Abstract
Objective
ADAMTS-7 and ADAMTS-12, two members of the ADAMTS (adisintegrin and metalloprotease with thrombospondin motifs) family, associate with and degrade COMP by binding to the EGF domain of COMP. Granulin-epithelin precursor (GEP) is a secreted growth factor that mediates tissue regeneration, tumorigenesis, and inflammation, and may play an important role in the pathogenesis of arthritis through mechanisms yet unknown. GEP also binds to the EGF domain of COMP. This study is to determine 1) whether there exists a protein interaction network between GEP, ADAMTS-7/-12 and COMP; 2) whether GEP interferes with the interactions between ADAMTS-7/-12 metalloproteinases and COMP substrate, including the cleavage of COMP; 3) whether GEP affects TNFα-mediated induction of ADAMTS-7/-12 expression and COMP degradation; and 4) whether GEP levels are altered during the progression of arthritis.
Methods
Yeast two-hybrid, in vitro GST pull down and co-immunoprecipitation assays were used to 1) examine the interactions between GEP, ADAMTS-7/-12 and COMP, and 2) map the binding sites required for the interactions between GEP and ADAMTS-7/-12; Immunoflouresence cell staining was performed to visualize the sub-cellular localization of GEP and ADAMTS-7/-12; an in vitro digestion assay was employed to determine whether GEP inhibits ADAMTS-7/-12-mediated digestion of COMP; The role of GEP in inhibiting TNFα-induced ADAMTS-7/-12 expression and COMP degradation in cartilage explants was also analyzed.
Results
GEP binds directly to ADAMTS-7 and ADAMTS-12 in vitro and in chondrocytes, and the four C-terminal TSP motifs of ADAMTS-7/-12 and each granulin unit of GEP mediate their interactions. Additionally, GEP co-localizes with ADAMTS-7 and ADAMTS-12 on the cell surface of chondrocytes. More importantly, GEP inhibits COMP degradation by ADAMTS-7/-12 in a dose-dependent manner through the following two mechanisms: a) competitive inhibition through direct protein-protein interactions with ADAMTS-7/-12 and COMP; and b) inhibition of TNFα-induced ADAMTS-7/-12 expression. Furthermore, GEP levels are significantly elevated in patients with either osteroarthritis or rheumatoid arthritis.
Conclusion
Our observations demonstrate a novel protein-protein interaction network between GEP growth factor, ADAMTS-7 and ADAMTS-12 metalloproteinases, and COMP extracellular matrix protein. Furthermore, GEP is a novel specific inhibitor of ADAMTS-7/-12-mediated COMP degradation, and may play a significant role in preventing the destruction of joint cartilage in arthritis.
Introduction
Arthritis is characterized by the breakdown of extracellular matrix components and subsequent loss of articular cartilage. Destruction of articular cartilage extracellular matrix (ECM) in arthritic joints is mediated by excessive proteolytic activity (1). Cartilage consists mainly of ECM with very few cells. The ECM is a network of proteins and macromolecules that provides both strength and nutrients for the cells. Cartilage oligomeric matrix protein (COMP), a prominent non-collagenous component of cartilage, accounts for approximately 1% of the wet weight of articular tissue. COMP is a 524-kDa pentameric, disulfide-bonded, multi-domain glycoprotein composed of approximately equal subunits (~110 kDa each) (2). COMP fragments have been detected in the cartilage, synovial fluid, and serum of patients with knee injuries, osteoarthritis and rheumatoid arthritis (3). In previous studies to identify the physiological enzymes responsible for COMP degradation, we performed a functional genetic screen, which led to the isolation of ADAMTS-7 and -12 as COMP-binding partners (4, 5). Subsequent studies showed that both ADAMTS-7 and ADAMTS-12 were able to digest COMP in vitro and that their levels were significantly elevated in arthritic cartilage and synovium compared to a normal controls (3–5).
ADAMTS-7 and -12 belong to the ADAMTS (adisintegrin and metalloprotease with thrombospondin motifs) metalloproteinase family. The ADAMTS family consists of secreted zinc metalloproteinases with a precisely ordered modular organization that includes at least one thrombospondin (TSP) type I repeat (6). Thus far, nineteen members of this family have been cloned and some of them have been implicated in disease (7). For instance, ADAMTS-13 mutants have a role in thrombotic thrombocytopenic purpura, which is characterized by reduced circulating platelets (8). Mutations in the ADAMTS-2 gene (procollagen I N-proteinase) cause Ehlers-Danlos syndrome Type VII C, a genetic condition characterized by defects in collagen synthesis (9). ADAMTS-2 is also mutated in bovine dermatopraxis (9). A number of ADAMTS members have been implicated in the breakdown of cartilage in osteoarthritis and rheumatoid arthritis, including ADAMTS-4 (aggrecanase 1), ADAMTS-5 (aggrecanase 2) (10), ADAMTS-7 (4) and ADAMTS-12 (5).
Granulin Epithelin Precursor (GEP), also known as PC-cell-derived growth factor (PCDGF), progranulin, acrogranin, or GP80, was first purified as a growth factor from conditioned tissue culture media (11). GEP is a 593-amino-acid secreted glycoprotein with an apparent molecular weight of 80 kDa (12), which acts as an autocrine growth factor. GEP contains seven-and-a-half repeats of a cysteine-rich motif (CX5–6CX5CCX8CCX6CCXDX2HCCPX4CX5–6C) in the order P-G-F-B-A-C-D-E, where A-G are full repeats and P is a half-motif. The C-terminal region contains the conserved sequence CCXDX2HCCP, has a metal binding site, and may be involved in a regulatory capacity. Notably, GEP undergoes proteolytic processing with the liberation of small, 6-kDa repeat units known as granulins (or epithelins), which retain biological activity (13); the peptides are active in cell growth assays and may help to mediate inflammation (14). Increasing evidence has also implicated GEP in the regulation of development, differentiation, and disease. It has been isolated as a differentially expressed gene in mesothelial differentiation (15), sexual differentiation of the brain (16), and macrophage development (17), as well as in rheumatoid arthritis and osteoarthritis (18). Mutations in GEP cause tau-negative frontotemporal dementia linked to chromosome 17 (19, 20). In addition, GEP has been shown to be a crucial mediator of wound response and tissue repair (21, 22).
Several GEP-associated proteins have been reported to affect the action of GEP in various processes (22). Among them, COMP associates with GEP and potentiates GEP-mediated chondrocyte proliferation (23). Recently ADAMTS-7 was also found to interact with GEP and inactivate GEP-mediated chondrogenesis (24). In this study we will determine (1) whether there exists a protein interaction network between GEP, ADAMTS-7/-12 and COMP, (2) whether GEP affects the interaction between ADAMTS-7/-12 and COMP, (3) whether and how GEP inhibits ADAMTS-7/-12-mediated degradation of COMP, and (4) whether GEP levels are altered in arthritis.
