Abstract
Background & Aims
Hepatic steatosis is associated with insulin resistance, but it is not clear whether increased intrahepatic triglyceride (IHTG) content causes the resistance or is a marker. Subjects with familial hypobetalipoproteinemia (FHBL) have high levels of IHTG because of a genetic defect in hepatic export of triglycerides, and provide a unique cohort to study the relationship between steatosis and insulin sensitivity.
Methods
One group of lean subjects with normal IHTG content (2.2%±0.6% of liver volume) (n=6), and 3 groups of overweight and obese subjects, matched for body-mass index, were studied: 1) normal IHTG content (3.3%±0.5%; n=6), 2) high IHTG content (21.4%±2.6%) due to nonalcoholic fatty liver disease (NAFLD; n=6), and 3) high IHTG content (18.1%±2.2%) due to FHBL (n=3). A hyperinsulinemic-euglycemic clamp procedure, in conjunction with glucose tracer infusion, was used to determine multi-organ insulin sensitivity.
Results
Hepatic insulin sensitivity (reciprocal of glucose rate of appearance [μmol · kgFFM−1 · min−1] × insulin [mU · L−1]) was greatest in the lean group (2.0±0.4); it was the same among subjects with FHBL (0.8±0.1) and the group with normal IHTG content, matched for body-mass index, (0.7±0.1), but greater than the NAFLD group (0.3±0.1) (P<.01). Muscle insulin sensitivity (percent increase in glucose uptake during insulin infusion) was greatest in the lean group (576%±70%). Muscle insulin sensitivity was similar in subjects with FHBL and those with normal IHTG (319%±77%, 326%±27%, respectively), but greater than the NAFLD group (145%±18%) (P<.01).
Conclusions
Steatosis is dissociated from insulin resistance in FHBL, which suggests that increased IHTG content is a marker, not a cause, of metabolic dysfunction.
Keywords: steatosis, insulin sensitivity, obesity, clamp
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a common complication of obesity.1 Excessive intrahepatic triglyceride (IHTG) content is associated with insulin-resistant glucose metabolism in both liver and skeletal muscle and impaired insulin-mediated suppression of lipolysis in adipose tissue. 2–6 In fact, we have found that IHTG is a better predictor of multi-organ insulin resistance than body mass index (BMI), percent body fat and visceral fat mass.7 However, it is not known whether excessive IHTG content causes insulin resistance or is simply a marker of systemic metabolic dysfunction.
Patients who have familial hypobetalipoproteinemia (FHBL) provide a unique opportunity for exploring the relationship between IHTG content and insulin action, because the genetic truncation of apolipoprotein B (apoB) impairs hepatic very-low density lipoprotein-triglyceride (VLDL-TG) export and causes an accumulation of IHTG.8 The amount of IHTG in patients with FHBL is about a 3-fold higher than healthy volunteers matched on age, sex, and BMI.9 The effect of steatosis induced by FHBL on insulin action is not clear, because of limited and potentially conflicting data from previous studies 10–12. In one study, insulin and glucose areas under the curve during an oral glucose tolerance test were ~50 % greater in non-obese subjects with FHBL than healthy non-obese volunteers, but the differences between groups were not statistically significant.10, 11 Data from another study found that insulin resistance, assessed by using the homeostasis model of insulin resistance (HOMA-IR), in non-obese subjects with FHBL was similar to values obtained in healthy control subjects, and lower than more obese subjects with NAFLD.12 We are not aware of any studies that evaluated specific organ insulin sensitivity in subjects with FHBL.
Therefore, the purpose of the present study was to determine whether increased IHTG content caused by a genetic defect in TG secretion, is associated with multi-organ insulin resistance, as reported in subjects who have increased IHTG content as part of typical NAFLD. A hyperinsulinemic-euglycemic clamp procedure, in conjunction with stable isotopically-labeled tracer infusion, was performed in overweight and obese subjects with FHBL and subjects matched on BMI who had either normal or increased IHTG content to assess hepatic and skeletal muscle insulin sensitivity. We hypothesized that insulin sensitivity would be better in subjects who have increased IHTG content because of FHBL than those who have typical NAFLD.
