Abstract
Background & Aims
Infection with H. pylori represses expression of the gastric H, K-ATPase α subunit (HKα), which could contribute to transient hypochlorhydria. CagL, a pilus protein component of the H. pylori type IV secretion system, binds to the integrin α5β1 to mediate translocation of virulence factors into the host cell and initiate signaling. α5β1 binds ADAM17, a metalloenzyme that catalyzes ectodomain shedding of receptor tyrosine kinase ligands. We investigated whether H. pylori-induced repression of HKα is mediated by CagL activation of ADAM17 and release of heparin-binding epidermal growth factor (HB-EGF).
Methods
HKα promoter and ADAM17 activity were measured in AGS gastric epithelial cells transfected with HKα promoter-reporter constructs or ADAM17-specific small interfering (si)RNAs and infected with H. pylori. HB-EGF secretion was measured by ELISA analysis and ADAM17 interaction with integrins were investigated by co-immunoprecipitation analyses.
Results
Infection of AGS cells with wild-type H. pylori or an H. pylori cagL-deficient isogenic mutant that also contained a wild-type version of cagL (P12ΔcagL/cagL) repressed HKα promoter-Luc reporter activity and stimulated ADAM17 activity. Both responses were inhibited by point mutations in the NF-κB binding site of HKα or by infection with P12ΔcagL. siRNA-mediated silencing of ADAM17 in AGS cells inhibited the repression of wild-type HKα promoter and reduced ADAM17 activity and HB-EGF production, compared to controls. Coimmunoprecipitation studies of AGS lysates showed that wild-type H. pylori disrupted ADAM17–α5β1 complexes.
Conclusions
During acute H. pylori infection, CagL dissociates ADAM17 from the integrin α5β1 and activates ADAM17-dependent, NF-κB–mediated repression of HKα. This might contribute to transient hypochlorhydria in patients with H. pylori infection.
Keywords: H. pylori; CagL; ADAM17; H,K-ATPase
Introduction
Acute Helicobacter pylori infection of the human gastric corpus reduces acid secretion causing transient hypochlorhydria which may facilitate bacterial colonization1, 2. Hypochlorhydria provokes superficial gastritis, which may progress to atrophic gastritis, intestinal metaplasia, dysplasia, and eventually carcinoma3–5. The mechanisms underlying H. pylori-mediated transient hypochorhydria are poorly understood. H. pylori expresses a type IV secretion system (T4SS) which forms a pilus to inject cytotoxin-associated gene A (CagA) protein into host cells6. T4SS proteins are encoded by a 40 kb genetic locus termed the cag pathogenicity island (PAI). CagL is a pilus protein that interacts with host cellular α5β1 integrins through its arginine-glycine-aspartate (RGD) motif, guiding proper positioning of the T4SS and translocation of CagA7. Consequent activation of host signaling pathways stimulates the transcription factor NF-κB and up-regulates inflammation-associated genes such as IL-8 and Cox28–12 Another important repercussion of NF-κB activation in gastric epithelial cells is transcriptional repression of H,K-ATPase, the enzyme responsible for acid secretion. We previously reported that H. pylori down-regulates H,K-ATPase α subunit (HKα) promoter-reporter constructs transfected into gastric epithelial AGS cells13 and that NF-κB p50 subunit homodimer binding to the HKα promoter underlies this transcriptional repression14.
ADAMs (a disintegrin and metalloprotease) are glycosylated transmembrane metalloproteases containing an N-terminal pro-domain, metalloenzyme domain, cysteine-rich disintegrin domain, and C-terminal cytoplasmic domain. ADAMs catalyze ectodomain cleavage of cell surface integral membrane proteins, generating soluble forms of TNFα and the EGF receptor (EGFR) ligands EGF and heparin-binding EGF (HB-EGF)15, 16. Disintegrin domains in many ADAMs possess RGD analogous acidic motifs that facilitate integrin binding, and numerous instances of ADAM family members binding to αnβ1 integrin heterodimers have been reported17, 18. ADAM17, also known as TACE (TNFα converting enzyme), was first identified by its generation of soluble TNFα from a membrane-bound precursor19. ADAM17 co-localizes with α5β1 integrin heterodimer in HeLa cells during cell migration20 although the significance of integrin binding in ADAM17 activation is not clearly understood.
Antral gastric biopsies from H. pylori-infected patients express more ADAM17 mRNA than normal controls21, and H. pylori-infected gastritis patients showed increased levels of the EGFR ligands EGF, amphiregulin and HB-EGF22, 23. EGFR expression and signaling is perturbed in head and neck, lung, breast, and bladder cancers24, 25, and H. pylori is known to up-regulate EGFR in gastric epithelial cells26. We hypothesized that H. pylori CagL displaces ADAM17 from α5β1 integrins, activating a signaling pathway that culminates in suppression of HKα gene transcription and expression. Data presented in this paper indicate that H. pylori infection of AGS cells causes a CagL-dependent dissociation of ADAM17 from α5β1 integrin, activation of the enzyme, cellular secretion of HB-EGF, and NF-κB-dependent repression of transfected HKα promoter activity. These results further elucidate H. pylori orchestration of host signal transduction mechanisms in favor of bacterial pathogenicity.
Materials and Methods
Cells, Bacteria and Reagents
Human gastric epithelial AGS cells (ATCC, Manassas, VA) were maintained in Ham’s F12 medium containing 10% FBS as described14. H. pylori wild-type (WT) strains P12 and 7.13 were grown on Brucella-agar plates at 37°C using a micro-aerophilic gas pack system (BD Biosciences, Sparks, MD). WT H. pylori strains 7.13, P12, P12ΔcagL-deficient isogenic mutant strain and complemented P12ΔcagL/cagL were grown on Brucella-agar plates7. Bacterial multiplicities of infection (MOI) were calculated as described14. E. coli strain BL21 expressing pET28 His-tagged CagL (H. pylori TIGR strain 26695) expression plasmid was grown overnight in LB containing kanamycin (60 μg/ml) and induced with IPTG (1 mM, 1.5 hours)7. Overexpressed CagL in the inclusion bodies was purified as described7. RGD and control peptides were from Enzo Life Sciences (Plymouth Meeting, PA). All reagents were of molecular biology grade.
HKα Promoter-Luc Reporter Plasmid Transfection
HKα promoter-Luc reporter constructs (HKα2179 and HKα206) were formed by independently integrating two nesting segments (2179 bp and 206 bp) of proximal human gastric HKα 5′-flanking sequence into the luciferase reporter plasmid pGL2-Basic Vector as described13. Point mutations at −159 bp (AΠC, forward strand; TΠG, reverse strand) and at −161 bp (GΠA, forward strand; CΠT, reverse strand) were introduced into the NF-κB1 site of both constructs using QuikChange Lightning Site-Directed Mutagenesis Kit (Stratagene)14, and mutagenesis was verified by dideoxy sequencing. AGS cells were co-transfected with wild-type or mutated HKα promoter-Luc reporter constructs and pMaxGFP for transfection efficiency control and normalization, and promoter-reporter activities were measured and normalized as described27. Transfected cells were incubated with recombinant (r)CagL protein or recombinant human (rh)ADAM17 (R&D Systems, Minneapolis, MN) in Ham’s F12, 10% FBS at concentrations and times as specified.
