Abstract
Jak2/Stat-mediated prolactin signaling culminates in Stat5a-DNA-binding. However, not all Jak2-dependent genes have Stat5 sites. Western analysis with inhibitors showed Jak2 is a proximal intermediate in prolactin-induced RUSH phosphorylation. Transfection assays with HRE-H9 cells showed the RUSH-binding site mediated the ability of prolactin to augment progesterone-dependent transcription of the RUSH gene. Jak2 inhibitors or targeted RUSH-site mutation blocked the prolactin effect. RUSH co-immunoprecipitated with phospho-Jak2 from nuclear extracts. Jak2 inhibitors abolished the nuclear pool of phospho-RUSH not the nuclear content of RUSH in HRE-H9 cells. Nucleolar-affiliated partners, e.g. nucleolin, were identified by μLC/MS/MS analysis of nuclear proteins that co-immunoprecipitated with RUSH/GST-RING. RUSH did not exclusively co-localize with fibrillarin to the nucleolus. MG-132 (proteasomal inhibitor) failed to block Tyrene CR4-mediated decrease in phospho-RUSH, and did not promote RUSH accumulation in the nucleolus. These studies authenticate prolactin-dependent Jak2 phosphorylation of RUSH, and provide functional implications on the RUSH network of nuclear interactions.
Keywords: Jak2, prolactin, HLTF, nucleolin, actin, HRE-H9 cells (rabbit endometrium)
1. Introduction
Prolactin initiates intracellular signaling by binding to cell-surface receptors with subsequent activation of associated kinases (Binart et al., 2010). Major signaling cascades include Jak2/Stat5a, Ras-Raf-MAPK, and PI-3K (Clevenger et al., 2003). In Jak2/Stat5a signaling, prolactin receptor binding triggers trans- and/or auto-phosphorylation of two Jak2 molecules constitutively associated with the membrane-proximal proline-rich Box 1 motif of the receptor (Hennighausen and Robinson, 2008). Phospho-Jak2 coordinates the tyrosine-phosphorylation of Stat5a (Li, 2008). Jak2/Stat5a-mediated prolactin signal transduction culminates in sequence-selective binding of Stat5a to previously silent milk protein genes in mammary gland. In this paradigm, Stat5a is an important mediator of prolactin action because Stat5a-deficient mice have reduced alveolar development and fail to lactate (Liu et al., 1997; Teglund et al., 1998).
In rabbit endometrium, prolactin augments the progesterone-dependent increase in transcription of the uteroglobin gene (Chilton et al. 1988; Kleis-SanFrancisco et al., 1993), the founding member of the SCGB1A1 family (Mukherjee et al., 2007). The search for responsible mediators culminated in the cloning and characterization of the RUSH transcription factors — a full length, progesterone-dependent, alpha isoform, and a truncated, estrogen-dependent, beta isoform (Hayward-Lester et al., 1996). RUSH-1α has seven (I, Ia, II–VI) motifs characteristic of SWI/SNF ATPases (Racki and Narlikar, 2008), a C3HC4-RING finger motif characteristic of E3 ubiquitin ligases (Deshaies and Joazeiro, 2009; Nagy and Dikic, 2010), and a HIRAN domain purported to recognize damaged DNA and stalled replication forks (Iyer et al., 2006). RUSH-1α protein is 95% similar and 91% identical to its human ortholog, HLTF, previously named SMARCA3. RUSH-1β protein is identical to full-length RUSH-1α through the RING-finger and for 33 amino acids thereafter. Then, because of an alternative splicing event, the protein extends for five unique amino acids and stops. Thus RUSH-1β is missing SWI/SNF-motifs IV–V–VI, but retains its protein interaction potential through the RING-finger motif.
Cyclic amplification and selection of targets identified the RUSH binding site (−126/−121) in the SCGB1A1 gene (Hewetson et al., 2002), and chromatin immunoprecipitation confirmed site-specific binding of RUSH-1α to that site in the transcriptionally active promoter in the absence of Stat5a binding sites (Hewetson et al., 2002). Transient transfection assays with mutated constructs and HRE-H9 cells, a rabbit uterine epithelial cell line (Li et al., 1989), showed the RUSH-binding site mediated the ability of prolactin to augment progesterone-dependent transcriptional activation of the uteroglobin gene. The identification of RUSH-1α as a nuclear effector of prolactin signals prompted speculation about a Jak/RUSH alternative to Jak/Stat signaling. The fact that RUSH is a phosphonuclear protein, and RUSH-DNA binding is mediated by tyrosine-phosphorylation (Hewetson et al., 2004) supported the hypothesis. Moreover, not all Jak2 regulated genes contain Stat5 binding sites (Dawson et al., 2009; Sattler and Griffin, 2009).
The C3HC4-RING finger of RUSH is a protein interaction domain (Mansharamani et al., 2001; Hewetson et al., 2008). It binds the transcription factors Egr-1 and c-Rel, and catalyzes DNA looping through its affiliation with these protein partners (Hewetson and Chilton, 2008). RUSH-1α mediates progesterone-dependent transcription of its own promoter through this DNA-looping mechanism. Northern blotting showed prolactin augments progesterone-dependent transcription of the RUSH gene in addition to the SCGB1A1 gene (Hayward-Lester et al., 1996).
In this study, Jak2 inhibitors — AG490, TG101209, Staurosporine, Jak inhibitor 1, Jak 2 inhibitor 2 and Tyrene CR4 — were used in conjunction with the PI-3 kinase inhibitor, Wortmannin, and the MAP kinase inhibitor, PD98059, in HRE-H9 cells to show that RUSH-1α is phosphorylated by Jak2 as a direct consequence of prolactin treatment. Western analysis, immunofluorescence microscopy, and transient transfection assays confirmed a functional Jak/RUSH pathway.
