PubMed] [Google Scholar]
- 22.Ahn CB, Cho ZH. A new phase correction method in NMR imaging based on autocorrelation and histogram analysis. IEEE Trans Med Imaging. 1987;6(1):32–36. doi: 10.1109/TMI.1987.4307795. [DOI] [PubMed] [Google Scholar]
- 23.Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 24.Skare S, Newbould RD, Clayton DB, et al. Clinical multishot DW-EPI through parallel imaging with considerations of susceptibility, motion, and noise. Magn Reson Med. 2007;57(5):881–890. doi: 10.1002/mrm.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griswold MA, Breuer F, Blaimer M, et al. Autocalibrated coil sensitivity estimation for parallel imaging. NMR Biomed. 2006;19(3):316–324. doi: 10.1002/nbm.1048. [DOI] [PubMed] [Google Scholar]
- 26.Bi X, Carr JC, Li D. Whole-heart coronary magnetic resonance angiography at 3 Tesla in 5 minutes with slow infusion of Gd-BOPTA, a high-relaxivity clinical contrast agent. Magn Reson Med. 2007;58(1):1–7. doi: 10.1002/mrm.21224. [DOI] [PubMed] [Google Scholar]
- 27.Bhat H, Lai P, Li D. Self-tracking of contrast kinetics for automatic triggering of contrast-enhanced whole-heart coronary magnetic resonance angiography. J Magn Reson Imaging. 2009;29(4):809–816. doi: 10.1002/jmri.21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aharon S, Oksuz O, Lorenz C. Simultaneous projection of multibranched vessels with their surroundings on a single image from coronary MRA. Proceedings of the 14th Annual Meeting of ISMRM; Seattle, WA, USA. 2006; p. 365. [Google Scholar]
- 29.Stuber M, Botnar RM, Fischer SE, et al. Preliminary report on in vivo coronary MRA at 3 Tesla in humans. Magn Reson Med. 2002;48(3):425–429. doi: 10.1002/mrm.10240. [DOI] [PubMed] [Google Scholar]
- 30.Buonocore MH, Gao L. Ghost artifact reduction for echo planar imaging using image phase correction. Magn Reson Med. 1997;38(1):89–100. doi: 10.1002/mrm.1910380114. [DOI] [PubMed] [Google Scholar]
- 31.Hennel F. Image-based reduction of artifacts in multishot echo-planar imaging. J Magn Reson. 1998;134(2):206–213. doi: 10.1006/jmre.1998.1502. [DOI] [PubMed] [Google Scholar]
- 32.Buonocore MH, Zhu DC. High spatial resolution EPI using an odd number of interleaves. Magn Reson Med. 1999;41(6):1199–1205. doi: 10.1002/(sici)1522-2594(199906)41:6<1199::aid-mrm16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 33.Buonocore MH, Zhu DC. Image-based ghost correction for interleaved EPI. Magn Reson Med. 2001;45(1):96–108. doi: 10.1002/1522-2594(200101)45:1<96::aid-mrm1014>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 34.Duerk JL, Simonetti OP. Theoretical aspects of motion sensitivity and compensation in echo-planar imaging. J Magn Reson Imaging. 1991;1(6):643–650. doi: 10.1002/jmri.1880010605. [DOI] [PubMed] [Google Scholar]
- 35.Broome DR, Girguis MS, Baron PW, et al. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol. 2007;188(2):586–592. doi: 10.2214/ajr.06.1094. [DOI] [PubMed] [Google Scholar]