ADAM17 Activity Measurement
AGS cells (3 × 104 cells/well) were plated overnight in 96-well plates, serum-deprived for 15–20 h and infected with WT H. pylori or corresponding mutant strains (MOI=50) for varying times. Cells were washed 3X with ice-cold phosphate-buffered saline (PBS) and overlaid with serum-free F12 medium (200 μl) containing quenched fluorogenic peptide substrate (10 μM; MCA-Pro-Leu-Ala-Gln-Ala-Val-Dpa-Arg-Ser-Ser-Ser-Arg-NH2; Cat. No. 616402, Calbiochem, CA). ADAM17 activity was measured spectrofluorometrically as the rate of change of relative fluorescence units (ΔRFU.s−1) as described16. rhADAM17 served as a positive control.
Small Interfering RNA (siRNA) Transfection
AGS cells (1.25 × 105, 75%–85% confluent) were incubated for 24 h with Dharmafect transfection reagent 1 (T-2001-07) and 100 nM pooled siRNAs against human ADAM17 (J-003453-05) or non-targeting siRNA (D-001810-03) (Dharmacon RNA Technology, Lafayette, CO). Effects of ADAM17-specific siRNA on HKα promoter activity were studied in AGS cells transiently-transfected with HKα2179 for 24 h and then infected with WT H. pylori strain P12 or P12ΔcagL (MOI=50, 8 h). Transcriptional activities of HKα2179-Luc constructs were measured as described27.
Real-time RT-PCR
Total RNA was isolated from AGS cells (80% confluent, serum-deprived for 15–20 h) using STAT-60 (Tel Test, Friendswood, TX) and reverse-transcribed using iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA). AGS cell ADAM17 mRNA was quantitated by RT-PCR using an iCycler iQ with iQ™ SYBR ® Green Super mix (Bio-Rad, Hercules, CA) and ADAM17-specific primer mix (PPH00343A; SuperArray Bioscience, Frederick, MD). Cellular β2-microglobin mRNA was used as a normalization control.
Immunoprecipitation and Immunoblotting
AGS cells (80% confluent, serum-deprived for 15–20 h) were infected (MOI=50, 1 h) with WT H. pylori strain P12 or P12ΔcagL, washed and harvested in ice-cold PBS, and centrifuged (520g, 5 min). Cell pellets were dissolved in Triton Lysis Buffer (Boston BioProducts, Worcester, MA) containing protease inhibitor cocktail (P8340, Sigma, St. Louis, MI) and Halt™ Phosphatase Inhibitor Cocktail (Thermo Scientific, Rockford IL) in a rotating shaker at 4°C for 30 min. Cell lysates were incubated overnight at 4°C with ADAM17-specific antibody (Santa Cruz Biotechnology, CA) or β1 integrin-specific antibody (AIIB2, developed by CH Damsky and NICHD, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA 52242) (5 μg antibody/mg lysate protein). After incubation at 4°C for 1 h with protein G-agarose beads, immunoprecipitated protein complexes were eluted with SDS-PAGE sample buffer and analyzed by immunoblotting as described16 using ADAM17- or β1 integrin-specific antibodies. Immunoprecipitations with non-immune IgG or protein G-agarose beads alone served as negative controls. ADAM17 protein levels following ADAM17-specific siRNA transfection and/or H. pylori infection were assessed by immunoblotting16, using ADAM17-specific antibody (QED, San Diego, CA) and p44/42 MAP kinase (ERK)-specific antibody (Cell Signaling Technology, Danvers, MA) or β-actin as gel loading controls. Immunoblots shown are representative of 3 replicates.
HB-EGF Shedding
AGS cells (80% confluent, serum-deprived for 15–20 h) were infected (MOI=50, 1 h) with WT H. pylori strain P12 or P12ΔcagL. Cellular supernatants were concentrated using 9 kDa cut-off protein concentrators (Thermo Scientific, Rockford, IL) and HB-EGF concentration was measured using HB-EGF DuoSet ELISA kit (R&D Systems, Minneapolis, MN).
Statistical Analysis
Statistical significance between treatment groups was determined by two-way ANOVA and Bonferroni post-test method using PRISM 4.0 (GraphPad, San Diego, CA). A P value <0.05 was defined as statistically significant.
Results
CagL Mediates H. pylori-induced Repression of HKα Promoter
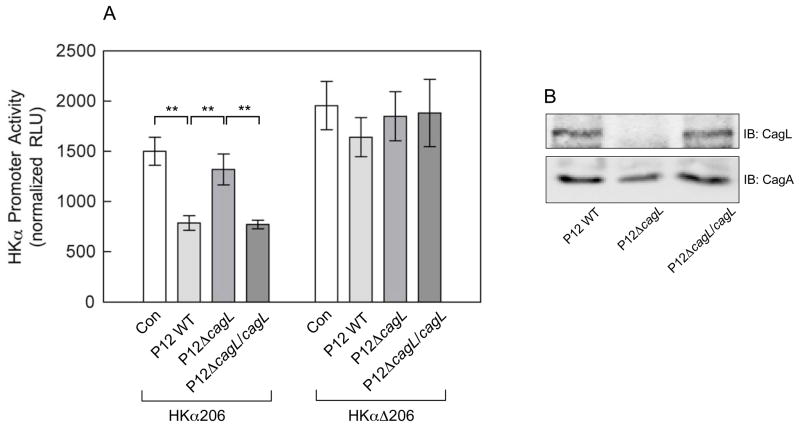
H. pylori represses HKα promoter activity in gastric epithelial cells by an NF-κB-dependent mechanism14. To test the hypothesis that CagL mediates this down-regulation of HKα expression, AGS cells were transfected with HKα promoter-Luc reporter constructs encompassing 206 bp of human HKα 5′-flanking sequence containing either the wild-type (HKα206) or 2 bp-mutated NF-κB binding site (HKαΔ206). The cells were then infected (MOI=50, 8h) with WT H. pylori strain P12, a cagL-deficient mutant strain (P12ΔcagL), or P12ΔcagL genetically-complemented with WT cagL (P12ΔcagL/cagL). Figure 1A shows that WT H. pylori and P12ΔcagL/cagL infection repressed HKα promoter activity by ~50%, while P12ΔcagL infection caused no significant repression, unambiguously implicating the cagL gene in HKα repression, and confirming that polar or other unrecognized mutations in P12ΔcagL play no role in this repression. Absence or presence of cagL in P12ΔcagL and P12ΔcagL/cagL was confirmed by immunoblotting (Figure 1B), and bacterial-AGS cell adhesion assay showed WT H. pylori to be marginally more adherent than P12ΔcagL (3019±118 vs 2610±116 cfu/103 cells, P=0.07). The mechanism through which CagL modulates HKα promoter activity depends on a functional NF-κB site in the promoter, because neither P12WT nor P12ΔcagL/cagL infections significantly affected HKαΔ206 activity (Figure 1A). Constitutive HKαΔ206 promoter activities in AGS cells were significantly higher than the wild-type promoter, suggesting that NF-κB exerts a tonic repression of HKα promoter activity in these cells. Subsequent experiments further investigated the mechanistic basis of CagL-mediated HKα repression.
Figure 1.
