Abstract
The incidence rate of acute kidney injury (AKI) is highest in elderly patients, who comprise an ever-growing segment of the population at large. AKI in these patients is associated with an increased risk of short-term and long-term death and chronic kidney disease, including end-stage renal disease. Whether AKI in older individuals carries a larger relative risk for these outcomes compared to younger individuals in unclear at this time. Other domains such as health-related quality of life may be mildly impacted after an episode of AKI. No effective therapies for AKI are currently available for wide-spread use. However, since the incidence of AKI is highest in the elderly and the phenotype is not discernibly different from AKI in all populations, future randomized controlled trials of interventions for AKI should be performed in the elderly population.
Index words: Acute renal failure, elderly, aged, epidemiology, outcomes, quality of life
Acute kidney injury (AKI; formally referred to as acute renal failure), is increasingly common in the population at large and is associated with significant morbidity, mortality, and health care costs. In hospitalized patients, the risk of death associated with AKI is elevated 3–6 fold compared to those without AKI.1 Despite significant advances in health-care technology over the past several years, the incidence of AKI appears to be increasing over time. This may be related to more aggressive medical and surgical therapies that result in stress to the kidney, the increasing number of comorbidites in the population that accumulate during increasing life-span, and the older age of the population at large. In the developed world, the increase in life expectancy has resulted in a continuous growth of the population over the age of 70 years.2 In fact, the segment of the population in which the incidence of AKI has been increasing the most rapidly is in those with advanced age. Thus, it is likely that it is the aged population that will yield the greatest potential for successful studies of possible interventions for AKI.
Epidemiology of AKI in the Elderly
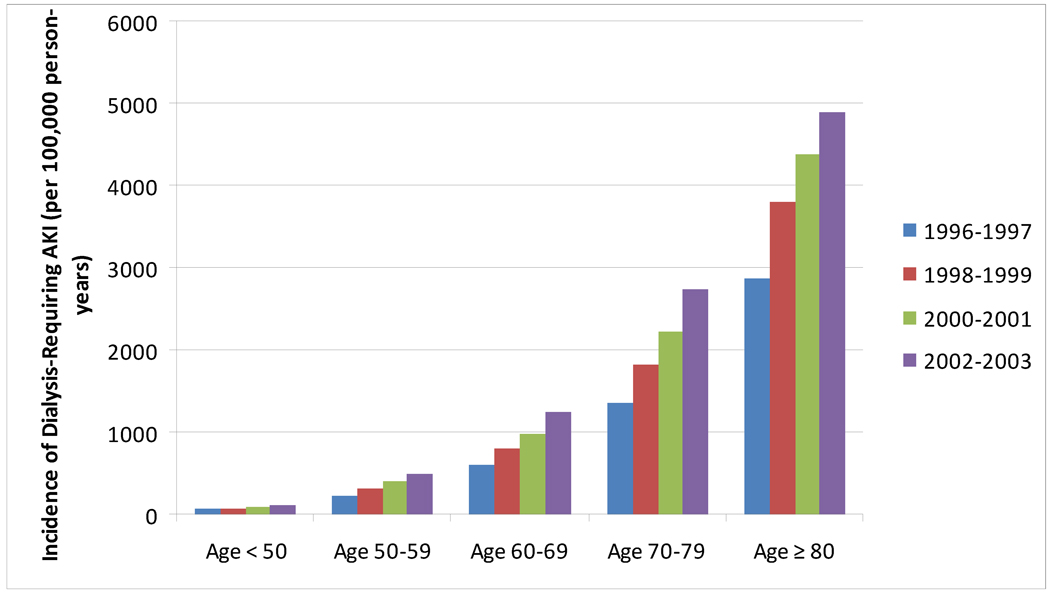
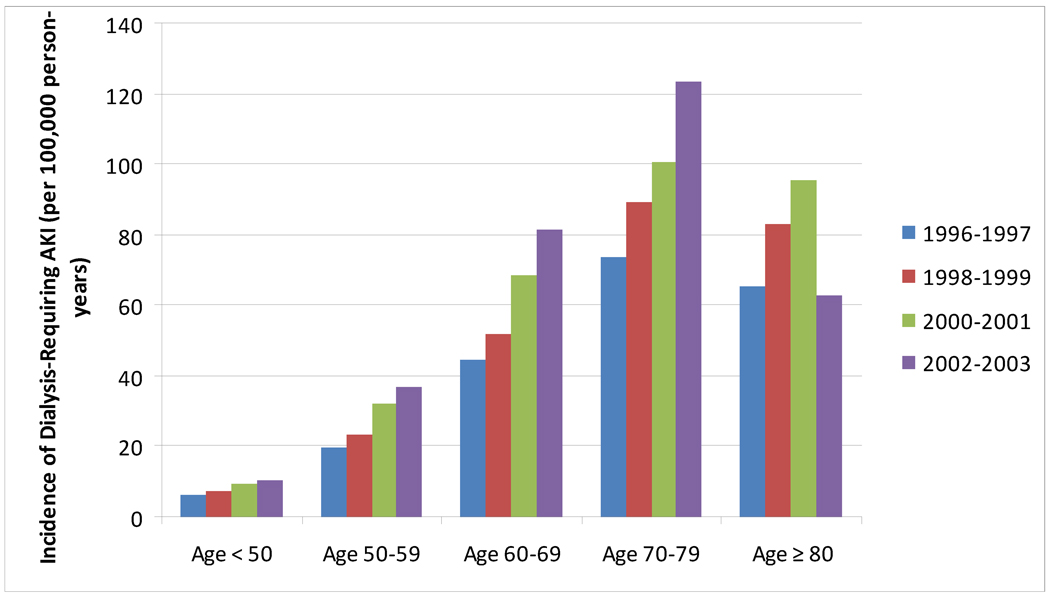
The age cutoff for “elderly” has conventionally been deemed age 65 or greater, and will be used as a general definition of “elderly” for the purposes of this review. Multiple studies in the literature have demonstrated that AKI is more common in elderly individuals3–6 and many have demonstrated a clear age-dependent relationship between AKI and older age.4, 7, 8 This relationship is not only evident in studies using International Classification of Diseases, Ninth Revision codes to define AKI, but also in large databases using inpatient and outpatient creatinine values to define AKI (Figure 1).8 The data demonstrate that the incidence rate of AKI is increasing over the past several years and that the incidence rate of severe AKI (requiring dialysis) is also increasing over time (Figure 2). Notable from these data is the discrepancy between the incidence rate of non-dialysis and dialysis-requiring AKI in patients aged 80 or greater. This likely represents a component of treatment bias (refusal of dialysis by patients, families, and/or health-care providers) rather than a higher incidence of less-severe phenotypes of AKI, however both phenomena may be present.
