Abstract
The default-mode network (DMN) consists of a set of brain areas preferentially activated during internally focused tasks. We used functional MRI to study the DMN in bipolar mania and acute schizophrenia. 17 bipolar disorder (BD), 14 schizophrenia (SZ) and 15 normal control (NC) subjects underwent 10-minute resting scans. The DMN was extracted using independent component analysis and template-matching; spatial extent and timecourse were examined. Both patient groups showed reduced DMN connectivity in the medial prefrontal cortex (mPFC) (BD:x=-2,y=54,z=-12; SZ:x=-2,y=22,z=18). BD subjects showed abnormal recruitment of parietal cortex (correlated with mania severity) while SZ subjects showed greater recruitment of the frontopolar cortex/basal ganglia. Both groups had significantly higher frequency fluctuations than controls (frequency × diagnosis:F(43,2)=3.183,p=0.05). We found ventral mPFC abnormalities in BD and dorsal mPFC abnormalities in SZ. The higher frequency of BOLD signal oscillations observed in patients suggests abnormal functional organization of circuits in both disorders. Further studies are needed to determine how these abnormalities are related to specific symptoms of each condition.
Keywords: Independent component analysis, mania, anterior cingulate cortex, basal ganglia
1. INTRODUCTION
Multiple positron emission tomography and functional magnetic resonance imaging (fMRI) studies have identified brain areas that are preferentially active in the absence of goal-directed activity and deactivate during the performance of sensorimotor or cognitive tasks (Fox et al., 2005; Greicius et al., 2004; Greicius et al., 2003; Shulman et al., 1997). These areas, including the lateral and medial parietal cortex, medial prefrontal cortex (PFC), and hippocampus, show synchronous activity patterns and have been called the default-mode network (DMN). Because these brain areas are implicated in “internally focused tasks” (Buckner et al., 2008), the DMN is hypothesized to subserve ongoing, or default, functions of the brain such as self-referential mental activity and autobiographic memory retrieval (Damoiseaux et al., 2006; Gusnard and Raichle 2001).
One useful approach for studying brain activity in the absence of cognitive or emotional tasks is independent component analysis (ICA), which resolves data signals into maximally-independent sources. This approach is similar to that used to resolve the voices of multiple individuals, as well as random noise, in a tape-recorded conversation. When applied to functional MRI (fMRI) data, ICA can detect signal changes due to motion or other artifacts, as well as neuronal activity (van de Ven et al., 2004). ICA produces spatial maps (components) within which voxels with stronger contributions to the component have increasingly similar blood oxygen level dependent (BOLD) signal timecourses. Application of ICA to fMRI datasets readily detects the DMN (Greicius et al., 2003), as well as several other neuronal networks (visual association, auditory, and sensory-motor) with characteristic low-frequency BOLD signal fluctuations (Beckmann et al., 2005). Detection of these biologically meaningful networks is particularly compelling because ICA examines BOLD signal coherence without prior assumptions about brain function. Studies using region of interest based approaches have confirmed and extended these findings in humans and primates, and indicate that there is a rich landscape of ongoing brain activity even “at rest” (Vincent et al., 2007).
Large scale neuronal networks with characteristic low-frequency BOLD signal fluctuations such as the DMN are of interest in psychiatry for two reasons: one can study neuronal connectivity in psychiatric illness by examining the spatial integrity of these networks; and one can probe the contribution of specific networks to psychopathology by examining BOLD signal fluctuations in each network. Both are relevant to research in bipolar disorder and schizophrenia, two common and debilitating psychiatric conditions characterized by white matter abnormalities and disrupted signaling across large scale neuronal networks (Kubicki et al., 2007; Hasler et al., 2006; Mohamed et al., 1999; Morrison-Stewart et al., 1991). Indeed, recent studies have identified abnormalities in the DMN, especially in the anterior cingulate/medial PFC region, at rest or during task performance in chronic medicated outpatients with schizophrenia (Garrity et al., 2007; Bluhm et al., 2007; Liu et al., 2006; Liang et al., 2006), bipolar disorder (Calhoun et al., 2007), and major depressive disorder (Greicius et al., 2007). Since these regions subserve attentional modulation and assessment of emotional salience (Öngür and Price 2000), abnormalities within the DMN may underlie cognitive and affective processing problems in psychiatric conditions. It is not currently clear whether specific regions within the DMN underlie distinct cognitive and affective abnormalities.
In this study, we focused on the spatial characteristics of the DMN in acutely ill patients, and carried out an exploratory analysis of its temporal features as well. We used ICA to identify the DMN component in bipolar disorder patients with acute mania, schizophrenia patients with acute psychosis, and matched healthy control subjects. We first compared the spatial extent of the DMN across groups. Significant differences in this analysis correspond to differences in the correlation of BOLD signal with that of the DMN, which does not necessarily reflect differences in activity level. Next, we used Fourier transformations of BOLD signal timecourse and compared oscillation frequency within the DMN across groups. To control for generalized abnormalities in resting-state activity, we carried out a parallel analysis on the primary visual cortex (V1) component. We reasoned that focusing on acute psychopathology would reveal both shared and distinct abnormalities in the DMN with those reported in stable outpatients. Psychopathology is chronic and unrelenting in schizophrenia so we hypothesized loss of medial PFC coherence with the DMN as previously reported in stable outpatients (Whitfield-Gabrieli et al., 2009; Camchong et al., 2009; Calhoun et al., 2007; Garrity et al., 2007; Bluhm et al., 2007). On the other hand, we predicted that acutely manic subjects would show loss of PFC coherence with DMN, although studies of stable outpatients with bipolar disorder have not reported this. We also predicted that both patient groups would show abnormalities in the frequency of BOLD signal fluctuation within the DMN reflecting dysregulation of cortical activity during acute illness.