Materials and Methods
Subjects
Three groups of overweight and obese subjects participated in this study: 1) normal IHTG content (≤5.5% of liver volume) (n=6, all women; age 43±3.8 y), 2) excessive IHTG content (>10% of liver volume) due to NAFLD (n=6, 2 men, 4 women, age 38.2±5.9 y), and 3) excessive IHTG content (>10% of liver volume) with FHBL due to APOB gene heterozygosis (n=3, 1 man, 2 women, age 59.7±2.9 y). A fourth group consisted of lean healthy individuals with normal IHTG content (≤5.5% of liver volume) (n=6, all women, age 56±1.1 y.). Fewer subjects were recruited to the FHBL group than the other groups because it is difficult to find eligible participants for this cohort. Subjects in the first three groups were matched on BMI, and the NAFLD and FHBL groups were also matched on IHTG content. All subjects completed a comprehensive medical evaluation, which included a 2-hour oral glucose tolerance test. No subject had any history or evidence of liver disease other than NAFLD, consumed more than 20 g/day of alcohol, had impaired glucose tolerance, diabetes or other serious illnesses. Subjects gave their written informed consent before participating in this study, which was approved by the Human Research Protection Office of Washington University School of Medicine.
Body composition analyses
Visceral adipose tissue (VAT) mass and IHTG content was determined by using magnetic resonance imaging and magnetic resonance spectroscopy and (Siemens, Erlanger, Germany) as we have described previously.13 Fat mass (FM) and fat-free mass (FFM) were determined by using dual-energy X-ray absorptiometry (Hologic QDR 4500, Waltham, MA).
Hyperinsulinemic-euglycemic clamp procedure
Subjects were admitted to the Clinical Research Unit at Washington University School of Medicine the night before the clamp procedure and consumed a standard meal at 1800 h. After subjects fasted overnight, a catheter was inserted into an antecubital vein to infuse tracer, insulin, and dextrose. Another catheter was inserted into a contralateral radial artery, to obtain blood samples. After a baseline blood sample was obtained to determine the background plasma glucose tracer-to-tracee ratio (TTR), a primed-continuous infusion of [6,6-2H2]glucose (priming dose: 22.5 μmol/kg; infusion rate: 0.25 μmol · kg−1 · min−1) was initiated. At 210 min, an insulin infusion was started (initiated with a 2-step priming dose of 160 mU/m2 per min for 5 min followed by 80 mU/m2 per min for 5 min) and maintained at a rate of 50 mU/m2 per min for 180 min. Dextrose (20%) was infused at a variable rate to maintain plasma glucose concentration at 100 mg/dL. The dextrose solution was enriched with [6,6-2H2]glucose (~2.5%) to minimize changes in plasma glucose TTR during the clamp procedure.14 The infusion of [6,6-2H2]glucose was stopped during the clamp procedure (from 210 to 390 min) to account for the expected decline in hepatic glucose production. Blood samples were taken every 10 min during the last 30 min of the basal period and the clamp procedure to determine plasma glucose and insulin concentrations and glucose basal and clamp TTRs.
Analyses of samples and calculations
Plasma glucose, insulin and apoB concentrations were measured by using an automated glucose analyzer (Yellow Spring Instruments Co, Yellow Springs, OH), a chemiluminescent immunometric assay (Immulite 1000), and immunonephelometry,15 respectively. Plasma glucose TTRs were determined by using electron impact ionization gas chromatography-mass spectroscopy (GC-MS; MSD 5973 system with capillary column; Hewlett-Packard; Palo Alto, CA), as previously described.16, 17
During steady-state conditions, total (endogenous and exogenous) glucose rate of appearance (Ra) in plasma is equal to glucose rate of disappearance (Rd), and was calculated by dividing the glucose tracer infusion rate by the average plasma glucose TTR during the last 30 min of the basal period and the hyperinsulinemic euglycemic clamp procedure. Endogenous glucose Ra was calculated by subtracting the known exogenous unlabeled glucose infusion rate from the total Ra. Skeletal muscle insulin sensitivity was assessed by calculating the relative increase from basal in glucose Rd during insulin infusion. Skeletal muscle insulin sensitivity was also assessed as the absolute increase in glucose Rd divided by the absolute increment in circulating insulin concentration during insulin infusion to adjust for potential differences in achieved insulin concentrations. Hepatic insulin sensitivity was assessed by the Hepatic Insulin Sensitivity Index (HISI), which is the inverse of the product of the basal hepatic glucose production rate (in μmol · kg FFM−1 · min−1) and the fasting plasma insulin concentration (in mU/L).18, 19
Statistical analysis
After evaluating normality and equal variance assumptions, analysis of variance was used to compare mean values across groups. When assumptions were violated, data transformations were explored and implemented when appropriate, with a log transformation being applied before triglyceride means were compared. When appropriate data transformations could not be identified, analysis of variance was applied nonparametrically to the ranks of the data. Within the framework of all analyses of variance, pairwise comparisons were performed using the appropriate statistical contrast. A P value <.05 was considered statistically significant. All data are presented as means ± SEM. Statistical analyses were performed using version 9.2 of SAS.