Figure 1.
Incidence Rates of Dialysis-Requiring acute kidney injury (AKI) between 1996 and 2003. The incidence rate of AKI is increasing over time in each stratum of age and the absolute incidence rates of AKI are highest in elderly individuals. Data from Kaiser Permanente of Northern California, as reported in Hsu et al. 4
Figure 2.
Incidence Rates of Dialysis-Requiring acute kidney injury (AKI) between 1996 and 2003. The incidence rate of AKI is increasing over time in each stratum of age through age 79. The incidence rates of dialysis-requiring AKI are highest in persons aged 70–79, however, the relationship between older age and higher rate of dialysis-requiring AKI does not hold for the oldest stratum (≥ 80). Data from Kaiser Permanente of Northern California, as reported in Hsu et al.4
Risk Factors for AKI in Elderly Individuals
The higher incidence of AKI in elderly persons can be potentially attributed to the following: A) comorbidities that accumulate with age may facilitate AKI (e.g., renovascular disease, congestive heart failure); B) comorbidities may necessitate procedures, drugs or surgery that function as kidney stressors and nephrotoxins; C) the kidney undergoes age-dependent structural and functional alterations over time (Box 1).9–22 The result of the latter is a reduced GFR at baseline and a diminished kidney reserve in the setting of pathophysiological challenges, lending elderly patients very vulnerable to acute stress and more likely to develop clinically relevant AKI.
Few studies in the published literature thoroughly attribute etiology to AKI. Few studies discriminate between acute tubular necrosis (ATN) and prerenal AKI effectively enough to draw meaningful conclusions about the true proportions of these kidney “syndromes”, although some studies have estimated that 40% of AKI in the elderly is due to ATN, and 30% due to prerenal causes.23, 24 Approximately one-quarter of AKI in elderly patients is due to obstruction.7, 24 Elderly individuals are also more likely to suffer from chronic kidney disease (CKD), congestive heart failure, hypertension, renovascular disease, diabetes, and are more likely to undergo surgery (especially cardiac and vascular surgery). Commensurate with these conditions and risks, elderly patients are more likely to be exposed to nephrotoxic contrast (during cardiac or vascular arteriography), exposed to angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and also to non-steroidal anti-inflammatory agents (NSAIDs) for osteoarthritis. The later two classes of agents (ACE inhibitors/ARBs and NSAIDs) modulate kidney autoregulation and increase the risk for hemodynamically-mediated AKI. Thus, the combination of changes in the aging kidney, the abnormalities of other organ systems, and the exposure to various pharmaceutical agents makes elderly individuals most susceptible for development of AKI.
Diagnosis of AKI in the Elderly
AKI is usually clinically diagnosed by an abrupt change in serum creatinine concentration. However, serum creatinine concentration is dependent on the steady state between creatinine release from muscles and excretion via the kidneys. Since muscle mass generally declines with age,25, 26 serum creatinine concentrations should fall with age, if true glomerular filtration rate (GFR) is left unchanged. After acute injury to the kidney that results in an abrupt decline in GFR, the rate and magnitude of rise in serum creatinine may be blunted in the elderly because of the smaller amount of muscle mass. Furthermore, a recent study of creatinine kinetics in AKI demonstrated that individuals with CKD will have a slower rate of rise in serum creatinine for the same reduction in GFR than people without CKD.27 Although the effects of older age on creatinine kinetics in AKI were not examined in this study, since the prevalence of CKD is higher in the elderly compared to younger individuals,28 on average, serum creatinine is likely not an ideal biomarker for AKI in the elderly because of delays to achieve a rise and peak serum creatinine concentration even with abrupt reductions in GFR. Over the past several years, the search for novel biomarkers of AKI (both serum and urine) to detect AKI earlier has been rampant.29 These novel biomarkers for AKI will be briefly reviewed below.
Cystatin C is an endogenous protease inhibitor produced at a constant rate by nucleated cells, and excreted exclusively by glomerular filtration. Serum levels are stable with changes in age, muscle mass, diet and physical activity and cystatin C is predominantly excreted by GFR. Herget-Rosenthal et al. demonstrated that a 50% rise in serum cystatin C performed well in elderly patients (mean age 70 ± 8) for diagnosing AKI both 2 or 1 days prior to clinical AKI as defined by a 50% increase in serum creatinine (area under receiver operating curves 0.82 and 0.97, respectively).30 These findings were similar in another study of patients in the intensive care unit.31 Serum cystatin C is used at some hospitals in the United States, but has not yet been adopted for widespread use for assessing acute and long-term changes in kidney function.