(A) CagL mediates H. pylori-induced repression of HKα promoter. AGS cells transfected with HKα206 or HKαΔ206 promoter-Luc reporter constructs were infected (MOI=50, 8h) with H. pylori WT P12, P12ΔcagL, or P12ΔcagL/cagL strains. HKα promoter activities in cell lysates were then measured as RLU of luciferin luminescence normalized to co-transfected GFP fluorescence (means, SD, n=3; **P<0.01). (B) Immunoblot of H. pylori WT P12, P12ΔcagL, and P12ΔcagL/cagL strain lysates probed with CagL-specific or CagA-specific (loading control) antibodies.
H. pylori Activates ADAM17 in AGS Cells
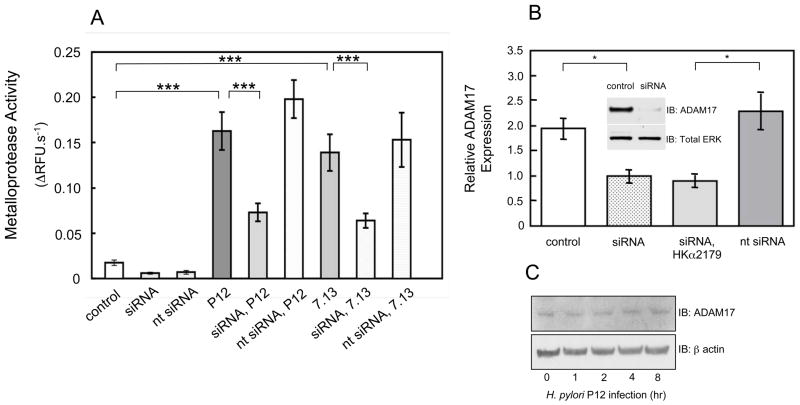
To determine the effect of the bacteria on metalloprotease activity, AGS cells were infected with H. pylori (MOI=50, 1 h) and metalloprotease activity was measured fluorometrically in ΔRFU.s−1 after medium replacement and addition of quenched fluorogenic substrate. WT H. pylori strains P12 or 7.13 increased metalloprotease activity ~9-fold and ~8-fold respectively compared to control (Figure 2A). However, P12 and 7.13 H. pylori infection of AGS cells transfected with ADAM17-specific siRNA increased metalloproteinase activity only 4-fold and 3.5-fold respectively. H. pylori-induced metalloprotease activation was comparable in cells transfected with nt siRNA and in untransfected cells. AGS cells and H. pylori alone showed low levels of metalloprotease activity (0.14 and 0.19 ΔRFU.s−1 respectively; not shown). AGS cell ADAM17 mRNA and ADAM17 protein levels were measured by real-time PCR and immunoblotting respectively 72 hrs after cell transfection with ADAM17-specific pooled siRNA comprising four individual siRNAs. Pooled siRNAs significantly repressed ADAM17 mRNA in AGS cells and also in AGS cells previously transfected with an HKα2179-Luc reporter plasmid, while nt siRNA transfection had no effect on ADAM17 expression (Figure 2B). AGS cell ADAM10 mRNA was unaffected by ADAM17-specific siRNA transfections (not shown). siRNA transfection also markedly repressed ADAM17 protein (92% by densitometric quantitation) compared to mock transfection (insert in Figure 2B). Total cell ADAM17 expression was unchanged over an 8 h time-course of H. pylori P12 infection (Figure 2C). Taken together, these data indicate that H. pylori infection of AGS cells causes activation of ADAM17.
Figure 2.
H. pylori activates ADAM17 in AGS cells. (A) AGS cells transfected with ADAM17-specific siRNA or non-targeting siRNA for 72 h were infected (MOI=50, 1 h) with WT H. pylori strains P12 or 7.13. After medium replacement, metalloproteinase activity was measured using a quenched fluorogenic oligopeptide substrate. Mock siRNA-transfected AGS cells served as a control (mean, SD, n=8; ***P<0.001). (B) ADAM17 siRNA-tranfected cells were subsequently transfected with HKα promoter-Luc reporter construct (HKα2179) and were then processed to extract RNA or lysed for immunoblot analysis. ADAM17 mRNA was measured 72 h after siRNA transfection using RT-PCR and β microglobin as the internal control (mean, SD, n=3; *P <0.05). (B insert) Immunoblot of ADAM17 protein in cell lysates 72 h after siRNA transfection (total ERK is the gel loading control. (C) Immunoblot of total cell ADAM17 expression over an 8 h time-course of H. pylori P12 infection.
CagL Mediates H. pylori-induced ADAM17 Activation in AGS Cells
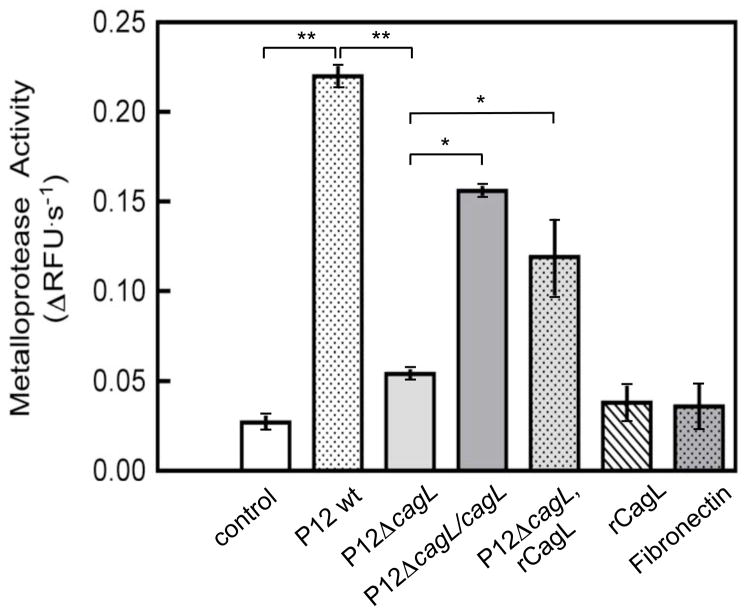
AGS cells were plated into untreated, rCagL-treated or fibronectin (FN)-treated 96-well plates and then infected with H. pylori (MOI=50, 8 h). Cellular ADAM17 activity was measured after medium replacement and addition of quenched fluorogenic substrate. WT H. pylori and P12ΔcagL/cagL infections induced a significantly greater increase in ADAM17 activity than P12ΔcagL mutant in AGS cells plated in untreated wells, and the presence of plate-adsorbed rCagL significantly restored ADAM17 activity in P12ΔcagL-infected cells (Figure 3). Plate-adsorbed FN (a natural ligand of integrins) or rCagL alone had no effect on ADAM17 activity, possibly due to adsorption-induced steric effects and/or low bound concentrations. These data indicate that H. pylori T4SS pilus CagL interaction with the host cell constitutes an extracellular signal to activate ADAM17. The finding that the P12ΔcagL mutant alone stimulated ADAM17 to a small extent implies that bacterial factors other than CagL are also involved in this activation.
Figure 3.
CagL mediates H. pylori-induced ADAM17 activation in AGS cells. AGS cells (3 × 104 cells/well) were incubated overnight in untreated, human recombinant (r) CagL-treated (5 μg/ml), or fibronectin-treated (5 μg/ml) 96-well plates, infected (MOI=50, 8 h) with WT H. pylori strain P12, P12 ΔcagL or genetically-complemented P12 ΔcagL/cagL, and after medium replacement cellular ADAM17 activity was measured using a quenched fluorogenic oligopeptide substrate (means, SD, n=8; *P<0.05, **P<0.01).
