Abstract
Background
Chronic HIV-1 infection is characterized by high levels of persistent immune activation. Both HIV-1-encoded TLR7/8 ligands and TLR ligands encoded by products of microbial translocation have been implicated in inducing and sustaining immune activation in infected individuals, but the consequences of simultaneous exposure to different TLR ligands is not well understood.
Objective
To examine the impact of pre-exposure of monocytes to HIV-1 encoded TLR8 ligands on their ability to respond to subsequent stimulation with microbial TLR2/4 ligands.
Method
Stable monocytic cell lines (THP1-Blue™-CD14 cells) or primary monocytes were stimulated with ligands for TLR2, TLR4 and TLR8, including chemically inactivated HIV-1, alone, or in sequential combinations. Responses by THP1 cells to TLR stimulation were quantified using quanti-blue colometric assay, and TLR-induced TNF-α production of primary monocytes was quantified by intracellular cytokine staining using flow cytometry.
Results
The exposure of monocytes to HIV-1 or HIV-1-derived TLR8 ligands sensitized these cells for TLR4 stimulation, resulting in a significant higher response to LPS compared to cells that were not pre-stimulated with TLR8 ligands or HIV-1.
Conclusion
TLR crosstalk can enhance the pro-inflammatory monocytes response to products of microbial translocation, and might play an important role in the modulation of immune function in HIV-1 infection.
Keywords: HIV-1, Toll-like receptors, monocytes, LPS, microbial translocation
Introduction
Chronic HIV-1 infection is characterized by strong persistent immune activation, and the level of immune activation has been identified as a significant predictor of HIV-1-disease progression [1, 2]. One widely accepted model of HIV-1 immuno-pathogenesis postulates that heightened immune activation results in accelerated activation and proliferation of memory-effector CD4+T-cells, leading to their deletion [3]. Recent studies investigating the mechanisms underlying HIV-1-associated immune activation have suggested that Toll-like receptors (TLRs) play a central role in mediating immune activation in HIV-1 infection [4–6].
TLRs recognize components of pathogens and initiate the pathogen-specific immune response [7, 8]. TLR ligands have been shown to modulate monocyte function [9] initiating signaling cascades leading to NF-κB activation and subsequent gene expression, which vary depending on which TLR has been activated [10]. TLR2 and TLR4 are involved in the recognition of microbial products [11, 12], while TLR7 and TLR8 sense single stranded RNA [7, 13–15]. It has been demonstrated that chronic HIV-1 infection is associated with elevated circulating levels of the TLR4 ligand LPS, an important component of Gram-negative bacteria that enter the circulation through microbial translocation in the immuno-compromised gut of HIV-1-infected individuals [16]. Furthermore, the single stranded RNA sequence of HIV-1 itself contains sequences that can serve as TLR7 and TLR8 ligands and directly stimulate pro-inflammatory cytokine production by dendritic cells and monocytes [13, 14, 17, 18]. Chronic stimulation of TLRs by products of microbial translocation and HIV-1-derived TLR ligands might therefore represent an important mechanism of immune activation in HIV-1 infection.
The consequences of stimulation through different TLR pathways for monocytes function are however not well understood. Recently published studies in the murine model and human studies suggest that the activation of one TLR pathway in a cell has a significant impact on the ability of that cell to respond to stimulation of a different TLR pathway [19–21], resulting in either enhancement or reduction of the response to a second TLR ligand (22). The current study examined the impact of pre-exposure of monocytes to HIV-1 virions, the genomic RNA of which contains TLR ligands, on their ability to respond to subsequent stimulation with microbial TLR2/4 ligands, and demonstrates significant enhancement of the monocytes response to LPS following prestimulation with HIV-1.
Materials and Methods
Study subjects
Samples from HIV-1 negative subjects enrolled at Massachusetts General Hospital were included in the studies. The study was approved by the Massachusetts General Hospital Institutional Review Board and the University of KwaZulu-Natal Biomedical Research Ethics Committee and each subject gave informed consent for participation.
THP1 cell culture
THP1-Blue™-CD14 (THP-1) cells are TLR-expressing human monocytic NF-kB-reporter cells expressing CD14 and various TLRs. Upon TLR stimulation, THP-1 cells activate transcription factors resulting in the secretion of embryonic alkaline phosphatase (SEAP), which is detected using Quanti-Blue reagent, as described by the manufacturer’s protocols (Invivogen, CA). THP1-Blue™-CD14 were maintained in medium consisting of RPMI1640, 10%FCS, 2mM L-glutamine, supplemented with 200µg/ml of zeocin and 10µg/ml of blasticidin. THP-1 cells (3×105 cells/well) were stimulated in 96-well flat-bottomed plates with either medium alone, or TLR ligands at the following concentrations: 2ul/ml of heat-killed Listeria monocytogenes (HKLM, TLR2 ligand), 0.1ug/ml of Lipopolysaccharide (LPS-EK, TLR4 ligand), or 0.5µg/ml CL075 (thiazoloquinolone derivative, TLR8 ligand,) all from Invivogen, CA (23) at 37°C and 5% CO2 for 18hours. The concentrations for TLR ligands and incubation periods were optimized in previous experiments (data not shown). TLR stimulation resulted in the activation of the NF-κβ pathway and the NF-κβ-dependent expression and secretion of the secreted alkaline phosphatase (SEAP) that was subsequently quantified using a Quanti-blue mix assay and read at 630nm according to the manufacturer’s protocol (Invivogen).