Results
Body composition and metabolic variables
IHTG content was higher in the FHBL and NAFLD groups than in the Normal IHTG and Lean groups (Table 1). Mean plasma apoB concentration in the FHBL group was almost 3-fold lower than the mean values in the Normal IHTG and NAFLD groups (25.5±5.3, 68.9±6.7 and 78.8±5.9 mg/dL, respectively; P<.01). Basal glucose concentration was similar in all 4 groups, but plasma insulin concentration was higher in the NAFLD group than in Normal IHTG, FHBL, and Lean groups (Table 1). LDL-cholesterol was significantly lower in FHBL, than in all other groups (Table 1).
Table 1.
Body composition and metabolic variables in the study subjects
| Lean | Normal IHTG | NAFLD | FHBL | P value† | |
|---|---|---|---|---|---|
| Body mass index (kg/m2) | 23.5±0.6* | 31.8±0.8 | 33.9±0.5 | 31.6±5.8 | 0.0003 |
| Visceral adipose tissue (cm3) | 956±158 | 818±146 | 1492±292 | 1466±97 | 0.07 |
| IHTG content (%) | 2.2±0.6** | 3.3±0.5** | 21.4±2.6 | 18.1±2.2 | <0.0001 |
| Glucose (mg/dL) | 93.2±2.3 | 94.7±3.1 | 94.2±2.8 | 99.2±4.0 | 0.64 |
| Insulin (mU/L) | 4.6±0.9** | 9.8±0.8 | 22.8±3.8** | 10.3±1.5 | <0.0001 |
| Free fatty acid (μmol/mL) | 0.60±0.03 | 0.42±0.04 | 0.60±0.06 | 0.54±0.08 | 0.07 |
| LDL-cholesterol (mg/dL) | 115±14** | 101±11** | 102±9** | 38±9 | 0.007 |
| HDL-cholesterol (mg/dL) | 56±5 | 53±5 | 46±6 | 50±9 | 0.65 |
| Triglyceride (mg/dL) | 91±14 | 95±14 | 207±41** | 60±8 | 0.0016 |
| ALT (IU/L) | 13.8±1.5 | 23.3±3.8 | 78.7±22.6* | 21.7±0.7 | 0.001 |
| AST (IU/L) | 17.3±0.8 | 18.7±1.0 | 39.8±9.8* | 21.3±2.4 | 0.03 |
IHTG=intrahepatic triglyceride; NAFLD=nonalcoholic fatty liver disease; FHBL=familial hypobetalipoproteinemia; AST=aspartate aminotransferase; ALT=alanine aminotransfase. Data are means±SEM.
P value represents significance of differences between any groups. Value significantly different from the corresponding value in the FHBL group;
P<.05,
P<.01.