ADAM17 Down-regulation Abrogates H. pylori-induced HKα Promoter Repression
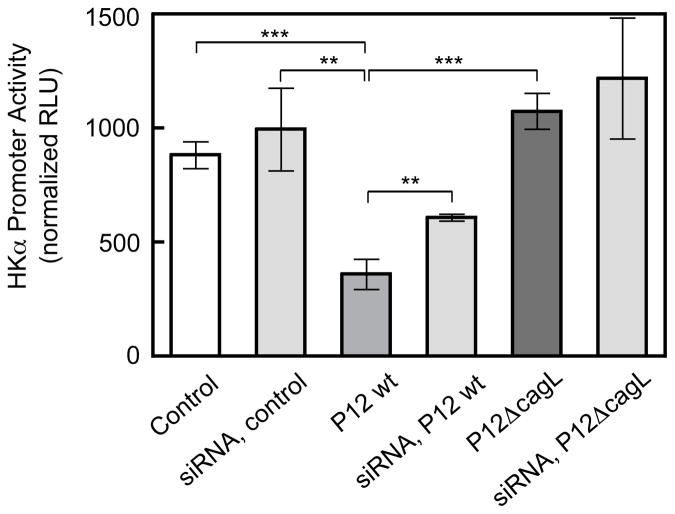
The coordinated roles of cagL and ADAM17 in repressing HKα gene expression were further investigated by measuring H. pylori-mediated HKα promoter activity in AGS cells with attenuated expression of ADAM17. AGS cells were transfected for 48 h with ADAM17-specific pooled siRNA or non-targeting siRNA, and then transfected for 24 h with HKα2179 promoter-Luc construct. The doubly-transfected AGS cells were infected with WT P12 H. pylori or P12ΔcagL (MOI=50, 8 h). As shown in Figure 4, ADAM17-specific pooled siRNA cell transfections had no significant effect on HKα2179 activity in mock-infected cells. HKα 2179 activity was repressed by 64% in control AGS cells infected with WT P12 H. pylori, and repressed by only 38% in ADAM17-silenced AGS cells. Inoculation of control AGS cells or ADAM17-silenced AGS cells with P12ΔcagL had no significant effect on HKα2179 activity. These data confirm that ADAM17 is a crucial component of the signaling pathway that links H. pylori infection of gastric epithelial cells to inhibition of HKα gene expression, and further emphasize the CagL-dependency of this repression.
Figure 4.
ADAM17 down-regulation abrogates H. pylori-induced HKα promoter repression. AGS cells were first transfected with ADAM17-specific pooled siRNAs and then with HKα2197 promoter-Luc constructs. The cells were then infected (MOI=50, 8 h) with WT H. pylori strain P12 or P12 ΔcagL. HKα promoter activity was measured as RLU of luciferin luminescence normalized to co-transfected GFP fluorescence (means, SD, n=3; **P<0.01, ***P<0.001).
CagL Mediates Dissociation of ADAM17 from β1 Integrin
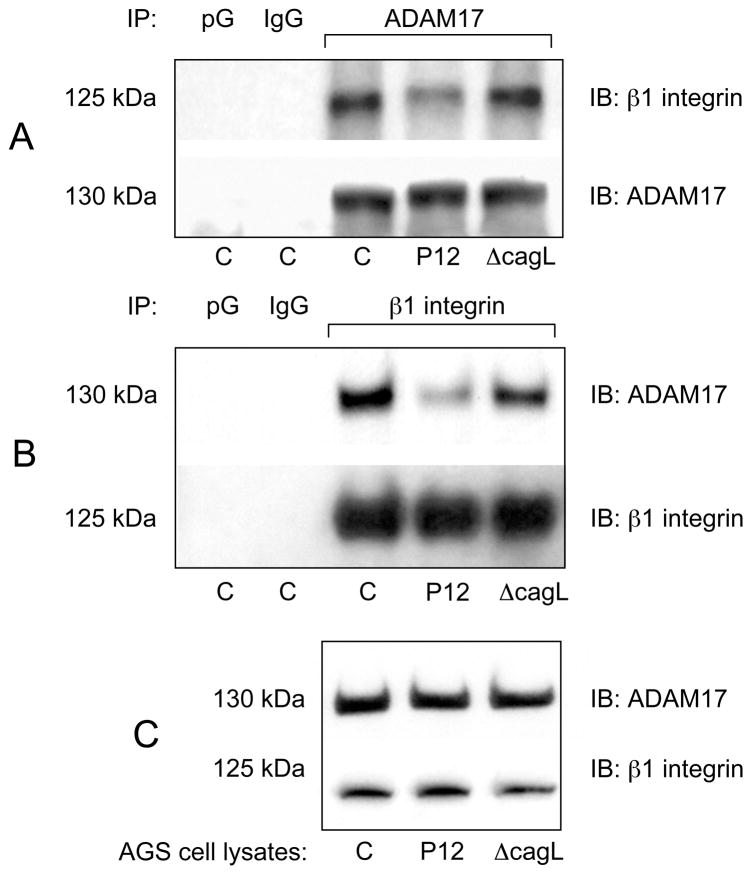
Because CagL has been reported to interact with host cell α5β1 integrin in the early stages of gastric epithelial cell infection by cagPAI-positive H. pylori7, we hypothesized that ADAM17 is bound to β1 integrin in non-infected AGS cells and that CagL may induce the dissociation of ADAM17 from this complex during infection. To test this idea, AGS cells were infected with WT H. pylori strain P12 or P12ΔcagL (MOI=50, 1 h), and the cell lysates were incubated with anti-ADAM17 antibody or with anti-β1 integrin antibody. The presence of bound β1 integrin or ADAM17 in the immunoprecipitated protein complexes was assessed by immunoblotting. As shown in Figure 5A, β1 integrin signal intensity was markedly reduced in ADAM17 immunoprecipitates of AGS cell lysates infected with WT P12 H. pylori, but not in lysates of cells infected with P12ΔcagL. Conversely, ADAM17 signal intensity was markedly reduced in β1 integrin immunoprecipitates of AGS cell lysates infected with WT P12 H. pylori, but not in lysates of cells infected with P12ΔcagL (Figure 5B). ADAM17 and β1 integrin immunoblots of corresponding immunoprecipitated lysates served as gel-loading controls (lower panels, Figure 5A and B). Figure 5C shows that total cell ADAM17 and β1 integrin were unaffected by H. pylori infection, indicating that H. pylori increases the ratio of active to inactive ADAM17 in AGS cells although the total cellular ADAM17 level remains unchanged. These data are consistent with binding of ADAM17 to β1 integrin in AGS cells, and with H. pylori-induced, CagL-dependent dissociation of ADAM17 from β1 integrin following exposure of AGS cells to H. pylori. Because WT H. pylori infection of AGS cells increased ADAM17 specific activity, the results are also consistent with H. pylori-induced ADAM17 dissociation from β1 integrin being accompanied by up-regulation of ADAM17 activity.
Figure 5.