In addition to stimulation with single TLR agonists, the effects of pre-stimulation or ‘priming’ of THP1-cells with one TLR ligand (TLR8, or TLR2, or TLR4) on the response to stimulation with a second TLR ligand was assessed. THPI-cells were pre-stimulated with 0.5µg/ml of CL075 or media alone in four separate wells. Supernatants from the THPI-cell culture were removed after 3hours incubation, the adhered cells washed twice with fresh RPMI, replenished with fresh media, and cultured with TLR2-HKLM, TLR4-LPS-EK, TLR8-CL075 (at similar concentrations indicated above), or media alone for a further 18hours, before NF-κβ activation was quantified using the Quanti-blue assay. In a subset of experiments, the TLR8 antagonist Pepinyl-MYD (Invivogen) was added to THP-1 cells (3×105cells/well) for 6hours prior to stimulation with CL075 to demonstrate that the pre-stimulation with CL075 was indeed mediated through TLR8.
In-vitro stimulation of PBMCs with TLR ligands
Fresh PBMCs from healthy individuals were separated from whole blood by Ficoll-Hypaque (Sigma). 1.5×106 PBMCs/ml in RPMI were cultured with at 2ul/ml of TLR2-HKLM, 0.1µg/ml of TLR4-LPS-EK, or 0.5µg/ml of TLR8-CL075. The effect of pre-stimulation of PBMCs was assessed using the TLR8 CL075 agonist, and also using aldrithiol-2-inactivated HIV-1 virus or micro-vesicle controls (MN strain, lot P4097; AIDS and Cancer Virus Program, National Cancer Institute). Fresh PBMCs were pre-stimulated with 0.5µg/ml of CL075, 0.7µg/ml of p24 (CA) aldrithiol-2 inactived virus (“AT-2 virus”), or vesicle control, or media alone. The cultured PBMCs were then washed twice with RPMI after 3hours of pre-stimulation, and supernatants from the PBMCs culture were removed and replenished with fresh media. The pre-stimulated PBMCs were then cultured with TLR2-HKLM, TLR4-LPS-EK, TLR8-CL075, AT-2 virus, vesicle control, or media alone (at the concentrations indicated above) for an additional 18hours. PBMCs that were pre-incubated with media, but no TLR agonists, acted as controls. Supernatants were collected for TNFα ELISA quantification according to the manufacturer’s instructions (SA biosciences). For the detection of intracellular cytokines using flow cytometric analysis, 5µg/ml brefeldin A (Sigma) was added to each tube after adding the TLR ligands. Stimulation for all assays was conducted at 37°C and 5% CO2.
Flow Cytometry
The intracellular cytokine content of monocytes was determined following 18hours of stimulation. Briefly, cells were stained for surface (CD3-Alexa700, CD19-Alexa700, CD56-Alexa700, CD14-allophycocyanin [APC]-Cy7) and intracellular marker TNF-PE-Cy7 [BD]). Monocytes were defined as CD3neg CD19neg CD56neg CD14+cells, and gates were set accordingly. Cells were fixed, permeabilized (Fix perm A&B; Caltag), and stained intracellularly with anti-TNFa-PE-Cy7. All samples were acquired on an LSRII (BD). The frequencies of cytokine-positive monocytes were determined by subsequent analysis using FlowJo software.
Statistical analyses
Statistical analyses and graphical presentation was done using Graphpad Prism 5 (Graphpad). Results are given as means with standard deviations or medians with ranges. Paired two-tailed Student t tests were used to test the statistical significance. Differences after comparisons were considered statistically significant if P <0.05.
