Abstract
Transactivation/transformation–domain associated protein (TRRAP) is a component of several multi-protein HAT complexes implicated in both transcriptional regulation and DNA repair. We recently identified Trrap, the murine ortholog of TRRAP, as an essential protein implicated in mitotic progression control, although its target genes are not known. In the present study, we analyzed the expression profiles of Trrap-responsive genes, using cDNA microarray in mitotic cells. From a panel of 17 664 transcript elements, we found that loss of Trrap leads to expression alteration of a large fraction of genes at mitotic stage. Functional classification of these genes indicates that Trrap influences a variety of cellular processes including cell cycle progression, cytoskeleton and cell adhesion, protein turnover, metabolism and signal transduction. The majority (71%) of differentially expressed genes was down-regulated in Trrap- deficient cells, whereas the rest were up-regulated, suggesting that Trrap may also play a role in transcriptional silencing. ChIP analysis revealed that Trrap might regulate gene expression by participating in acetylation of histone H4 and/or H3 depending on target genes and cell cycle stage. Our study indicates that Trrap regulates the expression of a wide range of genes in both quiescence and mitotic stages. Removal of the Trrap protein is associated with both increased and decreased gene expression.
INTRODUCTION
Many important cellular functions, including proliferation, cell cycle progression and checkpoint control, are guided by changes in gene expression and require the coordinated efforts of transcription machinery and chromatin-remodeling activities. Acetylation of specific lysine residues within the amino-terminal tails of core histones has been implicated in transcription regulation and other nuclear processes (1,2). The level of histone acetylation within a given region of the genome is maintained by varied recruitment of histone acetyltransferases (HATs) and histone deacetylates (HDACs). HAT enzymes are a part of large multiprotein complexes containing adaptors, activators and other associated proteins with unknown functions. It has recently become apparent that many transcription factors, including c-Myc and E2F, overcome the repressive effects of chromatin structure via recruitment of a multi-protein complex containing HAT activity to target genes (3–6).
TRRAP (Transactivation/transformation–domain associated protein), and its yeast ortholog Tra1p, has been identified as a subunit common to several HAT complexes such as PCAF/SAGA (Spt-Ada-Gcn5-acetyl-transferase), Tip60/NuA4 (nucleosomal acetyl-transferase of histone H4), TATA-binding protein (TBF)-free TAFII-containing complex (TFTC), SILK (novel SAGA-related complex) and SAGA-like (SLIK) complexes (7–15). Mutations within the COOH-terminus of Tra1p that disrupt its interaction with HAT complexes result in gene-specific transcriptional defects that correlate with lowered promoter-specific histone acetylation (9). Previous studies have established that TRRAP interacts with the transcription factors c-Myc and E2F (12,16–19). Chromatin immunoprecipitation (ChIP) studies have shown that TRRAP is recruited to the c-Myc target site in chromatin, thereby inducing local histone acetylation (20,21). TRRAP participates in the transcriptional regulation of the p53 target gene mdm2 through histone acetylation activity (22), although the specific HAT complex recruited by TRRAP to p53 target sites has not been identified. Altogether, these observations suggest that TRRAP plays a role in transcriptional regulation by modifying local chromatin through histone acetylation.
It remains unknown to what extent different TRRAP-containing complexes contribute to the transcriptional regulation of different genes and gene families. TRRAP is a subunit of two major classes of HAT complexes with distinct histone specificity (8,11,15). First class consists of PCAF, GCN5, TFTC and STAGA. These complexes contain the HAT enzymatic subunits PCAF or GCN5 and are shown to acetylate preferentially histone H3. Consistent with the essential role of TRRAP in these complexes, mutants of TRRAP homolog in yeast exhibit compromised H3 acetylation (9). The second class of TRRAP-containing complexes includes Tip60 complex containing the HAT enzymatic subunit Tip60 with preference for histone H4 (11,23). Acetylation of histone H4 at several gene promoters was shown to be mediated by TRRAP (9,20–23). Therefore, TRRAP may be involved in acetylation of both histone H3 and histone H4 depending on the gene promoter context. However, little is known if a change in preference for histone acetylation occurs at different cell cycle stages.
To study the biological function of TRRAP, we inactivated the murine homolog of TRRAP (Trrap) in embryonic fibroblast cells and in mouse germline using the ‘conditional’ knockout approach (24). Ablation of Trrap blocked cell proliferation, resulting in the loss of viability of ES cells and embryonic fibroblasts, as well as peri-implantation embryonic lethality in mice. By using an inducible Cre-loxP system, we demonstrated that loss of Trrap causes aberrant mitotic progression and a compromised mitotic checkpoint. Preliminary analysis of expression profiles of a limited number of genes revealed that Trrap might be important for the transcription of a subset of cell cycle regulators (24); however, use of unsynchronized cell populations limited an accurate analysis of the target genes. In order to study physiologically relevant targets of Trrap during mitosis and advance our understanding of the roles of Trrap in chromatin remodeling-mediated transcription, we performed fluorescence glass microarray analysis using Trrap-deficient and Trrap-containing cells.
MATERIALS AND METHODS
Cell culture
Mouse embryonic fibroblasts that are amenable to inducible deletion of Trrap by Cre-ER were described previously (24). Unless otherwise indicated, the cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), penicillin/streptomycin and glutamine. Deletion of Trrap was induced with 1 µM 4-hydroxy-tamoxifen (OHT) (Sigma, St Louis, MO), as described previously (24). Progression of the cells through the cell cycle was measured by flow cytometric analysis of propidium iodide-stained cells as described previously (24).
Microarrays, probe preparation and hybridization
cDNA microarray slides used in this study were fabricated in the microarray core facility (the Biooptics Department) at the Research Institute of Molecular Pathology (IMP), Vienna, Austria. Briefly, the microarrays were constructed using a set of PCR primer pairs specific for open reading frames (ORF), which were used to amplify each ORF of the mouse genome, purchased from Research Genetics (Huntsville, AL). Individual PCR products were verified as unique by gel electrophoresis and were purified before spotting on microscope slides using a high-precision robotic gridder. Each slide has 17 664 spots divided into eight blocks, including ∼8500 unique mouse cDNA sequence and 4000 ESTs. Total mRNAs were isolated from cells using Tri Reagent according to the manufacturer’s instructions (Sigma, St Louis, MO). Total RNAs were used to generate Cy3- or Cy5-labeled cDNA by reverse transcription using Cy3-UTP or Cy5-UTP (CyPack PA55322, Amersham Biosciences, Little Chalfont, UK) and the Superscript II (Invitrogen, Carlsbad, CA). The appropriate Cy3- and Cy5-labeled cDNA probes were combined with mouse COT-1 DNA. After denaturation, the probes were added to the microarrays that were placed in a hybridization chamber and incubated overnight (12–20 h) at 50°C.
Data normalization and analysis
After cohybridization to the microarrays, fluorescence images were captured at both emission wavelengths using a Genepix 4000A fluorescent scanner (Axon Instruments, Inc., Foster City, CA). The fluorescent intensities for both dyes (Cy3 and Cy5) were extracted and analyzed using Genepix Pro 3.0 microarray analysis software (Axon Instruments, Inc., Foster City, CA). Signal ratios between samples obtained from Trrap-containing (Cy3) versus Trrap-deficient (Cy5) cells were obtained after subtracting local background values. Normalization of the Cy3 to Cy5 fluorescent signals was determined by the global median normalization. Spots that were substandard on the scanned image were excluded. As a significance cut-off for the signal:background ratio we used 1.4, i.e. a spot was excluded from further analyses if it had a signal:background ratio <1.4 in both channels. All statistical analyses and evaluations were done using the BioConductor software package. The technical settings (including algorithms) used in our facility were normalized according to standard criteria documented previously (Bioconductor website: http://www.bioconductor.org/ and http://www.bioconductor.org/repository/devel/vignette/marrayNorm.pdf). Primary data on normalization of these genes are available upon request.