CagL mediates dissociation of ADAM17 from β1 integrin. AGS cells were infected (MOI=50, 1 h) with WT H. pylori strain P12 or P12 ΔcagL. Cell lysates were immunoprecipitated with (A) ADAM17-specific antibody followed by immunoblotting (IB) of IP protein with β1 integrin-specific antibody; ADAM17 IB served as loading control. Reverse IP was performed by immunoprecipitating the lysates with (B) β1 integrin-specific antibody followed by IB with ADAM17-specific antibody; β1 integrin IB served as loading control.. (C) Direct ADAM17 and β1 integrin IB of cell lysates served as a gel loading control and a positive control of antibody specificity respectively. Protein G beads (pG) and non-immune immunoglobulin Gs (IgG)-precipitated lysates served as negative controls. Mock-infected AGS cells are shown in lanes “C”.
CagL Mediates H. pylori-induced Secretion of HB-EGF from AGS Cells
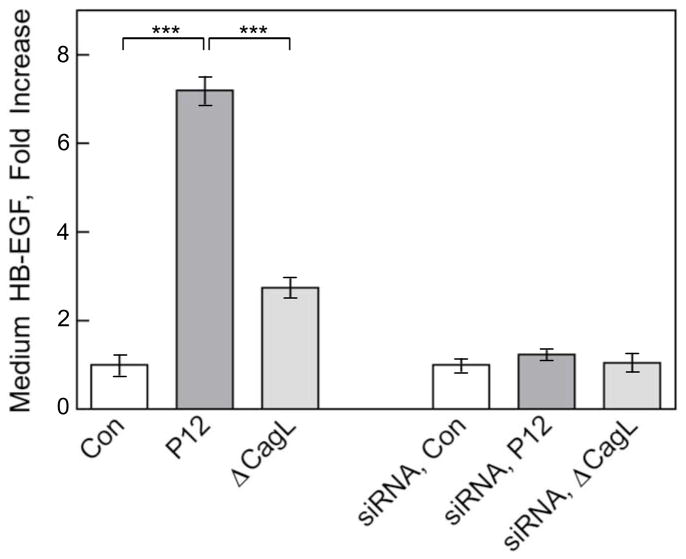
Given that ADAM17 releases membrane-bound precursors of EGFR ligands28, evidence was sought for H. pylori-mediated cleavage of heparin-binding epidermal growth factor (HB-EGF) from AGS cells in culture. Figure 6 shows that infection of AGS cells with WT H. pylori P12 or P12ΔcagL (MOI=50, 1 h) increased medium [HB-EGF] by 7-fold and 2.7-fold respectively, compared to mock-infected controls. siRNA depletion of ADAM17 expression in AGS cells abrogated H. pylori P12 and P12ΔcagL-mediated secretion of HB-EGF. Taken together with the data in Figure 2A, which shows that ADAM17 siRNA significantly blunts H. pylori-mediated ADAM17 activation, these data are consistent with CagL-dependent activation of ADAM17 causing AGS cell ectodomain shedding of HB-EGF.
Figure 6.
CagL mediates H. pylori-induced shedding of HB-EGF from AGS cells. AGS cells were infected (MOI=50, 1 h) with WT H. pylori strain P12 or P12 ΔcagL with or without previously silencing ADAM17 expression by siRNA. Culture medium supernatants were concentrated by centrifugal filtration and HB-EGF in the retentates was measured by ELISA (mean, SD, n=2; ***P<0.001).
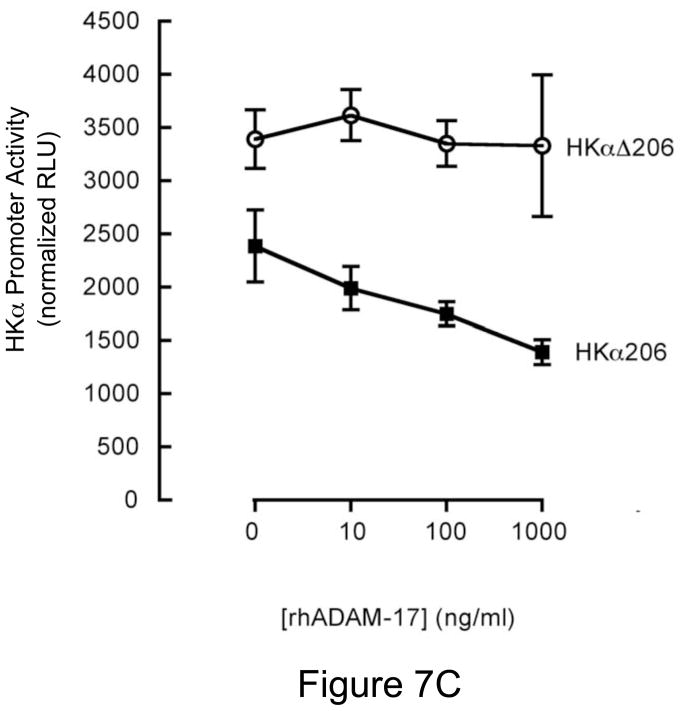
RGD-Peptide, rCagL and rhADAM17 Independently Repress HKα Promoter Activity
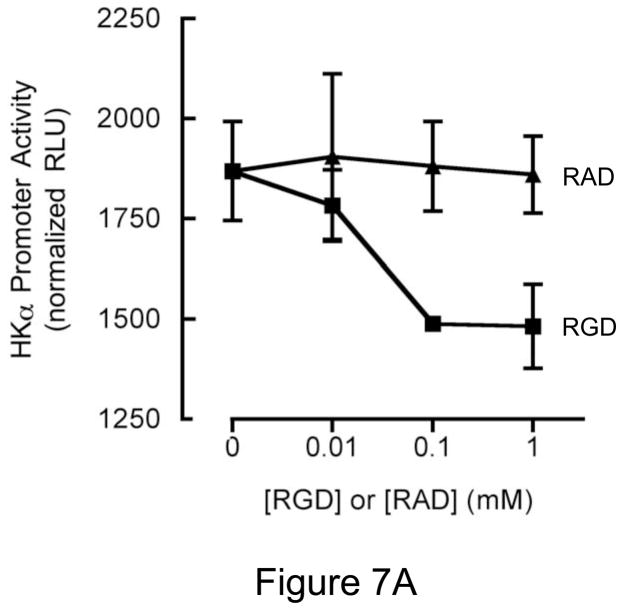
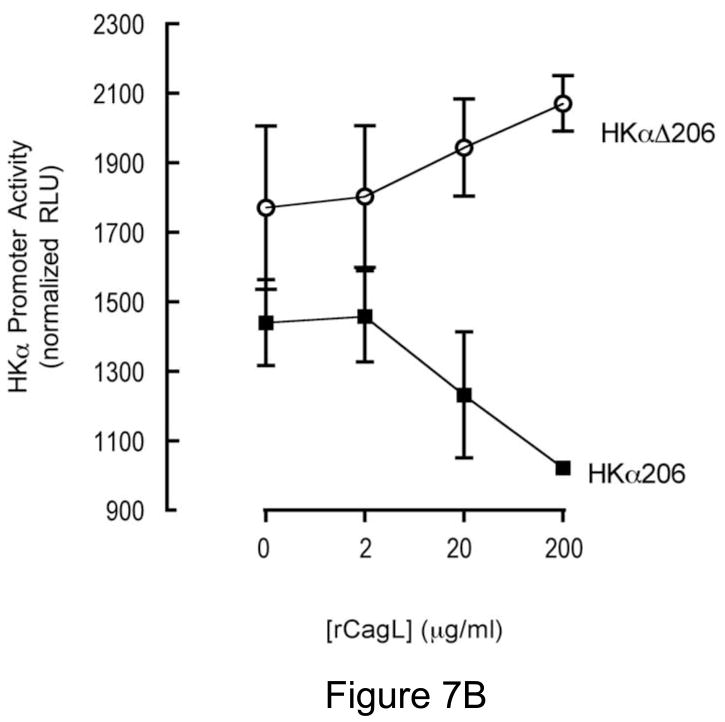
Given our finding that H. pylori-associated CagL activates host cell ADAM17 and represses HKα gene expression, and that CagL interaction with α5β1 integrins is mediated by an CagL N-terminal RGD motif7, we reasoned that treatment of AGS cells with synthetic RGD-containing peptide, rCagL, or rhADAM17 should repress transfected HKα promoter activity. To test these hypotheses, AGS cells were transfected with the HKα promoter-Luc constructs HKα206 or HKαΔ206. The cells were incubated for 8 h with increasing concentrations of RGD or RAD peptides, rCagL or rhADAM17, and HKα promoter activity was measured in cell lysates as RLU of luciferin luminescence. The RGD peptide, but not an RAD peptide used as a specificity control, caused a dose-dependent repression of transfected wild-type HKα promoter (Figure 7A). Co-incubation of AGS cells with rCagL also caused dose-dependent repression of transfected wild-type HKα promoter (Figure 7B). In contrast, constitutive activity of the NF-κB site-mutated HKα promoter (HKαΔ206) was higher than the wild-type promoter, and was stimulated with increased [rCagL]. Figure 7C shows that co-incubation of the wild-type HKα-transfected cells with enzymatically-active rhADAM17 (0.6 ΔRFU.s−1) resulted in dose-responsive repression of promoter activity, compared to untreated vehicle controls, whereas activity of the mutated HKα promoter remained relatively constant regardless of rhADAM17 concentration. Taken together, the data indicate that both CagL and ADAM17 participate mechanistically in H. pylori-mediated regulation of HKα promoter activity, and that the mechanism is dependent on the presence of a functional NF-κB binding site on the HKα promoter.