RESULTS
Pre-exposure of THP1-CD14 cells to TLR8 ligands modulates their responses to TLR2 and TLR4 ligands
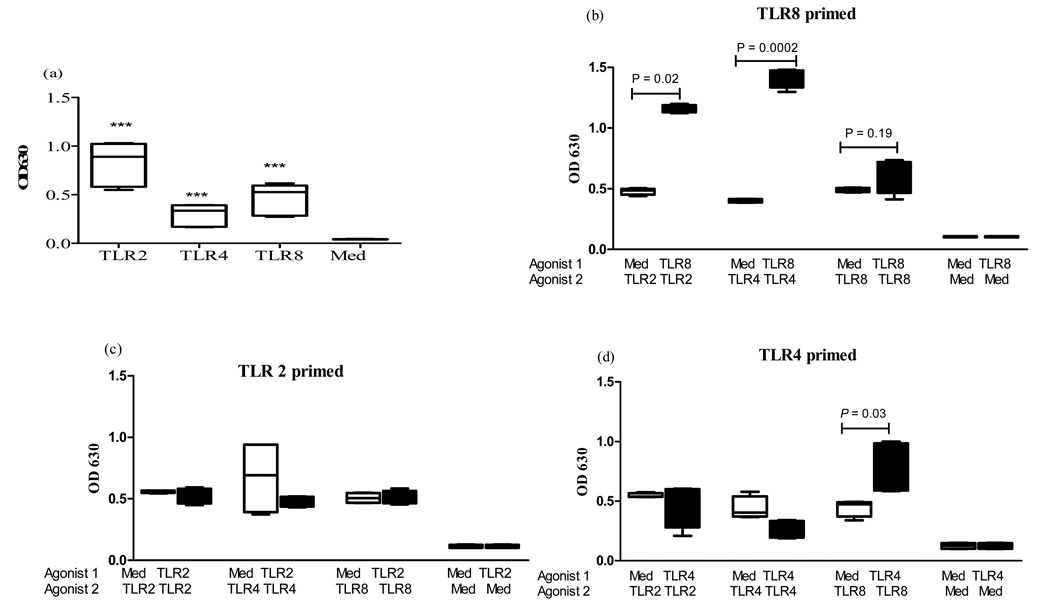
We initially stimulated THP1-cells with either medium alone, or different TLR ligands (TLR2-HKLM, TLR4-LPS-EK, or TLR8-CL075) to determine their ability to respond to these ligands. Upon stimulation with these TLR2/4 and 8 ligands, THP1-cells secreted considerable amounts of SEAP which was significantly detectable above background (TLR2; p = <0.0001, TLR4; p = 0.001, TLR8; p= <0.0001) using QUANTI-Blue™ assay (fig.1a). As previous studies have suggested that the sequence of exposure to TLR agonists may impact on the ability of cells to respond to subsequent stimulation [19], we subsequently studied the effects of pre-exposure of THP1-cells to a TLR8 agonist (agonist 1) on responses to TLR2 and TLR4 agonists (agonist 2) (fig 1b). The pre-stimulation effects on the THP1-cells were also assessed with a TLR2 or TLR4 ligand as the pre-stimulant agonist (agonist 1) (fig 1c&d). No significant differences were noted in the response to TLR4 or TLR8 ligands when cells were pre-stimulated with the TLR2 agonist (fig 1c). TLR4 pre-stimulation significantly increased the response to the TLR8 ligand CL075 (p= 0.03), resulting in a 2-fold increase compared to pre-incubation with media alone (fig 1d). However, the pre-exposure to TLR8 ligands modulated the ability of monocytes to respond to subsequent stimulation most dramatically, significantly increasing their responses to subsequent stimulation with TLR2 (3-fold, p=0.02) and TLR4 (5-fold, p=0.0002) ligands (fig 1b). In contrast, TLR8 ligands elicited no notable priming effects to stimulation with the same TLR8 agonist (p=0.19). This priming effect was significantly higher than the sum of TLR4 and TLR8 stimulation, suggesting that the effect was not simply additive, but that TLR8 prestimulation amplified the response to TLR4 ligands. Using the TLR8 antagonist Pepinyl-MYD (Invivogen), we furthermore demonstrate that addition of this antagonist to THP1 cells before exposing them to the TLR8 agonist significantly (p =0.0001) reduced the subsequent response to LPS (Average OD of 0.52 +/− 0.4 (SD)), compared to THP1 cells that were pre-stimulated with the TLR8 agonist in the absence of the antagonist prior to stimulation with LPS (average OD of 1.8 +/− 0.2 (SD)). In contrast, the antagonist did not decrease the response of THP1 cells to LPS in the absence of pre-stimulation with the TLR8 agonist. Taken together, these data using a monocytes derived cell line demonstrate that pre-exposure to TLR8 ligands can significantly enhance the subsequent response of monocytes to TLR2 and TLR4 ligands.
Figure 1. Responses of THPI-Blue™-CD14 cells to TLR stimulation.
(a) THP1 cells were incubated with the following TLR agonists for 18 hrs: 2ul/ml of TLR2-HKLM, 0.1µg/ml of TLR4-LPS-EK, 0.5 µg/ml TLR8-CL075, or media alone. Average and Standard Errors are shown. ***P< 0.001 compared to control medium (med) n = 6.