Several urinary biomarkers of AKI that reflect tubular injury rather than changes in GFR have been studied recently in the literature.29 The performance of the urinary biomarkers to diagnose AKI early has varied depending on the cohort studied, and one of the most important effect modifiers of performance may be due to age. For example, a recent meta-analysis of the performance of NGAL as a biomarker for the early diagnosis of AKI reveals some effect modification by age, in that the performance was better in children compared to adults. Thus far, discrepancies in the performance of other urinary biomarkers (e.g., interleukin 18, kidney injury molecule 1) in adults versus children32, 33 have not been as stark, although no single biomarker has been able to achieve excellent performance alone for the early diagnosis of AKI in older adults.34, 35 It is also unknown whether the performance of any AKI biomarkers differ in the old or very old compared to younger adults. Nevertheless, before urinary biomarkers become adopted into clinical practice, it is likely that at the very least, a panel of multiple biomarkers will be needed to diagnose AKI early and accurately in older adults.36
Outcomes of AKI in Elderly Individuals
While AKI may be more common in elderly adults, it is more important to understand whether AKI is associated with more severe or less severe consequences in this population.
Short-term Mortality After AKI
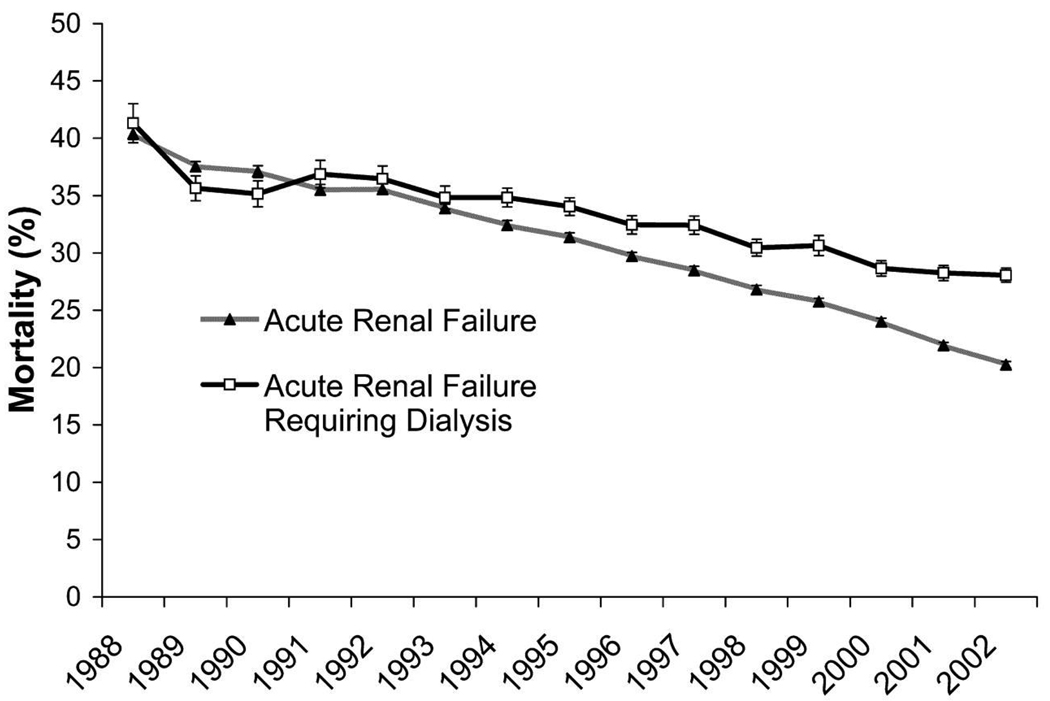
The in-hospital mortality rate for elderly patients with AKI ranges from 15–40%.8, 24 While the prevailing thought has been that the mortality associated with AKI has not improved over past decades, recent data suggests that survival is in fact improving over time (Figure 3).8, 37 Waikar et al. and Xue et al. both demonstrated that mortality associated with AKI decreased from the years 1988 to the year 2002 and the years 1992 to 2001, respectively, despite the fact that the severity of illness of these patients, as assessed by the Deyo-Charlson comorbidity index, increased over the same period of time.37 These data also demonstrated that in-hospital mortality declined in parallel in elderly patients with AKI.37 One study compared survival in older vs. younger patients with AKI and found that the relative risk for death associated with AKI for those aged 80 or older was not significantly greater than those aged less than 65.23 Thus, while the incidence of AKI is increasing in elderly patients,4 it appears that the immediate consequences associated with AKI are following trends similar to the outcomes witnessed with AKI in younger patients.
Figure 3.
In-hospital mortality of patients with acute renal failure and acute renal failure that required dialysis from 1988 to 2002. Error bars denote SE. Data from Nationwide Inpatient Sample.
Reproduced from Waikar et al37 with permission of the American Society of Nephrology.