Figure 7.
RGD peptide, rCagL and rhADAM17 independently repress HKα promoter activity. AGS cells were transfected with a WT HKα promoter-Luc reporter construct (HKα206) or an HKα206 construct with two point mutations in the NF-κB binding site (HKαΔ206). Transfected AGS cells were incubated for 8 h with (A) synthetic RGD or RAD-containing peptides; (B) recombinant CagL (rCagL); or (C) recombinant human ADAM17 (rhADAM17) and HKα promoter activity was measured as RLU of luciferin luminescence normalized to co-transfected GFP fluorescence (means, SD, n=3).
Discussion
This study sought to identify the mechanistic basis of H. pylori-induced repression of proton pump gene expression13, 14, 29. Prolonged inhibition of acid secretion leads to intestinal metaplasia, a premalignant lesion, and to gastric cancer. In susceptible individuals, the H. pylori-induced mucosal progression to premalignancy occurs in this setting of reduced acid secretion, which in addition to facilitating initial colonization also activates pro-inflammatory pathways involved in development of disease10, 30, 31. Animal studies have shown that hypochlorhydria is a permissive factor in gastric cancer, rather than an epiphenomenon. Gastrin-deficient (G−/−) mice became hypochlorhydric, their stomachs becoming chronically inflamed, progressing to fundic atrophy, intestinal metaplasia, dysplasia, and eventually antral carcinoma3. HKα knock-out mice are achlorhydric, have abnormal parietal cells, and have ciliated metaplasia associated with dysplasia32. This recapitulation of the phenotypic progression to gastric cancer, triggered not by H. pylori but by perturbation of gastric pH, provides an important rationale for clarifying mechanisms whereby H. pylori inhibits acid secretion.
The cagPAI and the secreted bacterial oncoprotein CagA6, 33 facilitate H. pylori gastric corpus colonization, rather than the antrum34, placing the bacteria close to parietal cells and potentially jeopardizing acid secretion. Another cagPAI gene product, CagL, is dispensable for gastric colonization, but through its interaction with α5β1 integrin facilitates binding of the H. pylori T4SS-pilus to the cell surface enabling transfer of CagA into the cytoplasm7. To further clarify mechanisms of H. pylori-induced hypochlorhydria, we investigated the hypothesis that CagL initiates signaling that culminates in NF-κB activation and HKα repression by inducing dissociation of ADAM17 from α5β1 integrin.
Experimental evidence presented here is consistent with this hypothesis. AGS cell infection with WT H. pylori or an H. pylori cagL-deficient isogenic mutant (P12ΔcagL) genetically-complemented with cagL (P12ΔcagL/cagL) repressed transfected HKα promoter-Luc reporter activity and stimulated AGS cell ADAM17 activity. Both responses were abrogated by P12ΔcagL infection or by point mutations in the HKα NF-κB binding site. siRNA silencing of ADAM17 in AGS cells abrogated WT H. pylori-mediated HKα promoter repression and significantly repressed ADAM17 enzymatic activity and HB-EGF secretion induced by WT H. pylori strains P12 and 7.13. Co-immunoprecipitation and immunoblotting of AGS lysates revealed WT H. pylori-dependent attenuation of ADAM17/β1 complexes. Recombinant CagL or ADAM17 added to AGS cells transfected with HKα promoter-Luc reporter construct induced a dose-responsive repression of HKα promoter activity, as did addition of an RGD-containing synthetic peptide. CagL has been reported to interact with α5β1 integrin through its RGD motif7, and competitive displacement of ADAM17 from α5β1 integrin at this motif, as suggested by our data, may constitute a novel mechanism of ADAM17 activation. α5β1 integrin complexes and associated ADAM17 are expressed on gastric epithelial cell basolateral membranes, separated from apical membrane-adherent H. pylori by tight junctions. However, H. pylori is known to disrupt cell-cell junctions, gaining access to intercellular basolateral spaces and consequent contact with integrins35, 36. Thus, T4SS CagL may play a dual role by positioning T4SS on the host cell basolateral membrane to facilitate CagA translocation, and concurrently dissociating ADAM17 from α5β1 integrin with resulting activation of ADAM17. Chronic H. pylori infection of human gastric mucosa increases ADAM17 mRNA levels21 which may also increase ADAM17 enzymatic activity, but we propose that in the acute phase of H. pylori infection, ADAM17 activation is regulated by CagL-mediated dissociation from α5β1 integrin.
H. pylori infection of gastric epithelial cells transactivates EGFR by metalloproteinase-mediated shedding of HB-EGF37, 38. H. pylori is also known to induce cell proliferation through EGFR transactivation by ADAM activation39. ADAM17 catalyzes the release of membrane-bound precursors of the receptor ligands TNFα, EGF, HB-EGF and amphiregulin, among others20, 28. These ligands are inflammatory mediators and pro-proliferation factors, interacting with their receptors to activate receptor tyrosine kinase signaling leading to ERK1/2 activation. H. pylori is a known activator of ERK-1/2-mediated NF-κB signaling cascade9, 26 and this study demonstrates that H. pylori-induced ADAM17 activation in AGS cells is accompanied by shedding of the potent EGFR ligand HB-EGF into the medium, a down-stream consequence of which would be ERK-1/2-mediated NF-κB activation. We have previously documented H. pylori-mediated, EGFR-dependent NF-κB mobilization using MEK1/2 inhibitors14, and that NF-κB p50 homodimer interaction with a cis-response element on the HKα promoter represses HKα gene transcription14. Consistent with this finding, the present study demonstrates that mutation of the core NF-κB binding site of the HKα promoter abrogates H. pylori cagL-mediated, rCagL-mediated, RGD oligo peptide-mediated, and rhADAM17-mediated repression of promoter activity. Mutation of the NF-κB binding site significantly increased the constitutive activity of transfected HKα promoter-reporter constructs, suggesting that in transfected AGS cells, NF-κB p50 homodimer activity is constitutively elevated and exerts a tonic repression of HKα promoter by interaction with the NF-κB binding site.