Materials and methods
Plasmid constructs
Yeast expression constructs
cDNAs encoding GEP (GenBank accession number NM_002087) and each granulin, was amplified using Pfu turbo DNA (Promega) polymerase with pcDNA3.1-GEP as a template. All the GEP primers are summarized below:
Primers used for amplifying GEP and its fragments.
| Fragment | Primers |
|---|---|
| GEP | Forward: 5′-ACGCGTCGACGATGTGGACCCTGGTGAGCTGGGTGG-3′ |
| Reverse:5′-AATGCGGCCGCTAATCACAGCAGCTGTCTCAAGGCTGG-3′ | |
| P granulin | Forward: 5′-ACGCGTCGACGACGCGGTGCCCAGATGGTCAGTTCTGC-3′ |
| Reverse:5′-AATGCGGCCGCTAAGGGCATCAACCTGGCAGGGGCCAC-3′ | |
| Granulin G | Forward 5′-ACGCGTCGACGTCTGCCGGCCACTCCTGCATC -3′ |
| Reverse 5′-AATGCGGCCGCAAAGCAGGATCGCCCGTCTGCACTG-3′ | |
| Granulin F | Forward 5′-ACGCGTCGACGATCCAGTGCCCTGATAGTCAG-3′ |
| Reverse 5′-AATGCGGCCGCAATGCGCGGGTGTGAACCAGGTC-3′ | |
| Granulin B | Forward 5′-ACGCGTCGACGCTGGCAAAGAAGCTCCCTGCCC-3′ |
| Reverse 5′-AATGCGGCCGCAAAGAGGCACTTACTCTGGATCAG-3′ | |
| Granulin A | Forward 5′-ACGCGTCGACGTGTGACATGGAGGTGAGCTGC -3′ |
| Reverse 5′-AATGCGGCCGCAAACAGGTACCCTTCTGCGTGTCAC-3′ | |
| Granulin C | Forward 5′-ACGCGTCGACGTGTGATAATGTCAGCAGCTGTCCC-3′ |
| Reverse 5′-AATGCGGCCGCAAAGCTTCCTCGCTGACACTGCCCC-3′ | |
| Granulin D | Forward 5′-ACGCGTCGACGTGTGACCAGCACACCAGCTGCCCG-3′ |
| Reverse 5′-AATGCGGCCGCCTCGCAGGATCGAGCCTTCACG -3′ | |
| Granulin E | Forward 5′-ACGCGTCGACTGTGGGGAAGGACACTTCTGC -3′ |
| Reverse 5′-AATGCGGCCGCAAACACTTGGTACCCCTGGCTGC-3′ |
Note that SalI and NotI restriction sites are underlined.
pDBleu and pPC86 (Life Technologies, Gaithersburg, MD) are fusion vectors for the linkage of proteins to the Gal4 DNA binding domain and the VP16 transactivation domain, respectively. cDNA inserts encoding GEP were cloned in-frame into the SalI/NotI sites of pDBleu to generate pDB-GEP. This procedure was repeated for the granulin units. GEP fragments and constructs are shown in Figure 2. The segments encoding series deletion mutants of ADAMTS-7/-12 were cloned in-frame into the SalI/NotI sites of the pPC86 vector to generate the indicated plasmids (4, 5).
Figure 2. Each granulin unit of GEP is sufficient to bind to ADAMTS-7 and ADAMTS-12.
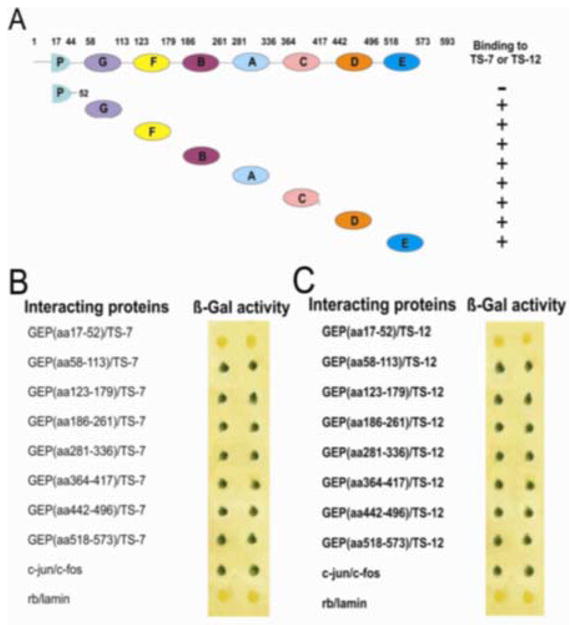
Panel A. Schematic structure of GEP constructs. The numbers refer to amino acid residues. The binding between the designated GEP granulin unit and ADAMTS-7 or ADAMTS-12 is indicated by “+” or “−”. Panel B. Interaction between ADAMTS-7 and various GEP granulin units. Independent yeast colonies were obtained by co-transforming each with two plasmids—one plasmid encoding a GEP granulin unit fused to VP16 and the other encoding ADAMTS-7 fused to Gal4. The transformants were transferred onto a nitrocellulose membrane, and β-galactosidase activity was determined. Due to their known interaction, c-jun and c-fos served as a positive control, whereas unrelated proteins rb and lamin served as a negative control. Results are shown in duplicate. Panel C. Interaction between ADAMTS-12 and various GEP granulin domains. Two plasmids encoding the GEP granulin and ADAMTS-12 fusion proteins were co-transfected into yeast. Protein-protein interactions were determined by assaying β-galactosidase activity.
GST-fusion protein
The bacterial expression vector pGEX-3X (Life Technologies) was used to produce recombinant glutathione S-transferase (GST) fusion proteins in E. coli. cDNA fragments encoding the EGF domain of COMP were inserted in-frame into the BamHI/EcoRI sites of pGEX-3X to generate the plasmid pGEX-EGF (4, 5).
All constructs were verified by nucleic acid sequencing; subsequent analysis was performed using Curatools (Curagen, New Haven, CT) and BLAST software (http://www.ncbi.nlm.nih.gov/BLAST).
Expression and purification of GST and His-tagged proteins
For expression of GST fusion proteins, plasmids pGEX3X and pGEX-EGF were transformed into DH5α E. coli (Life Technologies). Fusion proteins were affinity-purified on GSH-agarose beads, as described previously. His-GEP was purified by affinity chromatography using a HiTrap chelating column (Amersham Biosciences). Briefly, bacteria lysates supplemented with 20 mM HEPES (pH 7.5) and 0.5 M NaCl were applied to a HiTrap chelating column; the column was washed with HSB buffer (40 mM HEPES, pH 7.5, 1 M NaCl, and 0.05% Brij 35) containing 10 mM imidazole. His-GEP was eluted with HSB buffer containing 300 mM imidazole.
Assay of protein-protein interactions using the Y2H system
Two independent yeast colonies were analyzed for the interaction of two proteins, one of which was fused to the Gal4 DNA binding domain and the other to the VP16 transactivation domain. The procedures of Vojtek et al. and Hollenberg et al. were followed for 1) growing and transforming the yeast strain MAV203 with the selected plasmids and 2) detecting β-galactosidase activity and growth phenotypes on selective SD-leu−/trp−/his−/ura− 3AT+ plates (25, 26).
In vitro binding assay
For examination of the binding of ADAMTS-12 to GEP in vitro, Ni 2+-nitrilotriacetic acid-Sepharose beads were pre-incubated with either His or His-tagged GEP and then incubated with purified ADAMTS-12. Bound proteins were resolved by 12% SDS-PAGE and detected by Western blotting with anti-ADAMTS-12 antibodies (5, 27).
To examine whether GEP disrupts the interaction between ADAMTS-7/-12 and COMP, glutathione-Sepharose beads (50 ul) were pre-incubated with either purified GST (0.5 ug, serving as control) or GST-COMP-EGF, and then incubated with recombinant ADAMTS-7 or ADAMTS-12. Various amounts of purified GEP were then added into this system and incubated overnight. Bound proteins were resolved by 12% SDS-PAGE and detected by Western blotting with anti-ADAMTS-7 or anti-ADAMTS-12 antibodies (4, 5, 27).
Co-immunoprecipitation
Approximately 500 ug of cell extracts prepared from isolated human chondrocytes were incubated with anti-GEP (25 ug/ml, acrogranulin, N-19; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-ADAMTS-12 (25 ug/ml) (4, 5, 27), or control rabbit IgG (25 ug/ml) antibodies for 1 h, followed by incubation with 20 ul of protein A-agarose (Invitrogen) at 4°C overnight. After washing five times with immunoprecipitation buffer, bound proteins were released by boiling in 20 ul of 2× SDS loading buffer for 3 min. Released proteins were examined by Western blotting with anti-ADAMTS-12 or anti-GEP antibodies, using the ECL chemiluminescent system (Amersham Biosciences).