Long-term Mortality after AKI
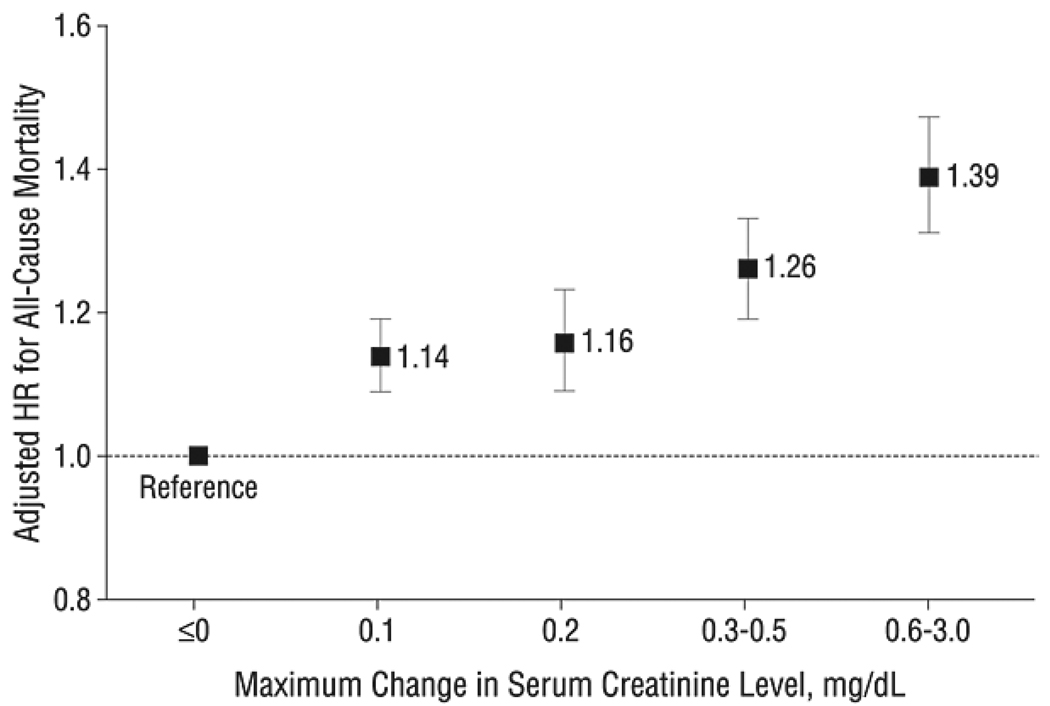
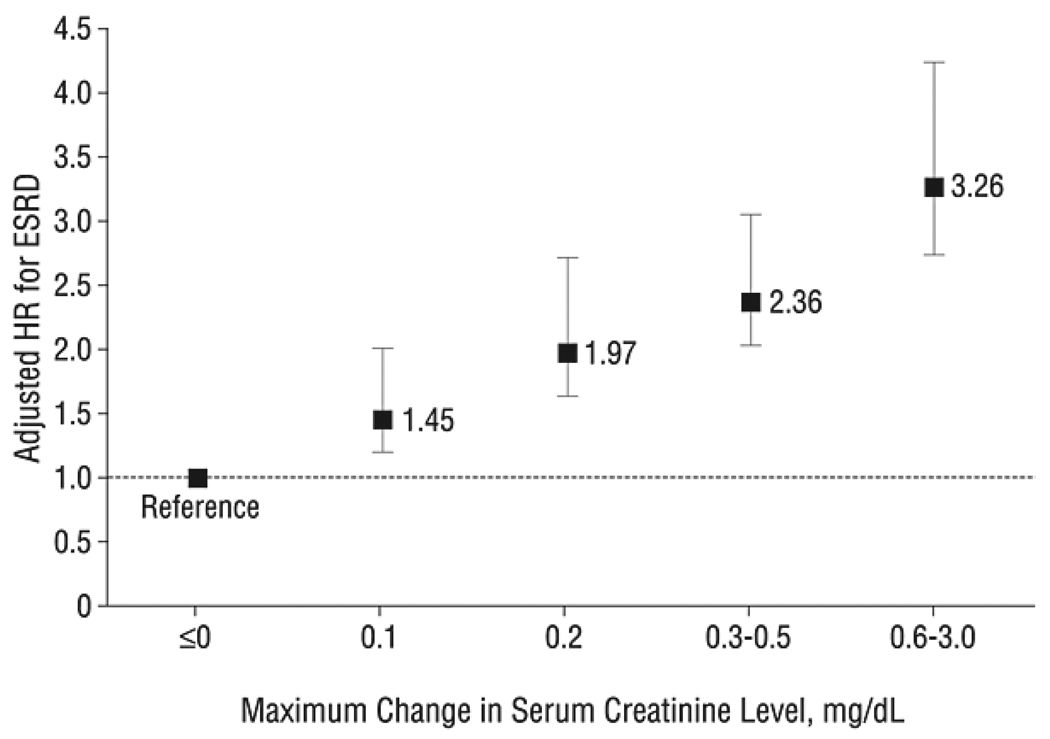
Until recently, few studies have specifically focused on long-term outcomes of AKI in elderly patients. Multiple studies published over the past few years have demonstrated that AKI is independently associated with an increased risk of long-term death.38–41 Even very small changes in creatinine (in elderly patients with acute myocardial infarction) were associated with long-term death, and greater changes are associated with greater risk of mortality (Figure 4).42, 43 In a study that examined survival in both young and old patients, AKI and older age were independently associated with the risk for long-term death.40 The magnitude of the adjusted hazard ratios for death with AKI decreased as age increased (adjusted hazard ratio 1.72, 1.4, 1.2 for age strata 46–60, 61–70, and ≥ 71, respectively, vs. age 18–45).40 No formal test for interaction was reported for the effect of age on the association between AKI and mortality, but this phenomenon is very notable. One hypothesis that may explain this phenomenon may be that the potential impact of AKI is diluted as one ages as the cumulative effect of other comorbidities overwhelms any direct consequences from decreased kidney function.
Figure 4.
Adjusted hazard ratios (HRs) and 95% confidence intervals for all-cause mortality according to maximum level of serum creatinine level increase during hospitalization. Data from Cooperative Cardiovascular Project, 1994 to 2004.
Reproduced from Newsome et al42 with permission of the American Medical Association.
A study that specifically examined outcomes in elderly patients with severe AKI found that the risk of death in patients with AKI requiring dialysis after cardiac surgery was not significantly different between older and younger patients (45% in those < 70 and 40% in those ≥ 70).44 Since the decision to institute hemodialysis is not based on stringent criteria and thus requires decision making by individual treating physicians, it is possible that either the older group was less likely to receive dialysis due to physician preference or the amount of injury to require dialysis was less in the older group. However, two of three severity of illness scoring systems used to assess these patients were not significantly higher in the younger group.
In summary, AKI in older patients, as in younger patients, appears to be independently associated with an increased risk of premature death. However, the contributing effect of AKI may be diluted in persons of older age.