Basal kinetics and insulin sensitivity
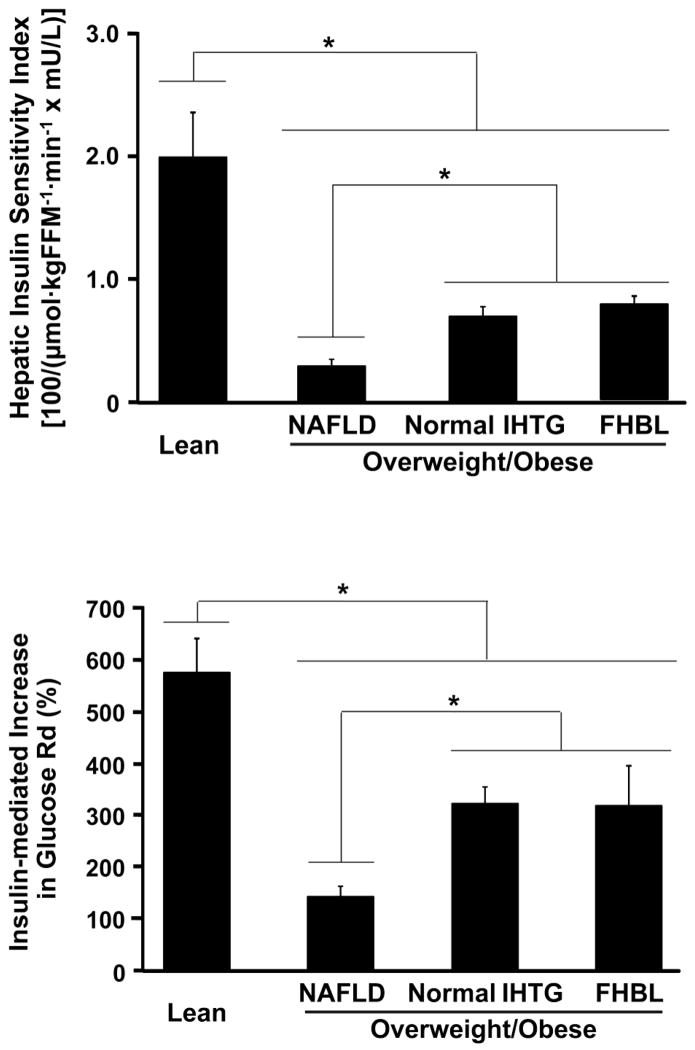
Hepatic insulin sensitivity was greatest in Lean subjects (Figure 1, top panel). Hepatic insulin sensitivity in subjects with FHBL was the same as in BMI-matched subjects with normal IHTG, but was double the value observed in those with NAFLD (P<.01), (Figure 1, top panel). Basal glucose Rd, expressed per kg of FFM, was not different between groups (14.3±0.3, 14.5±0.9, 14.8±0.5, and 15±0.9 μmol · kg FFM−1 · min−1 in Lean, FHBL, Normal IHTG and NAFLD, respectively). Insulin infusion during the hyperinsulinemic-euglycemic clamp procedure increased plasma insulin concentrations to 80.8±5.9, 87.6±9.5, 71.7±7.5, and 119.5±4.8 mU/L in Lean, Normal IHTG, FHBL, and NAFLD groups, respectively (P<.01, NAFLD vs other groups). Plasma glucose concentrations during the clamp procedure were 105.3±1.4, 104.9±2.7, 100.2±1.8 and 98.9±1.1 mg/dL in Lean, Normal IHTG, FHBL, and NAFLD groups, respectively. Free fatty acid (FFA) concentrations were not different between groups at baseline (Table 1). Plasma FFA concentrations decreased during insulin infusion to 0.02±0.01, 0.03±0.01, 0.03±0.01, and 0.08±0.01 μmol/ml in Lean, Normal IHTG, FHBL, and NAFLD groups, respectively (P<.01, NAFLD vs other groups). Glucose Rd increased during insulin infusion to 96.6±10.1, 60.4±10.6, 63.2±4.8, and 37.3±4.6 μmol · kg FFM−1 · min−1 in Lean, FHBL, Normal IHTG and NAFLD, respectively. The relative increase in glucose Rd during insulin infusion in subjects with FHBL was the same as in BMI-matched subjects with normal IHTG, but was double the value observed in those with NAFLD (P<.01) (Figure 1, bottom panel). The relative increase in glucose Rd during insulin infusion was greatest in Lean subjects.
Figure 1.