Co-immunoprecipitation of Jak2 with RUSH-1α confirmed a physical interaction between these phosphoproteins in nuclear extract (Hewetson et al., 2002). Additional nuclear protein partners, such as nucleolin (C23), were identified by μLC/MS/MS analysis of nuclear extract proteins that co-immunoprecipitated with RUSH. Like nucleolin, some partners also co-immunoprecipitated with GST-RING, confirming the RING motif mediates RUSH-protein binding, and some were identified as phosphonuclear proteins by μLC/MS/MS analysis of nuclear extract proteins that immunoprecipitated with antiphosphotyrosine antibodies. Confocal immunofluorescence images of HRE-H9 cells showed an even nuclear distribution of RUSH and phospho-Jak2. RUSH did not exclusively co-localize with fibrillarin to the nucleolus, and the proteasome inhibitor, MG-132, failed to promote nucleolar accumulation of RUSH. These new findings provide functional implications for the RUSH network of nuclear and phospho-nuclear proteins.
2. Material and Methods
2.1 Reagents and Tools
Ready Gel 5 % and 7.5% Tris-HCl precast polyacrylamide gels (161–1213 and 161–1154, respectively) were purchased from Bio-Rad (Hercules, CA). Gradipure Coomassie Brilliant Blue R-250 stain reagent was purchased from VWR (Sugar Land, TX). Thermo Scientific Pierce silver stain kit (formerly called SilverSNAP Silver Stain Kit II) was purchased Fisher Thermo Scientific (Rockford, IL). Promegestone (R5020) was purchased from Perkin Elmer Life Science Products, Inc. (Waltham, MA). Dr. A.F. Parlow, Scientific Director, National Hormone and Pituitary Program (Torrance, CA), provided the ovine prolactin (oPRL). Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) was the commercial source of Protein A/G PLUS-Agarose (sc-2003) beads, and antibodies for phosphorylated (p) Jak2 (sc-21870). SMARCA3 antibodies (ab17984), and two nucleolar markers — mouse monoclonal [38F3] to fibrillarin (ab4566) and rabbit polyclonal to fibrillarin (ab5821) — were purchased from Abcam Inc. (Cambridge, MA). Mouse anti-phosphotyrosine, 4G10® Platinum antibodies (05–1050) were purchased from Millipore (Billerica, MA). Invitrogen Corp. (Carlsbad, CA) was the source of Alexa-conjugated secondary antibodies — Alexa Fluor® 594 chicken anti-mouse IgG (A-21201), Alexa Fluor® 594 chicken anti-goat IgG (A-21468), Alexa Fluor® 488 chicken anti-rabbit IgG (A-21441), and ProLong Gold antifade reagent with DAPI.
The following Jak2 inhibitors were purchased from the EMD Biosciences (San Diego, CA): Tyrphostin AG490 (658401); Jak inhibitor 1 (420097); Jak 2 inhibitor II (420132); Tyrene CR4 (655230); the PI-3 kinase inhibitor, Wortmannin (681675); the MAK kinase inhibitor PD98059 (513000); and the proteasome inhibitor MG-132 (474790). Two additional Jak2 inhibitors included TG101209 purchased from Symansis (Auckland, New Zealand), and Staurosporine (S6942) purchased from Sigma-Aldrich (St. Louis, MO).
2.2 Animal treatments
All studies with virgin, adult New Zealand white rabbits were conducted according to the NIH Guidelines for the Care and Use of Laboratory Animals, as reviewed and approved by the Animal Care and Use Committee at Texas Tech University Health Sciences Center. Estrous animals were given sc injections of progesterone (3 mg/kg/day) for 5 days.
2.3 Nuclear extracts
Nuclei were isolated from the endometrium from progesterone treated rabbits (Kleis-SanFrancisco et al., 1993), and from R5020-treated HRE-H9 cells (Hewetson and Chilton, 2003) to induce RUSH-1α expression. Nuclear extract proteins were immunoprecipitated with anti-phosphotyrosine 4G10 Platinum antibodies (4 μl/reaction) or anti-phospho Jak2 (5 μl/reaction), resolved by SDS-PAGE, and processed for Western blotting with anti-SMARCA3 (1:1000) or anti-phospho Jak2 (1:1000), respectively, and species appropriate HRP-conjugated secondary antibodies.
2.4 Protein Sequence Analysis
Nuclear extract proteins were co-immunoprecipitated with RUSH (n = 3), GST-RING (n = 1), or anti-phosphotyrosine antibodies (n = 2). The use of the RING-motif in GST-pulldown assays has been described (Mansharamani et al., 2001; Hewetson and Chilton, 2008; Hewetson et al., 2008). Immunoprecipitated nuclear proteins were resolved by SDS/PAGE, and either Silver- or Coomassie-stained. One-dimensional gel slices were excised, dehydrated with acetonitrile, and subject to in-gel tryptic digestion. Protein sequence analysis was performed at the Harvard Microchemistry Facility and Proteomics Analysis Facility by microcapillary reverse-phase high performance liquid chromatography (HPLC) nano-electrospray tandem mass spectrometry (μLC/MS/MS) on a Finnigan LCQ DECA XP Plus quadrupole ion trap mass spectrometer (RUSH/HLTF immunoprecipitated samples, plus one 4-hour gradient to further identify peptides; anti-phosphotyrosine immunoprecipitated samples), or a Thermo LTQ-Orbitrap mass spectrometer (GST-RING samples). These instrument configurations are capable of acquiring individual sequence (MS/MS) spectra on-line at high sensitivity (<<<1 femtomole) for multiple peptides in the chromatographic run. These MS/MS spectra (or sequence) are then correlated with known sequences using the algorithm Sequest developed at the University of Washington (Eng et al., 1994), and programs developed in the Harvard Microchemistry laboratory (Chittum et al., 1998). MS/MS peptide sequences were reviewed for consensus with known proteins, and the results manually confirmed for fidelity.