2. METHODS
2.1. Subjects
Seventeen subjects with bipolar disorder (9M/8F, age 34.4 ± 12, age of onset 22.8 ± 8.7), fourteen with schizophrenia (8M/6F, age 42.3 ± 9.5, age of onset 22.3 ± 5.9) recruited from inpatient units at an academic psychiatric hospital, and fifteen healthy control subjects (9M/6F, age 37.9 ± 9.5) were included in this study. Demographic differences between groups were not statistically significant (Age: F(44,2)=3.09, p=0.06; gender: χ2=0.165, p=0.92). Diagnosis was determined using the Structured Clinical Interview for the DSM-IV (SCID) (First et al., 1995). Patients were assessed on the Young Mania Rating Scale (YMRS), the Positive and Negative Symptom Scale (PANSS), as well as the Montgomery-Asberg Depression Rating Scale (MADRS). One bipolar disorder patient was assessed clinically and using chart review only. The main analyses below were also repeated using age as covariate. The pattern of findings was similar (e.g. in anterior cingulate and parietal cortices) although details of cluster size and z-score coordinates varied somewhat.
Bipolar disorder patients were hospitalized with acute manic episodes (4 met criteria for mixed episode) and schizophrenia patients hospitalized with acute psychosis (7 met criteria for schizoaffective disorder, not currently in a mood episode). The symptom scale scores were PANSS: 58.6 ± 12.6, YMRS: 24.3 ± 7, MADRS: 11 ± 4 for the bipolar disorder group and PANSS: 84.3 ± 19.2, YMRS: 14.4 ± 9.1, and MADRS: 18.3 ± 7.7 for the schizophrenia group. Every bipolar disorder patient was taking lithium or anticonvulsant, and an antipsychotic at scan time, while all schizophrenia patients were taking an antipsychotic and 6 out of 14 were taking lithium or an anticonvulsant. Chlorpromazine equivalents of antipsychotic medication doses were 290.2 ± 182.4 for bipolar disorder and 497.6 ± 404.3 for schizophrenia groups.
Exclusion criteria included age outside the range of 18-65, substance abuse in the last month or lifetime history of substance dependence (nicotine use allowed), any neurological illness, positive pregnancy test or lactation, electroconvulsive therapy in the last 6 months, history of head trauma with loss of consciousness, and contraindications to MR scanning. The study was approved by the McLean Hospital Institutional Review Board, and all subjects gave written informed consent before participating in the study. To ensure that subjects understood the study, we conducted an informed consent survey, including simple questions about risks and benefits and the ability to withdraw consent. If the subject did not answer all questions correctly, the informed consent document was re-reviewed to ensure comprehension.
39 bipolar disorder patients, 18 schizophrenia patients and 15 controls were recruited for this study, but usable data were obtained from 17 bipolar disorder patients, 14 schizophrenia patients, and 15 healthy controls. Reasons for the high rate of attrition included patients’ inability to complete the scan, excessive motion during the scan, and substance use disorder revealed subsequent to the scan. Bipolar disorder subjects who did not complete the study (N=22) had markedly more severe psychotic symptoms (PANSS:82.0) as well as moderately more severe manic and depressed symptoms (YMRS=26.9; MADRS=18.4) than those who did.
2.2. Functional MRI Data Acquisition/Preprocessing
All scans were acquired on a 3T Siemens scanner using standard head coil and higher order automated shimming. A custom pillow reduced head movement. Subjects underwent a 10 minute fMRI scan in which they were instructed to “stay awake and not think of anything in particular” and to keep their eyes open. All subjects reported remaining awake during the scan.
After acquisition of high-resolution anatomical images, functional images were acquired with scan parameters as follows: repetition time 2.5 seconds, echo time 24 miliseconds, voxel size 3.5mm isotropic, interleaved slice acquisition, head-first prone slice encoding (to reduce susceptibility artifact), no skip, 35 coronal slices starting at the frontal pole and covering the entire brain, flip angle=90°. A total of 240 frames were collected over a 10:05 minute scan and the first two frames were discarded for magnetic field equilibration. Using SPM2 (http://www.fil.ion.ucl.ac.uk/spm), data were realigned to correct for motion, normalized into Talairach space, resliced, and smoothed with a 6mm Gaussian kernel. Data were also slice time corrected. Translational motion of all subjects was less than 2mm in all 3 dimensions except two control subjects who moved 2.5mm in one dimension. Mean displacement in the x-, y-, and z- dimensions were 0.01±0.18mm, 0.46±0.23mm, 0.11±0.55mm for healthy controls, 0.01±0.35mm, 0.38±0.30mm, 0.09±0.44mm for bipolar disorder, and 0.22±0.22mm, 0.36±0.23mm, 0.14±0.61mm for schizophrenia, respectively. Mean pitch, roll, and yaw were 0.002±0.007°, -0.001±0.005°, 0.002±0.003° for healthy controls, -0.002±0.012°, -0.002±0.006°, 0.001±0.006° for bipolar disorder, -0.002±0.009°, 0.000±0.008°, 0.005±0.004° for schizophrenia, respectively. There were no between-group differences in these six measures.
2.3. Functional MRI Data Analysis
Our data analysis approach consisted of three steps: identification of the DMN's spatial extent and BOLD signal timecourse in individual subjects using ICA and template-matching; comparison of DMN spatial extent across diagnostic groups using classical higher-level analysis; comparison of DMN BOLD signal timecourse across groups using ANOVAs.
240 preprocessed images from each subject were concatenated into single four-dimensional images and subjected to ICA using MELODIC (Multivariate Exploratory Linear Decomposition into Independent Components) Version ln(11), part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). MELODIC implements a Probabilistic ICA estimation (Beckmann and Smith 2004). Following masking of non-brain voxels, voxel-wise de-meaning and normalization of voxel-wise variance, data were whitened and projected into a multi-dimensional subspace using probabilistic Principal Component Analysis. The 4-dimensional data were decomposed into a set of timecourses and spatial maps by optimizing for non-Gaussian spatial source distributions using a fixed-point iteration technique (Hyvarinen 1999). Estimated components were divided by the standard deviation of the residual noise and thresholded by fitting a mixture model to the histogram of intensity values (Beckmann and Smith 2004). The resultant components are comprised of a spatial map of voxels, each assigned a z-score based on likelihood of belonging in the given component. Some components in this analysis represent artifact arising from motion, blood flow or other sources, while other components represent neural networks.
