Abstract
Background & Aims
Several studies have examined structural brain changes associated with chronic pain syndromes, including irritable bowel syndrome (IBS), but study sample sizes have been small and heterogeneous.
Methods
We used magnetic resonance imaging (MRI)-based techniques, voxel-based morphometry, and cortical thickness analysis, to examine brain anatomical differences in a relatively large, tightly screened sample of IBS patients (n=55); we compared data with that from healthy individuals (controls, n=48).
Results
IBS was associated with decreased gray matter density (GMD) in widespread areas of the brain, including medial prefrontal and ventrolateral prefrontal cortex, posterior parietal cortex, ventral striatum, and thalamus. Compared with controls, we observed increased GMD in patients with IBS in the pregenual anterior cingulate cortex and the orbitofrontal cortex, as well as trends in the posterior insula/secondary somatosensory cortex, (para)hippocampus, and left dorsolateral prefrontal cortex. In accounting for anxiety and depression, we found that several of the regions involved in affective processing no longer differed between patients with IBS and controls, whereas the differences in prefrontal and posterior parietal cortices remained. The areas of decreased GMD associated with IBS were largely consistent across clinical subgroups, based on predominant bowel habit and pain predominance of symptoms. There were no overall or regional differences in cortical thickness between patients with IBS and controls.
Conclusions
Changes in density of gray matter among regions involved in cognitive/evaluative functions are specifically observed in patients with IBS, whereas changes in other areas of the brain can be accounted for explained by levels of anxiety and depression.
Keywords: neuroimaging, VBM, neural, visceral
Introduction
Irritable bowel syndrome (IBS) is a complex, symptom-based disorder defined by recurrent abdominal pain or discomfort associated with alterations in bowel habits1. The syndrome is heterogeneous, with subgroups based on predominant bowel habits, and most bothersome symptoms. Increased anxiety (or anxiety disorders) is observed in the majority of patients2. As would be predicted with such a heterogeneous syndrome, results of functional neuro-imaging studies in IBS patients show variable results; nevertheless, increased regional activity in insula (INS) and anterior midcingulate cortex (aMCC) have been most commonly reported3.
IBS has been categorized as a “functional” pain syndrome, along with a number of other syndromes that have as a characteristic pain of unknown origin, including fibromyalgia, chronic low back pain, headache, and chronic vulvar pain. Although increased afferent drive may contribute to the hypersensitivity of these syndromes, accumulating evidence suggests that an alteration in descending pain modulation, and an associated alteration in cortico-limbic-pontine brain circuits may make an important contribution to the hypersensitivity4. Structural abnormalities in these regions have been identified in small populations of patients suffering from different persistent pain disorders (including fibromyalgia5-8, chronic low back pain9, 10, and headache/migraine11-13 and chronic vulvar pain14). Although decreases in gray matter size predominate, in some cases, increases in regional gray matter are observed and interpreted as a possible use-dependent hypertrophy14. The relationship of changes in gray matter density (GMD) with functional alterations and symptoms in pain disorders, and the underlying mechanisms remain incompletely understood. Regional GMD changes may be a trait of patients or could be consequence of disease through mechanisms such as neurotoxity or neuroinflammation (in the case of GMD decreases) or from use-related increases 15-18. Two studies have also reported morphometric differences between small samples of IBS patients and healthy controls, which included a reduction in GMD and cortical thickness (CT) in aMCC 19, 20. However, these studies did not determine which characteristics of IBS patients (e.g., pain, other IBS symptoms, anxiety, depression) contributed to the anatomical abnormalities.
In the current study, we attempted to dissect possible components of neuro-anatomical abnormalities in IBS patients by evaluating a large tightly screened sample of female IBS patients, for which we had information about multiple IBS symptoms, as well as measures of anxiety and depression. We hypothesized that: 1) IBS is associated with decreased gray matter in regions shown to have increased responsiveness to rectal distension and its expectation, including INS and ACC, possibly due to over-use excitotoxicity; 2) IBS is associated with an increase in gray matter in some regions, particularly in regions involved in cognitive and attentional modulation of interoceptive information, consistent with a role of these regions in central pain amplification; 3) depression, anxiety, and predominant symptom explain part of the anatomical differences between IBS and controls.
Materials and Methods
Subjects
The sample consisted of 106 women of whom 56 had IBS and 49 were age matched female controls from 3 different fMRI study protocols performed on the same MRI scanner. These studies were approved by the Office for the Protection of Research Subjects at the University of California, Los Angeles. Subjects for these studies were recruited by advertisement and from specialty clinics of the UCLA Division of Digestive Diseases. All patients were evaluated by 1 of 2 gastroenterologists at the UCLA Center for Neurobiology of Stress, experienced in the diagnosis of functional gastrointestinal disorders. IBS patients met ROME II criteria and were screened by a psychologist using a structured psychiatric interview (SCID) to exclude any patient with a recent DSM IV diagnosis of mood and affect disorders. Patients were also excluded if they were taking any centrally acting medications, such as antidepressants (TCAs, SSRIs, NSRIs), anxiolytics, or pain medications. Three subjects were excluded due to missing behavioural data (n=2) and poor MRI data (n=1).