2.5 Transient Transfection Assays
Cloning the RUSH fragment (−712/+90) into the luciferase reporter plasmid pGL-3-Basic, targeted mutagenesis of the RUSH site, and differential expression of the constructs in HRE-H9 cells has been described (Hewetson and Chilton, 2003, 2008). HRE-H9 cells are an SV-40 transformed rabbit uterine epithelial cell line derived from the endometrium of 3-month-old female rabbits treated with hCG (Li et al., 1989). Transient transfections were performed in 24-well dishes a minimum of 2 times in quadruplicate. Cells were treated (Hewetson and Chilton, 2003, 2008) with R5020 (10−8 M) in dimethyl sulfoxide (0.1% v/v) for 16–20 h ± Tyrene CR4 (800 nM) for 16–20 hours ± prolactin (10−8 M) for 5 min. pRUSH-LUC expression was normalized to co-transfected pRL-TK-LUC expression to control for transfection efficiency. Multiple ratio data were ranked (Conover and Iman, 1981) and the ranks between the groups were analyzed by Kruskal-Wallis (Nonparametric) ANOVA followed by Dunn's Multiple Comparisons Test (P < 0.05 significance level) using GraphPad InStat Version 3 for Macintosh (GraphPad Software, Inc.). The P value for the Kruskal-Wallis Test was P < 0.0001.
2.6 Immunofluorescence microscopy
Immunofluorescence microscopy was as previously described (Hewetson and Chilton, 2008). Briefly, HRE-H9 cells were grown on poly-D-lysine coated coverslips and treated ± R5020 (10−8 M) in dimethyl sulfoxide (0.1% v/v) for 16–20 h ± prolactin (10−8 M) for 2 or 5 min. Cells were fixed in cold methanol (−20 C) for 10 min followed by cold acetone (−20 C) for 1 min; rinsed in PBS [20 mM sodium phosphate, pH 7.5, and 0.9% sodium chloride]; quenched in 0.1 M glycine for 1 hr at room temperature; and blocked in 10% normal chicken serum with 0.3% Triton X-100. Cells were incubated with primary antibodies (1:500) overnight at 4 C. After three rinses in PBS, cells were incubated with species relevant Alexa Fluor® conjugated secondary antibodies (1:400) for 60 min at room temperature in the dark. Cells were rinsed in PBS and mounted in ProLong Gold antifade reagent with DAPI. Controls included cells that were not treated with hormones, and labeling reactions that excluded either primary or secondary antibodies.
Immunofluorescence was observed with an Olympus IX71 inverted microscope equipped for laser scanning confocal microscopy (LSCM). Images were acquired using Olympus Fluoview 500 software and multi — Argon, red HeNe, green HeNe, and blue diode — lasers. Initially, differential interference contrast (DIC) images were acquired in order to demarcate cell boundaries. Images were acquired for phospho-Jak2, RUSH, and DAPI. All images were imported into Metamorph 6.3 image analysis software (Molecular Devices, Sunnyvale, CA). Cell boundaries were traced over the DIC images using the region tools and transferred to the phospho-Jak2 and RUSH images. Phospho-Jak2 and RUSH images were thresholded. Nuclei were isolated using the “create regions around objects” function. The percent threshold area was determined using the “Show Region Statistics” menu. Values [n = 6–13; power calculation required 5 replicates per treatment (p<0.05)] were analyzed by Kruskal-Wallis (Nonparametric) ANOVA followed by Dunn's Multiple Comparisons Test (P < 0.05 significance level) using GraphPad InStat Version 3 for Macintosh (GraphPad Software, Inc.). The P value for the Kruskal-Wallis Test was P < 0.0002.
2.7 Immunoprecipitation and Western analysis
HRE-H9 cells from 12-well dishes were harvested in 1 ml RIPA lysis buffer [0.1% sodium dodecyl sulfate, 1% Nonidet P-40, 1% sodium deoxycholate, 2 mM EDTA, 0.15 M NaCl, 0.01 M sodium phosphate, 50 mM NaF, 2 mM sodium orthovanadata, 1 mM phenylmethylsulfonylfluoride, 5 μg/ml aprotinin, 1 μg/ml pepstatin A, and 2 μg/ml leupeptin] for 1 h at 4 C with constant tumbling, i.e. end-over-end mixing with a Lab-Quake rotator. Lysates were cleared of cellular debris by centrifugation (14,000 × g at 4 C for 30 minutes). Resultant supernatants were tumbled with primary antibodies for 1 h at 4 C, and Protein A/G Plus-Agarose beads (20μl/per reaction) were added for overnight (≥16 h) tumbling. Beads were washed 8 times in PBS. Proteins were extracted from beads, resolved on either 5% or 7.5% SDS-PAGE (minigels), and transferred to nitrocellulose. Molecular-mass markers were separated by electrophoresis and transferred with the samples. Membranes were incubated in blocking buffer, i.e. 1× TBST [20 mM Tris-HCl (pH 7.6), 150 mM NaCl, and 0.05% Tween 20] with 3% Carnation non-fat dry milk, for 60 min. Membranes were incubated with antibodies in antibody dilution buffer (1× TBST with 1% Carnation non-fat dry milk) overnight at 4°C. Membranes were washed 8 × 15 min in 1× TBST. Membranes were incubated with appropriate HRP-conjugated secondary antibodies in antibody dilution buffer for 1 hr at room temperature. Membranes were subsequently washed 8 times in 1× TBST. Specific signals were detected by chemiluminescence with the Immun-Star™ Western™ kit.