(b) The effects of pre-stimulation of THP1-CD14 cells with a TLR8 agonist on responses to TLR2 and TLR4. THP-1 cells were pre-stimulated with either media or a TLR8 ligand (0.5µg TLR8-CL075) for 3 hrs (agonist 1), washed, and subsequently stimulated with a second TLR agonist (agonist 2: 0.5µg/ml TLR8-CL075, 2ul/ml TLR2-HKLM, 0.1µg/ml of TLR4-LPS-EK or media alone, as indicated). Vertical boxes represent 25th to 75th percentiles with median values delineated with a horizontal bar in response to stimulation with agonist n = 6.
(c) The effects of pre-stimulation of THP1-CD14 cells with a TLR2 agonist on responses to TLR4 and TLR8 agonists. THP-1 cells were pre-stimulated with either media or a TLR2 ligand (2ul/ml of TLR2-HKLM) for 3 hrs (agonist 1), washed, and subsequently stimulated with a second TLR agonist (agonist 2: 0.5µg/ml TLR8-CL075, 2ul/ml TLR2-HKLM, 0.1µg/ml of TLR4-LPS-EK or media alone, as indicated). Vertical boxes represent 25th to 75th percentiles with median values delineated with a horizontal bar in response to stimulation with agonist 2 n = 6.
(d) The effects of pre-stimulation of THP1-CD14 cells with a TLR4 agonist on responses to TLR2 and TLR8 agonists.THP-1 cells were pre-stimulated with either media or a TLR4 ligand (0.1µg/ml of TLR4-LPS-EK) for 3 hrs (agonist 1), washed, and subsequently stimulated with a second TLR agonist (agonist 2: 0.5µg/ml TLR8-CL075, 2ul/ml TLR2-HKLM, 0.1µg/ml of TLR4-LPS-EK or media alone, as indicated). Vertical boxes represent 25th to 75th percentiles with median values delineated with a horizontal bar in response to stimulation with agonist 2 n = 6.
Pre-exposure to TLR8 ligands increases the cytokine response of primary monocytes to TLR2 and TLR4 ligands