CKD and ESRD after AKI
Although AKI has been classically felt to be a “reversible” condition that holds no long-term consequence for kidney function, several recent studies have demonstrated that AKI is independently associated with an increased risk for CKD and/or end-stage renal disease (ESRD) in survivors of hospitalization (Table 1 and Figure 5).38, 39, 42, 45 In regards to older age, one of the independent predictors of GFR after AKI is age at the time of AKI (coefficient −0.48, p < 0.001).46 In other words, the greater the age the lower the GFR over follow-up.
Table 1.
Studies Examining Development of CKD and/or ESRD in AKI and non-AKI Patients
| Study | Setting | No. | Mean Age |
Definitions of Groups |
Outcomes |
Comments related to Elderly | |||
|---|---|---|---|---|---|---|---|---|---|
| Death | ESRD | ||||||||
| Incidence Rate (person- years) |
Adjusted HR (95%CI) |
Incidence Rate (person- years) |
Adjusted HR (95% CI) |
||||||
| Wald et al.45 |
Population -based cohort |
17,367 | 62 | In-hospital dialysis Matched non-AKI |
101/1,000 108/1,000 |
0.95 (0.89–1.02) (ref) |
26/1,000 9/1,000 |
3.23 (2.7–3.86) (ref) |
Absolute risk for ESRD higher in older patients: ≥65, 9.5% in AKI vs 2.8% in non-AKI; <65, 7.4% in AKI vs 3.2% in non-AKI |
| Lower bound of 95% CI for adjusted HR in those older >65 does not appear to overlap upper bound of 95% CI for adjusted HR in those <65 (signifies presence of effect modification by age) |
|||||||||
| Lo et al.39 | Population -based cohort |
3773 | 63 | In-hospital dialysis Matched non-AKI |
NR | 2.3 (1.8–3.0) (ref) |
17/1,000†
479/1,000 |
28.1 (21.1–37.6)†
(ref) |
None |
| Newsome et al.42 |
Medicare –acute MI |
87094 | 77 | ΔSCr = 0 | 139.1/1,000 | 1.00 (ref) | 2.3/1,000 | 1.00 (ref) | Entire cohort “elderly”; no further age strata examined |
| ΔSCr = 0.1 | 145.5/1,000 | 1.14 | 2.3/1,000 | 1.45 | |||||
| ΔSCr = 0.2 | 157.0/1,000 | 1.16 | 3.6/1,000 | 1.97 | |||||
| ΔSCr = 0.3–0.5 | 193.6/1,000 | 1.26 | 6.3/1,000 | 2.36 | |||||
| ΔSCr = 0.6–3.0 | 274.9/1,000 | 1.39 | 20.0/1,000 | 3.26 | |||||
| Ishaniet al.38 |
Medicare | 233,803 | 79 | AKI by ICD-9 | 54.3%* | 2.48 (2.38–2.58) | 27.5/1,000 | 13.0 (11.0–16.0) | Incidence rate | adjusted HR (95% CI) for ESRD by age strata: 67–70 y: 8/1,000 | 1.00 (ref) 71–75 y: 6.9/1,000 | 0.87 (0.74–1.02) 76–80 y: 5.7/1,000 | 0.72 (0.61–0.85) 81–85y : 4.3/1,000 | 0.63 (0.52–0.76) ≥86 y: 1.9/1,000 | 0.36 (0.28–0.46) |
| AKI on CKD by ICD-9 |
64.3%* | 3.24 (3.08–3.4) | 101.5/1,000 | 41.2 (34.6–49.1) | |||||
2 year cumulative incidence of death
Outcome stage 4 or worse CKD (not ESRD)
Abbreviations: MI, myocardial infarction; AKI, acute kidney injury; SCr, serum creatinine; ICD-9, International Classification of Diseases, Ninth Revision; HR, hazard ratio, ESRD, end-stage renal disease; CI, confidence interval; CKD, chronic kidney disease; ref, reference.
Figure 5.
Adjusted hazard ratios (HRs) and 95% confidence intervals for end-stage renal disease (ESRD) according to maximum level of serum creatinine level increase during hospitalization. Data from Cooperative Cardiovascular Project, 1994 to 2004.
Reproduced from Newsome et al42 with permission of the American Medical Association.
Finally, elderly patients with pre-existing CKD and AKI are at the highest risk for ESRD.38 Do elderly individuals with AKI have a higher risk for CKD/ESRD than younger patients with AKI? Schmitt and colleagues sought to examine whether age impacts the ability to recover kidney function after an episode of AKI. In a systematic review and meta-analysis of data from 17 groups of investigators from studies of AKI, patients aged ≥ 65 years of age had a 28% greater risk of not recovering kidney function after AKI, whether assessed at the time of hospital discharge or soon thereafter.47 These data were unadjusted, and do not answer the question if the adjusted relative risk is higher in older vs. younger patients.
Data are conflicted as to whether the absolute relative risk for ESRD increases or decreases with older age. One study from Canada demonstrated that the absolute rates and adjusted hazard ratios for ESRD were higher in patients aged 65 or older with AKI compared to those aged less than 65,45 while a U.S. study demonstrated that the absolute rates and adjusted hazard ratios for ESRD decrease as age increases (Table 1).38 The reasons for these contradictory results are unclear. Both used administrative databases, which are prone to misclassification of exposure. The sensitivity for AKI diagnosis is actually higher in the elderly (41.4% for patients ≥ 75 years old vs. 32.6% for patients < 75 years old).48 If the risk of death after AKI is indeed elevated (as most studies have demonstrated), it is possible that the lower absolute and relative risks for ESRD with increased age witnessed in the study by Ishani et al. are confounded by competing risk for death, as the adjusted HR for death after AKI was greater than 2 in this study. Other factors which may be responsible for these contradictory results include various forms of treatment/allocation bias in the U.S. cohort, as those who are older and have more comorbidities may be less likely to be treated with maintenance life-sustaining renal replacement therapy (RRT).