3. Results
3.1 Western analysis
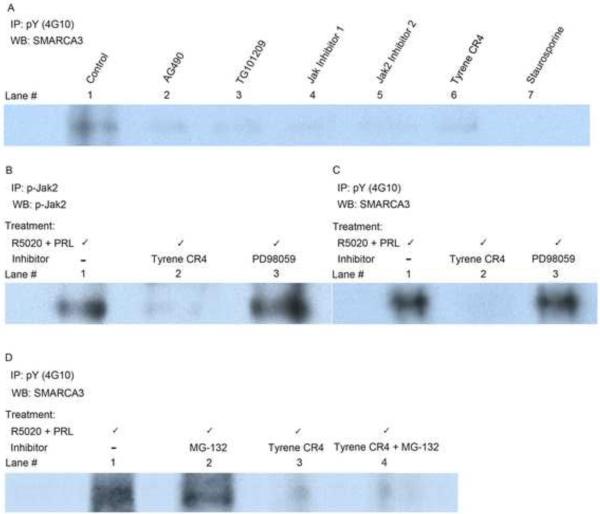
Previously we reported (Hewetson et al., 2004) RUSH is a tyrosine-phosphorylated protein only capable of binding DNA when phosphorylated. To test the hypothesis that RUSH is phosphorylated as a direct consequence of prolactin treatment, HRE-H9 cells were given R5020 (16–20 hours) to induce RUSH-1α and then exposed to prolactin for 2 minutes in the presence of individual Jak2 inhibitors — AG490, TG101209, Staurosporine, Jak inhibitor 1, Jak 2 inhibitor 2 and Tyrene CR4 — plus the PI-3 kinase inhibitor, Wortmannin, and the MAP kinase inhibitor, PD98059. Western analysis of whole cell extracts revealed that all inhibitors of Jak2 blocked phosphorylation of RUSH (Figure 1A). Inhibitors of MAPK and PI-3K pathways that can be activated by prolactin stimulation had no measurable effect on RUSH expression (data shown as positive controls in other figures).
Figure 1.
Jak2 tyrosine phosphorylates RUSH in vivo.
Panel A, Prolactin-induced phosphorylation of RUSH was evaluated by Western analysis of whole cell extracts from serum-starved HRE-H9 cells treated with R5020 for 16–20 hours ± Jak2-specific inhibitors + prolactin for 2 minutes. This treatment of HRE-H9 cells applies to all panels in this figure.
Panel B. Inhibition of prolactin-induced Jak2 phosphorylation with Tyrene CR4 eliminated catalytically active Jak2 from nuclear extracts of HRE-H9 cells. Treatment with PD98059 had no effect.
Panel C. Inhibition of prolactin-induced Jak2 phosphorylation by Tyrene CR4 eliminated phospho-RUSH from nuclear extracts of HRE-H9 cells. Treatment with PD98059 had no effect.
Panel D. Nuclear phospho-RUSH was not degraded by the proteasome in vivo. MG-132 did not block a Tyrene CR4-mediated decrease in the level of phospho-RUSH from nuclear extracts of HRE-H9 cells. IP, Immunoprecipitation; WB, Western blot; pY (4G10), phosphotyrosine; p-Jak2, phospho-Jak2; SMARCA3, RUSH.
3.2 Transient transfection assays
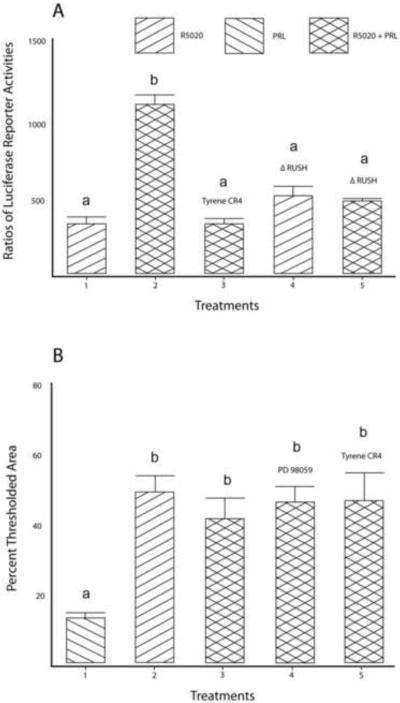
To show that Jak2-dependent phosphorylation of RUSH mediates the action of prolactin, control and hormone-dependent transcriptional activities were measured with either an intact RUSH construct (−712/+90) containing the promoter (−162/+90), included PRE-half site (−138/−133), and authentic RUSH site (−616/−611), or the same RUSH construct with a targeted mutation (Δ) in the RUSH site. The complete absence of Stat5 binding sites from this construct (−712/+90) was confirmed with MatInspector (Genomatix) Release Professional 8.01 (Cartharius et al., 2005), and with BIOBASE BKL TRANSFAC Suite. As shown in Figure 2A, prolactin increased (p < 0.001) progesterone-dependent transcription of the RUSH gene. The Jak2-inhibitor Tyrene CR4 completely blocked the prolactin-treatment effect. Progesterone-dependent transcription of ΔRUSH was comparable (p > 0.05) to the intact RUSH construct, but mutation of the RUSH site was highly deleterious (p < 0.001) to the prolactin effect. Either treatment with Tyrene CR4 or mutation of the RUSH site eliminated (p > 0.05) the prolactin-mediated increase in progesterone-dependent transcription of the RUSH gene.
Figure 2.