Indirect immunoflouresence cell staining
Cultures of human C28/I2 chondrocytes (kindly provided by Dr. Mary B. Goldring) were plated on glass coverslips coated with polylysine and grown in DMEM supplemented with 10% fetal bovine serum (Invitrogen) under an atmosphere of 5% CO2 at 37 °C. After reaching 80% confluence, the cells were fixed with cold acetone/methanol (1:1) for 20 min and washed twice in 4 °C phosphate buffered saline for 5 min. The samples were then air-dried. Following rehydration in phosphate-buffered saline and blocking with 20% goat serum in phosphate buffered saline for 30 min, the cells were incubated with primary antibodies (i.e. rabbit polyclonal anti-ADAMTS-7 or ADAMTS-12 antibodies (4, 5, 27) diluted 1:50 and polyclonal goat anti-GEP antibodies diluted 1:50) (Santa Cruz Biotechnology) at room temperature for 1 h. After being washed with phosphate-buffered saline, the coverslips were incubated with secondary antibodies (i.e. goat anti-rabbit IgG conjugated with fluorescein isothiocyanate diluted 1:100 and chick anti-goat IgG conjugated with rhodamine diluted 1:100) (Santa Cruz Biotechnology) for 1 h. The specimens were observed under a fluorescence microscope with the appropriate optical filters. Microscopic images were captured using the Image Program (Media Cybernetics) and an Olympus microscope.
In vitro digestion assay
Purified human COMP was incubated with recombinant intact ADAMTS-7(4) or ADAMTS-12(5) in a digestion buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM CaCl2, 2 mM ZnCl2, and 0.05% Brij-35, pH 7.5) (4, 5, 27). Various amounts of purified GEP were added to this system and incubated at 37°C for 12 h. The digested products, including intact COMP and COMP fragments, were resolved by 10% non-reducing SDS-PAGE, and released proteins were examined by Western blotting with polyclonal anti-COMP antibodies, using the ECL chemiluminescent system (Amersham Biosciences).
Knock-down of GEP by specific siRNA
The human chondrocyte cell line, C-28/12, was used as a model for analyzing the efficiency of knockdown by the siRNAs and for determining the consequences of knockdown of GEP on COMP degradation by ADAMTS-7 and ADAMTS-12. The cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS). The control vector pSUPER and the plasmid pSUPER-siGEP were co-transfected with the corresponding expression plasmid into C28/I2 cells using LipofectAMINE 2000 reagent (Invitrogen, Rockville, MD) and the levels of GEP was monitored using immumofluorescence cell staining. The data demonstrated that the siRNA 5′-GCCTATCCAAGAACTACAC-3′ was able to efficiently reduce the expression of human GEP. The C-28/12 cells were then transfected with ADAMTS-7, ADAMTS-12 expression plasmid, without or with pSUPER-siGEP, and cultured for 3 days, the media were collected and assayed by Western blotting with anti-COMP antibody.
Cartilage Explants
Human cartilage was cultured as described previously (27, 28) with approval of Institutional Review Boards (IRB# 12758). Briefly, human knee cartilage was dissected into fragments approximately 4 mm in diameter by punches 1- to 2-mm in thickness. The cartilage was dispensed into tissue-culture flasks (0.7 g/flask) and incubated overnight in control, serum-free medium Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) containing 25 mM HEPES, 2 mM glutamine, 100 μg/ml streptomycin, 100 IU/ml penicillin, 2.5 μg/ml gentamicin, and 40 units/ml nystatin. Fresh control medium (10 ml) with TNF-α (5 ng/ml) (in triplicate for statistical analysis) was then added (day 0). At day 2, the supernatants were harvested for COMP degradation analysis by Western blotting and RNA was extracted from the cartilage samples, as described below. In some cultures, antibodies against ADAMTS-7 and/or ADAMTS-12 (5 μg/ml of anti-ADAMTS-7 and/or ADAMTS-12 rabbit polyclonal antibodies) (4, 5) or 100 ng/ml of recombinant GEP, were added. At day 7, culture supernatants were harvested and COMP degradative fragments were resolved using Western blotting.
Histology and Immunohistochemistry
The articular cartilage from OA, RA and normal controls was harvested and fixed in 4% PBS buffered PFA at 4°C overnight, and then decalcified in 10% EDTA for 4 weeks. After the tissue was dehydrated and embedded in paraffin, 6 μm sections were cut. Sections were stained with Safranin O- fast green to assess the presence of proteoglycan.
In the case of Immunohistochemistry assay, tissue sections were deparaffinized by immersing into xylene, and rehydrated by graded ethanol, then treated with 0.1% trypsin for 30 min at 37°C. After blocking in 20% goat serum for 60 min at room temperature, sections were incubated with either anti-ADAMTS7 polyclonal antibody (4) (1:200 dilution), anti-ADAMTS-12 polyclonal antibody (5) (1:200 dilution), rabbit anti-COMP polyclonal antibody (5) (1:100 dilution), or anti-GEP polyclonal antibody (23) (1:100 dilution; Santa Cruz Biotechnology Inc.) at 4°C overnight and then a HRP-conjugated secondary antibody for 60 min at room temperature. The signal was detected by Vector Elite ABC Kit (Vectorstain Vector Lab, Inc.).
Statistical Test
Two-sampleStudent’s t-test was used to determinesignificant differences (p < 0.001) of the level of GEP between normal and arthritic cartilage.
Results
Interaction between GEP and ADAMTS-12 in yeast
A previous study revealed an association between ADAMTS-7 and GEP (24). Since ADAMTS-7 and 12 are members of the same family and share a similar domain organization and structure (29, 30), we first used a yeast-2-hybrid assay to determine whether GEP also binds to ADAMTS-12. Briefly, a plasmid encoding GEP-Gal4DBD fusion protein and a plasmid encoding ADAMTS-12-VP16AD fusion protein were co-transformed into the yeast strain MAV203. Plasmid pairs encoding c-jun/c-fos and rb/lamin fusion proteins were used as positive and negative controls, respectively. The assay revealed a positive interaction between GEP and ADAMTS-12, as verified by positive β-galactosidase activity as well as a positive growth phenotype on plates lacking histidine and uracil and containing 3AT (Figure 1A).
Figure 1. GEP associates with ADAMTS-12.
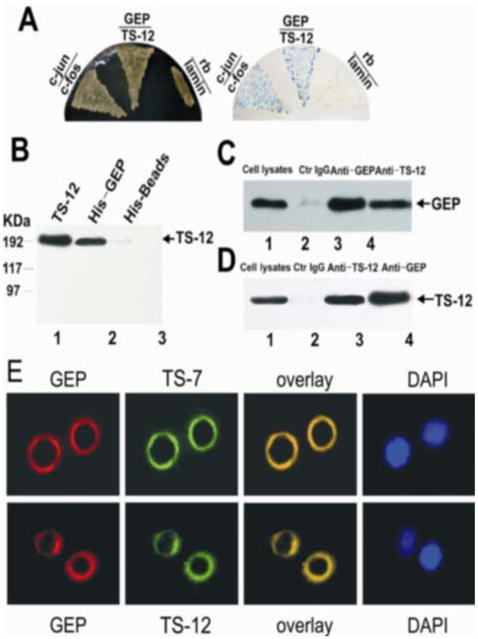
Panel A. GEP binds ADAMTS-12 in a yeast-2-hybrid assay. pPC86-ADAMTS-12 encoding ADAMTS-12 fused to VP16 (activation domain) in pPC86 and pDB-GEP encoding GEP fused to Gal4 (DNA-binding domain) in pDBleu, were co-transfected into yeast strain MAV203 and selected on SD-leu−/trp−/his−/ura−/3AT+ plates (left) and observed for β-galactosidase activity (right). c-jun and c-fos served as a positive control, whereas rb and lamin served as a negative control. Panel B. GEP directly binds to ADAMTS-12. His-GEP fusion protein was immobilized on His beads, incubated with recombinant ADAMTS-12, and resolved by immunoblotting with anti-ADAMTS-12 antibodies. ADAMTS-12 (lane 1) and His beads (lane 3) were used as positive and negative controls, respectively. Panel C-D. Co-immunoprecipitation of ADAMTS-12 and GEP from chondrocytes. Human chondrocyte extracts were incubated with control IgG, anti-GEP, or anti-ADAMTS-12 antibodies. Following precipitation, proteins were resolved by SDS-PAGE and detected with anti-GEP (C) or anti-ADAMTS-12 (D) antibodies. Cell lysates (lane 1) served as positive control. (E) GEP co-localizes with ADAMTS-7 and ADAMTS-12 on the cell surface of chondrocytes. C28/I2 human chondrocytes were stained with anti-GEP, anti-ADAMTS-7, or anti-ADAMTS-12 antibodies. The nuclei were stained with DAPI.