The ICA algorithm selected the optimal number of components for each subject, ranging from 32 to 57. The mean component numbers were 42.3, 44.8, and 42.9 for the control, bipolar disorder, and schizophrenia groups, respectively. Note that these component maps do not reflect areas of task-related blood flow change, but rather a collection of voxels with coherent activity. An automated two-step process was next used to select the component in each subject that most closely matched the DMN as previously described (Greicius et al., 2004). Briefly, a frequency filter was first applied to remove any components in which high-frequency signal (>0.1 Hz) constituted 50% or more of the total power in the Fourier spectrum. A template of the DMN (templates and software courtesy of Dr. Michael Greicius, Stanford University, Stanford, CA) was next used to select the “best-fit” component in each subject where the sum of z-scores inside the template minus the sum of z-scores outside it was maximal (Greicius et al., 2004). For each subject, we visually inspected all component spatial maps to ensure that the appropriate DMN was chosen by this procedure, and did not detect any discrepancies.
Once the DMN component was identified for all subjects, we carried out two types of analyses based on the spatial map and component timeseries. For the spatial extent analysis, DMN components were entered into a higher level analysis performed using FEAT (fMRI Expert Analysis Tool), version 5.4 (http://www.fmrib.ox.ac.uk/fsl). Between-group differences were examined using an ordinary least squares simple mixed-effects model. Z statistic images were thresholded at Z>3.09 (p<0.001, uncorrected) and spatial extent of 25 voxels. We chose this liberal statistical threshold because we used a relatively novel analytic approach (independent component analysis) where we could not predict effect sizes in between-group comparisons, and because we studied difficult clinical populations. Our group N's were sufficient but modest, limiting statistical power to detect between-group differences. The between-group analyses were masked with the DMN statistical map of the first group (e.g. NC>BD comparisons masked with the NC group map and BD>NC comparisons with the BD group map). Figure 1 depicts the mean group DMN images from the higher-level analysis. An identical analysis was next carried out using the V1 template instead of DMN. This analysis examined spatial extent differences in the V1 component as a control network (template courtesy of Dr. Michael D. Greicius, Stanford University, Stanford, CA). These analyses were not corrected for multiple comparisons.
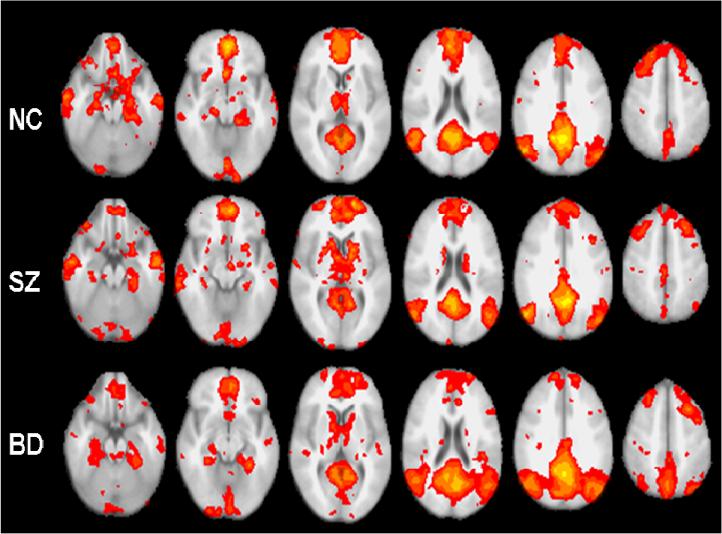
Figure 1.
Average DMN components in normal control, schizophrenia, and bipolar disorder groups mapped onto axial slices from the mean structural image of all study subjects. Despite the overall similar appearance of the components in the three groups, differences are apparent in the medial temporal lobe, PFC and parietal cortex.
We examined whether representative clusters identified as being significantly different between groups arose because one group had good coherence with the DMN in that cluster (i.e. positive z-scores), or because the other group had poor coherence (i.e. negative z-scores). For this purpose, we calculated mean z-scores of voxels in a cluster from individual DMN components using the Featquery toolbox within FEAT.
Since 7 out of 14 patients in the schizophrenia group were diagnosed with schizoaffective disorder, we carried out a separate higher level analysis to examine DMN differences between patients with schizophrenia and schizoaffective disorder. This analysis is underpowered and its results should be interpreted with caution. In addition, since all patients in the study were taking medication at scan time, we repeated the higher-level analysis for bipolar disorder and schizophrenia groups with de-meaned chlorpromazine (CPZ) equivalents as covariate to examine any antipsychotic medication effect on ICA results. We conducted a similar analysis with the schizophrenia group with de-meaned PANSS scores as covariate, and a third analysis with the bipolar disorder group with de-meaned YMRS scores as covariate.
For temporal characteristics analysis, the timecourse associated with each DMN component was transformed into the frequency domain using Fourier transformations. We averaged the resultant power spectra for each subject group, and average data points into bins of 0.02 Hz. We then carried out a repeated-measures analysis of variance (ANOVA) on the resulting histograms to explore differences in the power distribution between groups. To explore the impact of PANSS and YMRS scores, we created high and low score groups using a median split, and compared power distribution in these groups (N=8 and 6 for low- and high-PANSS subgroups within schizophrenia, respectively; N=9 and 8 for low- and high-YMRS subgroups within bipolar disorder, respectively). We also calculated a midpoint of power distribution (shifts in power spectrum towards higher frequencies lead to rightward shifts of midpoint) and correlated this midpoint with YMRS and PANSS scores.