Regardless, the risk for CKD/ESRD is elevated after AKI and these data are corroborated by data from experimental animals that demonstrates that AKI can accelerate or result in CKD/kidney fibrosis.49–51 Whether older age truly accentuates this relationship in humans is not clear, but other data from experimental animals suggests that aged tubular epithelial cells have diminished proliferative reserve and thus have impairment of repair after AKI.52 The impaired repair mechanism may due to multiple factors in aged kidneys including the following: A) Lower rate of cellular turnover; B) Lower levels of endothelial progenitor cells that can be recruited to replace damaged endothelial cells; C) Decreased expression of several growth factors.53
To summarize, the risk of CKD and ESRD is clearly elevated after AKI in older patients, even after adjusting for important covariates. Whether this relationship is truly causal as suggested by animal models, simply related to residual confounding, or due to AKI being a clinical manifestation of progressive CKD is unclear. Only a randomized controlled trial that demonstrates a reduction in the rate of AKI and a subsequent reduction of CKD/ESRD in that treatment group can answer this question.
Functional Outcomes
In elderly patients, the focus of medical care should often be quality of life (QoL) rather than quantity of life. Few studies have adequately assessed QoL and other issues pertinent to aged individuals in those who have experienced AKI. Most large, well-designed, longitudinal studies of elderly adults have studied the relationship between CKD and QoL, but not AKI and QoL.
Nobel et al. studied a very small group of patients in the intensive care unit at a university hospital that experienced and survived AKI that required RRT.54 The mean age of the participants at the time of enrollment/AKI was 52.5 years. Only 16 patients of the original 126 were alive after 15 years, and only 12 of the 16 completed SF-36 forms. In these 12 surviving patients with AKI, the investigators found that the overall physical health summary score and scores for seven of the health domains were reduced compared to population norms. However, the mental health summary score did not differ from the general population. While this study was one of the first to attempt to examine health-related QoL in association with AKI, the very small sample size, small spectrum of disease (only severe AKI), high rate of attrition, and relatively younger age of the cohort limit the generalizability to elderly patients with AKI.
Landoni et al. performed assessment of QoL in 22 patients that survived dialysis-requiring AKI and 30 case-matched non-AKI patients at a mean of 42 months after hospitalization.55 They measured QoL using the Medical Outcomes Study Short-Form general health survey, and found that there was a trend towards a smaller proportion of survivors from AKI rating their quality of life as “excellent”, 68.2% of AKI survivors rated their perceived general health as “very good” or “good”. There were no differences in the proportion that had limitations in daily activities (13.6% vs. 10% in AKI and non-AKI, respectively), or in the number that experienced pain during the last 4 weeks. Interestingly, 31.8% of AKI survivors had hearing impairment, compared with zero among the non-AKI survivors. Thus, this study would suggest that severe AKI does not lead to substantial decreases in QoL.
Ahlstrom et al. studied QoL in 153 survivors of AKI-RRT by administering the EuroQoL questionnaire at a median of 2.4 years after AKI.56 The investigators found that the score in AKI survivors was lower compared to age- and gender-matched general population. Age, modality, length of RRT, APACHE II, and SOFA scores were not correlated with the EuroQOL score. Intriguingly, compared with the general population, there was no difference in satisfaction with overall health (according to a visual analogue scale) in AKI survivors.
Korkiella et al. measured QoL 6 months after AKI requiring RRT. Functional ability, as assessed by the Activities of Daily Living score was fairly good at 6 months.57 The most common complaints were loss of energy and limited physical mobility. Unfortunately, these estimates may have been biased, as only 50% of survivors responded to the questionnaire.
Finally, Gopal et al measured QoL via the Nottingham Health Profile in 35 responders approximately 2.5 years after AKI requiring RRT.58 The mean age was 58.9 years. They found that 86.5% were satisfied with state of health, 60.6% said mobility affected, and 41.9% unable to walk 200 meters. Most survivors (94.5%) felt that their treatment was worthwhile and 91.2% felt that they would undergo the same treatment again if necessary. Unfortunately, there were not any non-AKI participants to which to compare these results. Furthermore, 39% of those with AKI did not respond. However, these results reiterate the findings of Ahlstrom et al.; despite lesser physical abilities after AKI, the majority of patients were still satisfied with their state of wellness. It is unclear whether a sense of gratitude after surviving a life-threatening experience overwhelmed any disappointment related to their actual functional state.
Prevention and Treatment of AKI in the Elderly
Unfortunately, despite many effective strategies to prevent and treat AKI in experimental animals, there still are few, if any proven strategies for humans. Thus, prevention strategies against AKI in elderly patients generally involve recognizing their increased vulnerability to AKI. Some of these strategies including avoiding nephrotoxic agents, ensuring adequate volume expansion prior to known stressors such as administration of intravenous contrast or nephrotoxic medications,59 and utilizing off-pump coronary artery bypass surgery60 in high risk individuals for AKI. GFR should be calculated using the Modification of Diet in Renal Disease (MDRD) Study equation or the CKD-EPI equation to determine higher risk status, although both equations may overestimate the prevalence of CKD in elderly patients.61, 62
Once AKI is established, general supportive measures such as hemodialysis should not be withheld based solely on old age, as the literature to date does not support inferior outcomes in elderly patients with dialysis-requiring AKI. Of course, rational decisions to withhold care based on the number and severity of comorbidities and potential for meaningful recovery with QoL should also be made in conjunction with the patient, family, and other health-care providers. Although not specific to elderly patients with AKI, the literature does not currently provide strong support for a specific modality63 or intensity of RRT during AKI.64, 65
Conclusions
The incidence of AKI is increasing over time and is most common in elderly individuals. This is due to many reasons, including the increased vulnerability of the kidney to stressors and insults with increasing age. Short-term survival in AKI appears to be improving over time, even in the elderly population. The long-term risk for death and CKD/ESRD after AKI is increased; however, it is not clear from the existing data whether older age significantly modifies the magnitude of relative risk compared to those without AKI in one direction or the other. AKI may result in mild decrements in functional status and health-related QoL; however, more studies that measure these domains are needed. Since the incidence of AKI in the elderly is so much more common, yet the phenotype and outcomes are not discernibly different from AKI in younger populations, the elderly population serves as a potentially fertile cohort in which to perform interventional studies for the prevention and treatment of AKI. Hopefully, this will lead to progress in terms outcomes in patients of all ages who are at risk for or experience AKI.