Jak2 affects transcription of the RUSH gene.
Panel A. Prolactin increased (p < 0.001) progesterone-dependent transcription of a RUSH reporter construct (compare lanes 1 and 2). Inhibition of Jak2 phosphorylation by Tyrene CR4 (compare lanes 2 and 3), or mutation of the RUSH binding site (compare lanes 4 and 5), completely blocked (p < 0.001) the ability of prolactin to augment progesterone-dependent transcription of the same construct. Mean (± SEM) values with the same letter designation are not significantly different (p > 0.05). Serum-starved HRE-H9 cells were treated with ± R5020 for 16–20 hours ± Inhibitor ± prolactin for 5 minutes. This treatment of HRE-H9 cells applies to all outcomes in this figure.
Panel B. Phosphorylation of Jak2 had no effect (p > 0.05) on the total nuclear content of progesterone-induced RUSH evaluated by quantitative LSCM (details in section 2.6 of the Materials and Methods. Mean (± SEM) values with the same letter designation are not significantly different (p > 0.05).
3.3 Nuclear RUSH and phospho-Jak2
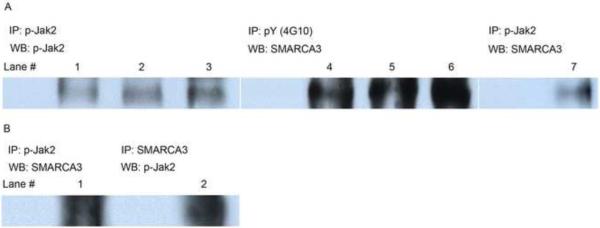
We previously reported nuclear co-localization of RUSH and Jak2 (Hewetson et al., 2002) in nuclear extracts from endometrium. Subcellular fractionation experiments demonstrated catalytically active Jak2 in nuclei isolated from endometrium (Figure 3A) and HRE-H9 cells (Figure 1B), and confirmed a physical affiliation between RUSH and catalytically active Jak2 (Figure 3A, 3B). Confocal immunofluorescence imaging corroborates a significant pool of phosphonuclear Jak2 in HRE-H9 cells (Figure 4A). Treatment of HRE-H9 cells with Tyrene CR4 abolished the nuclear pools of catalytically active Jak2 (Figure 1B) and phospho-RUSH (Figure 1C), but not the nuclear content of RUSH (Figure 2B) as evaluated by quantitative LSCM. As shown in Figure 1C, PD 98059 had no effect on the nuclear pool of phospho-RUSH. Moreover, neither Tyrene CR4 nor PD 98059 has any effect (p > 0.05) on the nuclear pool of RUSH (Figure 2B) confirming that nuclear Jak2 phosphorylates a progesterone-dependent pool of RUSH.
Figure 3.
Catalytically active Jak2 is present in the nucleus.
Panel A. Phospho-Jak2 (lanes 1–3) and phospho-RUSH (lanes 4–6) coexist in nuclear extracts from endometrial cells. Phospho-Jak2 and RUSH are physically affiliated (lane 7) in nuclear extracts from endometrial cells.
Panel B. Co-immunoprecipitation of phospho-Jak2 and RUSH confirmed the physical interaction between them in nuclear extracts from HRE-H9. IP, Immunoprecipitation; WB, Western blot; pY (4G10), phosphotyrosine; p-Jak2, phospho-Jak2; SMARCA3, RUSH.
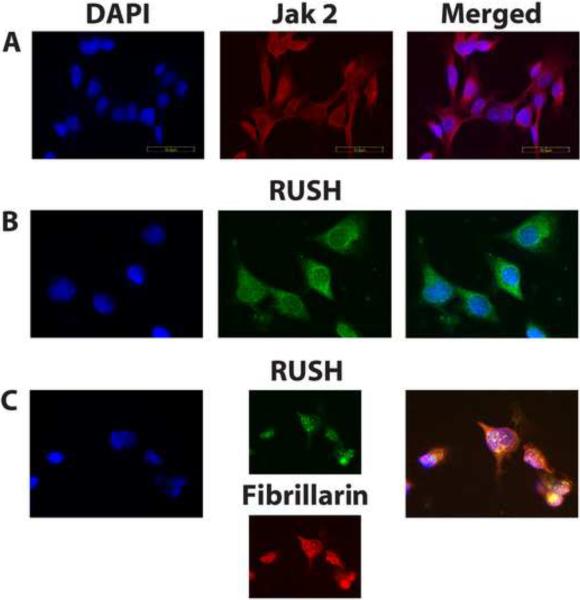
Figure 4.
Catalytically active Jak2 resides in the nucleus with RUSH.
Panel A. Confocal immunofluorescence images identify phospho-Jak2 (red) in both the nuclear and cytoplasmic compartments of serum-starved HRE-H9 cells treated with R5020 for 16–20 hours ± Jak2-specific inhibitors + prolactin for 2 minutes. This treatment of HRE-H9 cells applies to all panels in this figure. DAPI (4,6-diamidino-2-phenylindole) nuclear DNA staining is blue. This image corroborates the detection of nuclear phospho-Jak2 by Western analysis shown in Figure 1B. Scale bar, 50 μm, applies to all images in this figure.
Panel B. Confocal immunofluorescence images identify RUSH (green) in both the nuclear and cytoplasmic compartments of HRE-H9 cells.
Panel C. Merged confocal immunofluorescence images of RUSH (green) with fibrillarin (red) show nuclear RUSH does not co-localize (yellow) exclusively to nucleoli. Results are representative of seven independent experiments performed in duplicate.