Northern blot analysis
Probe preparation for northern blot analysis. Reverse transcription–PCR (RT–PCR) was performed on total RNA prepared from mouse embryonic fibroblasts. Two micrograms of RNA were used for cDNA synthesis using 0.5 µg oligo dT (Amersham Biosciences, Little Chalfont, UK) and 200 U of SuperScript™II RNase H– reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. PCR primers for individual genes were designed to generate a DNA fragment 300–700 bp in length. The following primer pairs were used for PCR amplification of RNA probes for each of the tested genes: for the Trrap gene, 5′-CGGGATCCATGAAGCTTCAC-3′ and 5′-AACTCTCCAGGGATCTCCAC-3′; for the antigen identified by monoclonal antibody Ki67 gene (Mki67), 5′-TAGAGGATCTGCCTGGCTTC-3′ and 5′-TGTCCTTGGTTGGTTCCTCC-3′; for the mammary tumor integration site 6 (Int6), 5′-TGGATCGGCACCTGGTCTTT-3′ and 5′-TCCCTGGTTGACTGCATCTG-3′; for nucleophosmin, 5′-CATGAGTCCTCTTAGGCCTC-3′ and 5′-TCCACTAATGTGCACAGGCC-3′; for the cyclin A2 gene (Ccna2), 5′-TCGCTGCATCAGGAAGACCA-3′ and 5′-GGCAGGCTGTTTACTGACTG-3′; for the eukaryotic translation elongation factor 1 alpha gene (ETEF1α), 5′-GACACGTAGA TTCCGGCAAG-3′ and 5′-CCGTTCTTGGAGATACCAGC-3′; for the eukaryotic translation elongation factor 1 beta gene (ETEF1β), 5′-GGTGCTCAACGATTACCTGG-3′ and 5′-GACTTCGCAACAACTGCAGG-3′; for the RNA polymerase 1–3 gene, 5′-TCAGGAGCTGGAGAGGAAAG-3′ and 5′-TTCCTTGCTTCGCTCAGTGG-3′; for the thymosin β4 gene, 5′-ACAAGAGAAGCAAGCTGGCG-3′ and 5′-TCA TCATCTCCCACCCAGCT-3′; for the ribosomal protein L27A gene (RP L27A), 5′-TAGGTCTTCTCTTGGCC TCC-3′ and 5′-GACTTCCTCAGTCTGGATGG-3′; for coatomer protein complex gene, subunit gamma 2 (Copg2), 5′-CACAAGCAGTCTGACCAAGG-3′ and 5′-CCAGCTCTCTTGCAGTACAG-3′; for the non-POU domain-containing octamer-binding protein (NonO), 5′-ATCAGCATCACCACCAGCAG-3′ and 5′-CCTTCCTCGGTCATCCACAA-3′; for the syndecan 2 (Sdc2), 5′-TTGGGCTTGATGGCCTGTGT-3′ and 5′-GGCTGCTAGAACTTCTGTCC-3′; for the Finkel-Biskis-Reilly murine sarcoma virus ubiquitously expressed gene (FBR-MuSV), 5′-ACATGCAGCTCTTTGTCCGC and 5′-CAACATTGACAAAGCGCCGG; for the Huntingtin interactin protein 2 (HIP-2), 5′-TCAAGGAGGTGCTGAAGAGC and 5′-GTTGATGCTCCTCAAGAGGC; for the von Hippel-Lindau binding protein 1 (VHL-BP-1), 5′-CTGGCCGATAACCTGTACTG and 5′-CATTCCTCACAGTTCTCGG; for the quaking (qk), 5′-TGGAAACGAAGGAGAAGCCG and 5′-TGTTGGCGTCTCTGTAGGTG. PCR was performed on 2 µl of cDNA sample using the corresponding primer pairs. The resulting DNA fragment was cloned into the pCR2.1 vector using the TA cloning system (Invitrogen, Carlsbad, CA). After sequence confirmation by DNA sequencing, all inserts were excised by EcoRI restriction digestion and purified using a gel purification kit (Qiagen, Courtaboeuf, France).
Northern blot analysis. Total RNA (10 µg) was electrophoresed using a 0.9% agarose gel in the presence of 6.7% formaldehyde and transferred to a Hybond N+ membrane (Amersham Biosciences, Little Chalfont, UK) in 10× SSC. 32P-labeled probes were made from 20–50 ng of DNA using random priming by the Prime-IT RmT kit according to the manufacturer’s instructions (Stratagene, La Jolla, CA). The probe hybridization and stripping buffers and conditions were as instructed by the membrane manufacturer.
Chromatin immunoprecipitation (ChIP) assay
Cells were cultured in 15-cm plates and synchronized as above. Formaldehyde cross-linking and chromatin immunoprecipitations were performed essentially as previously described (21). Briefly, cross-linked cells were harvested in SDS lysis buffer and disrupted by sonication. The lysates were immunoprecipitated with polyclonal antibodies specific for either acetylated histone H3 (Upstate Biotech, cat. no. 06-599) or acetylated histone H4 (Upstate Biotech, cat. no. 06-866). Input and immunoprecipitated DNAs were amplified by PCR and the PCR products were analyzed by agarose/ethidium bromide gel electrophoresis. For PCR reactions, we used 1/75 and 1/15 of the immunoprecipitated DNA with anti-acetyl-H3 and anti-acetyl-H4 antibodies, respectively. The primers used for the cyclin A2 promoter were: 5′-GGGCATAGAGACACGCCTTT-3′ (CycA2.FOR) and 5′-GCAGGAGCGTATGGATCTGA-3′ (CycA3.REV). The primers for the thymosin β4 promoter were: 5′-GGCTGTCCACTGGTCTGAAA-3′ (ThB4-3.FOR) and 5′-GAACCACATCGATGGCGGAA-3′ (ThB4-3.REV).
RESULTS
Experimental outline
Trrap is essential for proliferation and is a cofactor for several transcription factors (7–15,24), however its target genes have not been identified. Because loss of Trrap compromises mitotic progression (24) and because several yeast mutants lacking HAT exhibit G2-to-M progression defects (25–28), we sought to identify Trrap target genes associated with cell cycle progression. Cell lines with inducible deletion of Trrap permit us to conditionally inactivate Trrap (24). Upon addition of the inducer 4-hydroxy-tamoxifen (OHT), the Cre-ER fusion protein is translocated into the cell nucleus, resulting in the deletion of the Trrap gene by Cre recombinase (24). Efficiency of Trrap deletion was monitored by northern blot analysis which showed that Trrap transcripts were virtually absent in OHT-treated cells at the time points used for sample collection (see below).
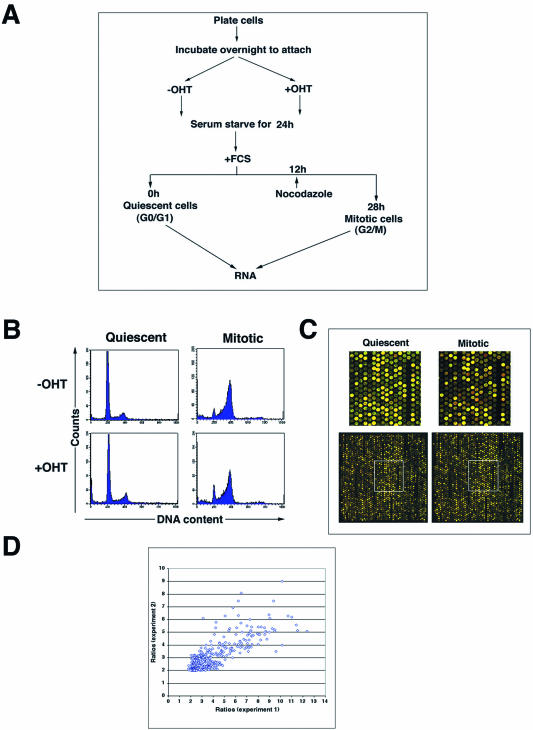
The experimental outline for cell synchronization is shown in Figure 1A. Trrap-containing cells (pre-incubated without OHT) and Trrap-deficient cells (pre-incubated with OHT for 24 h) were serum starved for 24 h resulting in an accumulation of G0/G1 (designated as ‘quiescent cells’, Fig. 1B). To obtain the mitotic population nocodazole (100 ng/ml) was added at 12 h after release from the G0/G1 block and were kept until cells were harvested (designated as ‘mitotic cells’, Fig. 1B). RNA was extracted from these cells and used to prepare probes to screen cDNA micro-arrays containing 17 664 mouse gene elements (Fig. 1C). This experimental design allowed us to monitor the expression profiles of a large number of known genes and as yet unnamed genes (ESTs) as potential targets of Trrap. We performed two independent experiments using identical protocols and the ratios of differentially expressed genes from both experiments showed a good correspondence (Fig. 1D). The results presented here are from one of the two experiments (Experiment 2).