3. RESULTS
3.1. Differences in DMN
3.1.1. Bipolar disorder vs. control subjects
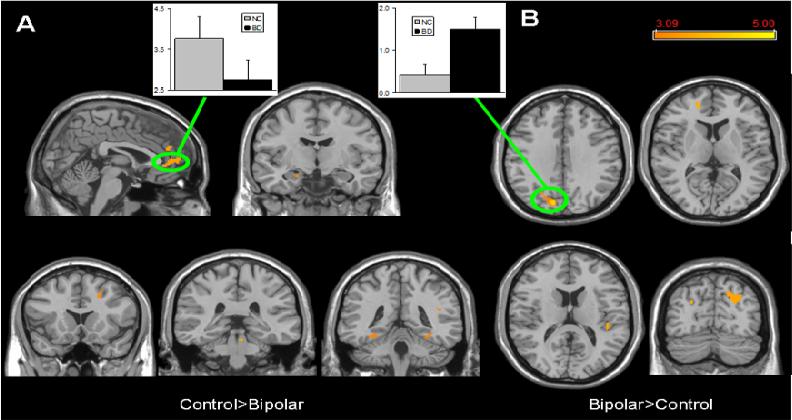
The Control>Bipolar comparison revealed several regions of significant difference, with the largest cluster in the medial PFC (Brodmann's Areas 10 and 32). In control but not bipolar disorder subjects, the DMN also included parts of the right premotor cortex, bilateral fusiform gyri, left hippocampus, and the pons (Table 1; Figure 2a). Mean Z-scores within the medial PFC cluster were 3.76±0.55 in controls and 2.75±0.48 in bipolar disorder (Figure 2a). By contrast, the left parietal cortex, left fusiform gyrus, right visual and auditory association cortices, left frontal polar cortex and the pons had significantly more coherence with the DMN in the Bipolar > Control comparison (Table 1; Figure 2b). Mean Z-scores in the left parietal cluster were 1.49±0.29 in bipolar disorder and 0.41±0.25 in controls (Figure 2b).
Table 1.
Significant differences in DMN spatial extent
| Brodmann's Area | Hemisphere | Voxels | Max Z | x (mm) | y (mm) | z (mm) | |
|---|---|---|---|---|---|---|---|
| NC>BP | |||||||
| Medial PFC | BA 10 | midline | 117 | 3.55 | -2 | 54 | -12 |
| BA32 | midline | 57 | 3.21 | -2 | 44 | 22 | |
| Pre-motor Cortex | BA 6 | R | 64 | 4.16 | 16 | 12 | 68 |
| Anterior Fusiform Gyrus | BA 36 | L | 38 | 3.37 | -24 | -14 | -34 |
| R | 105 | 3.61 | 24 | -12 | -28 | ||
| R | 25 | 3.45 | 14 | 0 | -24 | ||
| Hippocampus | L | 30 | 3.59 | -12 | -28 | -4 | |
| Pons | R | 50 | 3.62 | 14 | -28 | -32 | |
| BP>NC | |||||||
| Lateral Parietal Cortex | BA 7 | L | 46 | 3.46 | -32 | -74 | 34 |
| Frontal Polar Cortex | BA 10 | L | 45 | 3.19 | -18 | 48 | 8 |
| Visual Association Cortex | BA 19 | R | 153 | 4.49 | 10 | -80 | 32 |
| R | 105 | 3.28 | 22 | -80 | 32 | ||
| Posterior Fusiform Gyrus | BA 37 | L | 66 | 3.28 | -26 | -48 | -12 |
| Auditory Association Cortex | BA 41 | R | 49 | 3.81 | 42 | -28 | 14 |
| Pons | R | 35 | 3.3 | 4 | -34 | -22 | |
| NC>SZ | |||||||
| Dorsal Anterior Cingulate | BA 24 | midline | 59 | 3.45 | -2 | 22 | 18 |
| SZ>NC | |||||||
| Frontal Polar Cortex | BA 10 | L | 40 | 4.57 | -20 | 60 | 8 |
| Dorsolateral PFC | BA 9/46 | L | 35 | 3.92 | -36 | 24 | 32 |
| R | 59 | 4.1 | 16 | 6 | 14 | ||
| R | 33 | 3.51 | 26 | 6 | -2 | ||
| R | 27 | 3.33 | 16 | 14 | 6 | ||
| Basal Ganglia | R | 65 | 4.26 | 28 | -14 | -8 | |
| L | 26 | 3.46 | -14 | -16 | -2 | ||
| L | 199 | 4.2 | -18 | -12 | 16 | ||
| L | 78 | 3.25 | -14 | 6 | 4 | ||
Abbreviations as in the text
Figure 2.
Significant differences in DMN spatial extent between normal control and bipolar disorder subjects in whole brain analysis mapped onto parasagittal, coronal, and axial slices from a single subject's structural image in Talairach space; A) Normal control > Bipolar disorder; B) Bipolar disorder > Normal control. In each panel, average z-scores from a highlighted cluster are shown for normal control (gray) and bipolar disorder (black) groups. p≥0.001, uncorrected; spatial extent > 25 voxels.
3.1.2. Schizophrenia vs. control subjects
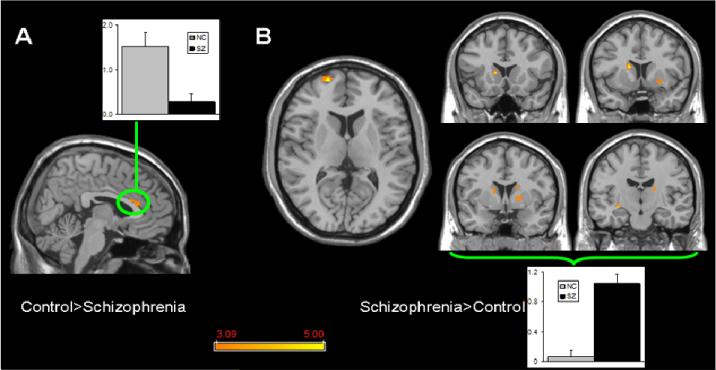
In the Control>Schizophrenia comparison, DMN spatial extent was significantly greater in the dorsal anterior cingulate cortex (Brodmann's Area 24) (Table 1; Figure 3a). Mean Z-scores in this cluster were 1.52±0.31 in controls and 0.27±0.19 in schizophrenia subjects (Figure 3a). The complementary Schizophrenia>Control comparison revealed that the left frontal polar cortex, right dorsolateral prefrontal cortex and multiple regions within the basal ganglia have greater coherence with the DMN (Table 1; Figure 3b). Mean Z-scores averaged across the basal ganglia clusters were 1.04±0.12 in schizophrenia subjects and 0.06±0.09 in controls (Figure 3b).