Questionnaires
Each subjects completed the Brief Symptom Inventory (BSI21}, the Hospital Anxiety and Depression Scale (HADS22) and the UCLA Digestive Disease Center Symptom Questionnaire (BIS23), and reported his or her most bothersome symptom and bowel habits (constipation, diarrhea, or alternating). Symptom severity was assessed on a 5-point scale from 0. None: no symptoms to 4. Very severe: markedly affects my lifestyle.
MRI
Brain images were acquired on a 3T MRI scanner (Siemens Allegra). First, a sagittal scout was used to position the head. Then each subject underwent a high-resolution 3D T1-weighted, sagittal, magnetization prepared rapid gradient echo (MPRAGE) [TR = 2.3ms, TE = 0.00285ms, flip angle 9, final resolution 1×1×1mm].
VBM and CTA preprocessing
We used the CIVET pipeline (v. 1.1.9) for all preprocessing of VBM and CTA data (http://wiki.bic.mni.mcgill.ca/index.php/CIVET). Details (including references) of these methods are provided in Supplementary Methods. For VBM, tissues were classified as gray matter (GM), white matter (WM), or CSF, and smoothed 8mm. Cortical thickness in mm was calculated at 81924 vertices for each brain and smoothed 20mm. Cerebellum was excluded from analyses.
Analysis
For whole brain tissue analyses, we extracted the mean gray matter density (GMD), white matter density (WMD), and CSF density, as well at the mean cortical thickness for each individual. We plotted these values against age and performed a one-way ANOVA to compare between groups (SPSS 16.0, SPSS Inc.).
We used SurfStat (http://www.stat.uchicago.edu/∼worsley/surfstat/) for VBM and CTA. We applied a general linear model (GLM) comparing groups, with age as a covariate of no interest. Separate GLMs comparing controls and IBS were run for CTA and VBM with the covariates: age; age + anxiety; age + depression; age + anxiety + depression. In addition for VBM, we performed separate analyses comparing subgroups of IBS patients with age as a covariate. The subgroups were based on bowel habits (constipation (n=15), diarrhea (n=17), alternating (n=19), or unspecified (n=5), or whether the most bothersome symptom was pain (pain predominant, n=17) or something else (non-pain predominant, n=38). However, all patients reported abdominal pain as one of their symptoms, and met the Rome II criteria24. For all analyses, corrections for multiple comparisons were performed using random field theory-based cluster analysis25. We set a threshold so that only contiguous voxels with a t-value of greater than 2.5 could be considered in the cluster analysis. Results from the GLM analyses are thus reported as corrected at the cluster level. For the CTA, we ran a threshold of threshold set at p<0.05 for the voxels to be included in the cluster analysis.
For brain regions known to process nociceptive information and interoception, including primary (S1) and secondary (S2) somatosensory cortices, INS, ACC, and PFC, brainstem, and thalamus, regions which are also most commonly observed to have different GMD between controls and chronic pain populations (e.g.14), we applied a liberal corrected threshold (p<0.1; note that the cluster contains voxels showing a minimum t-value of 2.5, so the uncorrected threshold at the voxel level is about p<0.01).
For each significant cluster from the GLM analysis between IBS and control groups removing age, we extracted the mean GMD for each subject for plotting results, and for the analyses below.
To compare the pain predominant (n=17) and non-pain predominant (n=38) groups, we computed GLMs between groups with age as a covariate for the average GMD in each significant cluster in the overall group comparison between IBS and controls.
To test for effects of disease duration and symptom severity, we performed bivariate correlation analyses between each of the variables IBS duration and IBS symptom severity and the GMD each significant cluster from the GLM analysis between IBS and control groups removing age.
Results
Patient characteristics
Subject characteristics (age) and behavioural variables (depression and anxiety) for IBS and controls are shown in Table 1. Mean ages (SD, range) for IBS and controls were 31.0 (12.3, 19-57) and 32.2 (10.1, 19-63), respectively. Of those 39 controls and 53 IBS who provided menses stage information, 5 controls and 4 IBS were postmenopausal. Patients had a wide range of disease duration (11.1+7.7, median 9.0; range: 1 to 34 years) and moderate IBS symptom severity (2.0+0.5, median 2.0; range 1 to 3 on a scale from 0 to 4).
Table 1. Subject characteristics.
Values are presented as mean (standard deviation). F- and p-values are from one-way ANOVA between IBS (n=55) and controls.
| IBS (n=55) | IBS-C (n=15) | IBS-D (n=17) | IBS-A (n=19) | Controls (n=48) | F-value (p-value) | |
|---|---|---|---|---|---|---|
| Age | 32.2 (12.3) | 35.0 (13.4) | 32.0 (5.46) | 31.2 (10.9) | 31.1 (12.3) | 0.243 (0.62) |
| Anxiety¥ | 5.89 (3.50) | 6.67 (3.67) | 5.24 (2.68) | 5.21 (3.16) | 3.49 (2.38) | 16.5 (<0.0001) |
| Depression¥ | 2.98 (2.77) | 3.27 (2.40) | 3.06 (2.81) | 2.21 (2.15) | 0.80 (0.922) | 27.4 (<0.00001) |
| IBS severityǂ | 2.00 (0.544) | 2.07 (0.704) | 2.00 (0.612) | 1.95 (0.405) | ||
| IBS duration (years) | 11.1 (7.73) | 16.5 (9.78) | 9.06 (6.06) | 8.47 (5.98) |
on a scale of 0 (None: no symptoms), to 4 (Very severe: markedly affects my lifestyle)
depression and anxiety were measured on the HADS; possible scores for each of anxiety and depresssion range from 0 to 21
IBS-C, IBS-D, and IBS-A, constipation, diarrhea, and alternating, respectively.