Figure 1.
(A) Experimental outline for synchronization of cells at different cell cycle phases. See text for details. Cells that were rendered quiescent by serum starvation were subsequently restimulated with serum for 28 h in the presence of nocodazole to obtain mitotic cells. (B) FACS analysis of cell cycle profiles of Trrap-containing (–OHT) and Trrap-deficient (+OHT) cells at quiescence and mitosis. Propidium iodide intensity (DNA content) is plotted against cell number (counts). (C) Comparison of expression profiles of Trrap-containing and Trrap-deficient cells. Total RNAs prepared from cells at the indicated time points were labeled with Cy5-dUTP (Trrap-deficient cells) or Cy3-dUTP (Trrap-containing cells) and competitive hybridization was carried out on microarrays. Red and green spots represent the genes that are down- and up-regulated, respectively, in Trrap-deficient cells as compared to Trrap-containing cells. Genes displaying equal levels of expression in both cell populations are represented by yellow spots. Only a part of a microarray slide (one block) and a zoomed detail (insets) of the corresponding block are shown. (D) Scatter plot of the fold ratios of differentially expressed genes from the two experiments conducted.
To control the effect of OHT on gene expression, a cell line transfected with an empty vector (PSG1; see also 24) was treated with or without OHT and gene expression profiles were analyzed by the cDNA array. With one exception (gene encoding PH domain-containing protein in retina, accession no. AI850671), we observed no significant expression differences between OHT-treated and untreated empty vector-transfected cells, suggesting that OHT did not alter general gene expression levels in these cells.
Expression profiles of genes in Trrap-deficient cells at quiescence
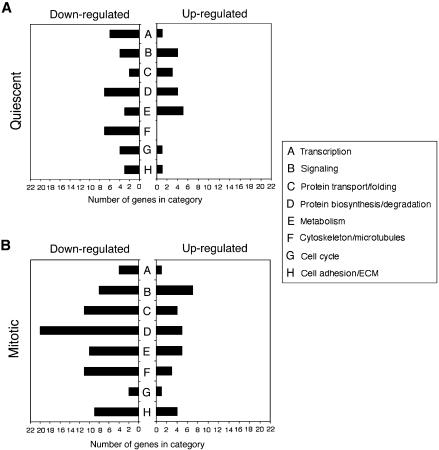
To investigate gene expression regulation by Trrap in quiescent state (G0/G1 phase), we first compared gene expression profiles of Trrap-deficient and Trrap-containing cells after serum starvation for 24 h (Fig. 1). Using a criterion difference in expression of at least 2-fold, we found 153 gene expression differences (0.86% of the total 17 664 entities present in the array), representing 67 known genes, between Trrap-containing and Trrap-deficient cells. One hundred of these entities, representing 46 known genes, were down-regulated (Table 1), and 53 entities, representing 21 known genes, were up-regulated in the Trrap-deficient cells (Table 2). The down-regulated genes in the Trrap-deficient cells are involved in a variety of cellular processes, including transcription, protein turnover, cytoskeleton organization, cell cycle, metabolism and adhesion (Fig. 2A). The majority of the up-regulated genes are those involved in metabolism, protein turnover and signaling (Fig. 2A).
Table 1. Genes down-regulated in Trrap-deficient cells in the G0/G1 phase of cell cycle.
| Accession no. | Gene description | Function | Ratio |
|---|---|---|---|
| AI839149 | procollagen, type I, alpha 1 | Cell adhesion/ECM | 2.2 |
| AI838652 | procollagen, type I, alpha 2 | Cell adhesion/ECM | 2.8 |
| AI841822 | utrophin | Cell adhesion/ECM | 2.1 |
| AI451073 | growth arrest and DNA-damage-inducible 45 alpha | Cell cycle | 2.6 |
| AI414879 | neuroblastoma ras oncogene | Cell cycle | 2.2 |
| AI414001 | polycystic kidney disease 2 | Cell cycle | 2.7 |
| AI528671 | septin 2 | Cell cycle | 2.2 |
| AI324640 | S-adenosylmethionine decarboxylase 1 | Cell growth/mainteinance | 2.1 |
| AI839002 | actin, alpha 1, skeletal muscle | Cytoskeleton/MBP | 2.1 |
| AI838959 | actin, alpha 2, smooth muscle, aorta | Cytoskeleton/MBP | 3.0 |
| AI323827 | capping protein alpha 1 | Cytoskeleton/MBP | 2.5 |
| AI325922 | cofilin 2, muscle | Cytoskeleton/MBP | 2.9 |
| AI835976 | erythrocyte protein band 4.1-like 3 | Cytoskeleton/MBP | 2.1 |
| AI835403* | thymosin, beta 4, X chromosome | Cytoskeleton/MBP | 2.1 |
| AI894247 | tubulin, beta 5 | Cytoskeleton/MBP | 2.0 |
| AI465143 | lectin, galactose binding, soluble 1 | Galactose binding lectin | 2.4 |
| AI842654 | lymphocyte antigen 6 complex | Immune system | 2.0 |
| AI326151 | glycine N-methyltransferase | Metabolism | 2.6 |
| AI447203 | phosphate cytidylyltransferase 1, choline, alpha isoform | Metabolism | 2.0 |
| AI451732 | propionyl Coenzyme A carboxylase, beta polypeptide | Metabolism | 2.0 |
| AI426498 | protein phosphatase 1, catalytic subunit, alpha isoform | Protein biosynthesis | 2.1 |
| AI325468 | protein phosphatase 1, regulatory (inhibitor) subunit 1A | Protein biosynthesis | 2.2 |
| AI323719 | suppressor of initiator codon mutations, related sequence 1 | Protein biosynthesis | 3.0 |
| AI326836 | calpain 6 | Protein degradation | 2.2 |
| AI835498 | cystatin C | Protein degradation | 2.7 |
| AI854847 | serine (or cysteine) proteinase inhibitor, clade E, member 2 | Protein degradation | 2.1 |
| AI836278 | ubiquitin conjugating enzyme E3A | Protein degradation | 2.1 |
| AI845673 | DnaJ (Hsp40) homolog, subfamily B, member 6 | Protein transport/folding | 2.1 |
| AI429549 | translocator of inner mitochondrial membrane 17 kDa, b | Protein transport/folding | 2.0 |
| AI841014 | guanine nucleotide binding protein, alpha stimulating | Signaling | 2.2 |
| AI893646 | protein tyrosine phosphatase, receptor type, K | Signaling | 2.0 |
| AI842115 | small GTPase, homolog (Saccharomyces cerevisiae) | Signaling | 2.1 |
| AI447373 | tubby-like protein 3 | Signaling | 2.1 |
| AI323619 | nuclear factor of kappa light chain gene enhancer in B-cells 1 | Transcription | 2.0 |
| AI324044 | nuclear, factor, erythroid derived 2, like 2 | Transcription | 2.4 |
| AI448329* | RNA polymerase 1–3 (16 kDa subunit) | Transcription | 3.2 |
| AI414288 | WW domain binding protein 5 | Transcription | 2.4 |
| AI837954 | zinc finger proliferation 1 | Transcription | 2.4 |
| AI844866 | zinc finger protein 216 | Transcription | 2.4 |
| AI323712 | ATPase, H+ transporting, lysosomal, beta 56/58 kDa isoform 2 | Transport systems | 3.6 |
| AI450702 | ATPase, Na+/K+ transporting, beta 3 polypeptide | Transport systems | 2.0 |
| AI415665 | calumenin | Transport systems | 2.9 |
| AI835793 | slit homolog 2 (Drosophila) | Transport systems | 2.0 |
| AI666768 | sodium channel, nonvoltage-gated 1 beta | Transport systems | 2.1 |
| AI385598 | solute carrier family 20, member 1 | Transport systems | 2.6 |
| AI426386 | synaptosomal-associated protein, 23 kDa | Unknown function | 2.6 |
Transcripts marked by asterisk were also analyzed by northern blotting.