Partial abrogation of H. pylori-induced HKα promoter repression in AGS cells expressing reduced levels of ADAM17 due to siRNA silencing (Figure 4), and the fact that the ΔcagL mutant strain also induces ADAM17 activation (although significantly less than WT H. pylori, Figure 3), indicate that while ADAM17 has a role in HKα gene repression, other CagL-dependent mechanisms are probably involved. The critical participation of CagL in binding of the H. pylori pilus to the cell surface, and the resultant capacitation of T4SS transfer functions, enable CagA, GM-tripeptide peptidoglycan and possibly other virulence factors to penetrate host cells. Interestingly, a yeast two-hybrid screen and GST-pulldown assays revealed that T4SS-associated CagY and CagA protein also bind to β1 integrin, which was proposed to facilitate the injection of CagA in an RGD-independent manner40. However, it remained unclear if and how binding of CagY and CagA to extracellular β1 integrin receptor could trigger intracellular signaling. Irrespective of the specific contribution of individual T4SS proteins (CagL, CagY and CagA) towards CagA injection, the present data from complementation of P12ΔcagL either genetically by WT cagL restoration or by extracellular addition of rCagL clearly demonstrate the crucial role of surface-exposed CagL in triggering signaling through β1 integrin and ADAM17. Following these interactions, intracellular CagA and GM-tripeptide both cause mobilization and nuclear localization of NF-κB, the former through activation of receptor tyrosine kinases that in turn up-regulate ERK-1/2 MAPK pathways9, 41, and the latter through interaction with the intracellular nuclear-oligomerization domain (Nod) 1 receptor, thereby inducing NF-κB activation in AGS cells42. The convergence of CagA, GM-tripeptide, and ADAM17 signaling at IκK complex degradation with emergence of NF-κB subunits allows for the formation of NF-κB p50 subunit homodimers, which have been shown to be necessary and sufficient for repression of HKα gene transcription14.
The extent to which the CagL-mediated repression of HKα promoter activity described here contributes to H. pylori-induced clinical hypochlorhydria is unclear. H pylori deploys at least two other mechanisms of acid inhibition during colonization. Vacuolating toxin secreted by H. pylori disrupts actin interaction with parietal cell apical membranes, preventing recruitment and fusion of H,K-ATPase-containing tubulovesicles and causing hypochlorhydria43. The inflammatory cytokine IL-1β, secreted by monocytes and neutrophils recruited by H. pylori-induced mucosal IL-8 production, also inhibits H,K-ATPase by impairing PI3K-mediated increases in [Ca2+]i44, 45. Indeed, host IL-1 gene polymorphisms causing increased IL-1β production in H. pylori infection magnify the risk of hypochlorhydria and gastric cancer46. Transcriptional repression of HKα contributing to H. pylori-induced acid inhibition would be consistent with the finding that H. pylori eradication results in increased H,K-ATPase expression47. We have also observed that HKα mRNA and H,K-ATPase levels in human gastric corpus biopsies are significantly depleted within 24 hours of WT H. pylori infection, but remained unaffected by infection with the ΔcagL mutant48.
In summary, this study demonstrates that H. pylori represses HKα transcriptional activity in gastric epithelial cells by inducing CagL-dependent ADAM17/β1 integrin dissociation, ADAM17 activation, HB-EGF secretion, and NF-κB binding to the HKα promoter. The marked perturbation of downstream signaling pathways caused by CagL interaction with the host cell constitutes a novel mechanism of H. pylori acid secretory pathogenesis, exemplifying how H. pylori orchestrates host cell signaling pathways to attain the goal of gastric colonization and persistence.
Acknowledgments
NIH DK070054 and ACS-IRG # 97-219-08 (to MG) and NIH DK064371 (to AS)
We thank Gary Shull for genomic DNA incorporating a portion of the human gastric H,K-ATPase α subunit 5′-flanking sequence, Richard M. Peek for provision of the wild-type H. pylori strain 7.13 and for valuable discussions, and Mamon Dey for skilled technical assistance.
Footnotes
Arindam Saha: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis.
Steffen Backert: Acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content.
Charles Hammond: Acquisition of data; statistical analysis; technical or material support.
Monika Gooz Study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; obtained funding; technical or material support.
Adam Smolka Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; technical or material support; study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graham DY, Alpert LC, Smith JL, et al. Iatrogenic Campylobacter pylori infection is a cause of epidemic achlorhydria. Am J Gastroenterol. 1988;83:974–80. [PubMed] [Google Scholar]
- 2.Morris A, Nicholson G. Experimental and accidental C. pylori infection in humans. In: Blaser MJ, editor. Campylobacter pylori in Gastritis and Peptic Ulcer Disease. New York: Igaku-Shoin; 1989. pp. 61–72. [Google Scholar]
- 3.Zavros Y, Eaton KA, Kang W, et al. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–66. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- 4.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 5.O’Toole D, Abdel-Latif MM, Long A, et al. Low pH and Helicobacter pylori increase nuclear factor kappa B binding in gastric epithelial cells: a common pathway for epithelial cell injury? J Cell Biochem. 2005;96:589–98. doi: 10.1002/jcb.20539. [DOI] [PubMed] [Google Scholar]
- 6.Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008;10:1573–81. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 7.Kwok T, Zabler D, Urman S, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862–6. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- 8.Mimuro H, Suzuki T, Tanaka J, et al. Grb2 Is a Key Mediator of Helicobacter pylori CagA Protein Activities. Molecular Cell. 2002;10:745–755. doi: 10.1016/s1097-2765(02)00681-0. [DOI] [PubMed] [Google Scholar]
- 9.Brandt S, Kwok T, Hartig R, et al. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci U S A. 2005;102:9300–5. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keates S, Hitti YS, Upton M, et al. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology. 1997;113:1099–109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Lim JW, Kim KH. Helicobacter pylori-induced expression of interleukin-8 and cyclooxygenase-2 in AGS gastric epithelial cells: mediation by nuclear factor-kappaB. Scand J Gastroenterol. 2001;36:706–16. doi: 10.1080/003655201300191969. [DOI] [PubMed] [Google Scholar]
- 12.Keates S, Keates AC, Warny M, et al. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag− Helicobacter pylori. J Immunol. 1999;163:5552–9. [PubMed] [Google Scholar]
- 13.Gooz M, Hammond CE, Larsen K, et al. Inhibition of human gastric H(+)-K(+)-ATPase alpha-subunit gene expression by Helicobacter pylori. Am J Physiol Gastrointest Liver Physiol. 2000;278:G981–91. doi: 10.1152/ajpgi.2000.278.6.G981. [DOI] [PubMed] [Google Scholar]