Although IBS had statistically significantly higher anxiety (5.9+3.5 vs 3.5+2.4; on a scale from 0- 21; p<0.001) and depression scores (3.0+2.8 vs 0.8+0.9; on a scale from 0 - 21; p<0.001) than controls, both individual values and means for both groups were at subclinical levels for anxiety and depression. Further characteristics of the sample subgroups are given in Table 1.
Whole brain tissue densities
Regardless of group, we found age-related decreases in overall GMD and cortical thickness, and age-related increases in CSF, as expected (Figure 1). No significant differences between groups were seen for these whole brain age-related changes (one-way ANOVA, p>0.5 for each tissue type).
Figure 1. Age-related brain changes.
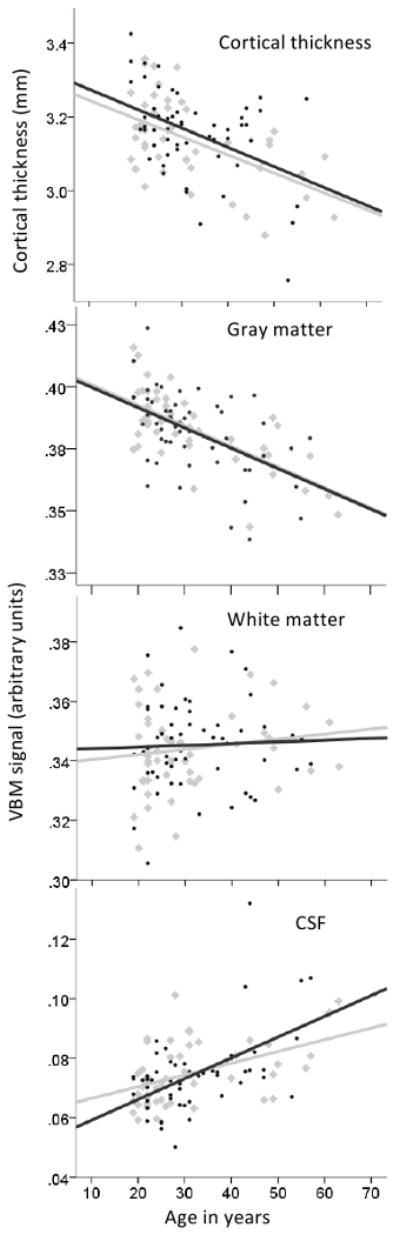
Scatter plots for IBS (black symbols and regression lines) and healthy controls (gray symbols and regression lines) are shown for average cortical thickness (CT), gray matter density (GMD), white matter density, and cerebrospinal fluid (CSF) density across the whole brain. Age-related decreases in GMD and CT are as expected, with no apparent differences between IBS and controls.
VBM
VBM results are summarized in Figures 2 and Table 2. Both decreases and increases in GMD in the IBS group compared to controls were observed.
Figure 2. VBM results.
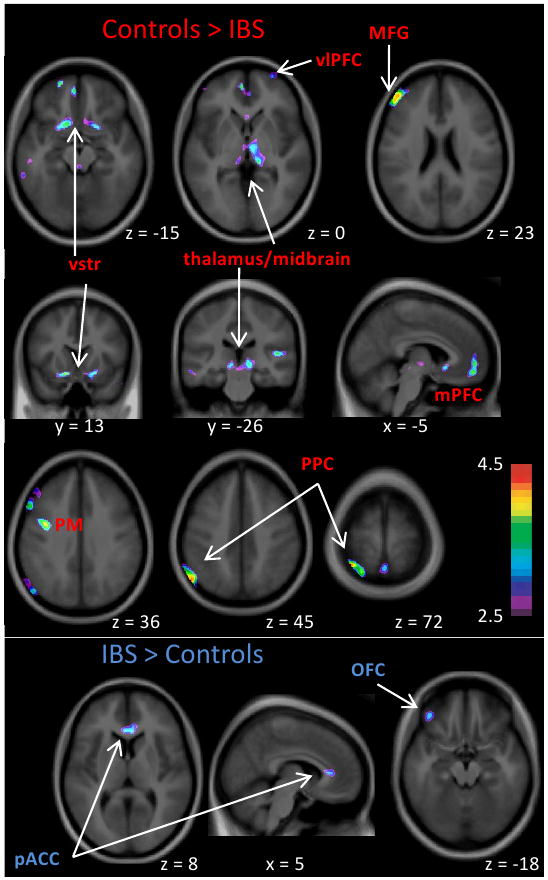
Significant GMD clusters from GLM comparing IBS and controls, with age as a covariate. Peak coordinates and correlations with behavioural variables are shown in Table 2. Results are displayed on a group average brain in stereotaxic (MNI) space. See table 2 for abbreviations. Colour bar shows t-value. Left side of image is left side of brain.
Table 2. Cluster corrected results from GLMs.