Box 1. Changes in the Aging Kidney
Reduction in total renal mass9
Glomerulosclerosis10
Reduction in active cortical parenchyma
Thickening of glomerular basement membrane11
Mesangial expansion12
Reduction in amount and length of tubules9
Thickening of large vessels’ walls12
Reduction in renal blood flow (10% per decade above age 40)13
Reduction in GFR (approximately 1ml/min/year above age 45)14–16
Blunted nitric oxide production17
Decreased maximum osmolality18
Increased susceptibility to apoptosis19
Abbreviations: GFR, glomerular filtration rate; EGF, Epidermal growth factor; IGF-1, Insulinlike growth factor 1; VEGF, vascular endothelial growth factor.
Acknowledgements
Support: Dr Coca is funded by the career development grant K23DK08013 from the National Institutes of Health, by the Hartford Foundation Center of Excellence in Aging at Yale Subspecialty Scholar Award, and by the American Society of Nephrology-ASP Junior Development Award in Geriatric Nephrology.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The author declares that he has no relevant financial interests.
REFERENCES
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005 Nov;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Manton KG, Vaupel JW. Survival after the age of 80 in the United States, Sweden, France, England, and Japan. N Engl J Med. 1995 Nov 2;333(18):1232–1235. doi: 10.1056/NEJM199511023331824. [DOI] [PubMed] [Google Scholar]
- 3.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007 Apr;18(4):1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007 Jul;72(2):208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagshaw SM, George C, Bellomo R. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11(3):R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006 Jan;1(1):43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 7.Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. Bmj. 1993 Feb 20;306(6876):481–483. doi: 10.1136/bmj.306.6876.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006 Apr;17(4):1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 9.Lindeman RD, Goldman R. Anatomic and physiologic age changes in the kidney. Exp Gerontol. 1986;21(4–5):379–406. doi: 10.1016/0531-5565(86)90044-6. [DOI] [PubMed] [Google Scholar]
- 10.McLachlan MS, Guthrie JC, Anderson CK, Fulker MJ. Vascular and glomerular changes in the ageing kidney. J Pathol. 1977 Feb;121(2):65–78. doi: 10.1002/path.1711210202. [DOI] [PubMed] [Google Scholar]
- 11.Darmady EM, Offer J, Woodhouse MA. The parameters of the ageing kidney. J Pathol. 1973 Mar;109(3):195–207. doi: 10.1002/path.1711090304. [DOI] [PubMed] [Google Scholar]
- 12.Tauchi H, Tsuboi K, Okutomi J. Age changes in the human kidney of the different races. Gerontologia. 1971;17(2):87–97. doi: 10.1159/000211811. [DOI] [PubMed] [Google Scholar]
- 13.Hollenberg NK, Adams DF, Solomon HS, Rashid A, Abrams HL, Merrill JP. Senescence and the renal vasculature in normal man. Circ Res. 1974 Mar;34(3):309–316. doi: 10.1161/01.res.34.3.309. [DOI] [PubMed] [Google Scholar]
- 14.Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest. 1950 May;29(5):496–507. doi: 10.1172/JCI102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985 Apr;33(4):278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 16.Rowe JW, Andres R, Tobin JD, Norris AH, Shock NW. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976 Mar;31(2):155–163. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- 17.Reckelhoff JF, Manning RD., Jr Role of endothelium-derived nitric oxide in control of renal microvasculature in aging male rats. Am J Physiol. 1993 Nov;265(5 Pt 2):R1126–R1131. doi: 10.1152/ajpregu.1993.265.5.R1126. [DOI] [PubMed] [Google Scholar]
- 18.Rowe JW, Shock NW, DeFronzo RA. The influence of age on the renal response to water deprivation in man. Nephron. 1976;17(4):270–278. doi: 10.1159/000180731. [DOI] [PubMed] [Google Scholar]
- 19.Qiao X, Chen X, Wu D, et al. Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci. 2005 Jul;60(7):830–839. doi: 10.1093/gerona/60.7.830. [DOI] [PubMed] [Google Scholar]
- 20.Chou JS, Reiser IW, Porush JG. Aging and urinary excretion of epidermal growth factor. Ann Clin Lab Sci. 1997 Mar-Apr;27(2):116–122. [PubMed] [Google Scholar]
- 21.Tran KT, Rusu SD, Satish L, Wells A. Aging-related attenuation of EGF receptor signaling is mediated in part by increased protein tyrosine phosphatase activity. Exp Cell Res. 2003 Oct 1;289(2):359–367. doi: 10.1016/s0014-4827(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Bennett SA, Ingram RL, Sonntag WE. Decreases in growth hormone receptor signal transduction contribute to the decline in insulin-like growth factor I gene expression with age. Endocrinology. 1995 Oct;136(10):4551–4557. doi: 10.1210/endo.136.10.7664676. [DOI] [PubMed] [Google Scholar]
- 23.Pascual J, Liano F. Causes and prognosis of acute renal failure in the very old. Madrid Acute Renal Failure Study Group. J Am Geriatr Soc. 1998 Jun;46(6):721–725. doi: 10.1111/j.1532-5415.1998.tb03807.x. [DOI] [PubMed] [Google Scholar]
- 24.Akposso K, Hertig A, Couprie R, et al. Acute renal failure in patients over 80 years old: 25-years' experience. Intensive Care Med. 2000 Apr;26(4):400–406. doi: 10.1007/s001340051173. [DOI] [PubMed] [Google Scholar]
- 25.Moretti C, Frajese GV, Guccione L, et al. Androgens and body composition in the aging male. J Endocrinol Invest. 2005;28(3 Suppl):56–64. [PubMed] [Google Scholar]
- 26.Lynch GS. Update on emerging drugs for sarcopenia - age-related muscle wasting. Expert Opin Emerg Drugs. 2008 Dec;13(4):655–673. doi: 10.1517/14728210802544476. [DOI] [PubMed] [Google Scholar]