Table 2. Genes up-regulated in Trrap-deficient cells in the G0/G1 phase of cell cycle.
| Accession no. | Gene description | Function | Ratio |
|---|---|---|---|
| AI852485* | syndecan 2 | Cell adhesion/ECM | 2.6 |
| AI850226 | CDC-like kinase | Cell cycle | 2.0 |
| AI854574 | thyroid autoantigen 70 kDa | DNA repair | 2.4 |
| AI854743 | adenylate kinase 3 alpha like | Metabolism | 2.1 |
| AI845273 | aspartate-beta-hydroxylase | Metabolism | 2.2 |
| AI843601 | fatty acid Coenzyme A ligase, long chain 3 | Metabolism | 2.1 |
| AI854177 | nudix (nucleoside diphosphate linked moiety X)-type motif 5 | Metabolism | 2.5 |
| AI844688 | serine racemase | Metabolism | 2.1 |
| AI847441 | protease (prosome, macropain) 26S subunit, ATPase 5 | Protein biosynthesis | 2.0 |
| AI851588 | ribosomal protein L23 | Protein biosynthesis | 2.2 |
| BC002014 | ribosomal protein S7 | Protein biosynthesis | 2.0 |
| AI528869 | Tripeptidyl peptidase II | Protein degradation | 2.1 |
| AI851468* | coatomer protein complex, subunit gamma 2 | Protein transport/folding | 2.0 |
| AI847098 | ERO1-like (S.cerevisiae) | Protein transport/folding | 2.2 |
| AI851542 | vacuolar protein sorting 41 (yeast) | Protein transport/folding | 2.1 |
| AI528859 | discs, large homolog 3 (Drosophila) | Signaling | 2.3 |
| AI465474 | oncostatin receptor | Signaling | 2.1 |
| AI846385 | RAB23, member RAS oncogene family | Signaling | 2.0 |
| AI854838 | tyrosine 3-monoox./tryptophan 5-monooxyg. activ. protein | Signaling | 2.4 |
| AI854349 | serum/glucocorticoid regulated kinase | Stress response | 2.1 |
| AI850641 | nuclear factor I/B | Transcription | 2.0 |
Transcripts marked by asterisk were also analyzed by northern blotting.
Figure 2.
Distribution of differentially expressed genes in Trrap-deficient cells. Numbers of down- and up-regulated genes per functional category in quiescent state (A) and in mitosis (B) were shown.
Differentially expressed genes between Trrap-containing and Trrap-deficient cells in mitotic cells
Because Trrap-deficient cells exhibit mitotic progression defects (24) and because mutations in several Trrap- containing HAT complexes cause G2-to-M progression phenotype (24–28), we examined the transcription profiles of Trrap-deficient cells after synchronization at mitosis by nocodazole (Fig. 1). We found that 169 gene elements were down-regulated, representing 113 known genes (Table 3), whereas 68 gene elements, representing 42 known genes, were up-regulated (Table 4).
Table 3. Genes down-regulated in Trrap-deficient cells at mitosis.
| Accession no. | Gene description | Ratio |
|---|---|---|
| Cell adhesion/extracellular matrix | ||
| AI573427 | catenin beta | 2.6 |
| AI838312 | Decorin | 2.9 |
| AI528687 | integrin beta 1 (fibronectin receptor beta) | 2.1 |
| AI838730 | popeye 3 | 3.6 |
| AI845452 | procollagen, type XI, alpha 2 | 5.6 |
| AI838929 | secreted acidic cysteine rich glycoprotein | 2.1 |
| AI847805 | secreted phosphoprotein 1 | 3.1 |
| AI838607 | thrombospondin 1 | 2.7 |
| AI842847 | tissue inhibitor of metalloproteinase | 2.4 |
| Cell cycle | ||
| AI849379 | calmodulin 2 | 2.5 |
| AI851373* | cyclin A2 | 2.9 |
| Cell proliferation | ||
| AI428398* | antigen identified by monoclonal antibody Ki 67 | 5.1 |
| Cytoskeleton/microtubules-based processes | ||
| AI844634 | actin, alpha 1, skeletal muscle | 3.0 |
| AI838959 | actin, alpha 2, smooth muscle, aorta | 4.2 |
| AI836381 | actin-like | 2.1 |
| AI837758 | capping protein alpha 2 | 2.8 |
| AI836876 | cofilin 1, non-muscle | 4.1 |
| AI848700 | kinesin family member 5B | 4.4 |
| AI850620 | matrin 3 | 2.1 |
| AI839417 | Moesin | 3.4 |
| AI465254 | t-complex testis expressed 1 | 5.3 |
| AI835403* | thymosin, beta 4, X chromosome | 3.2 |
| AI839893 | tubulin alpha 1 | 3.4 |
| DNA replication | ||
| AI447692 | origin recognition complex, subunit 2 homolog | 2.8 |
| Metabolism | ||
| AI836694 | ATP synthase, H+ transport, mitoch. F1 complex, gamma | 2.5 |
| AI839368 | carbonyl reductase 1 | 5.4 |
| AI841389 | enolase 1, alpha non-neuron | 6.3 |
| AI836205 | expressed in non-metastatic cells 4, protein (NM23-M4) | 2.2 |
| AI661450 | isocitrate dehydrogenase 2 (NADP+), mitochondrial | 3.7 |
| AI451732 | propionyl Coenzyme A carboxylase, beta polypeptide | 2.2 |
| AI836137 | pyruvate kinase 3 | 4.8 |
| AI850042 | stearoyl-Coenzyme A desaturase 2 | 2.4 |
| AI845316 | stearoyl-Coenzyme A desaturase 2 | 2.3 |
| AI853172 | UDP-glucose ceramide glucosyltransferase-like | 2.3 |
| Protein biosynthesis/degradation | ||
| AI840661* | eukaryotic translation elongation factor 1 alpha 1 | 4.3 |
| AI835282* | eukaryotic translation elongation factor 1 beta 2 | 4.1 |
| AI842443 | eukaryotic translation initiation factor 2 alpha kinase 1 | 5.0 |
| AI845502 | eukaryotic translation initiation factor 3 | 4.4 |
| AI848116 | eukaryotic translation initiation factor 4, gamma 2 | 3.1 |
| AI644523 | eukaryotic translation initiation factor 4E | 3.3 |
| AI847325* | Finkel-Biskis-Reilly murine sarcoma virus ubiq. expressed | 5.4 |
| AI323816* | mammary tumor integration site 6 | 7.5 |
| AI847420 | ribosomal protein L21 | 3.2 |
| AI854758 | ribosomal protein L27 | 2.3 |
| AI850051* | ribosomal protein L27a | 4.8 |
| AI841501 | ribosomal protein S3a | 4.9 |
| AI835625 | ribosomal protein S6 | 5.1 |
| AI854799 | ribosomal protein, large, P1 | 2.2 |
| AI841978 | calpain 4 | 2.5 |
| AI838658 | cathepsin B | 2.7 |
| AI842912 | COP9 (constitutive photomorphogenic, subunit 2 | 4.1 |
| AI843127* | huntingtin interacting protein 2 | 3.8 |
| AI836652 | ubiquitin B | 3.0 |
| AI835914 | ubiquitin-like 1 (sentrin) activating enzyme E1B | 2.6 |
| Protein transport/folding | ||
| AI843174 | ADP-ribosylation-like factor 6 interacting protein | 2.2 |
| AI841066 | DnaJ (Hsp40) homolog, subfamily A, member 1 | 4.8 |
| AI834803 | DnaJ (Hsp40) homolog, subfamily B, member 12 | 2.8 |
| AI841358 | heat shock 70 kDa protein 8 | 2.7 |
| AI841577 | heat shock protein, 74 kDa, A | 3.4 |
| AI838924 | heat shock protein, 84 kDa 1 | 2.2 |