Direct binding of GEP to ADAMTS-12 in vitro and in vivo
To further verify the interaction between GEP and ADAMTS-12, an in vitro binding assay was performed (Figure 1B). His-GEP conjugated to beads, but not His alone, was able to pulldown ADAMTS-12. Since only purified protein was used in these assays, the interaction between GEP and ADAMTS-12 is direct.
Previous studies involving co-immunoprecipitation assays have demonstrated that ADAMTS-7 binds to GEP in native human chondrocytes (24). We sought to determine whether this is also true for ADAMTS-12. Briefly, human chondrocyte extracts were incubated with anti-GEP antibody, anti-ADAMTS-12 antibody, or IgG (control). The immunoprecipitated complexes were subjected to reducing SDS-PAGE and detected using anti-GEP antibodies (Figure 1C) or anti-ADAMTS-12 antibodies (Figure 1D). GEP and ADAMTS-12 were detected in complexes immunoprecipitated by either anti-ADAMTS-12 or anti-GEP antibodies, but not control IgG, thus demonstrating that ADAMTS-12 binds to GEP in chondrocytes.
GEP co-localizes with ADAMTS-7 and ADAMTS-12 in the pericellular matrix of chondrocytes
Given that both ADAMTS-7 and ADAMTS-12 interact with GEP, we sought to verify whether all three proteins are co-localized within the same sub-cellular compartment. Indirect immunoflouresence cell staining of primary human chondrocytes revealed that both ADAMTS-7 and 12 are co-localized with GEP in the pericellular matrix of isolated adult human chondrocytes (Figure 1E). These findings are consistent with a specific binding interaction between ADAMTS-7 (24), ADAMTS-12 and GEP.
Each granulin repeat unit is sufficient for binding to ADAMTS-7 and ADAMTS-12
GEP undergoes proteolytic processing with the liberation of ~6 kDa repeating units known as granulins, which retain at least some of the biologic activity of full-length GEP (13). These peptides are active in cell growth assays and may be mediators of inflammation (14, 31). Since both ADAMTS-7 and 12 can bind to full-length GEP, we sought to determine whether this interaction also holds true for each repeat granulin using a filter-based β-galactosidase assay. Briefly, a plasmid encoding a partial or single granulin unit fused to Gal4DBD was co-transfected into yeast along with a plasmid encoding either ADAMTS-7-VP16AD (Figure 2B) or ADAMTS-12-VP16AD (Figure 2C) fusion protein. The associations between ADAMTS-7/-12 and various granulin units are summarized in Figure 2A. Our conclusion from this set of assays is that individual granulin units, such as granulin A, granulin B, granulin C, granulin D, granulin E, granulin F, and granulin G, but not partial granulin (P), are each sufficient to bind to ADAMTS-7 and ADAMTS-12.
Four C-terminal TSP motifs of ADAMTS-7 or ADAMTS-12 are necessary and sufficient for association with GEP
To identify the specific motif(s) in ADAMTS-7 and ADAMTS-12 that are responsible for GEP binding, we generated multiple deletion constructs, each expressing a unique ADAMTS-7 (Figure 3A) or ADAMTS-12 (Figure 3C) deletion mutant. Each plasmid encoded a fragment of ADAMTS-7 or ADAMTS-12 fused to VP16AD. Each plasmid was then co-transfected with GEP-Gal4DBD into yeast; positive protein-protein interactions were indicated by the presence of β-galactosidase activity. For ADAMTS-7, only constructs containing the four-C-terminal TSP repeats resulted in a positive interaction with GEP (Figure 3B). Interestingly, constructs containing only two of these C-terminal TSP repeats failed to interact with GEP. GEP interaction was still preserved in the construct containing no other domain except for the four TSP repeats. Similar findings were observed for ADAMTS-12 (Figure 3D). Taken together, these data suggest that the four C-terminal TSP repeats of ADAMTS-7 and 12 are both necessary and sufficient for their interaction with GEP.
Figure 3. GEP selectively binds to four C-terminal TSP motifs of ADAMTS-7/12.
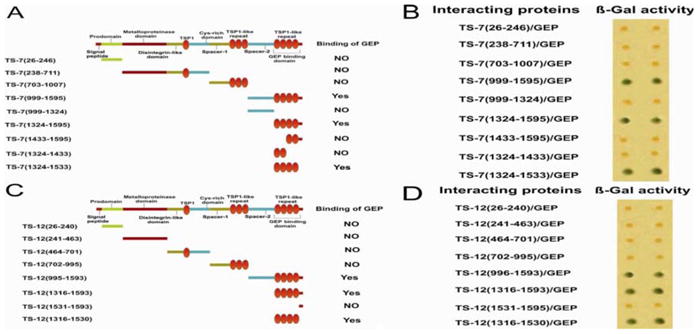
Panel A. Schematic of ADAMTS-7 constructs. Each label (left column) corresponds to a segment of ADAMTS-7—the numbers refer to amino acid residues. The binding between each ADAMTS-7 segment and GEP is indicated. Panel B. Interaction between GEP and ADAMTS-7 domains. pPC86-ADAMTS-7 and pDB-GEP were co-transformed into yeast and β-galactosidase activity was determined (see Methods and Materials). Results are shown in duplicate. Panel C. Schematic structure of ADAMTS-12 constructs. Each label (left column) corresponds to a segment of ADAMTS-7—the numbers refer to amino acid residues. Panel D. Interaction between GEP and ADAMTS-12 domains. pPC86-ADAMTS-12 and pDB-GEP were co-transformed into yeast and β-galactosidase activity was determined (see Methods and Materials). Results are shown in duplicate.
GEP disrupts the interaction between ADAMTS-7/-12 and COMP
Previous reports have demonstrated that the four C-terminal motifs of ADAMTS-7 and ADAMTS-12 are also responsible for their interactions with COMP (4, 5). In addition, both GEP and ADAMTS-7/-12 bind to COMP via the same EGF domain of COMP (4, 5, 23). These findings led us to investigate whether GEP interferes with the ability of ADAMTS-7 to bind and digest COMP. For this purpose, a GST pull-down assay was performed. In agreement with our previous report, GST fused to the EGF domain of COMP (GST-EGF), but not GST alone, efficiently pulled down ADAMTS-7 (lanes 1–2 in Figure 4A). However, the addition of GEP resulted in the dose-dependent inhibition of this interaction (Figure 4A). Similar inhibition by GEP was also observed for ADAMTS-12 and GST-EGF (Figure 4C). Taken together, these data indicate that GEP is able to disturb the interactions between ADAMTS-7/-12 and COMP in a dose dependent manner.
Figure 4. GEP disrupts the interaction between COMP and ADAMTS-7/-12.