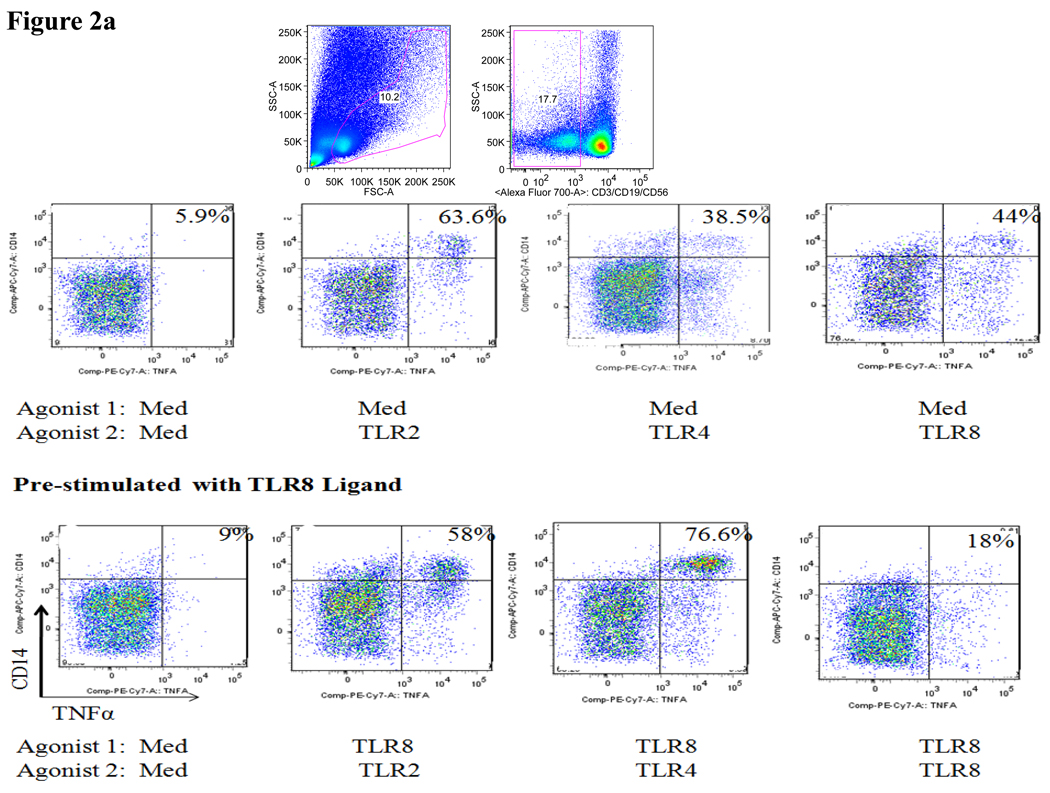
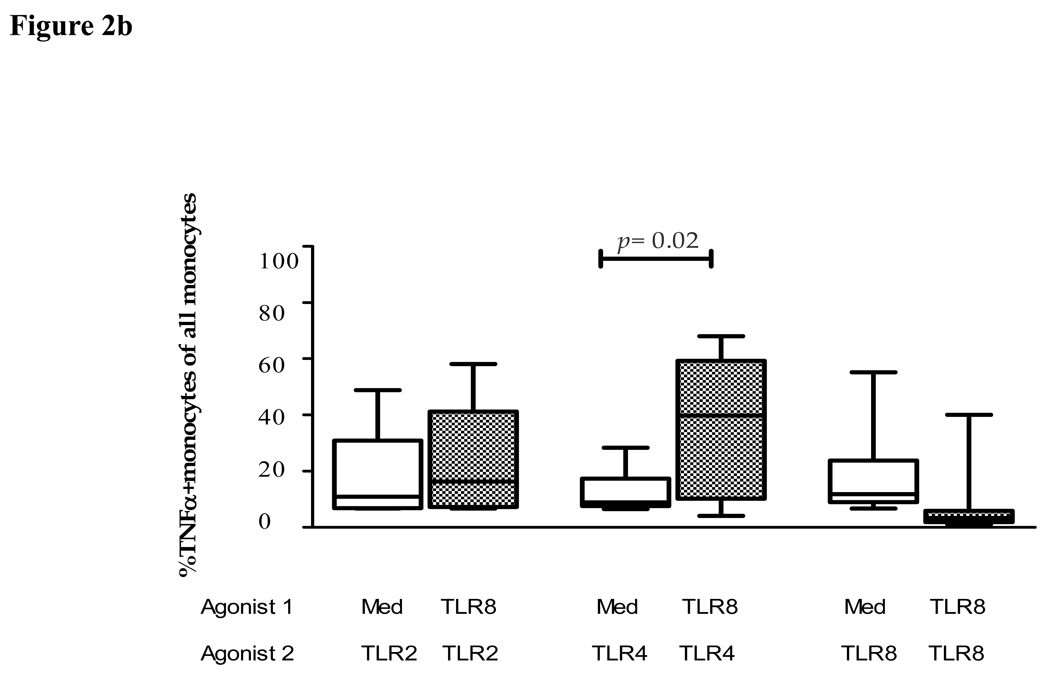
We next extended these initial studies to assess the effects of pre-exposure to TLR8 agonist on the subsequent response to TLR2 and TLR4 ligands by primary monocytes. Multiparameter flow cytometric analysis was employed to characterize the cytokine production by primary monocytes using intracellular cytokine staining. Representative flow cytometry plots showed robust TNFα production by monocytes after stimulation with TLR2, TLR4 and TLR8 ligands alone compared to stimulation with medium alone (fig 2a). The effect of pre-stimulation of PBMCs with the TLR8 agonist CL075 was subsequently assessed. TLR8 ‘primed’ PBMCs were washed and then cultured with TLR2-HKLM, TLR4-LPS-EK, TLR8-CL075, or media alone. PBMCs pre-cultured with medium alone were used as controls. The percentage TNFα+ monocytes in response to the respective TLR agonists following pre-stimulation with medium alone or the TLR8 agonist CL075 revealed that pre-exposure to TLR8 agonists significantly (p=0.02) increased monocytes responses to TLR4, but not TLR2 ligand (fig 2a&b). Once again, this priming effect was significantly higher than the sum of TLR4 and TLR8 stimulation alone, demonstrating that TLR8 pre-stimulation was amplifying responses to TLR4 ligands. In contrast, the percentage TNFα+ monocytes following pre-stimulation with TLR8 agonist CL075 followed by re-stimulation with the TLR8 agonist revealed a decreased monocyte response. In conclusion, robust TNFα production by monocytes is observed after stimulation with TLR2, TLR4 and TLR8 ligands, and responses to TLR4, but not TLR2 ligands, are significantly increased by pre-exposure to TLR8 agonists.