| AI841851 | heat shock protein, 86 kDa 1 | 2.4 |
| AI852527 | importin beta | 3.2 |
| AI836583 | tetratricopeptide repeat domain | 3.9 |
| AI429549 | translocator of inner mitochondrial membrane 17 kDa, b | 3.9 |
| AI325940 | von Hippel-Lindau binding protein 1 | 4.4 |
| RNA binding/processing | ||
| AI840802 | 5′-3′ exoribonuclease 2 | 2.7 |
| AI449004 | heterogeneous nuclear ribonucleoprotein A2/B1 | 2.5 |
| AI848331 | heterogeneous nuclear ribonucleoprotein U | 3.5 |
| AI323810* | nucleophosmin 1 | 6.0 |
| AI852094 | poly A binding protein, cytoplasmic 1 | 3.0 |
| AI849916 | polymyositis/scleroderma autoantigen 1 | 3.3 |
| AI429316* | quaking | 4.4 |
| AI838842 | RNA binding motif protein 3 | 2.7 |
| AI840449 | small nuclear ribonucleoprotein D1 | 3.8 |
| Signaling | ||
| AI834923 | guanine nucleotide binding protein, beta 2, related seq. 1 | 2.1 |
| AI836495 | guanosine diphosphate (GDP) dissociation inhibitor 3 | 5.4 |
| AI836571 | protein kinase C and casein kinase substrate in neurons 3 | 3.0 |
| AI843656 | protein kinase C, delta | 3.1 |
| AI323807 | RHO, GDP dissociation inhibitor (GDI) beta | 2.0 |
| AI845078 | small protein effector 1 of Cdc42 | 3.1 |
| AI849063 | transforming growth factor beta 1 induced transcript 4 | 2.5 |
| AI850808 | tyrosine 3-monoox./tryptophan 5-monoox. activ. protein | 2.9 |
| Transcription | ||
| AI845473 | DNA methyltransferase 3A | 3.2 |
| AI450641 | ets homologous factor | 4.8 |
| AI851714 | paired related homeobox 1 | 3.7 |
| AI448329* | RNA polymerase 1–3 (16 kDa subunit) | 4.0 |
| DNA binding | ||
| AI448424 | paternally expressed gene 3 | 4.3 |
| Integral membrane proteins | ||
| AI838302 | Cd63 antigen | 4.0 |
| AI854515 | CD9 antigen | 3.1 |
| AI845046 | trans-golgi network protein 1 | 2.5 |
| AI845774 | transmembrane 4 superfamily member 1 | 4.7 |
| AI847962 | transmembrane 4 superfamily member 2 | 3.7 |
| AI842908 | Wolfram syndrome 1 homolog (human) | 6.9 |
| Transport systems | ||
| AI845042 | annexin A5 | 2.2 |
| AI415665 | calumenin | 6.1 |
| AI449517 | ferritin light chain 1 | 2.5 |
| AI841957 | gamma-aminobutyric acid receptor, subunit alpha 6 | 2.5 |
| AI451073 | growth arrest and DNA-damage-inducible 45 alpha | 2.8 |
| AI836363 | prolyl 4-hydroxylase, beta polypeptide | 2.2 |
| AI450790 | sideroflexin 1 | 3.0 |
| AI844775 | thioredoxin | 2.4 |
| Neuronal function | ||
| AI849905 | neurofilament, medium polypeptide | 3.1 |
| Immune system | ||
| AI323595 | β-cell receptor-associated protein 31 | 2.1 |
| Galactose binding lectin | ||
| AI465143 | lectin, galactose binding, soluble 1 | 3.8 |
| AI450263 | lectin, galactose binding, soluble 6 | 4.0 |
| Blood coagulation factor | ||
| AI464520 | coagulation factor II (thrombin) receptor | 2.6 |
| Unknown function | ||
| AI450318 | ethanol decreased 2 | 2.9 |
| AI850738 | f-box only protein 30 | 5.1 |
| AI666723 | pellino 1 | 3.6 |
| AI838759 | postsynaptic protein Cript | 2.8 |
| AI838689 | protein tyrosine phosphatase 4a2 | 3.2 |
| AI845040 | translationally regulated transcript (21 kDa) | 2.3 |
| AI845630 | tyrosine 3-monoox./tryptophan 5-monoox. activ. prot., zeta | 7.5 |
Transcripts marked by asterisk were also analyzed by northern blotting.
Table 4. Genes up-regulated in Trrap-deficient cells at mitosis.
| Accession no. | Gene description | Ratio |
|---|---|---|
| Cell adhesion/ECM | ||
| AI851778 | connective tissue growth factor | 2.1 |
| AI841659 | matrix metalloproteinase 13 | 2.0 |
| AI448993 | procollagen, type XVIII, alpha 1 | 6.3 |
| AI854113* | syndecan 2 | 2.3 |
| Cell cycle | ||
| AI852430 | peripheral myelin protein, 22 kDa | 2.1 |
| Cell growth/maintenance | ||
| AI842277 | insulin-like growth factor binding protein 3 | 2.3 |
| Cell proliferation/differentiation | ||
| AI843746 | fibroblast growth factor inducible 14 | 2.5 |
| Cytoskeleton/MBP | ||
| AI851494 | kinesin heavy chain member 2 | 2.1 |
| AI853411 | microtubule-associated protein 7 | 2.1 |
| AI854333 | tropomyosin 1, alpha | 2.1 |
| DNA repair | ||
| AI848686 | damage specific DNA binding protein 1 (127 kDa) | 2.3 |
| Metabolism | ||
| AI846372 | asparagine synthetase | 2.3 |
| AI842131 | ATP synthase, H+ transport., mitoch. F0 complex, subunit c | 2.0 |
| AI836445 | stearoyl-Coenzyme A desaturase 2 | 2.3 |
| AI852717 | succinate-CoA ligase, GDP-forming, alpha subunit | 2.4 |
| Protein biosynthes./degradation | ||
| AI851255 | cathepsin B | 2.1 |
| AI836333 | nitrilase 1 | 2.0 |
| AI845068 | proteasome (prosome, macropain) 26S subunit, ATPase 2 | 2.0 |
| AI851441 | proteasome (prosome, macropain) subunit, alpha type 4 | 2.1 |
| AI844521 | proteasome (prosome, macropain) subunit, alpha type 6 | 2.7 |
| Protein transport/folding | ||
| AI854749 | chaperonin subunit 3 (gamma) | 2.4 |
| AI851468* | coatomer protein complex, subunit gamma 2 | 2.2 |
| AI846861 | N-ethylmaleimide sensitive fusion protein | 2.1 |
| AI851315 | SEC61, alpha subunit 2 (S. cerevisiae) | 2.0 |
| RNA binding/processing | ||
| AI851199* | non-POU-domain-containing, octamer-binding protein | 3.8 |
| AI854330 | ribonuclease P2 | 2.4 |
| Signaling | ||
| AI844216 | abl-interactor 1 | 2.0 |
| AI853544 | guanine nucleotide releasing factor 2 | 2.7 |
| AI850105 | leukemia-associated gene | 2.1 |
| AI465474 | oncostatin receptor | 2.2 |
| AI854805 | raf-related oncogene | 2.3 |
| AI847155 | ribosomal protein S6 kinase, 90 kDa, polypeptide 4 | 2.4 |
| AI853288 | Wnt1 responsive Cdc42 homolog | 2.2 |
| Transcription | ||
| AI852906 | circadian locomoter output cycles kaput | 2.3 |
| Transport systems | ||
| AI842352 | centrin 2 | 2.5 |
| AI842091 | trans-golgi network protein 2 | 2.4 |
| Unknown function | ||
| AI846269 | 5-azacytidine induced gene 2 | 2.2 |
| AI849996 | latexin | 3.0 |
| AI850263 | lysosomal membrane glycoprotein 2 | 2.0 |
| AI854112 | retinoid-inducible serine caroboxypetidase | 2.5 |
| AI852294 | REV1-like (S. cerevisiae) | 2.8 |
| AI851503 | tumor differentially expressed 1 | 2.6 |
Transcripts marked by asterisk were also analyzed by northern blotting.