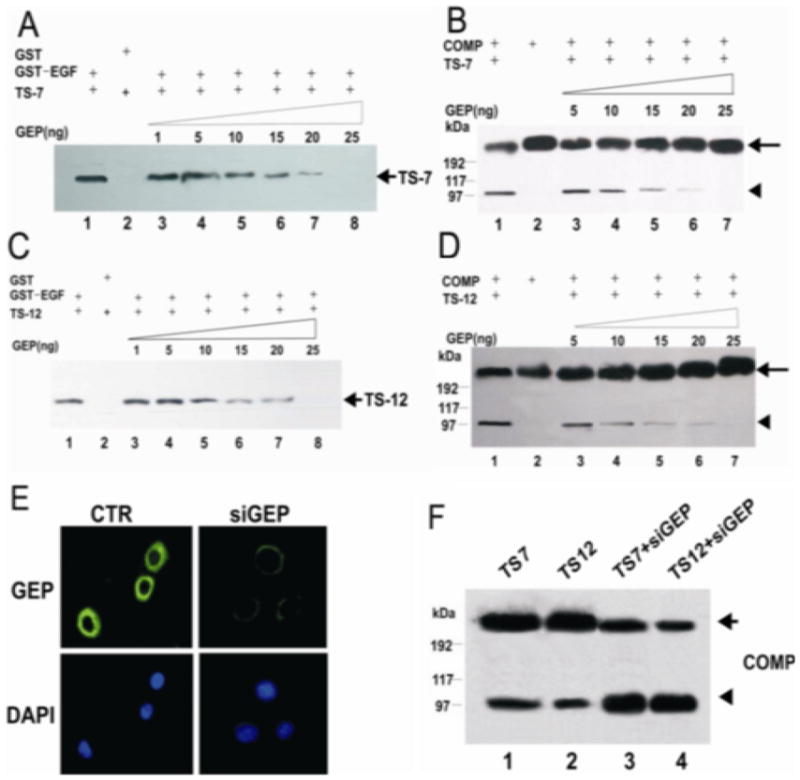
Panel A&C. GEP prevents ADAMTS-7/-12 from binding COMP. GST fused EGF-domain of COMP was immobilized on glutathione-Sepharose beads, and incubated with ADAMTS-7 (panel A) or ADAMTS-12 (panel C) in the presence of increasing amounts of GEP, as indicated. Bound proteins were examined by immunoblotting with anti-ADAMTS-7 (panel A) or anti-ADAMTS-12 (panel C) antibodies. Panel B&D. GEP protects COMP from degradation by ADAMTS-7/-12. COMP was incubated with ADAMTS-7 (panel B) or ADAMTS-12 (panel D), along with various amounts of GEP, as indicated. COMP cleavage products were resolved using non-reducing SDS-PAGE and anti-COMP antiserum. Intact COMP protein is denoted by arrows, while COMP fragments are denoted by arrowheads. Panel E. siRNAs repress GEP expression. Immortalized human chondrocytes, C-28/I2, transfected with either pSUPER (CTR), or pSUPER-GEP siRNA (siGEP) were stained with anti-GEP. The nuclei were stained with DAPI. Panel F. Knockdown of GEP increases COMP degradation. C-28/12 cells were transfected with a plasmid encoding ADAMTS-7 or ADAMTS-12, and pSuper-GEP siRNA (siGEP), as indicated. After 3 days, the medium was analyzed by reducing SDS-PAGE using an anti-COMP antibody.
GEP acts as a specific inhibitor of COMP degradation by ADAMTS-7 and ADAMTS-12
Joint destructive processes, such as OA and RA, are associated with the breakdown of cartilage extracellular matrix proteins, such as COMP, a process that may be mediated by matrix metalloproteinases, such as ADAMTS-7 and 12. The finding that GEP is able to disrupt the interaction between ADAMTS-7/-12 and COMP suggests that GEP may protect COMP degradation by these metalloproteinases. GEP granulin units are already known to function as inhibitors of degradative enzymes, such as thrombin (22, 32). In order to determine whether GEP protects COMP from degradation by ADAMTS-7, we employed an in vitro digestion assay (Figure 4B). Purified human COMP was incubated with purified ADAMTS-7 in a specialized buffer (see Methods and Materials). In the absence of GEP, ADAMTS-7 successfully digested COMP into smaller fragments (lane 1 in Figure 4B). However, the addition of purified GEP inhibited this degradation in a dose-dependent manner, resulting in lower levels of COMP fragments and higher levels of intact COMP protein (Figure 4B). Similar results were observed for ADAMTS-12 (Figure 4D). Taken together, these data indicate that GEP is able to protect COMP from digestion by ADAMTS-7 and 12 metalloproteinases.
To further verify the importance of GEP in inhibiting the degradation of COMP by ADAMTS-7 and ADAMTS-12, we sought to suppress GEP gene expression in human chondrocytes using the siRNA approach. We identified 19-nucleotide gene-specific sequences for GEP, and then generated pSUPER-siGEP construct encoding siRNA targeting this specific gene sequence. Immunofluorescent cell staining with human C-28/I2 chondrocytes transfected with pSUPER-siGEP or pSUPERvector demonstrated that expression of the specific siRNA efficiently reduced the level of endogenous GEP protein (Figure 4E). Next we examined whether the siRNA knockdown of GEP would affect COMP degradation. The C-28/I2 chondrocytes were transfected with ADAMTS-7, ADAMTS-12 expression plasmid, without or withpSUPER-siGEP for 3 days. Western blotting with anti-COMP antibody (Figure 4F) showed the intensity of the COMP fragment was increased in the media collected from the cells transfected with pSUPER-siGEP. These results further indicated that GEP was an inhibitor of COMP degradation.
GEP inhibits TNFα-induced ADAMTS-7/-12 expression
TNFα is the central proinflammatory cytokine that plays a cardinal role in joint destructive processes such as OA and especially RA. Previous studies have found that TNFα upregulates the expression of cartilage matrix degrading enzymes, such as ADAMTS-7 and ADAMTS-12 (27). We sought to examine whether GEP inhibits the TNFα-mediated induction of ADAMTS-7/-12 expression. To test this, human cartilage explants were cultured in the presence of TNFα and varying amounts of GEP for 2 days in serum-free medium. Real-time PCR was then used to analyze ADAMTS-7 and 12 expression levels. The results show that the presence of GEP led to a significant decrease in TNFα-induced ADAMTS-7 and ADAMTS-12 expression (Figure 5A, 5B). In the presence of 100 ng/ml of GEP, ADAMTS-7 and 12 expression levels returned to near baseline.
Figure 5. GEP inhibits TNFα-induced ADAMTS-7/-12 expression and COMP degradation.
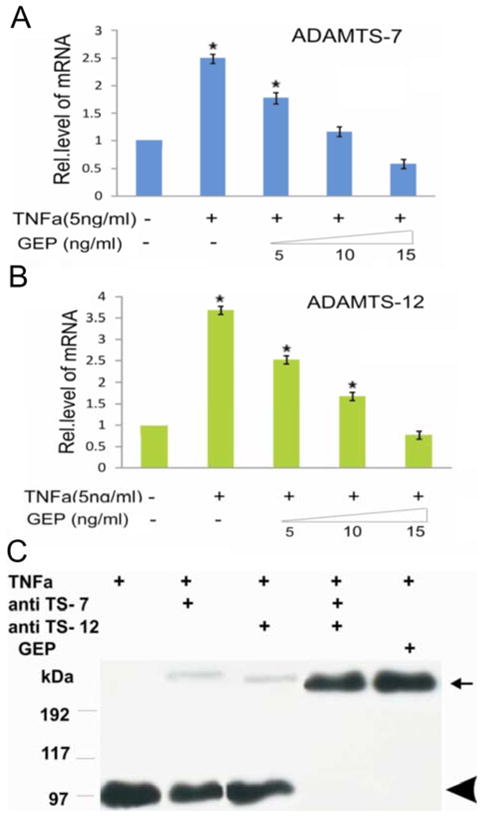
Panel A & B. GEP inhibits TNFα-induced ADAMTS-7 and ADAMTS-12 mRNA expression. Human cartilage explants were cultured in the presence of 5 ng/ml of TNFα and various amounts of GEP for 2 days. ADAMTS-7 and ADAMTS-12 mRNA levels were determined by real-time PCR. The units are arbitrary and the leftmost bar in each group indicates a relative level of 1. ***, p<0.05 vs. untreated controls. Panel C. GEP inhibits TNFα-mediated COMP degradation. OA cartilage explants were cultured in the presence of 5 ng/ml of TNFα with either anti-ADAMTS-7/-12 blocking antibodies or 100 ng/ml recombinant GEP, as indicated, for 7 days. The media were analyzed with non-reducing SDS-PAGE and anti-COMP antibodies. Intact COMP and its degradative fragments are indicated by arrows and arrowheads respectively.