- 14.Saha A, Hammond CE, Trojanowska M, et al. Helicobacter pylori-induced H,K-ATPase {alpha}-subunit gene repression is mediated by NF-{kappa}B p50 homodimer promoter binding. Am J Physiol Gastrointest Liver Physiol. 2008;294:G795–807. doi: 10.1152/ajpgi.00431.2007. [DOI] [PubMed] [Google Scholar]
- 15.Moss ML, Jin SL, Milla ME, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–6. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 16.Gooz M, Gooz P, Luttrell LM, et al. 5-HT2A receptor induces ERK phosphorylation and proliferation through ADAM-17 tumor necrosis factor-alpha-converting enzyme (TACE) activation and heparin-bound epidermal growth factor-like growth factor (HB-EGF) shedding in mesangial cells. J Biol Chem. 2006;281:21004–12. doi: 10.1074/jbc.M512096200. [DOI] [PubMed] [Google Scholar]
- 17.Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci. 1999;112 (Pt 21):3603–17. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- 18.Bridges LC, Bowditch RD. ADAM-Integrin Interactions: potential integrin regulated ectodomain shedding activity. Curr Pharm Des. 2005;11:837–47. doi: 10.2174/1381612053381747. [DOI] [PubMed] [Google Scholar]
- 19.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 20.Bax DV, Messent AJ, Tart J, et al. Integrin {alpha}5{beta}1 and ADAM-17 Interact in Vitro and Co-localize in Migrating HeLa Cells. J Biol Chem. 2004;279:22377–22386. doi: 10.1074/jbc.M400180200. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura T, Tomita T, Dixon MF, et al. ADAMs (a disintegrin and metalloproteinase) messenger RNA expression in Helicobacter pylori-infected, normal, and neoplastic gastric mucosa. J Infect Dis. 2002;185:332–40. doi: 10.1086/338191. [DOI] [PubMed] [Google Scholar]
- 22.Tuccillo C, Manzo BA, Nardone G, et al. Up-regulation of heparin binding epidermal growth factor-like growth factor and amphiregulin expression in Helicobacter pylori-infected human gastric mucosa. Dig Liver Dis. 2002;34:498–505. doi: 10.1016/s1590-8658(02)80108-6. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Gabazza EC, Imoto I, et al. Vacuolating cytotoxin A is associated with increased thrombin generation in gastric mucosa. Helicobacter. 2005;10:323–31. doi: 10.1111/j.1523-5378.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 24.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 25.Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33:369–85. doi: 10.1053/j.seminoncol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Keates S, Keates AC, Katchar K, et al. Helicobacter pylori induces up-regulation of the epidermal growth factor receptor in AGS gastric epithelial cells. J Infect Dis. 2007;196:95–103. doi: 10.1086/518440. [DOI] [PubMed] [Google Scholar]
- 27.Saha A, Hammond CE, Gooz M, et al. IL-1beta modulation of H,K-ATPase {alpha}-subunit gene transcription in Helicobacter pylori infection. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1055–1061. doi: 10.1152/ajpgi.00338.2006. [DOI] [PubMed] [Google Scholar]
- 28.Sahin U, Weskamp G, Kelly K, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–79. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saha A, Hammond CE, Gooz M, et al. The role of Sp1 in IL-1beta and H. pylori-mediated regulation of H,K-ATPase gene transcription. Am J Physiol Gastrointest Liver Physiol. 2008;295:G977–86. doi: 10.1152/ajpgi.90338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merchant JL. Inflammation, atrophy, gastric cancer: connecting the molecular dots. Gastroenterology. 2005;129:1079–82. doi: 10.1053/j.gastro.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 31.Peek RM, Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233–48. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- 32.Spicer Z, Miller ML, Andringa A, et al. Stomachs of Mice Lacking the Gastric H,K-ATPase alpha -Subunit Have Achlorhydria, Abnormal Parietal Cells, and Ciliated Metaplasia. J Biol Chem. 2000;275:21555–21565. doi: 10.1074/jbc.M001558200. [DOI] [PubMed] [Google Scholar]
- 33.Ohnishi N, Yuasa H, Tanaka S, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci U S A. 2008;105:1003–8. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rieder G, Merchant JL, Haas R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology. 2005;128:1229–42. doi: 10.1053/j.gastro.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 35.Amieva MR, Vogelmann R, Covacci A, et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–4. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wessler S, Backert S. Molecular mechanisms of epithelial-barrier disruption by Helicobacter pylori. Trends Microbiol. 2008;16:397–405. doi: 10.1016/j.tim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Keates S, Sougioultzis S, Keates AC, et al. cag+ Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J Biol Chem. 2001;276:48127–34. doi: 10.1074/jbc.M107630200. [DOI] [PubMed] [Google Scholar]
- 38.Wallasch C, Crabtree JE, Bevec D, et al. Helicobacter pylori-stimulated EGF receptor transactivation requires metalloprotease cleavage of HB-EGF. Biochem Biophys Res Commun. 2002;295:695–701. doi: 10.1016/s0006-291x(02)00740-4. [DOI] [PubMed] [Google Scholar]
- 39.Joh T, Kataoka H, Tanida S, et al. Helicobacter pylori-Stimulated Interleukin-8 (IL-8) Promotes Cell Proliferation Through Transactivation of Epidermal Growth Factor Receptor (EGFR) by Disintegrin and Metalloproteinase (ADAM) Activation. Digestive Diseases and Sciences. 2005;50:2081–2089. doi: 10.1007/s10620-005-3011-0. [DOI] [PubMed] [Google Scholar]
- 40.Jimenez-Soto LF, Kutter S, Sewald X, et al. Helicobacter pylori type IV secretion apparatus exploits beta1 integrin in a novel RGD-independent manner. PLoS Pathog. 2009;5:e1000684. doi: 10.1371/journal.ppat.1000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higashi H, Nakaya A, Tsutsumi R, et al. Helicobacter pylori CagA induces Ras-independent morphogenetic response through SHP-2 recruitment and activation. J Biol Chem. 2004;279:17205–16. doi: 10.1074/jbc.M309964200. [DOI] [PubMed] [Google Scholar]
- 42.Viala J, Chaput C, Boneca IG, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–74. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 43.Wang F, Xia P, Wu F, et al. Helicobacter pylori VacA disrupts apical membrane-cytoskeletal interactions in gastric parietal cells. J Biol Chem. 2008;283:26714–25. doi: 10.1074/jbc.M800527200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallace JL, Cucala M, Mugridge K, et al. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. Am J Physiol. 1991;261:G559–64. doi: 10.1152/ajpgi.1991.261.4.G559. [DOI] [PubMed] [Google Scholar]
- 45.Schepp W, Dehne K, Herrmuth H, et al. Identification and functional importance of IL-1 receptors on rat parietal cells. Am J Physiol. 1998;275:G1094–105. doi: 10.1152/ajpgi.1998.275.5.G1094. [DOI] [PubMed] [Google Scholar]
- 46.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 47.Osawa H, Kita H, Ohnishi H, et al. Helicobacter pylori eradication induces marked increase in H+/K+-adenosine triphosphatase expression without altering parietal cell number in human gastric mucosa. Gut. 2006;55:152–7. doi: 10.1136/gut.2005.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saha A, Hammond CE, Beeson C, et al. Helicobacter pylori represses proton pump expression and inhibits acid secretion in human gastric mucosa. Gut. 2010 doi: 10.1136/gut.2009.194795. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]