The largest groups of down-regulated genes in mitotic cells were those involved in protein turnover, adhesion, protein transport and folding, cytoskeleton and microtubule-based processes, metabolism and signaling (Table 3 and Fig. 2B). Only a few cell cycle-associated genes were down-regulated in Trrap-deficient cells during this stage. Trrap seems to regulate the gene expression of cyclin A2, a major regulator of cell cycle progression through S and G2/M phase, but not of cyclin B1 (Table 3 and data not shown). The majority of up-regulated genes consisted of those implicated in protein turnover, signaling, metabolism and cell adhesion (Table 4 and Fig. 2B). It is interesting to note that out of a total of 223 differentially expressed genes, only a small fraction (11 genes) overlapped between quiescent cells and mitotic cells. These results suggest that Trrap regulates distinct sets of genes in mitotic cells compared to quiescent cells.
Verification of microarray results by northern blot analysis
To verify the microarray results using an independent method, we chose 17 differentially expressed genes (Tables 1 and 2) for northern blot analysis. In the selection of these genes, we were guided by the criteria of broad representation of different gene classes and expression levels as well as the availability of RNA probes. In all cases, the northern blots confirmed the microarray results. As shown in Figure 3, thymosin β4, ribosomal protein L27A (RP L27A), eukaryotic translation elongation factor 1 beta (ETEF1β), mammary tumor integration site 6 (Int6), nucleophosmin 1 (nucleoph.1), cyclin A2 (Ccna2), eukaryotic translation elongation factor 1 alpha (ETEF1α), RNA polymerase 1–3 (RNA pol.1–3), Finkel-Biskis-Reilly murine sarcoma virus ubiquitously expressed (FBR-MuSV), huntingtin interactin protein 2 (HIP-2), von Hippel-Lindau binding protein 1 (VHL-BP-1), quaking (qk), coatomer protein complex subunit gamma 2 (Copg2), non-POU-domain-containing octamer-binding protein (NonO), syndecan 2 (Sdc2), antigen identified by monoclonal antibody Ki67 (Mki67), followed patterns of expression similar to those seen with the microarrays (Table 5). Trrap was used as a control to verify deletion of the gene.
Figure 3.
Confirmation of differentially expressed genes in microarray analysis by northern blot analyses. Ten micrograms of total RNA was used for each sample of the 17 differentially expressed genes. The blots were reused by stripping off the probes after each hybridization. The following probes were used: (A) thymosin β4; RP L27A (ribosomal protein L27A); (B) ETEF 1β (eukaryotic translation elongation factor 1 beta 2); Int6 (mammary tumor integration site 6); nucleoph. 1 (nucleophosmin 1); Ccna2 (cyclin A2); (C) ETEF 1α (eukaryotic translation elongation factor 1 alpha 1); RNA pol. 1–3 (RNA polymerase 1–3); Trrap; (D) FBR-MuSV (Finkel-Biskis-Reilly murine sarcoma virus ubiquitously expressed); HIP-2 (huntingtin interactin protein 2); VHL-BP-1 (von Hippel-Lindau binding protein 1); qk (quaking); (E) Copg2 (coatomer protein complex, subunit gamma 2); NonO (non-POU-domain- containing, octamer-binding protein); Sdc2 (syndecan 2); (F) Mki67 (antigen identified by monoclonal antibody Ki67). RNA loading was checked by non-specific cross-reacting antigen (NCA) probe and ethidium bromide staining of the gels (18 S rRNA and 28S rRNA).
Table 5. Comparison of microarray intensity ratios and northern blot densitometry ratios.
| Gene description | Ratio Microarray | Northern blota |
|---|---|---|
| Quiescent cells | ||
| Down-regulated genes | ||
| thymosin, beta 4, X chromosome | 2.1 | 2.4 |
| RNA polymerase 1–3 (16 kDa subunit) | 3.2 | 1.4 |
| Up-regulated genes | ||
| syndecan 2 | 2.6 | 2.2 |
| coatomer protein complex, subunit gamma 2 | 2.0 | 2.9 |
| Mitotic cells | ||
| Down-regulated genes | ||
| cyclin A2 | 2.9 | 3.0 |
| antigen identified by monoclonal antibody Ki 67 | 5.1 | 4.2 |
| thymosin, beta 4, X chromosome | 3.2 | 2.5 |
| eukaryotic translation elongation factor 1 alpha 1 | 4.3 | 2.3 |
| eukaryotic translation elongation factor 1 beta 2 | 4.1 | 2.2 |
| Finkel-Biskis-Reilly murine sarcoma virus ubiq. expressed | 5.4 | 3.3 |
| mammary tumor integration site 6 | 7.5 | 3.4 |
| ribosomal protein L27a | 4.8 | 3.3 |
| huntingtin interacting protein 2 | 3.8 | 2.1 |
| nucleophosmin 1 | 6.0 | 3.3 |
| quaking | 4.4 | 1.9 |
| RNA polymerase 1–3 (16 kDa subunit) | 4.0 | 2.1 |
| Up-regulated genes | ||
| syndecan 2 | 2.3 | 1.8 |
| coatomer protein complex, subunit gamma 2 | 2.2 | 2.7 |
| non-POU-domain-containing, octamer-binding protein | 3.8 | 2.6 |
aRatio of densitometric intensities normalized to the NCA control.
Absence of Trrap affects differentially acetylation of histones at the promoters of target genes
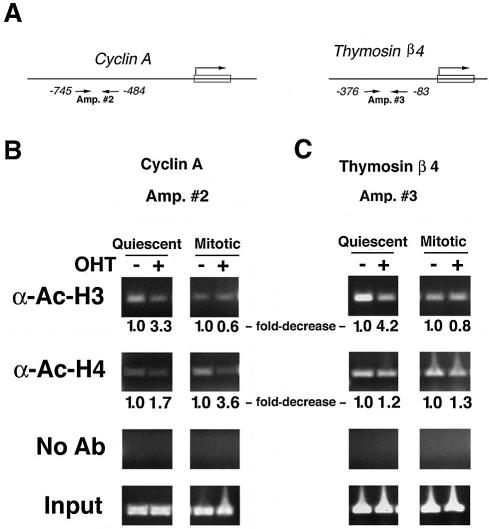
Because Trrap is believed to regulate target genes through participation in acetylation of H3 or H4 histones (9,12,20,21), we attempted to investigate the mode of gene expression regulated by Trrap in these genes. To test whether acetylation of histones H3 or H4 at the gene promoters is affected in cells lacking Trrap during cell cycle progression, we performed ChIP assays on several differentially expressed genes with antibodies specific to either acetylated histone (Fig. 4, and data not shown). While loss of Trrap induced a decrease in both histone H3 and H4 acetylation at the cyclin A2 promoter in the quiescent cells, only acetylation of histone H4 was affected in mitotic cells (Fig. 4B), suggesting that Trrap is preferentially involved in HAT recruitment to histone H4 during mitosis. The promoter of thymosin β4 exhibited an acetylation defect specifically associated with histone H3 in quiescent cells but not in mitotic cells. However, acetylation of histone H4 seemed to be only slightly affected in both quiescent and mitotic cells (Fig. 4C). These two examples suggest that Trrap modulates acetylation of different histones depending on cell cycle stage, most likely through selective recruitment of specific HAT complex.
Figure 4.
Histone acetylation at the gene promoters is affected in Trrap-deficient cells. (A) Schematic representation of the mouse cyclin A2 and thymosin beta 4 promoters. Small arrows indicate the primers used in PCR amplification reactions. (B and C) ChIP analysis of histone acetylation at the cyclin A2 and thymosin beta 4 promoters. Immunoprecipitated DNA was analyzed by PCR using primers recognizing the cyclin A2 promoter (B) and the thymosin beta 4 promoter (C). Mock immunoprecipitations (reactions lacking antibodies) were performed as a control (No Ab). Input corresponds to PCR reactions containing 1.25% of the total amount of chromatin used in immunoprecipitation reactions. Fold-decrease in acetylation levels in Trrap-deficient cells was calculated as a ratio of densitometric intensities between Trrap-containing cells (–OHT) and Trrap-deficient cells (+OHT) after normalization to the corresponding input. The acetylation level in Trrap-containing cells was set as 1.0.