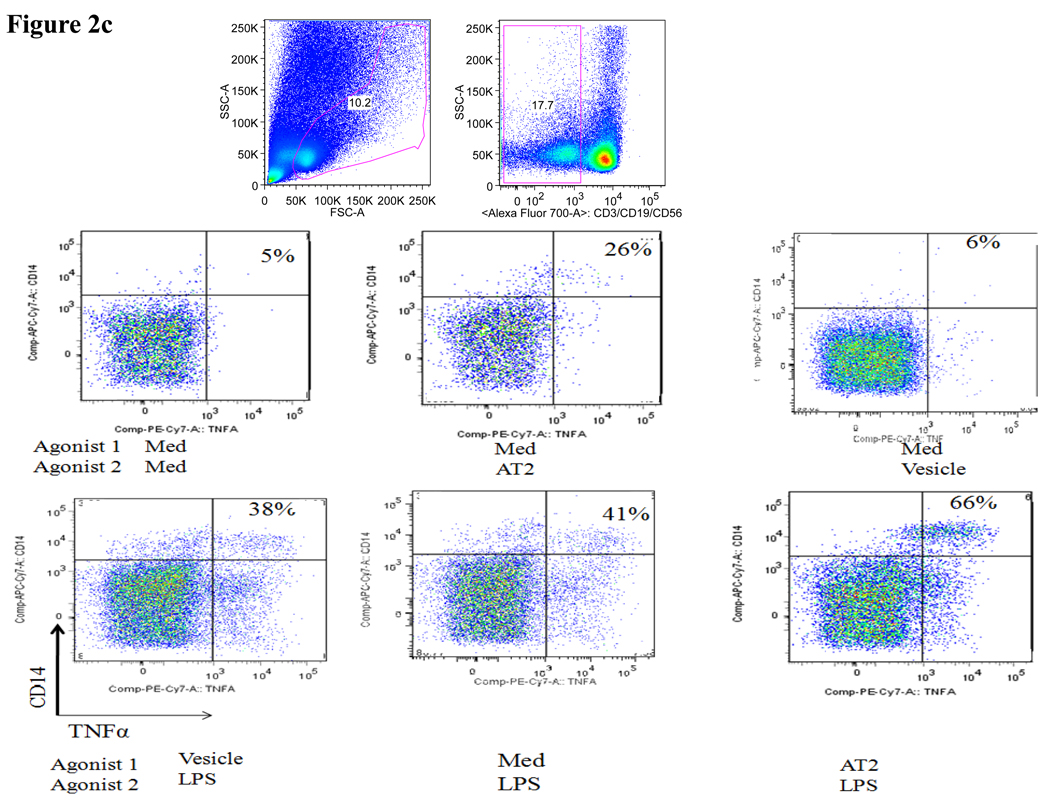
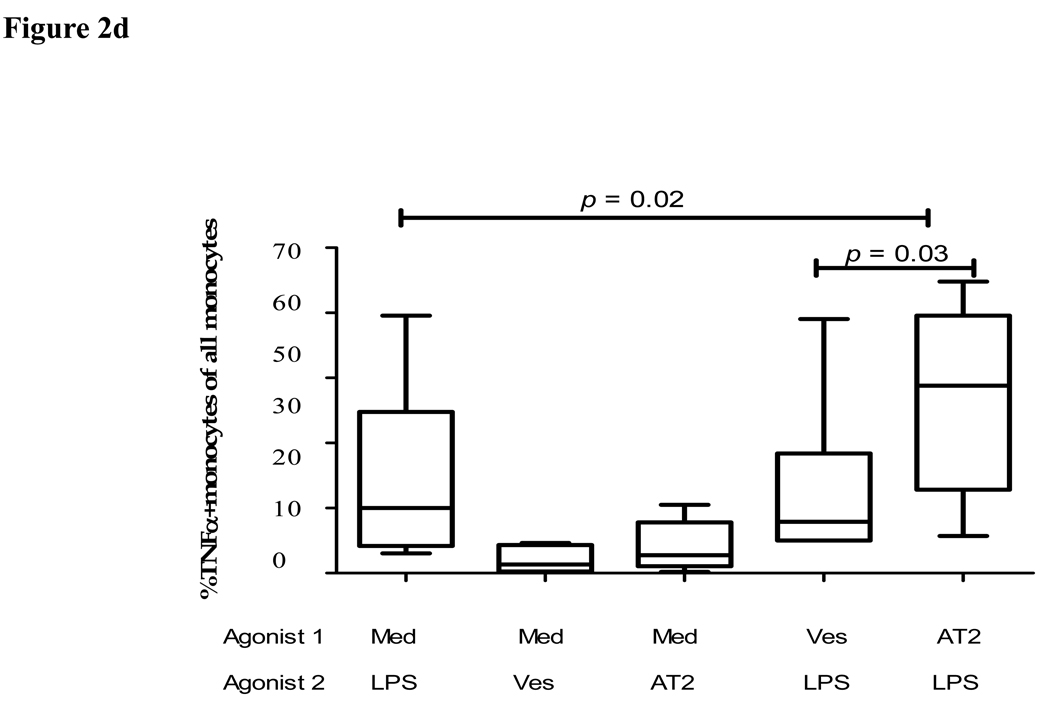
Figure 2. Impact of pre-exposure to TLR8 ligands on cytokine response of primary monocytes to TLR2 and TLR4 ligands.