3.4 μLC/MS/MS analysis of nuclear proteins
RUSH-interacting proteins, and their posttranslational modifications, were identified by μLC/MS/MS analysis. Nuclear extract proteins that co-immunoprecipitated with RUSH were resolved by SDS-PAGE. One-dimensional gel slices were in gel digested with trypsin, and resulting peptide mixtures were evaluated by μLC/MS/MS analysis. Authentic RUSH protein partners were identified (Table I) in at least two of three independent experiments with high stringency, i.e. a minimum of two high-scoring peptides per protein to achieve a false positive rate for protein identification < 0.1%. In actuality, the average number of high-scoring peptides per RUSH partner was nine. When the list of high-stringency binding partners was compared with proteins that also bound the RING domain, three candidates with 2–3 high-scoring peptides were identified (Table I). Eight RUSH protein partners were identified as phosphonuclear proteins, and nine were recognized members of the nucleolar proteome database (Ahmad et al., 2009; Andersen et al., 2005). Table I contains conclusive evidence that RUSH affiliates with nuclear actin.
Table 1.
RUSH-protein partners identified by μLC/MS/MS analysis.
| Tyrosine-phosphorylated | Nucleolar-affiliated | |
|---|---|---|
| A. Partners that bind the RING-finger motif | ||
| Nucleolin | √ | √ |
| Ribosomal protein L10 | √ | |
| HSP 70kDa protein 9B | ||
| B. Heterogeneous nuclear ribonucleoproteins | ||
| hnRNP U isoform | √ | |
| hnRNP A1 | √ | |
| hnRNP A3 isoform 1 | ||
| C. Histones | ||
| Core Histone macro-H2A.1 | √ | |
| Histone H1.1 | √ | |
| D. Chaperones | ||
| HSP 90α | √ | √ |
| HSP 90β | √ | √ |
| Endoplasmin (GRP94) | √ | |
| Nucleophosmin | √ | √ |
| E. RNA Helicases | ||
| RNA Helicase II/Gu protein | √ | |
| DEAD-box polypeptide 17 isoform p82 | √ | √ |
| F. DNA Replication | ||
| DNA Topoisomerase I | √ | |
| G. Splicing Factors | ||
| Splicing factor 3a, subunit 1 | √ | |
| H. Transcription regulator | ||
| Actin |
3.5 RUSH and nucleolin
The affiliation of RUSH with nucleolin, which is found in nucleoli and nucleoplasm, prompted an inquiry about the intracellular availability of RUSH. As shown in Figure 4B, endogenous RUSH is uniformly distributed throughout the nucleus and cytoplasm. Merged confocal immunofluorescence images of RUSH with the nucleolar marker protein fibrillarin showed RUSH in the nucleolus as well as the nucleoplasm (Figure 4C).
Proteasome inhibitors such as MG-132 are known to extend the activity of Jak2/Stat signaling by protecting tyrosine-phosphorylated proteins from degradation by the proteasome (Ungureanu et al., 2002; Yu and Burakoff, 1997; Nilsson et al., 2006). However, as shown in Figure 1D, MG-132 failed to block a Tyrene CR4-mediated decrease in the level of nuclear phospho-RUSH. Not surprising, MG-132 also failed to promote the nucleolar accumulation of RUSH (data not shown).
4. Discussion
The current model of RUSH regulation integrates RUSH phosphorylation by Jak2 as a direct consequence of prolactin signaling with the previous finding that RUSH is a phosphonuclear protein whose DNA binding ability is mediated by tyrosine-phosphorylation (Hewetson et al., 2004). Transfection assays with RUSH reporter constructs allowed us to correlate the loss of Jak2-mediated RUSH phosphorylation with the inability of prolactin to augment progesterone-dependent transcription. Jak/RUSH signaling independent of the Jak/Stat pathway is supported by the Dawson et al (2009) report that ~ 35% or 14 of 40 Jak2-regulated genes do not contain Stat5 binding sites.
A nuclear pool of catalytically active Jak2 means Jak2 is available to tyrosine phosphorylate protein targets in close proximity to their nuclear sites of action. In general, phosphorylation-mediated signaling is characterized by a dynamic relationship between protein participants in which site-specific and time-specific phosphorylation/dephosphorylation events occur sequentially to achieve an outcome (Zhang et al., 2005). Compelling examples of functions controlled by nuclear Jak2 include: 1) phosphorylation of NF1-C2 prevents its proteasomal degradation in mammary epithelial cells (Nilsson et al., 2006); 2) phosphorylation of histone H3 at tyrosine residue 41 (H3Y41) releases transcriptional repressor heterochromatin protein 1α (HP1α) resulting in transcriptional activation of Imo2 in hematopoietic cells (Dawson et al., 2009).
The NetPhos 2.0 Server predicts seven tyrosine phosphorylation sites in RUSH. Two of them (Y93 and Y195) are in the DNA-binding domain (amino acids 40–231). Since tyrosine phosphorylation is required for DNA-binding, these sites are the most likely candidates for site-specific validation/quantification of tyrosine-phosphorylation, and the generation of site-specific-phospho-antibodies with which to evaluate tyrosine phosphorylation by Jak2. Although it is unknown if the human (HLTF) and mouse (Hltf) orthologs are Jak2 targets, a context-dependent search of the sequences containing Y93 and Y195 with the BLAST 2.2.23 (SwissProt) program (Altschuel et al., 1997) showed the sites are 100% conserved in all three species.