DISCUSSION
In the present study, we took advantage of genome-wide expression analysis (cDNA microarrays) combined with an inducible deletion technique (Cre/loxP system) and identified genes whose expression is regulated by Trrap during cell cycle progression. Our analysis demonstrates that the expression of many genes is down-regulated due to loss of Trrap, whereas a smaller number of genes are up-regulated. This is consistent with the notion that Trrap is an important regulator of gene transcription throughout cell cycle progression. Functional classification of these genes indicates that Trrap target genes influence a variety of cellular processes including protein turnover, cell cycle progression, DNA repair, metabolism, signal transduction, cytoskeleton and cell adhesion (Fig. 2). Therefore, the severe and complex effects of Trrap loss on cellular functions are unlikely to be reflected by any single target gene. A more likely scenario is that Trrap coordinates expression of a number of gene families, thereby linking transcription, DNA replication, protein turnover and cell cycle control.
TRRAP is a subunit common to many HAT complexes that are believed to facilitate gene transcription (3–6). Two pieces of evidence suggest that Trrap plays a role in progression through cell cycle transitions. First, TRRAP associates with c-Myc and E2F that regulate key genes implicated in G1-to-S and G2-to-M cell cycle transitions (29–33). Second, Trrap-deficient cells exhibited mitotic defects (24). Many of the Trrap-regulated genes show clear connections to the main Trrap partners, c-Myc and E2F. Trrap regulates the expression of translation elongation factors, translation initiation factors, transcription elongation factors and ribosomal proteins, groups of genes known to be regulated by c-Myc (30). Therefore, some important effects of c-Myc on cells may be mediated by Trrap. Furthermore, the Trrap-regulated genes identified in this study function in DNA replication, apoptosis and DNA repair, cellular processes known to be dependent on E2F function (33,34).
Despite the fact that Trrap interacts with c-Myc and E2F, many Trrap-regulated genes do not show an obvious connection to the main outcome of c-Myc and E2F activities (30,33,34). For example, Myc-mediated apoptosis appears to be Trrap independent (17,35). Thus the sum of the effects at the transcriptional level seen with loss of c-Myc and E2F appears to be less than the overall effects of Trrap loss, suggesting that Trrap functions in multiple cellular processes that only partly overlap with those of c-Myc and E2F. Consistent with this may be the observation that Trrap-deficient cells display a more severe phenotype than those of cells lacking either c-Myc and E2F1 alone (24,36–38).
Since TRRAP interacts with many chromatin-remodeling complexes (7–15), loss of Trrap probably affects recruitment of HAT complexes to the promoter of these genes. This alteration would inevitably affect promoter-specific chromatin-modifying activities, resulting in compromised gene expression. Indeed, in mitotic cells, Trrap appears to be involved in specific acetylation of histone H4, consistent with the previous observations that Trrap recruits HAT complex with affinity towards histone H4 (20,21,23). However, in quiescent cells, Trrap may be involved in the regulation of the promoters of differentially expressed genes through modification of both histone H3 and H4 (cyclin A2), or specifically through modification of H3 (thymosin β4). Interestingly, although acetylation of histone H3 at cyclin A2 promoter is decreased in Trrap-deficient cells in quiescent state (Fig. 4B), we observed no apparent changes in cyclin A2 expression (Fig. 3B). This may be due to virtually undetectable levels of cyclin A2 in quiescent cells. Further studies are needed to clarify the lack of association between histone H3/H4 acetylation and gene expression in quiescent state. Nevertheless, our data suggest that Trrap’s involvement in acetylation of histone H3 versus histone H4 may depend on cell cycle stage and promoter context.
In addition to direct involvement of Trrap in gene expression through recruitment of HAT complexes to the gene promoter, it is also possible that the loss of Trrap indirectly affects the expression of certain genes due to the compromised expression of critical players that regulate general transcription. Indeed, our study shows that Trrap regulates expression of genes that are themselves involved in regulation of transcription (e.g. RNA polymerase 1–3 and ets homologous factor) (Tables 1 and 2). Finally, due to the limitations of the present approach, it is not possible to determine whether a specific Trrap-containing HAT complex is responsible for expression regulation of a particular gene or gene family.
Interestingly, many genes (total of 63 known genes) were up-regulated in Trrap-deficient cells, suggesting a negative regulation by Trrap. The mechanism of the up-regulation of certain genes in Trrap-deficient cells is currently unknown. It is possible that loss of Trrap affects the silencing function of certain HAT members, for example some MYST family members (39,40). Alternatively, up-regulation of gene expression in the absence of Trrap may be attributable to the compromised expression of putative repressor genes.
In summary, by combining microarray technology and inducible deletion of the Trrap gene in quiescent and mitotic cells this study represents an initial attempt to identify the multiple pathways regulated by Trrap. Full documentation on genome-wide expression pattern regulated by Trrap would provide information on precise cellular pathways involving Trrap and chromatin remodeling in controlling cell cycle progression, cell proliferation and checkpoints.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Herbert Auer for help with microarray techniques and Nadege Moreno for preparation of RNA probes. We also thank Janet Hall, Sean Tavtigian, and Emmanuel Lazaridis for reading the manuscript and helpful suggestions. Further thanks are due to John Cheney and Trudy Perdrix-Thoma for editing the manuscript. This work was supported in part by the Association pour la Recherche sur le Cancer (l’ARC), France. The research at the IMP was partly supported by the Austrian Research Foundation.
REFERENCES
- 1.Cairns B.R. (2001) Emerging roles for chromatin remodeling in cancer biology. Trends Cell. Biol., 11, S15–S21. [DOI] [PubMed] [Google Scholar]
- 2.Harbour J.W. and Dean,D.C. (2000) The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev., 14, 2393–4209. [DOI] [PubMed] [Google Scholar]
- 3.Hassan A.H., Neely,K.E., Vignali,M., Reese,J.C. and Workman,J.L. (2001) Promoter targeting of chromatin-modifying complexes. Front. Biosci., 6, D1054–D1064. [DOI] [PubMed] [Google Scholar]
- 4.Marmorstein R. (2001) Protein modules that manipulate histone tails for chromatin regulation. Nature Rev., 2, 422–432. [DOI] [PubMed] [Google Scholar]
- 5.Narlikar G.J., Fan,H.Y. and Kingston,R.E. (2002) Cooperation between complexes that regulate chromatin structure and transcription. Cell, 108, 475–487. [DOI] [PubMed] [Google Scholar]
- 6.Peterson C.L. and Workman,J.L. (2000) Promoter targeting and chromatin remodeling by the SWI/SNF complex. Curr. Opin. Genet. Dev., 10, 187–192. [DOI] [PubMed] [Google Scholar]
- 7.Allard S., Utley,R.T., Savard,J., Clarke,A., Grant,P., Brandl,C.J., Pillus,L., Workman,J.L. and Cote,J. (1999) NuA4, an essential transcription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related cofactor Tra1p. EMBO J., 18, 5108–5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brand M., Yamamoto,K., Staub,A. and Tora,L. (1999) Identification of TATA-binding protein-free TAFII-containing complex subunits suggests a role in nucleosome acetylation and signal transduction. J. Biol. Chem., 274, 18285–18289. [DOI] [PubMed] [Google Scholar]