The column labels indicate the factors used as covariates. IBS-C (n=15), IBS-D (n=17), IBS-A (n=19), pain predominant (n=17), and non-pain predominant (n=38) refer to GLMs in which these subgroups are compared to controls, with age as a covariate.
| age | age/anx | age/dep | age/anx/dep | IBS-C | IBS-D | IBS-A | pain | non-pain | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cluster | side | Peak x,y,z | T-val | vol | pval | vol | pval | vol | pval | vol | pval | pval | pval | pval | pval | pval | ||
| control>IBS | ||||||||||||||||||
| PPC | left | -38 | -53 | 69 | 4.45 | 6550 | 0.00002 | 11046 | 0.00000 | 19935* | 0.00000 | 4006 | 0.00037 | 0.02250 | 0.00018 | 0.03706 | ||
| MFG | left | -47 | 46 | 18 | 4.32 | 5407 | 0.00007 | 8291 | 0.00000 | 14158* | 0.00000 | 1368 | 0.01989 | 0.03333 | 0.02720 | 0.01029 | 0.00014 | |
| thalamus bilat | left | -12 | -29 | -4 | 3.66 | 4901 | 0.00012 | 205 | 0.32757 | 1637 | 0.01218 | 0.06771 | 0.00189 | 0.12237 | ||||
| right | 13 | -29 | -2 | 3.36 | 1399 | 0.01876 | 0.00028 | 0.00141 | ||||||||||
| temporal | right | 30 | 26 | -36 | 3.72 | 3668 | 0.00057 | 5001 | 0.00011 | 7259 | 0.00001 | 3144 | 0.00116 | |||||
| vstr bilat | left | -14 | 14 | -13 | 3.94 | 3042 | 0.00134 | 0.00203 | 0.24716 | |||||||||
| right | 22 | 12 | -12 | 3.47 | 0.00097 | 0.00081 | ||||||||||||
| mPFC | left | -7 | 55 | 9 | 4.56 | 2244 | 0.00442 | 2003 | 0.00653 | 14158* | 0.00000 | 0.02640 | 0.00153 | |||||
| precun | right | 8 | -77 | 64 | 3.72 | 2227 | 0.00454 | 7479 | 0.00001 | 19935* | 0.00000 | 3887 | 0.00043 | 0.02692 | ||||
| temporal | left | -59 | -30 | -11 | 3.39 | 1905 | 0.00768 | 4707 | 0.00015 | 0.00605 | 0.00089 | |||||||
| PM6 | left | -38 | 0 | 37 | 4.02 | 1363 | 0.02008 | 1417 | 0.01814 | 1344 | 0.02081 | 0.01538 | 0.01825 | |||||
| vlPFC | right | 27 | 67 | -4 | 3.56 | 1303 | 0.02250 | 2651 | 0.00237 | 0.00354 | ||||||||
| occipital | left | -21 | -79 | 11 | 4.70 | 1095 | 0.03392 | 1272 | 0.02389 | 969 | 0.04409 | 0.04818 | ||||||
| STG | right | 46 | -27 | 11 | 3.52 | 1001 | 0.04120 | 1671 | 0.01147 | 1924 | 0.00744 | |||||||
| Fr pole | left | -21 | 60 | -15 | 3.73 | 982 | 0.04289 | 14158* | 0.00000 | 4162 | 0.00030 | 0.00209 | 0.00153 | |||||
| putamen | left | -31 | -11 | 11 | 2.95 | 888 | 0.05250 | 1396 | 0.01887 | |||||||||
| vlPFC | left | -39 | 40 | -9 | 3.68 | 768 | 0.06873 | 14158* | 0.00000 | 0.04337 | ||||||||
| vlPFC | right | 55 | 38 | 26 | 3.09 | 703 | 0.08001 | 4921 | 0.00012 | |||||||||
| IBS>control | ||||||||||||||||||
| pACC | left | -3 | 30 | 9 | 3.14 | 1156 | 0.03000 | 1078 | 0.03512 | 0.01838 | 0.00027 | |||||||
| OFC | left | -41 | 38 | -22 | 3.53 | 983 | 0.04280 | 0.00064 | ||||||||||
| dLPFC | left | -42 | 19 | 34 | 3.91 | 745 | 0.07249 | 0.02648 | ||||||||||
| Hc/pHc | left | -38 | -59 | -14 | 4.02 | 740 | 0.07334 | 923 | 0.04865 | 2390 | 0.00352 | 0.03333 | 0.00960 | |||||
| Hc/pHc | right | 31 | -42 | -19 | 3.44 | 641 | 0.09291 | 926 | 0.04834 | |||||||||
| Hc/pHc | left | -30 | -32 | -22 | 3.40 | 639 | 0.09337 | 2390 | 0.00352 | 0.00960 | ||||||||
| S2/pINS | right | 46 | -26 | 23 | 3.27 | 621 | 0.09761 | 0.01308 | ||||||||||
Peak x,y,z, coordinates in MNI/ICBM standard for analysis with age as covariate; Tvalue refers to that peak. vol, volume in mm3; pval, corrected (cluster level) p-value; PPC, posterior parietal cortex; thalamus bilat, bilateral thalamus, midbrain; vStr bilat, bilateral ventral striatum; vlPFC, ventrolateral prefrontal cortex; mPFC, medial prefrontal cortex; precun, precuneus; PM6, premotor Brodmann area 6; sTG, superior temporal gyrus; pACC, pregenual ACC; OFC, orbitofrontal cortex; dlPFC, dorsolateral prefrontal cortex; Hc/pHc, hipposcampus/parahippocampal gyrus; S2/pINS, secondary somatosensory cortex, posterior insula.
indicates that cluster contains multiple regions.