Hepatic insulin sensitivity calculated as the product of glucose Ra in plasma during the basal stage of the clamp procedure (top panel), muscle insulin sensitivity assessed as the relative increase in glucose rate of disappearance (Rd) during insulin infusion (bottom panel) in subjects with familial hypobetalipoproteinemia (FHBL), normal intrahepatic triglyceride (IHTG) content and nonalcoholic fatty liver disease (NAFLD) and Lean controls (Lean). Values are means±SEM. *P<.01.
Discussion
Although NAFLD is common in obese persons and is associated with multi-organ insulin resistance6, 7, 20, 21 it is not known whether steatosis causes insulin resistance or whether insulin resistance is responsible for IHTG accumulation. In the present study, we attempted to dissect the relationship between steatosis and insulin action by evaluating obese subjects who had steatosis because of FHLB. These patients often have an accumulation of IHTG because of a genetic impairment in secreting VLDL.8, 10 Our data demonstrate that hepatic and skeletal muscle insulin sensitivity in overweight and obese subjects with FHBL is greater than subjects with NAFLD, matched on BMI, VAT volume and IHTG content. Moreover, hepatic and skeletal muscle insulin sensitivity in obese subjects with FHBL was the same as in obese subjects with normal IHTG and lower visceral adiposity. However, obese subjects with either normal IHTG content or high IHTG content due to FHBL are still insulin resistant compared with lean subjects. These data demonstrate a dissociation between steatosis and insulin resistance, and support the concept that increased IHTG content in obese subjects is a marker, not a cause, of metabolic dysfunction.
Dissociation between hepatic steatosis and insulin resistance has previously been observed in genetically and pharmacologically manipulated mouse models. Overexpression of hepatic diacylglycerol acyltransferase, which stimulates triglyceride synthesis,22 deletion of hepatic microsomal triglyceride transfer protein, which prevents the assembly and secretion of VLDL-triglyceride,23 and pharmacological blockade of hepatic fatty acid β-oxidation24 cause hepatic steatosis, without hepatic or skeletal muscle insulin resistance. The dissociation between IHTG content and insulin resistance observed in our obese subjects with FHBL suggests that either steatosis is a consequence of metabolic dysfunction or other factors associated with NAFLD, such as hepatic inflammation,25 endoplasmic reticulum stress,26 intracellular lipid intermediates,27 or as yet unidentified metabolites, are responsible for insulin resistance.
In summary, the results from our small series of subjects demonstrate that intrahepatic accumulation of TG does not necessarily cause insulin resistance, and suggest that intracellular TG itself is likely inert. However, in the appropriate setting, such as standard NAFLD, increased IHTG represents systemic metabolic dysfunction. Our observation in subjects with genetically-induced hepatic steatosis is analogous to the dissociation between elevated intramyocellular triglycerides and skeletal muscle insulin resistance observed in endurance-trained athletes.28 These data have important implications regarding the mechanisms responsible for the pathophysiology associated with ectopic fat distribution, and challenges the current concept that increased intracellular TG itself causes cellular metabolic dysfunction.
Acknowledgments
The authors wish to thank Bruce Patterson, Adewole Okunade, Freida Custodio, and Jennifer Shew for their technical assistance, the staff of the Clinical Research Unit for their help in performing the studies, and the study subjects for their participation.
This study was supported by National Institutes of Health grants DK 37948, DK 56341 (Nutrition and Obesity Research Center), RR024992 (Clinical and Translational Science Award), and RR-00954 (Biomedical Mass Spectrometry Resource). No conflicts of interests exist.