Understanding RUSH function is found in the context-dependent interactions of RUSH with its protein partners. However rudimentary, our knowledge of those partners — identity, criteria for partner choice, and function — was advanced by the use of μLC/MS/MS analysis of nuclear proteins that co-immunoprecipitated with RUSH. The human ortholog HLTF is one of 489 proteins known to flux through the nucleolus (Andersen et al., 2005). RUSH, like nucleolin, lacks the nucleolar localization (NoLS) signal – [(R/K)(R/K)X(R/K)] – that appears once or as multiple copies in peptides directed to or retained in the nucleolus (Emmott and Hiscox, 2009). Moreover, like nucleolin, RUSH physically affiliates with nucleophosmin (Table 1), which contains a NoLS, and is purported to target nucleolin to the nucleolus (Li et al., 1996). Physical interactions between RUSH and nucleolin raise the question of whether or not nucleolin, which possesses a histone chaperone activity (Angelov et al., 2006; Mongelard and Bouvet, 2007), enhances the chromatin remodeling activity of RUSH.
The C3HC4-RING motif in RUSH is a protein interaction domain (Mansharamani et al., 2001; Hewetson and Chilton, 2008; Hewetson et al., 2008). It binds the transcription factors Egr-1 and c-Rel, and catalyzes DNA looping through its affiliation with these protein partners (Hewetson and Chilton, 2008). RUSH mediates progesterone-dependent transcription of its own promoter through this DNA-looping mechanism. Moreover, the C3HC4-RING motif in RUSH binds a rabbit-specific ATP11B isoform (an atypical Type-IV P-type ATPase) purported to have a role in subnuclear trafficking of transcription factors with RING motifs (Mansharamani et al., 2001). The discontinuous PVITHC-HAKCPL sequence in the RING-finger binds a sequence conserved in all ATP11B isoforms (Hewetson et al., 2008). Fine mapping CLIPS™-constrained RING peptides that maintain the 3D-conformation of individual peptides showed the sequence HAKAPL-TH is essential for binding, and 100% conserved in all orthologs. Whether or not the discontinuous binding site is a universal protein-binding site with functional relevance to other species, and human disease, is unknown at this time.
This report of a physical affiliation between RUSH and actin in the nucleus is the first account of a physical affiliation between any HLTF ortholog and actin. As bona fide members of the nuclear compartment (Louvet and Percipalle, 2009), actin and actin-binding proteins are involved in gene transcription (Farrants, 2008) in association with ATP-dependent chromatin-remodeling (SWI/SNF) complexes (Zheng et al., 2009). A major function of actin is to behave as an allosteric regulator in the remodeling of macromolecular assemblies such as chromatin remodeling factors or transcription complexes (Zheng et al., 2009).
Collectively these studies focus our attention on previously unrecognized RUSH nuclear partners known to have important cellular functions. Identifying both stable and transiently interacting protein partners will help us to better understand the importance of the RING domain. The extent to which RING-protein interactions are evolutionarily conserved highlights their functional importance.
Acknowledgements
We thank Larry Starr, Medical Photography, TTUHSC, for artwork; and the NIH (HD29457) for support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad Y, Boisvert FM, Gregor P, Cobley A, Lamond AI. NOPdb: Nucleolar proteome database-2008 update. Nucleic Acids Res. 2009;37:D181–D184. doi: 10.1093/nar/gkn804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JS, Lam YW, Leung AK, Ong SE, Lyon CE, Lamond AI, Mann M. Nucleolar proteome dynamics. Nature. 2005;433:77–83. doi: 10.1038/nature03207. [DOI] [PubMed] [Google Scholar]
- Angelov D, Bondarenko VA, Almagro S, Menoni H, Mongélard F, Hans F, Mietton F, Studitsky VM, Hamiche A, Dimitrov S, Bouvet P. Nucleolin is a histone chaperone with FACT-like activity and assists remodeling of nucleosomes. EMBO J. 2006;25:1669–1679. doi: 10.1038/sj.emboj.7601046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binart N, Bachelot A, Bouilly J. Trends Endocrinol. Metab. Vol. 21. 2010. Impact of prolactin receptor isoforms on reproduction; pp. 362–368. [DOI] [PubMed] [Google Scholar]
- Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21:2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- Chilton BS, Mani SK, Bullock DW. Servomechanism of prolactin and progesterone in regulating uterine gene expression. Mol. Endocrinol. 1988;2:1169–1175. doi: 10.1210/mend-2-12-1169. [DOI] [PubMed] [Google Scholar]
- Chittum HS, Lane WS, Carlson BA, Roller PP, Lung FT, Lee BJ, Hatfield DL. Rabbit β-globin is extended beyond its UGA stop codon by multiple suppressions and translational reading gaps. Biochemistry. 1998;37:10866–10870. doi: 10.1021/bi981042r. [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr. Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Rank transformation as a bridge between parametric and nonparametric statistics. Am. Stat. 1981;35:124–129. [Google Scholar]
- Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- Emmott E, Hiscox JA. Nucleolar targeting: the hub of the matter. EMBO Rep. 2009;10:231–238. doi: 10.1038/embor.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng JK, McCormick AL, Yates JR., III An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass. Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- Farrants AK. Chromatin remodeling and actin organization. FEBS Lett. 2008;582:2041–2050. doi: 10.1016/j.febslet.2008.04.032. [DOI] [PubMed] [Google Scholar]