Decreased GMD in IBS
The group GLM with age as a covariate of no interest revealed decreased GMD clusters in IBS relative to controls in left posterior parietal cortex (PPC; BA 40/7) and precuneus, bilateral temporal lobe (right ventral temporal pole, left middle and inferior temporal gyri); bilateral vlPFC (BA 10) and medial PFC (mPFC, BA 10), left middle frontal gyrus (MFG, BA 46), bilateral thalamus/midbrain (this cluster consisted primarily of bilateral ventral medial thalamus, which extended ventrally to posterior medial midbrain (which contains the periaqueductal gray (PAG)), bilateral ventral striatum, premotor cortex (BA 6), and a small cluster in occipital cortex.
Increased GMD in IBS
Clusters with greater GMD in IBS than controls included midline pregenual ACC (pACC, BA 24/32) and left orbitofrontal cortex (OFC, BA 11). At a lower threshold (p<0.1 corrected), other areas with increased GMD included bilateral hippocampus/parahippocampus (Hc/pHc), right S2/posterior insula cortex (S2/pINS), and left dlPFC (BA 9). The increased S2/pINS and Hc/pHc GMD are shown in Supplementary Figure 1 as T-maps masked by clusters.
Covariate with anxiety and depression
Results of analyses that included the addition of anxiety, depression, or anxiety and depression as covariates (along with age in each case) for IBS versus controls, are summarized in Table 2. Inclusion of either anxiety or depression as covariates abolished the group differences in bilateral ventral striatum, and left OFC. Inclusion of anxiety and depression together as covariates additionally removed the group differences in bilateral thalamus/midbrain and left pACC. However, even after controlling for depression and anxiety, group effects between IBS and controls remained in left PPC, bilateral temporal cortices, and left MFG.
IBS symptom based subgroups
Comparing pain predominant (n = 17) and non-pain predominant groups demonstrated lower GMD in the pain predominant subgroup (F1,32 = 7.11, p=0.010) in the dlPFC cluster that was found to have greater GMD in IBS than controls. A comparison of the dlPFC decrease compared to controls in Figure 4 (far right column) indicates that only the non-pain predominant group showed this GMD increase. Also shown in Figure 4 are areas of reduced GMD in all IBS compared with controls that are apparently driven by either the pain predominant group (vlPFC, vStr) or the non-pain predominant group (thalamus/midbrain, mPFC).
Figure 4. GMD differences between pain predominant and non-pain predominant groups.
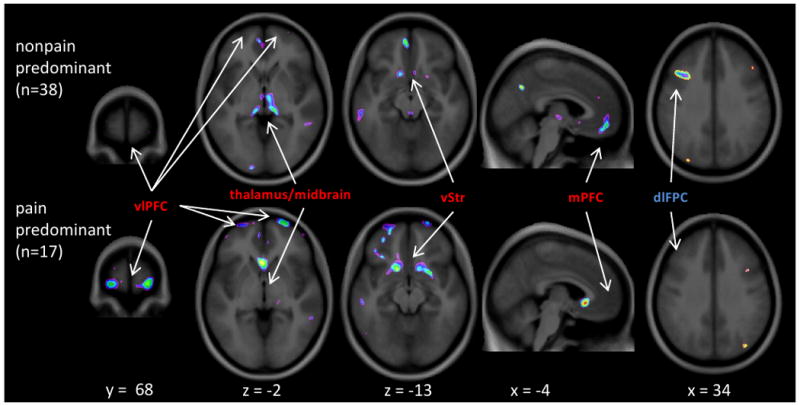
Examples of regions that differ between IBS subgroups based on most bothersome symptom (Pain, or Nonpain, which includes any other symptom). The clusters in red font are those that showed decreased GMD overall in IBS versus controls, whereas the blue is where IBS had increased GMD relative to controls. The figure clearly illustrates that the pain predominant group contributed the vlPFC and ventral striatum differences, while the non-pain predominant group contributed the differences in the thalamus/midbrain, mPFC, and dlPFC. The image is thresholded the same way as figures 2 and 3 (i.e. t>2.5 up to t=4.5). No masking has been applied to the images (i.e. whole brain results shown).
The results for GLM and cluster analyses for the subgroups of IBS subjects based on bowel habits are summarized in Table 2. Of note is that the IBS-C does not appear to contribute to the decrease in thalamic GMD relative to controls. In general, the findings of decreased or increased GMD relative to controls is consistent across all bowel habit subgroups, with the exception of the thalamus, the OFC, and pACC, regions in which the constipation-predominant group does not differ from controls.
We found only one significant (p<0.05, uncorrected) correlation between either IBS severity or IBS symptom duration and any of the average gray matter values for the clusters from the group (removing age) GLM. This was a small negative correlation between IBS duration and the dlPFC cluster GMD (r = -0.28, p<0.05). This correlation was found to only be in the non-pain predominant group (r = -0.35, p<0.05) and not in the pain group (r = -0.22, p=0.40).
CTA
We found no significant differences between groups regardless of the covariates entered.
Discussion
Here we report morphometric brain differences between IBS patients and controls, in terms of regional increases and decreases in gray matter density, while no differences in total gray matter density or cortical thickness were observed. Even though some similarities with previously published results from other patient populations were observed, many of the involved regions were different, and a number of regions with increased gray matter density were observed. Results from both cortical thickness and voxel-based morphometry analyses indicated that whole brain gray matter density and cortical thickness decreased in both IBS and controls with age, but there were no differences between groups. Highly significant regional differences were seen with voxel-based morphometry, but not with cortical thickness analysis.