Abbreviations
- IHTG
intrahepatic triglycerides
- BMI
body mass index
- apoB
apolipoprotein B
- FHBL
familial hypobetalipoproteinemia
- VLDL-TG
very-low density lipoprotein-triglyceride
- NAFLD
nonalcoholic fatty liver disease
- HISI
hepatic insulin sensitivity index
- FFM
fat free mass
Footnotes
Study concept and design AA, EF, GS, SK; acquisition of data AA, EF, MK; analysis and interpretation of data AA, EF, SK, KS, PY; drafting of the manuscript AA, SK; critical revision of the manuscript for important intellectual content GS, EF, MK, PY; study supervision SK.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic and clinical implications. Hepatology. 2009 doi: 10.1002/hep.23280. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 3.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 4.Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564–1572. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 5.Reeds DN, Yarasheski KE, Fontana L, Cade WT, Laciny E, DeMoss A, et al. Alterations in liver, muscle, and adipose tissue insulin sensitivity in men with HIV infection and dyslipidemia. Am J Physiol Endocrinol Metab. 2006;290:E47–E53. doi: 10.1152/ajpendo.00236.2005. [DOI] [PubMed] [Google Scholar]
- 6.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schonfeld G, Lin X, Yue P. Familial hypobetalipoproteinemia: genetics and metabolism. Cell Mol Life Sci. 2005;62:1372–1378. doi: 10.1007/s00018-005-4473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yue P, Tanoli T, Wilhelm O, Patterson B, Yablonskiy D, Schonfeld G. Absence of fatty liver in familial hypobetalipoproteinemia linked to chromosome 3p21. Metabolism. 2005;54:682–688. doi: 10.1016/j.metabol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Schonfeld G, Patterson BW, Yablonskiy DA, Tanoli TS, Averna M, Elias N, et al. Fatty liver in familial hypobetalipoproteinemia: triglyceride assembly into VLDL particles is affected by the extent of hepatic steatosis. J Lipid Res. 2003;44:470–478. doi: 10.1194/jlr.M200342-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J Lipid Res. 2004;45:941–947. doi: 10.1194/jlr.M300508-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Lonardo A, Lombardini S, Scaglioni F, Carulli L, Ricchi M, Ganazzi D, et al. Hepatic steatosis and insulin resistance: does etiology make a difference? J Hepatol. 2006;44:190–196. doi: 10.1016/j.jhep.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Frimel TN, Deivanayagam S, Bashir A, O’Connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–138. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 14.Finegood DT, Pacini G, Bergman RN. The insulin sensitivity index. Correlation in dogs between values determined from the intravenous glucose tolerance test and the euglycemic glucose clamp. Diabetes. 1984;33:362–368. doi: 10.2337/diab.33.4.362. [DOI] [PubMed] [Google Scholar]
- 15.Contois J, McNamara JR, Lammi-Keefe C, Wilson PW, Massov T, Schaefer EJ. Reference intervals for plasma apolipoprotein A-1 determined with a standardized commercial immunoturbidimetric assay: results from the Framingham Offspring Study. Clin Chem. 1996;42:507–514. [PubMed] [Google Scholar]
- 16.Patterson BW, Zhao G, Klein S. Improved accuracy and precision of gas chromatography/mass spectrometry measurements for metabolic tracers. Metabolism. 1998;47:706–712. doi: 10.1016/s0026-0495(98)90035-x. [DOI] [PubMed] [Google Scholar]
- 17.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 18.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 19.Gastaldelli A, Miyazaki Y, Pettiti M, Buzzigoli E, Mahankali S, Ferrannini E, et al. Separate contribution of diabetes, total fat mass, and fat topography to glucose production, gluconeogenesis, and glycogenolysis. J Clin Endocrinol Metab. 2004;89:3914–3921. doi: 10.1210/jc.2003-031941. [DOI] [PubMed] [Google Scholar]
- 20.Vega GL, Chandalia M, Szczepaniak LS, Grundy SM. Metabolic correlates of nonalcoholic fatty liver in women and men. Hepatology. 2007;46:716–722. doi: 10.1002/hep.21727. [DOI] [PubMed] [Google Scholar]
- 21.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 22.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Minehira K, Young SG, Villanueva CJ, Yetukuri L, Oresic M, Hellerstein MK, et al. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J Lipid Res. 2008;49:2038–2044. doi: 10.1194/jlr.M800248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grefhorst A, Hoekstra J, Derks TG, Ouwens DM, Baller JF, Havinga R, et al. Acute hepatic steatosis in mice by blocking beta-oxidation does not reduce insulin sensitivity of very-low-density lipoprotein production. Am J Physiol Gastrointest Liver Physiol. 2005;289:G592–598. doi: 10.1152/ajpgi.00063.2005. [DOI] [PubMed] [Google Scholar]
- 25.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 26.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]