Figure 3.
Significant differences in DMN spatial extent between normal control and schizophrenia subjects in whole brain analysis mapped onto parasagittal, coronal, and axial slices from a single subject's structural image in Talairach space; A) Normal control > Schizophrenia where the only finding is in the anterior cingulate cortex; B) Schizophrenia > Normal control where multiple clusters are seen throughout the basal ganglia. Average z-scores are shown for normal control (gray) and schizophrenia (black) groups from a highlighted cluster in (A), and from a group of clusters in the basal ganglia in (B). p≥0.001, uncorrected; spatial extent > 25 voxels.
3.1.3. Bipolar disorder vs. schizophrenia subjects
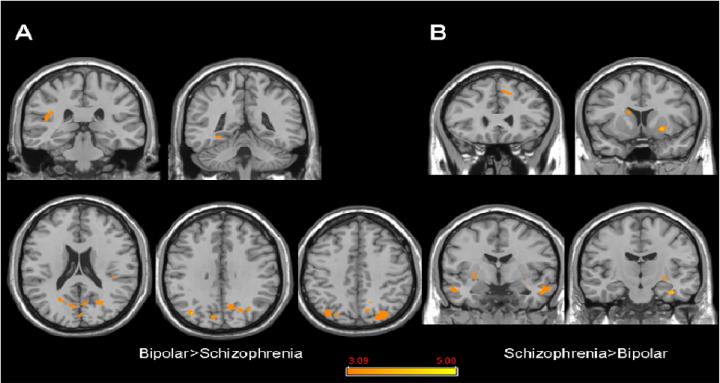
In the Bipolar>Schizophrenia comparison, parts of the lateral parietal cortex, parietal operculum, and primary and association visual cortex bilaterally, as well as the left posterior fusiform gyrus showed significantly more coherence with the DMN (Table 2; Figure 4a). In the complementary Schizophrenia>Bipolar comparison, the DMN included the left frontal polar cortex, right fusiform gyrus, temporal association cortices bilaterally, as well as parts of the basal ganglia bilaterally (Table 2; Figure 4b).
Table 2.
Significant differences in DMN spatial extent between patient groups
| Brodmann's Area | Hemisphere | Voxels | Max Z | x (mm) | y (mm) | z (mm) | |
|---|---|---|---|---|---|---|---|
| BD>SZ | |||||||
| Lateral parietal cortex | BA 7 | L | 600 | 3.99 | -32 | -74 | 34 |
| R | 45 | 3.51 | 18 | -52 | 44 | ||
| Parietal operculum | BA 40 | L | 52 | 3.53 | -42 | -30 | 18 |
| Posterior fusiform gyrus | BA 37 | L | 43 | 3.39 | -30 | -44 | -10 |
| Primary visual cortex | BA 17 | L | 39 | 3.14 | -6 | -84 | 6 |
| Visual association cortex | BA 18 | midline | 66 | 3.44 | 0 | -86 | 18 |
| BA 19 | R | 255 | 3.5 | 8 | -80 | 32 | |
| BA 19 | R | 254 | 4.02 | 36 | -76 | 34 | |
| SZ>BD | |||||||
| Frontal polar cortex | BA 10 | L | 29 | 3.56 | -18 | 62 | 6 |
| Superior frontal gyrus | BA 8 | R | 34 | 3.13 | 10 | 26 | 44 |
| R | 29 | 3.12 | 48 | 18 | 48 | ||
| Anterior fusiform gyrus | BA 20 | R | 54 | 4.33 | 36 | -16 | -28 |
| Medial temporal gyrus | BA 21 | L | 130 | 3.49 | -56 | -4 | -22 |
| BA 21 | R | 108 | 3.6 | 52 | -6 | -24 | |
| Basal ganglia | L | 137 | 4.11 | -24 | 8 | -8 | |
| L | 57 | 4.05 | -28 | -10 | -6 | ||
| R | 34 | 3.41 | 12 | 10 | 22 | ||
| R | 30 | 3.41 | 24 | -2 | -6 | ||
| R | 26 | 3.64 | 26 | -14 | -8 | ||
Abbreviations as in the text
Figure 4.
Significant differences in DMN spatial extent between bipolar disorder and schizophrenia subjects in whole brain analysis mapped onto coronal and axial slices from a single subject's structural image in Talairach space; A) Bipolar disorder > Scizophrenia; B) Schizophrenia > Bipolar disorder. p≥0.001, uncorrected; spatial extent > 25 voxels.
In a separate analysis, we compared the DMN between patients with schizophrenia and schizoaffective disorder and found no significant differences.
3.2. Differences in DMN timecourse
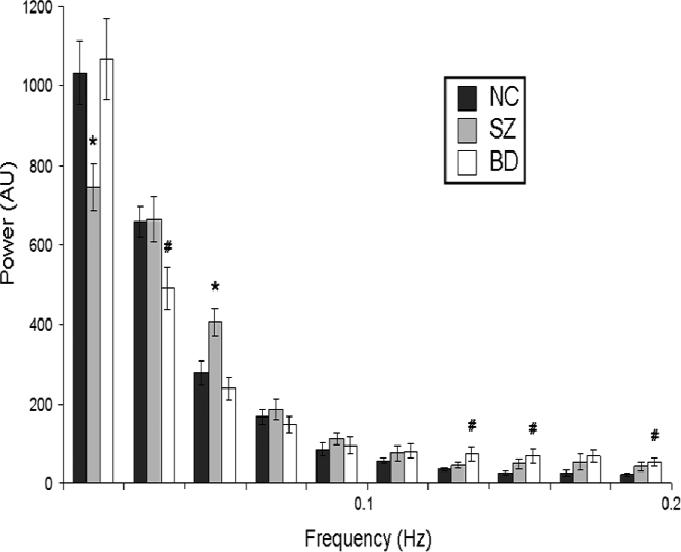
Figure 5 shows the distribution of power in the frequency domain for BOLD signal fluctuations in the DMN component for all three groups. Repeated-measures ANOVA showed a highly significant main effect of frequency (F(43,1)=881.584, p<0.001), but no main effect of diagnosis (i.e. all groups had similar total power). There was a significant frequency × diagnosis interaction (F(43,2)=3.183, p=0.05), indicating that the power was distributed differently for the 3 groups. ANOVAs with Scheffe's post-hoc tests at each 0.02Hz frequency bin showed that the schizophrenia group had significantly less power than controls at the 0-0.02Hz range, and significantly more power at the 0.04-0.06Hz range. The bipolar disorder group, by contrast, had reduced power in the 0.02-0.04Hz range, and increased power in the 0.12-0.14, 0.14-0.16 and 0.18-0.20Hz ranges.