(a) Representative flow cytometry plots show the percentage TNFα+ monocytes (upper right quadrant) in response to the respective TLR agonists following prestimulation with medium alone (top) or following prestimulation with the TLR8 agonist CL075 (0.5µg/ml TLR8-CL075, bottom).
(b) The figure shows the percentage of TNFα+CD14+ monocytes in 10 study subjects in response to the respective TLR agonists following prestimulation with medium alone (clear bars) or following prestimulation with the TLR8 agonist CL075 (0.5µg/ml TLR8-CL075, shaded bars). Vertical boxes represent 25th to 75th percentiles with median values delineated with a horizontal bar.
(c) Representative flow cytometry plots show the percentage TNFα+ monocytes (upper right quadrant) in response to LPS, AT-2 virus and its control vesicle following pre-stimulation with medium alone (top) or following pre-stimulation with the AT-2 virus or control vesicle (bottom).
(d) The figure shows the percentage of TNFα+CD14+ monocytes in 10 study subjects in response to LPS, AT-2 virus or vesicle control (agonist 2) following pre-stimulation with medium alone, or following prestimulation with the AT-2 virus or the vesicle control (agonist 1), as indicated. Vertical boxes represent 25th to 75th percentiles with median values delineated with a horizontal bar.
Pre-exposure to AT-2 virus increases the cytokine response of primary monocytes to the TLR4 ligand LPS
To further assess the impact of pre-exposure to HIV-1-derived TLR ligands on the subsequent response of the primary human monocytes to microbial LPS, we stimulated monocytes with AT-2 inactivated HIV-1, as described previously (18), and observed robust TNFα production compared to vesicle and medium control (fig 2c&d). Similar to TLR8 ligands, pre-stimulation with the AT-2 virus significantly enhanced the subsequent response of monocytes to LPS compared to cells that were pre-exposure to medium alone (p=0.02) or vesicle control (p=0.03) prior to stimulation with LPS. Furthermore, the response of monocytes to LPS following pre-stimulation with AT-2 virus was also significantly (2-fold) higher than the sum of the responses to LPS and AT-2 virus alone (p=0.035) (fig 2d). In line with these data, pre-stimulation with the TLR8 ligands significantly (p = 0.03) increased the subsequent amount of secreted TNFα produced in response to LPS stimulation quantified by ELISAs in the culture supernatants (average of 15 pg TNFα/ml +/− 1.1 (SD) in the absence of pre-stimulation versus 58 pg TNFα/ml +/− 2.8 (SD) following pre-stimulation with the TLR8 ligand CL075, p < 0.03). In conclusion, these data demonstrate that pre-exposure to HIV-1 significantly enhances the response of monocytes to microbial TLR4 ligands such as LPS.
Discussion
Stimulations of the TLR7/8 pathway by HIV-1 ssRNA derived ligands and the TLR4 pathways by products for microbial translocation have been both implicated in mediating the activation of the immune system during chronic HIV-1 infection, and resulting immuno-pathogenesis [16, 18]. However, little is known about how these two pathways might influence each other. Here we demonstrate that exposure to HIV-1 or HIV-1 derived TLR8 ligands significantly increases the production of pro-inflammatory cytokines by monocytes in response to subsequent stimulation with the TLR4 ligand LPS. These data suggest that the combination of HIV-1 replication and microbial translocation in chronically HIV-1 infected individuals might not only additively increase the activation of the immune system, but might actually amplify these effects.
The observation that stimulation through TLR8 amplifies the pro-inflammatory cytokine response of monocytes in response to products of microbial translocation, such as LPS, has important implication for HIV-1 pathogenesis, as the levels of immune activation during chronic HIV-1-infection have been strongly associated with the loss of CD4+ T cells, and consecutive disease progression (25). The increased sensitivity of monocytes to LPS in the setting of TLR8 stimulation also helps to explain the observation that HIV-1-associated immune activation decreases rapidly and significantly following the initiation of antiretroviral therapy and resulting reduction of viral load [27, 28], even before reconstitution of the immune system and eventual reduction of microbial translocation [27], as the decrease in the levels of HIV-1-encoded TLR8 ligands will render monocytes less responsiveness to circulating levels of LPS. Taken together, this data support a model in which TLR crosstalk plays an important role in the modulation of immune function in HIV-1 infection, and in the ability of the infected host to respond to stimulation by a variety of TLR ligands it is exposed to, either through intestinal microbial translocation, other opportunistic pathogens, or HIV-1 itself.
Acknowledgments
These studies were supported by the Howard Hughes Medical Institute through the Kwa-Zulu Natal Research Institute for TB and HIV and the Doris Duke Charitable Foundation and supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN266200400088C.