- 27.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009 Mar;20(3):672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 29.Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008 May;73(9):1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- 30.Herget-Rosenthal S, Marggraf G, Husing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004 Sep;66(3):1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 31.Ahlstrom A, Tallgren M, Peltonen S, Pettila V. Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol. 2004 Nov;62(5):344–350. doi: 10.5414/cnp62344. [DOI] [PubMed] [Google Scholar]
- 32.Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008 Apr;73(7):863–869. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006 Jul;70(1):199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 34.Liangos O, Tighiouart H, Perianayagam MC, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009 Sep;14(6):423–431. doi: 10.1080/13547500903067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009 May;4(5):873–882. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary Biomarkers for Sensitive and Specific Detection of Acute Kidney Injury in Humans. Clin Transl Sci. 2008 Dec;1(3):200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006 Apr;17(4):1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 38.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009 Jan;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009 Oct;76(8):893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009 May;249(5):851–858. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 41.Hobson CE, Yavas S, Segal MS, et al. Acute Kidney Injury Is Associated With Increased Long-Term Mortality After Cardiothoracic Surgery. Circulation. 2009 May 12;119(18):2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 42.Newsome BB, Warnock DG, McClellan WM, et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008 Mar 24;168(6):609–616. doi: 10.1001/archinte.168.6.609. [DOI] [PubMed] [Google Scholar]
- 43.Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med. 2008 May 12;168(9):987–995. doi: 10.1001/archinte.168.9.987. [DOI] [PubMed] [Google Scholar]
- 44.Van Den Noortgate N, Mouton V, Lamot C, et al. Outcome in a post-cardiac surgery population with acute renal failure requiring dialysis: does age make a difference? Nephrol Dial Transplant. 2003 Apr;18(4):732–736. doi: 10.1093/ndt/gfg043. [DOI] [PubMed] [Google Scholar]
- 45.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. Jama. 2009 Sep 16;302(11):1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 46.Ponte B, Felipe C, Muriel A, Tenorio MT, Liano F. Long-term functional evolution after an acute kidney injury: a 10-year study. Nephrol Dial Transplant. 2008 Dec;23(12):3859–3866. doi: 10.1093/ndt/gfn398. [DOI] [PubMed] [Google Scholar]
- 47.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis. 2008 Aug;52(2):262–271. doi: 10.1053/j.ajkd.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for Acute Renal Failure. J Am Soc Nephrol. 2006 Jun;17(6):1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 49.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007 May 2;72(2):151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 50.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004 Jan;13(1):1–7. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001 Nov;281(5):F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 52.Schmitt R, Marlier A, Cantley LG. Zag Expression during Aging Suppresses Proliferation after Kidney Injury. J Am Soc Nephrol. 2008 Dec;19(12):2375–2383. doi: 10.1681/ASN.2008010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmitt R, Cantley LG. The impact of aging on kidney repair. Am J Physiol Renal Physiol. 2008 Jun;294(6):F1265–F1272. doi: 10.1152/ajprenal.00543.2007. [DOI] [PubMed] [Google Scholar]
- 54.Noble JS, Simpson K, Allison ME. Long-term quality of life and hospital mortality in patients treated with intermittent or continuous hemodialysis for acute renal and respiratory failure. Ren Fail. 2006;28(4):323–330. doi: 10.1080/08860220600591487. [DOI] [PubMed] [Google Scholar]
- 55.Landoni G, Zangrillo A, Franco A, et al. Long-term outcome of patients who require renal replacement therapy after cardiac surgery. Eur J Anaesthesiol. 2006 Jan;23(1):17–22. doi: 10.1017/S0265021505001705. [DOI] [PubMed] [Google Scholar]
- 56.Ahlstrom A, Tallgren M, Peltonen S, Rasanen P, Pettila V. Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med. 2005 Sep;31(9):1222–1228. doi: 10.1007/s00134-005-2681-6. [DOI] [PubMed] [Google Scholar]
- 57.Korkeila M, Ruokonen E, Takala J. Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med. 2000 Dec;26(12):1824–1831. doi: 10.1007/s001340000726. [DOI] [PubMed] [Google Scholar]
- 58.Gopal I, Bhonagiri S, Ronco C, Bellomo R. Out of hospital outcome and quality of life in survivors of combined acute multiple organ and renal failure treated with continuous venovenous hemofiltration/hemodiafiltration. Intensive Care Med. 1997 Jul;23(7):766–772. doi: 10.1007/s001340050407. [DOI] [PubMed] [Google Scholar]
- 59.Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002 Feb 11;162(3):329–336. doi: 10.1001/archinte.162.3.329. [DOI] [PubMed] [Google Scholar]
- 60.Nigwekar SU, Kandula P, Hix JK, Thakar CV. Off-Pump Coronary Artery Bypass Surgery and Acute Kidney Injury: A Meta-analysis of Randomized and Observational Studies. Am J Kidney Dis. 2009 Sep;54(3):413–423. doi: 10.1053/j.ajkd.2009.01.267. [DOI] [PubMed] [Google Scholar]
- 61.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 62.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vinsonneau C, Camus C, Combes A, et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006 Jul 29;368(9533):379–385. doi: 10.1016/S0140-6736(06)69111-3. [DOI] [PubMed] [Google Scholar]
- 64.Palevsky PM, Zhang JH, O'Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008 Jul 3;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009 Oct 22;361(17):1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]