Figure 5.
Power spectra of DMN in normal control, schizophrenia, and bipolar disorder subjects (dark grey, light grey and white bars, respectively). The relative power of each frequency range is represented on the y-axis in arbitrary units (AU); frequency bins of 0.02Hz each are shown on the x-axis. (* indicates significant schizophrenia-healthy control diference; # indicates significant bipolar disorder- healthy control difference)
3.3. Medication and Symptom Scale Analyses
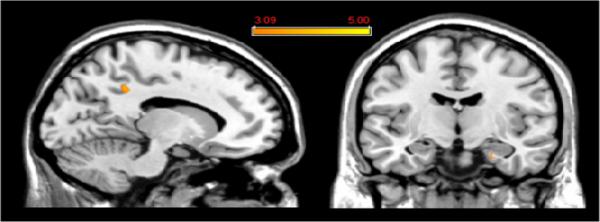
Coherence with the DMN did not correlate with CPZ equivalents or with PANSS scores for any brain region when these were entered into the higher-level analysis as a covariate (data not shown). By contrast, parts of the medial parietal cortex and parahippocampal gyrus showed coherence with the DMN correlated with YMRS scale scores (Table 3; Figure 6). The parietal cortex cluster identified in this analysis is proximate but anterior to that identified in the Bipolar>Control analysis (both in Brodmann's Area 7); there was no overlap between them.
Table 3.
Correlations with symptom scale scores
| Brodmann's Area | Hemisphere | Voxels | Max Z | x (mm) | y (mm) | z (mm) | |
|---|---|---|---|---|---|---|---|
| YMRS Correlation | |||||||
| Medial Parietal Cortex | BA 7 | L | 49 | 4.23 | -14 | -42 | 38 |
| Parahippocampal Gyrus | BA 36 | R | 34 | 3.16 | 24 | -14 | -26 |
Figure 6.
Two clusters showed a significant correlation between coherence with the DMN and YMRS scores among patients with bipolar mania. These are shown mapped onto a coronal and a sagittal slice from a single subject's structural image in Talairach space; see also Table 3. p≥0.001, uncorrected; spatial extent > 25 voxels.
There was no difference in power distribution of BOLD signal timecourse between high/low YMRS score subgroups within the bipolar disorder group or between high and low PANSS score subgroups within the schizophrenia group, although this analysis is underpowered because of small sample sizes. No significant correlations were detected between the midpoint of power distribution and YMRS scores in the bipolar disorder group, and PANSS scores in the schizophrenia group.
3.4. V1 Analyses
We conducted a parallel analysis on the same dataset using the V1 components. One cluster within V1 showed significant difference in the Bipolar>Control spatial analysis (32 voxels, maximal Z score=3.54, coordinates of peak activation at -18, -70, 18). There were no significant differences in V1 component spatial extent or timecourse in the Control>Bipolar, Schizophrenia>Control, Control>Schizophrenia, Bipolar>Schizophrenia, or Schizophrenia>Bipolar comparisons.
4. DISCUSSION
Neuronal networks showing low frequency BOLD signal fluctuations at rest are involved in important ongoing brain functions. In this study, we focused on the DMN because brain regions within this network may be particularly relevant to the origin and experience of mood and psychotic symptoms (Buckner et al., 2008; Williamson 2007; Greicius et al., 2007). In particular, the pattern of DMN abnormalities in acutely ill subjects is interesting to contrast with previous studies of stable outpatients because acute psychopathology may be associated with more pronounced abnormalities in brain activity. Recent findings indicate that the DMN is active when individuals are attendant to internally-focused tasks, and that each node within the network subserves specific functions related to this general role (Buckner et al., 2008). Our findings confirm previous reports that the DMN is a robust feature of brain activity identifiable in every subject. They also confirm our hypothesis that the mPFC is a major locus of shared abnormality in the DMN in schizophrenia and bipolar disorder. Broadly speaking, our results suggest that spontaneous oscillations in large-scale neuronal circuits are abnormal in psychiatric conditions, possibly underlying aspects of psychopathology.
In addition, each condition was also characterized by distinct abnormalities. Bipolar disorder was characterized by a reduction of coherence in several nodes within the DMN including the hippocampus, fusiform gyrus and pons, as well as abnormal recruitment of pontine, lateral parietal, and occipital regions into the DMN. Subjects with this condition also showed increased coherence of activity in the primary visual cortex in the V1 component analysis. Since participants were instructed to keep their eyes open during the scan, the V1 component was a particularly relevant control region. On the other hand, the schizophrenia group recruited a node in frontal polar cortex into the DMN, as well as multiple regions within the basal ganglia (caudate, putamen, globus pallidus) bilaterally. For bipolar disorder and schizophrenia, brain regions where DMN was more coherent than the control group were also more coherent than the other patient group (lateral parietal and visual cortices in bipolar disorder, and frontal polar cortex and basal ganglia in schizophrenia). Notable exceptions to this pattern included the insula which emerged in the Bipolar>Schizophrenia but not Bipolar>Control comparison, and temporal regions (fusiform and middle temporal gyri) and superior frontal gyrus which emerged in the Schizophrenia>Bipolar but not Schizophrenia>Control comparison.