Even though significant changes in gray matter density were observed in multiple regions, we limit our discussion to the regions implicated in our two main a priori hypotheses, and compare them to published results in the literature from patient populations with various persistent pain disorders.
Hypothesis 1. IBS is associated with decreased gray matter in regions shown to have increased responsiveness to rectal distension and its expectation
This hypothesis was based on the concept that morphometric changes observed in key regions of the so-called “pain matrix” in various patient populations with persistent visceral or somatic pain (including IBS) may be a consequence of chronic nociceptive input from the periphery9, 11, 13, 26, possibly involving glutamate induced excitotoxicity, or other neurodegenerative processes. Increased visceral afferent signalling to the brain as a consequence of peripheral or central sensitization in IBS pathophysiology has been suggested, and some, but not all studies have shown that IBS patients indeed show greater activation in insula and cingulate cortices in response to an acute visceral stimulus3, or to expectation of visceral stimuli27. However, in the current study, there was no significant gray matter density reduction observed in either cingulate or insula cortices. Our results are in contrast to findings of Davis and colleagues in a smaller sample of IBS patients19, which included decreased cortical thickness in right anterior midcingulate cortex and bilateral anterior insula, and decreased gray matter density in aMCC and thalamus. In a recent study of a different sample, the same group reported cortical thinning in the aMCC. In the aINS, cortical thickness was related to symptom duration, with thinning only seen in patients with short duration of symptoms20. In contrast, but similar to our findings, Schweinhardt et al. did not observe morphometric differences in insula and anterior cingulate cortex in a study of female patients with provoked vestibulodynia, a form of chronic vulvar pain14. They speculated that the absence of gray matter density reductions in these regions may have been unique to their study population, in which pain is not constant but only experienced during stimulation, e.g. during intercourse. In this sense lower abdominal pain in IBS is analogous to pain in provoked vestibulodynia, since it is not constant (in contrast to functional abdominal pain syndrome28) but only occurs in association with bowel movements and/or food intake.
In summary, we did not confirm our hypothesis of gray matter density decreases within the interoceptive network; in fact, we observed a non-significant increase (IBS>control) of gray matter density in right S2/posterior insula (Supplementary Figure 1). S2/pINS is considered the primary interoceptive cortex, and is activated during noxious and thermal stimulation29. Because of its role in interoceptive processing, individuals with increased pINS gray matter density may be more vulnerable to central amplification of normal interoceptive input. It should be noted that for these regions about which we had a priori hypotheses, we applied a lower statistical threshold. This increases the chance of type I statistical error, although our use of cluster analysis still gives us much confidence in the results.
Hypothesis 2. IBS is associated with an increase in gray matter in some regions, particularly in regions involved in cognitive and attentional modulation of interoceptive information
This hypothesis was based on the concept that regional gray matter density changes may be a genetically determined or acquired trait of IBS patients which is positively correlated with function of involved brain circuits. Evidence for such a positive correlation between cortical volume and function has been provided from different lines of investigation18, 30. Based on this hypothesis, gray matter density reductions would be expected to be associated with reduced functionality and vice versa.
We found gray matter density reductions in several prefrontal regions, in particular bilateral ventrolateral prefrontal cortex. The right vlPFC plays a role in cognitive modulation of pain31, and in placebo analgesia32. Previous studies reported compromised engagement of right vlPFC in IBS patients27, 33 and it is intriguing to speculate that reduced gray matter density in this area provides a neuroanatomical basis for compromised control of emotion and pain perception. In support of this hypothesis is our finding that VBM reduction in vlPFC was contributed exclusively by the subgroup of patients who identified pain as their most bothersome symptom (see below).
Similar to findings in several other populations of chronic pain sufferers5, 26, we found reduced gray matter density in medial prefrontal cortex. mPFC activity has been associated with antihyperalgesia34, and release of endogenous opioids in mPFC has been demonstrated during tonic pain35. The reduced gray matter density in mPFC might therefore be related to a reduced ability to engage this pain inhibition system. Inhibitory interactions between mPFC and dorsolateral prefrontal cortex have been reported, and an alteration in these interactions has been implicated in low back pain patients36. Decreases in dlPFC gray matter density have been reported in patients with low back pain9, arthritic hip pain37 and migraine38. We found a gray matter density increase in a small area of dlPFC in the patient group (in the non-pain predominant group), as well as decreased gray matter density in premotor cortex and middle frontal gyrus.
We also found highly significant reductions in thalamus/midbrain gray matter density (including periaqueductal gray), and which were independent of anxiety and depression symptoms. As the PAG plays a prominent role in descending pain modulation, and alterations in the engagement of endogenous pain modulation systems has been suggested as a possible mechanism contributing to central pain amplification in IBS33, 39, 40,these gray matter density reductions in the PAG region may be related to compromised descending modulation of pain.
Hypothesis 3. Depression, anxiety, and predominant symptom explain part of the anatomical differences between IBS and controls
Emotional modulatory regions and the effects of depression and anxiety
Emotional factors, in particular anxiety and increased stress responsiveness play an important role in the modulation of chronic pain2, 41. An increased prevalence of anxiety disorders and depression has been reported both in patients and in non-healthcare seeking individuals who meet symptom criteria for IBS2. We showed that controlling for anxiety and depression eliminated many of the regions considered to be part of an emotional arousal circuitry, including ventral striatum, medial thalamus/midbrain, mPFC, pregenual anterior cingulate cortex, and orbitofrontal cortex. In a previous study in IBS patients, the decreased gray matter density in the anterior/medial thalamus in IBS patients19 may in fact have been related to subclinical levels of anxiety or depression.