We then sought to determine whether GEP inhibits TNFα-induced COMP degradation in cartilage. To answer this, we cultured human cartilage explants for 7 days with TNFα in the presence of anti-ADAMTS-7 blocking antibodies, anti-ADAMTS-12 blocking antibodies, or recombinant GEP. COMP degradation was visualized with Western blotting using anti-COMP antibodies. In accordance with a previous report (27), TNFα treatment resulted in COMP degradation (lane 1 in Figure 5C), while anti-ADAMTS-7 and anti-ADAMTS-12 blocking antibodies inhibited COMP degradation (27), suggesting that both ADAMTS-7 and ADAMTS-12 are important for TNFα-mediated COMP degradation. Interestingly, the addition of recombinant GEP resulted in the complete inhibition of TNFα-induced COMP degradation in cartilage.
GEP levels are significantly elevated in diseased cartilage
Although the data from our study seem to suggest that GEP protects COMP from digestion by ADAMTS-7 and 12, it is unclear whether this plays a role in human disease. To examine this question, we performed real-time PCR using articular cartilage extracts from human subjects. Samples of healthy adult articular cartilage were obtained from the knees of four deceased individuals who had suffered from diseases unrelated to arthritis (from the Musculoskeletal Transplant Foundation). Arthritic cartilage was obtained from 12 patients undergoing elective total knee arthroplasty for end-stage arthritis; OA articular cartilage (Kellgren-Lawrence Grade 3 or 4) was obtained from the distal femora of 8 patients and RA cartilage (American College of Rheumatology Stage III and IV disease) was obtained from the knees of 4 patients who fulfilled the revised criteria of the American College of Rheumatology for the diagnosis of RA (33). GEP mRNA was significantly upregulated in both OA and RA cartilage (p <.001) (Figure 6A).
Figure 6. Increased expression of GEP in arthritic cartilage.
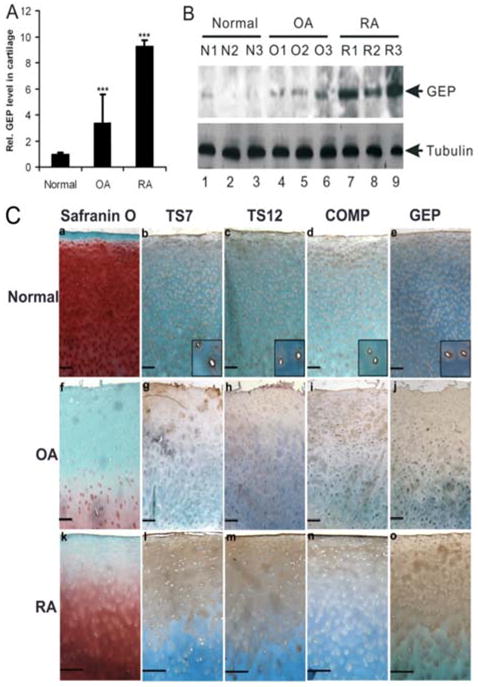
Panel A. Expression of GEP mRNA in normal, OA, and RA cartilage, assayed by real-time PCR. RNA was extracted from knee cartilage of 4 patients without arthritis, 8 patients with OA, and 4 patients with RA. The units are arbitrary and the relative unit of GEP expression for non-arthritic cartilage was set to 1. ***, p<001. Panel B. Expression of GEP protein in normal, OA and RA cartilage, assayed by Western Blotting. Total cartilage extracts from 3 normal, 3 OA, and 3 RA patients were resolved using 10% SDS-PAGE and probed with anti-GEP and anti-tubulin (internal control) antibodies. Panel C. Histological and immunostaining assays for ADAMTS-7 (TS7), ADAMTS-12 (TS12), COMP and GEP of normal, OA, and RA cartilage. The cartilage specimens were assayed using Safranin O-fast green staining (a, f, k), immunohistochemistry for TS7 (b, g, l), TS12 (c, h, m), COMP (d, i, n) or GEP (e, j, o). The inserts show higher magnification views of chondrocytes. Bar=100μm.
To assess GEP protein expression in OA and RA cartilage, we next performed western blot analysis. Total cartilage extracts from 3 normal, 3 OA, and 3 RA patients were resolved using 10% SDS-PAGE and probed with anti-GEP and anti-tubulin (internal control) antibodies (Figure 6B). Consistent with the expression pattern of GEP mRNA, cartilage from arthritis patients (especially RA patients) contained elevated GEP levels as compared to normal cartilage.
To further verify these findings, we then performed histological and immunohistochemical assays (Figure 6C). Safranin O-Fast green staining showed that proteoglycan was greatly reduced in OA and moderately reduced in RA, loss of articular cartilage layers and surface fibrillation can be seen in OA cartilage (a,f,k). We further examined the expression of ADAMTS7, ADAMTS-12, COMP and GEP using immunohistochemistry. These four molecules were positive in the pericellular matrices of all zones in normal cartilage (b, c, d, and e), and colocalized on the surface of articular chondrocytes (see inserts in the b, c, d and e); however, their localizations were mainly in the territorial and interteritorial matrices of superficial zone of the diseased cartilage. In addition, the levels of ADAMTS-7, ADAMTS-12 and GEP were moderately increased in OA cartilage (g, h, i and j), and markedly increase in RA cartilage (l, m, n and o).
Discussion
Arthritis is a disease characterized by the proteolytic degradation of cartilage extracellular matrix components with the subsequent loss of articular cartilage and bone. This process is regulated by a host of cytokines and inflammatory mediators. Our study involves one component of cartilage extracellular matrix, namely COMP. Although the function of COMP is not completely understood, it appears to mediate chondrocyte attachment as well as stabilize the extracellular matrix of articular cartilage by interacting with matrix components, such as type II collagen, type IX collagen, aggrecan, and fibronectin (34, 35). Fragments of COMP, which are thought to result from the degradative action of proteolytic enzymes, have been detected in the diseased cartilage, synovial fluid, and serum of patients with post-traumatic knee injuries, primary OA, and RA, suggesting that COMP degradation may play a key role in human disease (3).