4.1. Mania
In mania, the DMN is characterized by abnormalities in key nodes of the limbic system (mPFC and hippocampus) where abnormalities have been documented in bipolar disorder (Öngür and Price 2000). At the same time, lateral parietal areas show synchronous activity with the DMN, and a nearby although not identical region is correlated with the level of manic symptomatology (as measured by the YMRS). Reduced coherence in limbic regions and increased coherence in other cortical regions may be consistent with the dysregulated emotional processing and increased goal directed activities seen in mania (Strakowski et al., 2005; Phillips et al., 2003). Consistent with the involvement of this brain region in bipolar disorder, manic episodes have been reported following lesions of the parietal cortex (Fenn and George 1999). The finding of greater than normal coherence within the V1 component in bipolar disorder suggests that the inappropriate recruitment of posterior cortical regions into large-scale neural networks is not restricted to the DMN in this condition. This suggestion is also consistent with previous findings of visual processing abnormalities in bipolar disorder (Chen et al., 2006; Miller et al., 2003).
The only other study of DMN in bipolar disorder and schizophrenia classified these two conditions using the DMN and a temporal lobe network, and did not compare the DMN between groups (Calhoun et al., 2007). A quantitative assessment revealed weaker DMN increases in the posterior cingulate and bilateral parietal cortices in bipolar disorder as compared with controls, but direct comparison with our data is not possible since bipolar disorder subjects in that study were not in a manic episode.
4.2. Schizophrenia
In our study, schizophrenia was associated with deficient recruitment of the anterior cingulate gyrus into the DMN, consistent with a large schizophrenia literature (Benes 1998). In addition, there was a striking recruitment of multiple basal ganglia regions bilaterally into the DMN in schizophrenia. Circuit-level abnormalities in the basal ganglia have been reported in schizophrenia (Chang et al., 2007; Menon et al., 2001) and may be involved in problems of dynamic adjustment of control and cognitive sequencing (Kerns et al., 2008)(Kerns, et al. 2008). The abnormal recruitment of basal ganglia regions in schizophrenia is likely coupled with a loss of recruitment of the anterior cingulate cortex because the two are anatomically closely connected. The two findings may relate through a breakdown in executive functioning in schizophrenia. Finally, the frontal polar cortex was strongly recruited into the DMN in schizophrenia. This brain region is implicated in integrating the outcomes of two or more cognitive operations in pursuit of behavioral goals (Ramnani and Owen 2004), but more work is needed to confirm abnormalities in this process in schizophrenia.
Several previous studies have examined the DMN in schizophrenia. Two studies measuring the temporal homogeneity of BOLD signal reported reductions in coherence of activity in the cerebral cortex and cerebellum (Liang et al., 2006; Liu et al., 2006). These studies used region-of-interest based analyses and cannot be compared with the current work. Another study using a functional connectivity approach examined the DMN in schizophrenia, and reported reduced connectivity among dorsomedial PFC, parietal, and temporal regions of the DMN, which was interpreted as “functional disintegration” (Zhou et al., 2007). Reduced integration of brain activity across areas was also reported by a study of the dorsolateral PFC and striatum (Salvador et al., 2007), and by a study of posterior cingulate cortex connectivity with prefrontal, temporal, and parietal cortex and the cerebellum (Bluhm et al., 2007). Finally, another study used ICA to study outpatients with schizophrenia during a simple auditory oddball task (Garrity et al., 2007). In that study, unlike in ours, ventromedial PFC and anterior cingulate cortex were more coherent with the DMN in schizophrenia than in control subjects. There are many differences among these studies, including those in data acquisition details (TR, TR, voxel size), statistical approaches (seed-based functional connectivity, ICA, other temporal homogeneity approaches), patient population (acute inpatient vs. outpatient), and task instructions (cognitive tasks vs. rest). In addition, many studies do not account for the potential effects of psychotropic medications on BOLD signal oscillations. It is clear that functional integration across nodes of the DMN is deficient in schizophrenia but methodological differences preclude further conclusions.
4.3. Timecourse abnormalities
In an exploratory analysis, we also identified alterations in BOLD signal timecourse within the DMN in bipolar disorder and schizophrenia. Spontaneous neuronal oscillations are a major feature of brain networks, and they support the representation and consolidation of information (Buzsaki and Draguhn 2004). Low frequency oscillations are dysregulated in bipolar disorder and schizophrenia, suggesting significant abnormalities in underlying brain circuits and increased noise in the network. Bipolar disorder subjects showed a more extreme pattern, where very low frequency power was reduced and high frequency (>0.1 Hz) power was increased. There was no relationship between manic or psychotic symptoms and timecourse abnormalities, perhaps due to small sample size or because timecourse abnormality is a trait-like feature of mania.
4.4. Limitations
This study has a modest sample size because we studied acutely ill patients in 3 groups, which presented logistic difficulties. There was a high attrition rate especially in the mania group, and the patients who could not complete the study were somewhat more symptomatic than those who could. Nonetheless, the YMRS score of our manic patients was over 24, indicating that the completer patients were still highly symptomatic. Also, all patients were medicated at scan time in our study and a role for psychotropic medications in our findings cannot be ruled out. Nonetheless, two factors suggest that medication effects were not prominent in our study: both patient groups were on similar medications, but they showed different DMN abnormalities; and there was no relationship between our findings and CPZ equivalents. Another limitation of our study is the diagnostic heterogeneity in the schizophrenia group, where 7 out of 14 subjects were diagnosed with schizoaffective disorder. We cannot rule out the possibility that schizoaffective disorder patients show a differential pattern of DMN abnormalities which we are confounding with schizophrenia, but we do not think this is the case for two reasons. First, we studied patients who were hospitalized with acute psychosis and not currently in a mood episode, indicating that all patients in this group were phenomenologically similar. Second, a subgroup analysis between schizophrenia and schizoaffective disorder patients did not reveal significant differences, although this was an underpowered analysis.