Financial support:
These studies were supported by the Howard Hughes Medical Institute through the KwaZulu-Natal Research Institute for TB and HIV (K-RITH) and the Doris Duke Charitable Foundation, and supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN266200400088C.
Role of each author:
MWM performed the experiments and wrote the manuscript, JJC helped with the development, planning and optimization of the assays, JDL provided the AT-2 virus, TN helped with the design of the studies and the preparation of the manuscript, MA designed the studies and wrote the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none declared
Presented in part: 5th IAS meeting, Cape Town 2009
References
- 1.Deeks SG, Walker BD. The immune response to AIDS virus infection: good, bad, or both? J Clin Invest. 2004;113:808–810. doi: 10.1172/JCI21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giorgi JV, Fahey JL, Smith DC, Hultin LE, Cheng HL, Mitsuyasu RT, Detels R. Early effects of HIV on CD4 lymphocytes in vivo. J Immunol. 1987;138:3725–3730. [PubMed] [Google Scholar]
- 3.Fahey JL, Taylor JM, Manna B, Nishanian P, Aziz N, Giorgi JV, Detels R. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS. 1998;12:1581–1590. doi: 10.1097/00002030-199813000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Chang JJ, Altfeld M. TLR-mediated immune activation in HIV. Blood. 2009;113:269–270. doi: 10.1182/blood-2008-10-184598. [DOI] [PubMed] [Google Scholar]
- 5.Douek D. HIV disease progression: immune activation, microbes, and a leaky gut. Top HIV Med. 2007;15:114–117. [PubMed] [Google Scholar]
- 6.Haynes BF. Gut microbes out of control in HIV infection. Nat Med. 2006;12:1351–1352. doi: 10.1038/nm1206-1351. [DOI] [PubMed] [Google Scholar]
- 7.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Bekeredjian-Ding I, Roth SI, Gilles S, Giese T, Ablasser A, Hornung V, et al. T cell-independent, TLR-induced IL-12p70 production in primary human monocytes. J Immunol. 2006;176:7438–7446. doi: 10.4049/jimmunol.176.12.7438. [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 11.Akamine M, Higa F, Arakaki N, Kawakami K, Takeda K, Akira S, Saito A. Differential roles of Toll-like receptors 2 and 4 in in vitro responses of macrophages to Legionella pneumophila. Infect Immun. 2005;73:352–361. doi: 10.1128/IAI.73.1.352-361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 13.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 14.Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, et al. 5'-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 16.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 17.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol. 2007;178:1164–1171. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- 20.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165:7096–7101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 22.Lester RT, Yao X-D, Blake BT, McKinnon LR, Kaul R, Wachihi C, Jaoko W, Plummer FA, Rosenthal KL. Toll-like receptor expression and responsiveness are increased in viraemic HIV-1 infection. AIDS. 2008;22:685–694. doi: 10.1097/QAD.0b013e3282f4de35. [DOI] [PubMed] [Google Scholar]
- 23.Gorden KB, Gorski KS, Gibson S, Kedl R, Kieper W, Qiu X, Tomai M, Alkan S, Vasilakos J. Synthetic TLR Agonists Reveal Functional Differences between Human TLR7 and TLR8. J Immunol. 2005;174:1259–1269. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 24.Lee PI, Ciccone EJ, Read SW, Asher A, Pitts R, Douek DC, et al. Evidence for translocation of microbial products in patients with idiopathic CD4 lymphocytopenia. J Infect Dis. 2009;199:1664–1670. doi: 10.1086/598953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meier A, Bagchi A, Sidhu HK, Alter G, Suscovich TJ, Kavanagh DG, et al. Upregulation of PD-L1 on monocytes and dendritic cells by HIV-1 derived TLR ligands. AIDS. 2008;22:655–658. doi: 10.1097/QAD.0b013e3282f4de23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giorgi JV, Landay A. HIV infection: diagnosis and disease progression evaluation. Methods Cell Biol. 1994;42(Pt B):437–455. doi: 10.1016/s0091-679x(08)61089-4. [DOI] [PubMed] [Google Scholar]
- 26.Gray CM, Schapiro JM, Winters MA, Merigan TC. Changes in CD4+ and CD8+ T cell subsets in response to highly active antiretroviral therapy in HIV type 1-infected patients with prior protease inhibitor experience. AIDS Res Hum Retroviruses. 1998;14:561–569. doi: 10.1089/aid.1998.14.561. [DOI] [PubMed] [Google Scholar]
- 27.Lempicki RA, Kovacs JA, Baseler MW, Adelsberger JW, Dewar RL, Natarajan V, et al. Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients. Proc Natl Acad Sci U S A. 2000;97:13778–13783. doi: 10.1073/pnas.250472097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]