Given the potential importance of COMP degradation, attention has been turned to studying the enzymes that interact with COMP. Purified COMP is digested by several matrix metalloproteinases (MMPs) in vitro, including MMP-1, MMP-3, MMP-9, MMP-13, MMP-19 and MMP-20 (36). ADAMTS-4, ADAMTS-7 and ADAMTS-12 are members of the ADAMTS metalloproteinase family, and are able to digest COMP (4, 5, 37). Both ADAMTS-7 and ADAMTS-12 are elevated in arthritic cartilage (4, 5). Furthermore, the expression of both ADAMTS-7 and 12 is induced by the presence of inflammatory cytokines, such as TNFα (27). Concurrently, TNFα is also able to induce COMP degradation, suggesting that the TNF-induced digestion of COMP may be mediated, at least in part, by these two metalloproteinases (27). Our finding that anti-ADAMTS-7 and anti-ADAMTS-12 blocking antibodies inhibit TNFα-induced COMP degradation (Figure 5C) (27) reveals that this is indeed the case. More interestingly, we observed that GEP inhibits the TNFα-induced expression of both ADAMTS-7 and 12, and in doing so, GEP was able to prevent COMP degradation. This supports the notion that GEP may act to protect cartilage from the destructive actions of ADAMTS-7 and 12. Note that data were generated based on human articular cartilage tissues at the end stage of the disease, whether these results may represent and interpret the situation of the early stages of arthritis, particularly OA, remain to be delineated. A key observation from our data is that the fragments resulting from in vitro ADAMTS-7/-12-mediated COMP degradation are similar in size to the fragments observed in osteoarthritis as well as to the fragments observed in our cell-based assays (27). This serves as a point of correlation for our in vitro and in vivo data, and suggests that our observations regarding ADAMTS-7/-12-mediated COMP degradation may certainly be relevant to the pathogenesis of arthritis.
GEP is a secreted glycoprotein, which acts as an autocrine growth factor and is highly expressed in chondrocytes (23). GEP undergoes proteolytic processing with the liberation of ~6 kDa repeating units known as granulins, which retain at least some of the biologic activity of full-length GEP (13). GEP is involved in various biological and pathological processes, including rheumatoid arthritis and osteoarthritis (18), macrophage development (17), mesothelial differentiation (15), wound healing and tissue repair (21, 22), and cancer progression (13, 38). GEP is a potent stimulator of chondrocyte proliferation, a property that requires the binding of COMP (23). Our data demonstrate that the expression of GEP is significantly increased in osteoarthritic cartilage. Additionally, GEP levels are even higher in rheumatoid arthritis (Figure 6). This strongly suggests that GEP may play a role in the inflammatory component of arthritis pathogenesis, and also supports the concept that arthritic chondrocytes may exhibit increased anabolic activity, including the release of growth factors (39, 40). Although expression levels of ADAMTS-7, ADAMTS-12 and GEP are all increased in arthritis (Figure 6C), it may be that elevated GEP levels are not sufficient to completely neutralize the presence of increased ADAMTS enzymes, which in turn lead to COMP degradation.
In this study we demonstrate that both ADAMTS-7 and 12 interact with GEP and its repeat granulin units, as evidenced by data from yeast-2-hybrid, co-immunoprecipiation, and in vitro binding assays (Figure 1, 2). This interaction occurs via the four C-terminal TSP motifs of ADAMTS-7 and ADAMTS-12 (Figure 3). ADAMTS-4 and ADAMTS-5 lacking this domain fails to interact with GEP in a yeast two-hybrid assay (not shown). We also found that GEP is able to disturb the binding of ADAMTS-7/-12 to COMP, and thus inhibits the digestion of COMP by ADAMTS-7/- 12. ADAMTS-7, ADAMTS-12, and GEP all bind to the same EGF-domain of COMP (4, 5, 23), and both GEP and COMP bind to the four C-terminal TSP motifs of ADAMTS-7/-12 (Figure 3) (4, 5), suggesting that GEP competitively inhibits the binding of ADAMTS-7/-12 to COMP. In addition, GEP, ADAMTS-7, ADAMTS-12, and COMP co-localize in the pericellular matrices in human normal articular cartilage (Figure 6C). Collectively, these four molecules are intertwined in a protein-protein interaction network that could play a key role in the pathogenesis of arthritis.
Every granulin unit of GEP is able to efficiently bind to ADAMTS-7 and ADAMTS-12 (Figure 2). This is of particular significance since GEP granulins can retain biological activity, are active in cell growth assays, and may play an active role in inflammation (13). GEP is composed of seven (A-G) and a half (p) tandem repeats of a granulin/epithelin motif containing twelve cysteines (41). In many cases, mammalian GEP is secreted as an intact mitogenic protein, with the component granulins released after proteolytic processing (42). Granulin domains A, B, C and D were isolated from human inflammatory cells (43). The granulin/epithelin family displays a complex interplay of agonistic and antagonistic effects on mammalian cell growth in vitro. In some systems, the granulin peptides stimulate cell proliferation but in other systems, they act as inhibitors of mitosis (44). At present, only granulin A/epithelin 1 and granulin B/epithelin 2 have displayed biological activity. The actions of the other granulins (C, D, E, F, and G) are unknown.
In summary, we have established that GEP binds to both ADAMTS-7 and ADAMTS-12 in cartilage. Subsequent characterization of this novel association shows that GEP regulates ADAMTS-7 and ADAMTS-12 at the following two levels: a) GEP inhibits TNFα-induced ADAMTS-7 and ADAMTS-12 expression, and b) GEP disrupts the binding and cleavage of COMP by ADAMTS-7 and ADAMTS-12 via direct protein-protein interactions. These findings will not only lead to a better understanding of the actions of metalloproteinases, growth factors, and matrix proteins in cartilage, but may provide us with promising therapeutic targets, including GEP or its derivatives, for preventing and treating diseases of cartilage destruction.
Acknowledgments
We thank Dr. Mary B. Goldring for providing human C28/I2 chondrocytes. This work was aided by NIH research grants AR050620, AR053210, AG029388 and a grant from Arthritis National Research Foundation.
Abbreviations
- MMP
matrix metalloproteinase
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin type 1 motifs; PC-cell-derived growth factor (PCDGF)
- COMP
cartilage oligomeric matrix protein
- GEP
granulin-epithelin precursor
- OA
osteoarthritis
- RA
rheumatoid arthritis
- IGF
insulin-like growth factor
- FGF
fibroblast growth factor, TGF-β, transforming growth factor-β
- TNFα
Tumor Necrosis Factor α
- Il-1β
interleukin-1β
- SLPI
secretory leukocyte protease inhibitor
- IGF-IR
insulin-like growth factor receptor
- PCR
polymerase chain reaction
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
- 1.Salzet M. Leech thrombin inhibitors. Curr Pharm Des. 2002;8(7):493–503. doi: 10.2174/1381612023395664. [DOI] [PubMed] [Google Scholar]
- 2.Morgelin M, Engel J, Heinegard D, Paulsson M. Proteoglycans from the swarm rat chondrosarcoma. Structure of the aggregates extracted with associative and dissociative solvents as revealed by electron microscopy. J Biol Chem. 1992;267(20):14275–84. [PubMed] [Google Scholar]
- 3.Neidhart M, Hauser N, Paulsson M, DiCesare PE, Michel BA, Hauselmann HJ. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br J Rheumatol. 1997;36(11):1151–60. doi: 10.1093/rheumatology/36.11.1151. [DOI] [PubMed] [Google Scholar]