Another important limitation in this study: psychomotor agitation and autonomic dysregulation (e.g. tachycardia/ tachypnea) are common in bipolar mania and may generate BOLD signal changes which do not reflect oscillations in neuronal activity. Motion parameters did not vary significantly between groups in our study, indicating this is not a likely. On the other hand, we did not collect eye movement data in our scans, and group differences in eye movements may underlie our Brodmann's Area 7 findings in mania, since this region is involved in the planning and modulation of eye movements (Buneo and Andersen 2006). In addition, we did not collect heart or respiratory rate data. Tachycardia/tachypnea could drive coherent BOLD signal changes in multiple cortical areas and explain some findings. It is unlikely, however, that autonomic differences would lead to both reductions in coherence in the PFC and increases in coherence in posterior cortical areas.
4.5. DMN abnormalities in psychiatric conditions
Abnormalities in the brain system subserving internally focused activity in schizophrenia and bipolar disorder may have important implications for understanding the emergence of aberrant mental states. This approach is promising because most psychopathology is not task-related, but rather arises spontaneously during interactions with the environment. Therefore, networks that modulate these spontaneous interactions are suitable foci of inquiry. For example, these findings may arise from abnormalities in long-tract signaling (Kubicki et al., 2007) and a breakdown in activity integration among multiple brain areas, ultimately manifesting as disordered processing of emotion, thought, or perception. Our current understanding of large-scale neural networks, however, is not sufficient to make predictions about which DMN abnormalities contribute to which clinical presentations. To this end, we need studies of large-scale neural networks which examine network characteristics under clinically relevant experimental conditions (Greicius 2008).
Additional insights may come from the divergence between the previous findings of significantly increased coherence between anterior cingulate cortex and the DMN in schizophrenia during an auditory oddball task (Garrity et al., 2007) and our findings of significantly reduced coherence in the same region for the same disorder during rest. It is possible that schizophrenia patients recruit the anterior cingulate cortex into the DMN too well during task performance and not enough during rest, i.e. they are unable to “turn it off” during tasks and to “turn it on” at rest. This hypothesis can be tested in studies of the same subjects’ DMN during rest and tasks of varying difficulty.
Acknowledgments
We thank Dr. Michael D. Greicius for sharing DMN/V1 templates and data analysis software, and for helpful discussions.
Funding: This study was funded by grant 5R01MH058681-05 (Dr. Renshaw) and 1K23MH079982-01A1 (Dr. Öngür) from the National Institute of Mental Health; Shervert Frazier Research Institute at McLean Hospital (Dr. Cohen).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr. Öngür has received research support from Sanofi-Aventis. Dr. Renshaw is a consultant to Novartis, GlaxoSmithKline, Roche and Kyowa Hakko and has received research support from Eli Lilly. The authors declare no conflicts of interest with this work.
REFERENCES
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society London B Biological Sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions in Medical Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Benes FM. Model generation and testing to probe neural circuitry in the cingulate cortex of postmortem schizophrenic brain. Schizophrenia Bulletin. 1998;24:219–230. doi: 10.1093/oxfordjournals.schbul.a033322. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophrenia Bulletin. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44:2594–2606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Human Brain Mapping. 2008;29:1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Macdonald AW, 3rd, Bell C, Mueller BA, Lim KO. Altered Functional and Anatomical Connectivity in Schizophrenia. Schizophrenia Bulletin. 2009 doi: 10.1093/schbul/sbp131. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage. 2007;34:1253–1269. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Sheremata S, Holzman PS. Bipolar and schizophrenic patients differ in patterns of visual motion discrimination. Schizophrenia Research. 2006;88:208–216. doi: 10.1016/j.schres.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn D, George K. Post-stroke mania late in life involving the left hemisphere. Australian and New Zealand Journal of Psychiatry. 1999;33:598–600. doi: 10.1080/j.1440-1614.1999.00539.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders. New York State Psychiatric Institute, Biometrics Research; New York: 1995. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. American Journal of Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology. 2008;24:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biological Psychiatry. 2006;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Transactions Neural Networks. 1999;10:626–634. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biological Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. Journal of Psychiatry Reseach. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Z, Liang M, Hao Y, Tan L, Kuang F, Yi Y, Xu L, Jiang T. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- Menon V, Anagnoson RT, Glover GH, Pfefferbaum A. Functional magnetic resonance imaging evidence for disrupted basal ganglia function in schizophrenia. American Journal of Psychiatry. 2001;158:646–649. doi: 10.1176/appi.ajp.158.4.646. [DOI] [PubMed] [Google Scholar]
- Miller SM, Gynther BD, Heslop KR, Liu GB, Mitchell PB, Ngo TT, Pettigrew JD, Geffen LB. Slow binocular rivalry in bipolar disorder. Psychological Medicine. 2003;33:683–692. doi: 10.1017/s0033291703007475. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Paulsen JS, O'Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Archives of General Psychiatry. 1999;56:749–754. doi: 10.1001/archpsyc.56.8.749. [DOI] [PubMed] [Google Scholar]
- Morrison-Stewart SL, Williamson PC, Corning WC, Kutcher SP, Merskey H. Coherence on electroencephalography and aberrant functional organisation of the brain in schizophrenic patients during activation tasks. British Journal of Psychiatry. 1991;159:636–644. doi: 10.1192/bjp.159.5.636. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Salvador R, Martinez A, Pomarol-Clotet E, Sarro S, Suckling J, Bullmore E. Frequency based mutual information measures between clusters of brain regions in functional magnetic resonance imaging. Neuroimage. 2007;35:83–88. doi: 10.1016/j.neuroimage.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Fiez JA, Miezin FM, Raichle ME, Petersen SE. Common Blood flow changes across visual tasks: l. Increases in subcortical structures and cerebellum, but not in non-visual cortex. Journal of Cognitive Neuroscience. 1997;9:624–647. doi: 10.1162/jocn.1997.9.5.624. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Molecular Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- van de Ven VG, Formisano E, Prvulovic D, Roeder CH, Linden DE. Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Human Brain Mapping. 2004;22:165–178. doi: 10.1002/hbm.20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson P. Are anticorrelated networks in the brain relevant to schizophrenia? Schizophrenia Bulletin. 2007;33:994–1003. doi: 10.1093/schbul/sbm043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophrenia Research. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]