- 9.Brown C.E., Howe,L., Sousa,K., Alley,S.C., Carrozza,M.J., Tan,S. and Workman,J.L. (2001) Recruitment of HAT complexes by direct activator interactions with the ATM-related Tra1 subunit. Science, 292, 2333–2337. [DOI] [PubMed] [Google Scholar]
- 10.Grant P.A., Schieltz,D., Pray-Grant,M.G., Yates,J.R.,III and Workman,J.L. (1998) The ATM-related cofactor Tra1 is a component of the purified SAGA complex. Mol. Cell, 2, 863–867. [DOI] [PubMed] [Google Scholar]
- 11.Ikura T., Ogryzko,V.V., Grigoriev,M., Groisman,R. Wang,J., Horikoshi,M., Scully,R., Qin,J. and Nakatani,Y. (2000) Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell, 102, 463–473. [DOI] [PubMed] [Google Scholar]
- 12.McMahon S.B., Wood,M.A. and Cole,M.D. (2000) The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc. Mol. Cell. Biol., 20, 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pray-Grant M.G., Schieltz,D., McMahon,S.J., Wood,J.M., Kennedy,E.L., Cook,R.G., Workman,J.L., Yates,J.R.,III and Grant,P.A. (2002) The novel SLIK histone acetyltransferase complex functions in the yeast retrograde response pathway. Mol. Cell. Biol., 22, 8774–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saleh A., Schieltz,D., Ting,N., McMahon,S.B., Litchfield,D.W., Yates,J.R.,III, Lees-Miller,S.P, Cole,M.D. and Brandl,C.J. (1998) Tra1p is a component of the yeast Ada.Spt transcriptional regulatory complexes. J. Biol. Chem., 273, 26559–26565. [DOI] [PubMed] [Google Scholar]
- 15.Vassilev A., Yamauchi,J., Kotani,T., Prives,C., Avantaggiati,M.L., Qin,J. and Nakatani,Y. (1998) The 400 kDa subunit of the PCAF histone acetylase complex belongs to the ATM superfamily. Mol. Cell, 2, 869–875. [DOI] [PubMed] [Google Scholar]
- 16.Deleu L., Shellard,S., Alevizopoulos,K., Amati,B. and Land,H. (2001) Recruitment of TRRAP required for oncogenic transformation by E1A. Oncogene, 20, 8270–8275. [DOI] [PubMed] [Google Scholar]
- 17.Dugan K.A., Wood,M.A. and Cole,M.D. (2002) TIP49, but not TRRAP, modulates c-Myc and E2F1 dependent apoptosis. Oncogene, 21, 5835–5843. [DOI] [PubMed] [Google Scholar]
- 18.Kulesza C.A., Van Buskirk,H.A., Cole,M.D., Reese,J.C., Smith,M.M. and Engel,D.A. (2002) Adenovirus E1A requires the yeast SAGA histone acetyltransferase complex and associates with SAGA components Gcn5 and Tra1. Oncogene, 21, 1411–1422. [DOI] [PubMed] [Google Scholar]
- 19.McMahon S.B., Van Buskirk,H.A. Dugan,K.A., Copeland,T.D. and Cole,M.D. (1998) The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell, 94, 363–374. [DOI] [PubMed] [Google Scholar]
- 20.Bouchard C., Dittrich,O., Kiermaier,A., Dohmann,K., Menkel,A., Eilers,M. and Luscher,B. (2001) Regulation of cyclin D2 gene expression by the Myc/Max/Mad network: Myc-dependent TRRAP recruitment and histone acetylation at the cyclin D2 promoter. Genes Dev., 15, 2042–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank S.R., Schroeder,M., Fernandez,P., Taubert,S. and Amati,B. (2001) Binding of c-Myc to chromatin mediates mitogen-induced acetylation of histone H4 and gene activation. Genes Dev., 15, 2069–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ard P.G., Chatterjee,C., Kunjibettu,S., Adside,L.R., Gralinski,L.E. and McMahon,S.B. (2002) Transcriptional regulation of the mdm2 oncogene by p53 requires TRRAP acetyltransferase complexes. Mol. Cell. Biol., 22, 5650–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank S.R., Parisi,T., Taubert,S., Fernandez,P., Fuchs,M., Chan,H.M., Livingston,D.M. and Amati,B. (2003) MYC recruits the TIP60 histone acetyltransferase complex to chromatin. EMBO Rep., 4, 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herceg Z., Hulla,W., Gell,D., Cuenin,C., Lleonart,M., Jackson,S. and Wang,Z.Q. (2001) Disruption of Trrap causes early embryonic lethality and defects in cell cycle progression. Nature Genet., 29, 206–211. [DOI] [PubMed] [Google Scholar]
- 25.Apone L.M., Virbasius,C.M., Reese,J.C. and Green,M.R. (1996) Yeast TAF(II)90 is required for cell-cycle progression through G2/M but not for general transcription activation. Genes Dev., 10, 2368–2380. [DOI] [PubMed] [Google Scholar]
- 26.Choy J.S., Tobe,B.T., Huh,J.H. and Kron,S.J. (2001) Yng2p-dependent NuA4 histone H4 acetylation activity is required for mitotic and meiotic progression. J. Biol. Chem., 276, 43653–43662. [DOI] [PubMed] [Google Scholar]
- 27.Clarke A.S., Lowell,J.E., Jacobson,S.J. and Pillus,L. (1999). Esa1p is an essential histone acetyltransferase required for cell cycle progression. Mol. Cell. Biol., 19, 2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Megee P.C., Morgan,B.A. and Smith,M.M. (1995) Histone H4 and the maintenance of genome integrity. Genes Dev., 9, 1716–1727. [DOI] [PubMed] [Google Scholar]
- 29.Coller H.A., Grandori,C. TamayoP., Colbert,T., Lander,E.S., Eisenman,R.N. and Golub,T.R. (2000) Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling and adhesion. Proc. Natl Acad. Sci. USA, 97, 3260–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Q.M., Malek,R.L., Kim,S., Chiao,C., He,M., Ruffy,M., Sanka,K., Lee,N.H. Dang,C.V. and Liu,E.T. (2000) Identification of c-myc responsive genes using rat cDNA microarray. Cancer Res., 60, 5922–5928. [PubMed] [Google Scholar]
- 31.Ishida S., Huang,E., Zuzan,H., Spang,R., Leone,G., West,M. and Nevins,J.R. (2001) Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol. Cell. Biol., 21, 4684–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukas C., Sorensen,C.S., Kramer,E., Santoni-Rugiu,E., Lindeneg,C., Peters,J.M., Bartek,J. and Lukas,J. (1999) Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature, 401, 815–818. [DOI] [PubMed] [Google Scholar]
- 33.Polager S., Kalma,Y., Berkovich,E. and Ginsberg,D. (2002) E2Fs up-regulate expression of genes involved in DNA replication, DNA repair and mitosis. Oncogene, 21, 437–446. [DOI] [PubMed] [Google Scholar]
- 34.Kalma Y., Marash,L., Lamed,Y. and Ginsberg,D. (2001) Expression analysis using DNA microarrays demonstrates that E2F-1 up-regulates expression of DNA replication genes including replication protein A2. Oncogene, 20, 1379–1387. [DOI] [PubMed] [Google Scholar]
- 35.Nikiforov M.A, Chandriani,S., Park,J., Kotenko,I., Matheos,D., Johnsson,A., McMahon,S.B. and Cole,M.D. (2002) TRRAP-dependent and TRRAP-independent transcriptional activation by Myc family oncoproteins. Mol. Cell. Biol., 22, 5054–5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Field S.J., Tsai,F.Y., Kuo,F., Zubiaga,A.M., Kaelin,W.G.,Jr, Livingston,D.M., Orkin,S.H. and GreenbergM.E. (1996) E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell, 85, 549–561. [DOI] [PubMed] [Google Scholar]
- 37.Mateyak M.K., Obaya,A.J., Adachi,S. and Sedivy,J.M. (1997) Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ., 8, 1039–1048. [PubMed] [Google Scholar]
- 38.Yamasaki L., Jacks,T., Bronson,R., Goillot,E., Harlow,E. and Dyson,N.J. (1996) Tumor induction and tissue atrophy in mice lacking E2F-1. Cell, 85, 537–548. [DOI] [PubMed] [Google Scholar]
- 39.Ehrenhofer-Murray A.E., Rivier,D.H. and Rine,J. (1997) The role of Sas2, an acetyltransferase homologue of Saccharomyces cerevisiae, in silencing and ORC function. Genetics, 145, 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takechi S. and Nakayama,T. (1999) Sas3 is a histone acetyltransferase and requires a zinc finger motif. Biochem. Biophys. Res. Commun., 266, 405–410. [DOI] [PubMed] [Google Scholar]