- Hayward-Lester A, Hewetson A, Beale EG, Oefner PJ, Doris PA, Chilton BS. Cloning, characterization and steroid-dependent posttranscriptional processing of RUSH-1α and β, two uteroglobin promoter-binding proteins. Mol. Endocrinol. 1996;10:1335–1349. doi: 10.1210/mend.10.11.8923460. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes & Dev. 2008;22:711–721. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewetson A, Hendrix EC, Mansharamani M, Lee VH, Chilton BS. Identification of the RUSH consensus-binding site by cyclic amplification and selection of targets: demonstration that RUSH mediates the ability of prolactin to augment progesterone-dependent gene expression. Mol. Endocrinol. 2002;16:2101–2112. doi: 10.1210/me.2002-0064. [DOI] [PubMed] [Google Scholar]
- Hewetson A, Chilton BS. An Sp1-NF-Y/Progesterone receptor DNA binding-dependent mechanism regulates progesterone-induced transcriptional activation of the rabbit RUSH/SMARCA3 gene. J. Biol. Chem. 2003;278:40177–40185. doi: 10.1074/jbc.M303921200. [DOI] [PubMed] [Google Scholar]
- Hewetson A, Moore SL, Chilton BS. Prolactin signals through RUSH/SMARCA3 in the absence of a physical association with Stat5a. Biol. Reprod. 2004;71:1907–1912. doi: 10.1095/biolreprod.104.031435. [DOI] [PubMed] [Google Scholar]
- Hewetson A, Wright-Pastusek AE, Helmer RA, Wesley KA, Chilton BS. Conservation of inter-protein binding sites in RUSH and RFBP, an ATPIIB isoform. Mol. Cell. Endocrinol. 2008;292:79–86. doi: 10.1016/j.mce.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewetson A, Chilton BS. Progesterone-dependent DNA looping between RUSH/SMARCA3 and Egr-1 mediates repression by c-Rel. Mol. Endocrinol. 2008;22:813–822. doi: 10.1210/me.2007-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer LM, Babu MM, Aravind L. The HIRAN domain and recruitment of chromatin remodeling and repair activities to damaged DNA. Cell Cycle. 2006;5:775–782. doi: 10.4161/cc.5.7.2629. [DOI] [PubMed] [Google Scholar]
- Kleis-SanFrancisco S, Hewetson A, Chilton BS. Prolactin augments progesterone-dependent uteroglobin gene expression by modulating promoter-binding proteins. Mol. Endocrinol. 1993;7:214–223. doi: 10.1210/mend.7.2.8469234. [DOI] [PubMed] [Google Scholar]
- Li WI, Chen CL, Chou JY. Characterization of a temperature-sensitive β-endorphin-secreting transformed endometrial cell line. Endocrinology. 1989;125:2862–2867. doi: 10.1210/endo-125-6-2862. [DOI] [PubMed] [Google Scholar]
- Li WX. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol. 2008;18:545–551. doi: 10.1016/j.tcb.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Busch RK, Valdez BC, Busch H. C23 interacts with B23, a putative nucleolar-localization-signal-binding protein. Euro. J. Biochem. 1996;237:153–158. doi: 10.1111/j.1432-1033.1996.0153n.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Louvet E, Percipalle P. Chapter 3 Transcriptional control of gene expression by actin and myosin. Int. Rev. Cell Mol. Biol. 2009;272:107–147. doi: 10.1016/S1937-6448(08)01603-1. [DOI] [PubMed] [Google Scholar]
- Mansharamani M, Hewetson A, Chilton BS. Cloning and characterization of an atypical type IV P-type ATPase that binds to the RING motif of RUSH transcription factors. J. Biol. Chem. 2001;276:3641–3649. doi: 10.1074/jbc.M004231200. [DOI] [PubMed] [Google Scholar]
- Mongelard F, Bouvet P. Nucleolin: a multiFACeTed protein. Trends Cell Biol. 2007;17:80–86. doi: 10.1016/j.tcb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Mukherjee AB, Zhang Z, Chilton BS. Uteroglobin: A steroid-inducible immunomodulatory protein that founded the secretoglobin superfamily. Endocr. Rev. 2007;28:707–725. doi: 10.1210/er.2007-0018. [DOI] [PubMed] [Google Scholar]
- Nagy V, Dikic I. Biol. Chem. Vol. 391. 2010. Ubiquitin ligase complexes: from substrate selectivity to conjugational specificity; pp. 163–169. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Bjursell G, Kannius-Janson M. Nuclear Jak2 and transcription factor NF1-C2: a novel mechanism of prolactin signaling in mammary epithelial cells. Mol. Cell. Biol. 2006;26:5663–5674. doi: 10.1128/MCB.02095-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racki LR, Narlikar GJ. ATP-dependent chromatin remodeling enzymes: two heads are not better, just different. Curr. Opin. Genet. Dev. 2008;18:137–144. doi: 10.1016/j.gde.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M, Griffin JD. JAK2 gets histone H3 rolling. Cancer Cell. 2009;16:365–366. doi: 10.1016/j.ccr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- Ungureanu D, Saharinen P, Junttila I, Hilton DJ, Silvennoinen O. Regulation of Jak2 through the ubiquitination-proteasome pathway involves phosphorylation of Jak2 on Y1007 and interaction with SOCS-1. Mol. Cell. Biol. 2002;22:3316–3326. doi: 10.1128/MCB.22.10.3316-3326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.l., Burakoff SJ. Involvement of proteasomes in regulating Jak-STAT pathways upon interleukin-2 stimulation. J. Biol. Chem. 1997;272:14017–14020. doi: 10.1074/jbc.272.22.14017. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wolf-Yadlin A, Ross PL, Pappin DJ, Rush J, Lauffenburger DA, White FM. Time-resolved mass spectrometry of tyrosine phosphorylation sites in the epidermal growth factor receptor signaling network reveals dynamic modules. Mol. Cell. Proteomics. 2005;4:1240–1250. doi: 10.1074/mcp.M500089-MCP200. [DOI] [PubMed] [Google Scholar]
- Zheng B, Han M, Bernier M, Wen JK. Nuclear actin and actin-binding proteins in the regulation of transcription and gene expression. FEBS J. 2009;276:2669–2685. doi: 10.1111/j.1742-4658.2009.06986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]