Pain as the most bothersome symptom
In order to explore if morphometric brain changes in IBS may be related to clinical characteristics of these patients, we looked at subgroups of patients based on symptom duration, IBS symptom severity, pain predominance (patients who described pain as the most bothersome of their symptoms), and bowel habits. Visceral sensitivity – which is not a constant trait of IBS patients and was only evaluated in a small subset of the current sample – was not used in these analyses. Only pain-predominant patients showed reductions in ventral prefrontal pain inhibitory regions (vlPFC), as well as in the ventral striatum. In contrast, those who identified a symptom other than pain as most bothersome, showed gray matter density reductions in dlPFC, mPFC and thalamus/midbrain, the latter including the PAG. Based on the implications of all these regions in different cortico-limbic-pontine modulatory systems33, 36, we suggest that differential alterations in these systems may result in different predominant symptoms. However, this hypothesis will have to be tested in larger samples. The fact that no major correlations with symptom duration was observed, argues against the concept that prolonged altered signalling from the gut may induce secondary reductions in regional brain volumes. The question of whether the observed volumetric changes are related to primary alterations brain activity, or if they are a consequence of altered visceral signalling to the brain, could be addressed by studies looking at gray matter density changes in patients undergoing successful cognitive behavioural approaches, or in studies of asymptomatic relatives of IBS patients.
Conclusions
The findings in this large sample of female IBS patients with moderate symptom severity suggest that morphometric alterations occur primarily in brain networks concerned with attention and emotion modulation, as well as in cortio-limbic pontine pain modulatory systems, and to a smaller degree in networks processing interoceptive information (as suggested by the increased gray matter density of pINS). Future studies in much larger samples of patients are required to correlate cognitive (attention, hypervigilance, catastrophizing), affective (trait anxiety), and pain measures with the observed morphmetric brain changes. The finding that changes in the attentional and emotional networks as well as in the primary interoceptive cortex were not related to disease duration or severity suggests the possibility that these structural changes are endophenotypes of IBS, which may also be detectable in asymptomatic relatives.
Supplementary Material
Figure 3. Covariates with anxiety and depression.
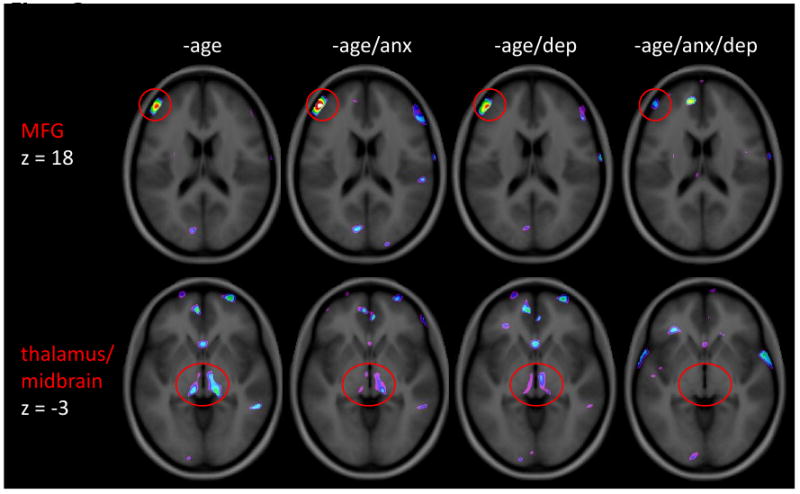
Examples of regions affected by adding covariates anxiety and depression to the GLM. The vlPFC cluster shown on the top row was significant in all GLMs, regardless of the covariate(s). The bottom row compares the thalamus cluster, which is eliminated when anxiety and depression are included as covariates. The image is thresholded the same way as figure 2 (i.e. t>2.5 up to t=4.5). No masking has been applied to the images (i.e. whole brain results shown).
Acknowledgments
Grant support: Supported by National Institutes of Health grants DK 64530 (EAM), AT 00268 (EAM) and DK 48351 (EAM), DK 073451-01(KT), DK 071626-04 (JSL), DK 084169-01 (JSL). DAS is supported by a fellowship from the Canadian Institute of Health Research.
Abbreviations
- Hc
hippocampus
- pHc
parahippocampal gyrus
- IBS-C/D/A
irritable bowel syndrome (patient) – constipation/diarrhea/alternating
- m/vl/dlPFC
medial/ventrolateral/dorsolateral prefrontal cortex
- (p)ACC
(pregenual) anterior cingulate cortex
- PPC
posterior parietal cortex
- MFG
middle frontal gyrus
- OFC
orbitofrontal cortex
- S2/pINS
secondary somatosensory cortex/posterior insula
- aINS
anterior insula
- (a)MCC
(anterior) midcingulate cortex
- VBM
voxel-based morphometry
- GMD
gray matter density
- CT(A)
cortical thickness (analysis)
Footnotes
Disclosures: EAM has received research support from Avera Pharmaceuticals and GlaxoSmithKline. DAS, no conflicts of interest exist. JBL, no conflicts of interest exist. JAB, no conflicts of interest exist. TK, no conflicts of interest exist. BDN, no conflicts of interest exist. MCB, no conflicts of interest exist.