- 4.Liu CJ, Kong W, Ilalov K, Yu S, Xu K, Prazak L, et al. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. FASEB J. 2006;20(7):988–90. doi: 10.1096/fj.05-3877fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu CJ, Kong W, Xu K, Luan Y, Ilalov K, Sehgal B, et al. ADAMTS-12 associates with and degrades cartilage oligomeric matrix protein. J Biol Chem. 2006;281(23):15800–8. doi: 10.1074/jbc.M513433200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int J Biochem Cell Biol. 2004;36(6):981–5. doi: 10.1016/j.biocel.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386(Pt 1):15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413(6855):488–94. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 9.Colige A, Sieron AL, Li SW, Schwarze U, Petty E, Wertelecki W, et al. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet. 1999;65(2):308–17. doi: 10.1086/302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malfait AM, Liu RQ, Ijiri K, Komiya S, Tortorella MD. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002;277(25):22201–8. doi: 10.1074/jbc.M200431200. [DOI] [PubMed] [Google Scholar]
- 11.Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56(4):607–17. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 12.Anakwe OO, Gerton GL. Acrosome biogenesis begins during meiosis: evidence from the synthesis and distribution of an acrosomal glycoprotein, acrogranin, during guinea pig spermatogenesis. Biol Reprod. 1990;42(2):317–28. doi: 10.1095/biolreprod42.2.317. [DOI] [PubMed] [Google Scholar]
- 13.Davidson B, Alejandro E, Florenes VA, Goderstad JM, Risberg B, Kristensen GB, et al. Granulin-epithelin precursor is a novel prognostic marker in epithelial ovarian carcinoma. Cancer. 2004;100(10):2139–47. doi: 10.1002/cncr.20219. [DOI] [PubMed] [Google Scholar]
- 14.Lu R, Serrero G. Inhibition of PC cell-derived growth factor (PCDGF, epithelin/granulin precursor) expression by antisense PCDGF cDNA transfection inhibits tumorigenicity of the human breast carcinoma cell line MDA-MB-468. Proc Natl Acad Sci U S A. 2000;97(8):3993–8. doi: 10.1073/pnas.97.8.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X, Gulyas M, Hjerpe A. Mesothelial differentiation as reflected by differential gene expression. Am J Respir Cell Mol Biol. 2004;30(4):510–8. doi: 10.1165/rcmb.2003-0266OC. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Nishiahara M. Granulin precursor gene: a sex steroid-inducible gene involved in sexual differentiation of the rat brain. Mol Genet Metab. 2002;75(1):31–7. doi: 10.1006/mgme.2001.3274. [DOI] [PubMed] [Google Scholar]
- 17.Barreda DR, Hanington PC, Walsh CK, Wong P, Belosevic M. Differentially expressed genes that encode potential markers of goldfish macrophage development in vitro. Dev Comp Immunol. 2004;28(7–8):727–46. doi: 10.1016/j.dci.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Justen HP, Grunewald E, Totzke G, Gouni-Berthold I, Sachinidis A, Wessinghage D, et al. Differential gene expression in synovium of rheumatoid arthritis and osteoarthritis. Mol Cell Biol Res Commun. 2000;3(3):165–72. doi: 10.1006/mcbr.2000.0211. [DOI] [PubMed] [Google Scholar]
- 19.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 20.Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442(7105):920–4. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 21.He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nat Med. 2003;9(2):225–9. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Nathan C, Jin W, Sim D, Ashcroft GS, Wahl SM, et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111(6):867–78. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- 23.Xu K, Zhang Y, Ilalov K, Carlson CS, Feng JQ, Di Cesare PE, et al. Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J Biol Chem. 2007;282(15):11347–55. doi: 10.1074/jbc.M608744200. [DOI] [PubMed] [Google Scholar]
- 24.Bai XH, Wang DW, Kong L, Zhang Y, Luan Y, Kobayashi T, et al. ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor. Mol Cell Biol. 2009;29(15):4201–19. doi: 10.1128/MCB.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollenberg SM, Sternglanz R, Cheng PF, Weintraub H. Identification of a new family of tissue-specific basic helix-loop- helix proteins with a two-hybrid system. Mol Cell Biol. 1995;15(7):3813–22. doi: 10.1128/mcb.15.7.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vojtek AB, Hollenberg SM, Cooper JA. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74(1):205–14. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 27.Luan Y, Kong L, Howell DR, Ilalov K, Fajardo M, Bai XH, et al. Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthritis Cartilage. 2008;16(11):1413–20. doi: 10.1016/j.joca.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276(44):41059–63. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 29.Liu CJ. The role of ADAMTS-7 and ADAMTS-12 in the pathogenesis of arthritis. Nat Clin Pract Rheumatol. 2009;5(1):38–45. doi: 10.1038/ncprheum0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin EA, Liu CJ. The Emerging Roles of ADAMTS-7 and ADAMTS-12 Matrix Metalloproteinases. Open Acess Rheumatology: Research and Review. 2009 doi: 10.2147/oarrr.s6264. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanocco-Marani T, Bateman A, Romano G, Valentinis B, He ZH, Baserga R. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999;59(20):5331–40. [PubMed] [Google Scholar]
- 32.Hong SJ, Kang KW. Purification of granulin-like polypeptide from the blood-sucking leech, Hirudo nipponia. Protein Expr Purif. 1999;16(2):340–6. doi: 10.1006/prep.1999.1077. [DOI] [PubMed] [Google Scholar]
- 33.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 34.Chen FH, Herndon ME, Patel N, Hecht JT, Tuan RS, Lawler J. Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J Biol Chem. 2007;282(34):24591–8. doi: 10.1074/jbc.M611390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen FH, Thomas AO, Hecht JT, Goldring MB, Lawler J. Cartilage Oligomeric Matrix Protein/Thrombospondin 5 Supports Chondrocyte Attachment through Interaction with Integrins. J Biol Chem. 2005;280(38):32655–61. doi: 10.1074/jbc.M504778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stracke JO, Fosang AJ, Last K, Mercuri FA, Pendas AM, Llano E, et al. Matrix metalloproteinases 19 and 20 cleave aggrecan and cartilage oligomeric matrix protein (COMP) FEBS Lett. 2000;478(1–2):52–6. doi: 10.1016/s0014-5793(00)01819-6. [DOI] [PubMed] [Google Scholar]
- 37.Dickinson SC, Vankemmelbeke MN, Buttle DJ, Rosenberg K, Heinegard D, Hollander AP. Cleavage of cartilage oligomeric matrix protein (thrombospondin-5) by matrix metalloproteinases and a disintegrin and metalloproteinase with thrombospondin motifs. Matrix Biol. 2003;22(3):267–78. doi: 10.1016/s0945-053x(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez EM, Mongiat M, Slater SJ, Baffa R, Iozzo RV. A novel interaction between perlecan protein core and progranulin: potential effects on tumor growth. J Biol Chem. 2003;278(40):38113–6. doi: 10.1074/jbc.C300310200. [DOI] [PubMed] [Google Scholar]
- 39.Fukui N, Zhu Y, Maloney WJ, Clohisy J, Sandell LJ. Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-alpha in normal and osteoarthritic chondrocytes. J Bone Joint Surg Am. 2003;85-A(Suppl 3):59–66. doi: 10.2106/00004623-200300003-00011. [DOI] [PubMed] [Google Scholar]
- 40.Sandell LJ. Anabolic factors in degenerative joint disease. Curr Drug Targets. 2007;8(2):359–65. doi: 10.2174/138945007779940142. [DOI] [PubMed] [Google Scholar]
- 41.Baba T, Hoff HB, 3rd, Nemoto H, Lee H, Orth J, Arai Y, et al. Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells. Mol Reprod Dev. 1993;34(3):233–43. doi: 10.1002/mrd.1080340302. [DOI] [PubMed] [Google Scholar]
- 42.Xu SQ, Tang D, Chamberlain S, Pronk G, Masiarz FR, Kaur S, et al. The granulin/epithelin precursor abrogates the requirement for the insulin-like growth factor 1 receptor for growth in vitro. J Biol Chem. 1998;273(32):20078–83. doi: 10.1074/jbc.273.32.20078. [DOI] [PubMed] [Google Scholar]
- 43.Sparro G, Galdenzi G, Eleuteri AM, Angeletti M, Schroeder W, Fioretti E. Isolation and N-terminal sequence of multiple forms of granulins in human urine. Protein Expr Purif. 1997;10(2):169–74. doi: 10.1006/prep.1997.0726. [DOI] [PubMed] [Google Scholar]
- 44.Plowman GD, Green JM, Neubauer MG, Buckley SD, McDonald VL, Todaro GJ, et al. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. J Biol Chem. 1992;267(18):13073–8. [PubMed] [Google Scholar]