Author contributions: DA Seminowicz: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript; statistical analysis. JB Labus: study concept and design; acquisition of data; critical revision of the manuscript; obtained funding. JA Bueller: acquisition of data; technical support. K Tillisch: acquisition of data; critical revision of the manuscript; obtained funding. BD Naliboff: acquisition of data; critical revision of the manuscript; obtained funding. MC Bushnell: study concept and design; analysis and interpretation of data; critical revision of the manuscript. EA Mayer: study concept and design; drafting of the manuscript; critical revision of the manuscript; obtained funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Naliboff BN, Rhudy JL. Anxiety in functional pain disorders. In: Mayer EA, Bushnell MC, editors. Functional pain syndromes: presentation and pathophysiology. Seattle: IASP Press; 2009. pp. 185–214. [Google Scholar]
- 3.Labus JS, Vianna EP, Tillisch K, Naliboff B, Mayer EA. Brain response during pelvic visceral distension in healthy controls and patients with irritable bowel syndrome: a quantitative meta analysis. Neurogastroenterol Motil. 2009;21(Suppl 1):80. [Google Scholar]
- 4.Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? The Journal of Pain. 2009;10:1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia - A voxel-based morphometry study. Pain. 2007;132:S109–S116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Lutz J, Jager L, de QD, Krauseneck T, Padberg F, Wichnalek M, Beyer A, Stahl R, Zirngibl B, Morhard D, Reiser M, Schelling G. White and gray matter abnormalities in the brain of patients with fibromyalgia: A diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58:3960–3969. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- 8.Hsu MC, Harris RE, Sundgren PC, Welsh RC, Fernandes CR, Clauw DJ, Williams DA. No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain. 2009;143:262–267. doi: 10.1016/j.pain.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt-Wilcke T, Leinisch E, bauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Wilcke T, Leinisch E, Straube A, Kampfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- 12.DaSilva AFM, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007;69:1990–1995. doi: 10.1212/01.wnl.0000291618.32247.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Suh SI, Seol HY, Oh K, Seo WK, Yu SW, Park KW, Koh SB. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28:598–604. doi: 10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- 14.Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140:411–419. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teutsch S, Herken W, Bingel U, Schoell E, May A. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage. 2008;42:845–849. doi: 10.1016/j.neuroimage.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 17.Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, Diamant NE. Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology. 2008;70:153–154. doi: 10.1212/01.wnl.0000295509.30630.10. [DOI] [PubMed] [Google Scholar]
- 20.Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in IBS: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 21.Derogatis L. The Brief Symptom Inventory: administration, scoring, and procedures manual. Minneapolis, MN: National Computer Systems, Inc.; 1993. [Google Scholar]
- 22.Zigmond AS, Snaith RR. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23.Munakata J, Naliboff B, Harraf F, Kodner A, Lembo T, Chang L, Silverman DH, Mayer EA. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112:55–63. doi: 10.1016/s0016-5085(97)70219-1. [DOI] [PubMed] [Google Scholar]
- 24.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45:II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz N, dmiraal-Behloul F, Arkink EB, Kruit MC, Schoonman GG, Ferrari MD, van Buchem MA. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008;48:1044–1055. doi: 10.1111/j.1526-4610.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 27.Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Reduced Brainstem Inhibition during Anticipated Pelvic Visceral Pain Correlates with Enhanced Brain Response to the Visceral Stimulus in Women with Irritable Bowel Syndrome. J Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clouse RE, Mayer EA, Aziz Q, Drossman DA, Dumitrascu DL, Moennikes H, Naliboff BD. Functional abdominal pain syndrome. In: Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE, editors. Rome III: The Functional Gastrointestinal Disorders. Third. McLean, Virginia: Degnon Associates, Inc.; 2006. pp. 557–594. [Google Scholar]
- 29.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 30.Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Pacheco J, Quinn BT, van der Kouwe A, Greve DN, Blacker D, Albert MS, Killiany RJ, Fischl B. Detection of cortical thickness correlates of cognitive performance: reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 2008;39:10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends in Cognitive Sciences. 2008;12:306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, Mayer EA. The neural correlates of placebo effects: a disruption account. Neuroimage. 2004;22:447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 33.Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Seifert F, Bschorer K, De Col R, Filitz J, Peltz E, Koppert W, Maihofner C. Medial prefrontal cortex activity is predictive for hyperalgesia and pharmacological antihyperalgesia. J Neurosci. 2009;29:6167–6175. doi: 10.1523/JNEUROSCI.4654-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 36.Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic Pain and the Emotional Brain: Specific Brain Activity Associated with Spontaneous Fluctuations of Intensity of Chronic Back Pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain Gray Matter Decrease in Chronic Pain Is the Consequence and Not the Cause of Pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocca MA, Ceccarelli A, Falini A, Colombo B, Tortorella P, Bernasconi L, Comi G, Scotti G, Filippi M. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37:1765–1770. doi: 10.1161/01.STR.0000226589.00599.4d. [DOI] [PubMed] [Google Scholar]
- 39.Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:3699–3704. doi: 10.3748/wjg.v13.i27.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25:7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage. 2009;47:994. doi: 10.1016/j.neuroimage.2009.05.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


