Abstract
Active transport along the axon is critical to the neuron. Motor-driven transport supplies the distal synapse with newly synthesized proteins and lipids, and clears damaged or misfolded proteins. Microtubule motors also drive long-distance signaling along the axon via signaling endosomes. While positive signaling initiated by neurotrophic factors has been well-studied, recent research has focused on stress signaling along the axon. Here, the connections between axonal transport alterations and neurodegeneration are discussed, including evidence for defective transport of vesicles, mitochondria, degradative organelles, and signaling endosomes in models of Amyotrophic Lateral Sclerosis, Huntington's, Parkinson's and Alzheimer's disease. Defects in transport are sufficient to induce neurodegeneration, but recent progress suggests that changes in retrograde signaling pathways correlate with rapidly progressive neuronal cell death.
Active axonal transport maintains extended neuronal processes
The unique morphology of neurons, highly polarized cells with extended axons and dendrites, makes them particularly dependent on active intracellular transport. The transport of proteins, RNA, and organelles over long distances requires molecular motors that operate along the cellular cytoskeleton (Glossary).
Two major roles for axonal transport are supply/clearance and long-distance signaling. Supply of newly synthesized proteins and lipids to the distal synapse maintains axonal activity, while misfolded and aggregated proteins are cleared from the axon by transport to the cell soma for efficient degradation [1]. Active transport of mitochondria also supplies local energy needs [2]. The second major role for active transport is the communication of intracellular signals from the distal axon to the soma, allowing the neuron to respond to changes in environment. While defects in either supply or clearance can readily be predicted to be deleterious to the health of the neuron, there has been a growing appreciation that the propagation of stress signaling along the axon may be a key neurodegenerative pathway leading to cell death [3,4].
Here, we focus on recent progress linking defects in fast axonal transport to the pathogenesis of neurodegenerative diseases (reviewed in Table I). Observations from cellular and animal models have provided evidence for multiple alterations in axonal transport, including impaired organelle motility, defects in degradative pathways, impaired neurotrophic signaling, and elevated stress signaling. While it is likely that several cellular pathways may contribute to distal degeneration, recent progress suggests that changes in the balance of signaling along the axon, from survival to stress signaling, may be a critical component of rapidly progressive neuronal cell death.
TABLE I. Neurodegenerative diseases linked to defects in axonal transport.
| Disease | Clinical Features* | Genetics | Proteins Implicated | Axonal Transport Disrupted? | Evidence | Ref |
|---|---|---|---|---|---|---|
| Amyotrophic Lateral Sclerosis | Upper+ and Lower++ motor neuron | Multi-factorial | SOD1 | Yes | Mutant SOD1 expression inhibits axonal transport early in disease. | [17, 3] |
| Huntington's Disease | Chorea, psychiatric and cognitive dysfunction | Autosomal dominant | Huntingtin | Yes | Expression of the pathogenic HTT causes axonal transport defects in fly models. Striatal neurons are selectively vulnerable to transport defects induced by mutant HTT in mouse models. | [18,101] |
| Alzheimer's Disease | Memory impairment, dementia | Multi-factorial | Tau, amyloid-β | Yes | Axonal swellings observed in mouse and fly models. Reductions in kinesin I promote amyloid deposition. | [102] |
| Parkinson's Disease | Rest tremor, rigidity, bradykinesia and gait disturbance | Multi-factorial | α-synuclein | Yes | α-synuclein mutants associated with PD induce reduced transport in cultured neurons. | [21] |
| Hereditary Motor Neuropathy, type VIIB (HMN7B) [also known as distal Spinal and Bulbar Muscular Atrophy (dSBMA)] | Lower motor neuron+ (hands>feet), prominent bulbar symptoms | Autosomal dominant | p150Glued | Not significantly affected | No significant defects observed in sciatic nerve ligation assays, but organelle accumulations observed in motor neurons in mice expressing mutant p150Glued. | [13,15] |
| Perry Syndrome | Parkinsonism, weight loss, hypoventilation, depression | Autosomal dominant | p150Glued | Not determined | Mutations recently characterized | [16] |
| Charcot –Marie-Tooth, type 2B | Length-dependent sensory and motor axonal neuropathy | Autosomal dominant | Rab 7 | Equivocal | Rab7 recruits effectors and/or motors to late endosomes/lysosomes. | [32] |
| Charcot–Marie-Tooth, type 2A | Length-dependent sensory and motor axonal neuropathy | Autosomal dominant | Kinesin (KIF1B) | Equivocal | Decreased synaptic vesicle proteins in sciatic nerve extracts of KIF1B-/-□mice. | [103] |
| Hereditary Spastic Paraplegia, type 10 | Lower extremity weakness and spasticity | Autosomal dominant | Kinesin (KIF5A) | Equivocal | Slower cargo velocity in-vitro | [104] |
Represents classical disease features.
Upper motor neurons features include: weakness, hyperreflexia, and spasticity
Lower motor neuron features include: weakness, muscle atrophy and fasciculations
SOD1 (superoxide dismutase), HTT (huntingtin)
Mutant motors link axonal transport defects to neurodegeneration
Direct evidence implicating axonal transport defects in the pathogenesis of neurodegeneration has come from the identification of mutations in the motors that drive axonal transport. Defects in kinesin-mediated anterograde transport would be predicted to lead to either synaptic defects or axonal dieback due to inadequate supply of new proteins and lipids from the soma to the distal synapse. While relatively few degenerative diseases have been directly linked to mutations in kinesin motors [1], potentially due to functional redundancy in the extended kinesin superfamily, targeted disruption of kinesin function is sufficient to induce neurodegeneration (reviewed in [5]).
In contrast, an increasing number of models link defects in components of the retrograde transport pathway to neurodegenerative disease (Fig. 1). Disruption of the dynein-dynactin motor complex that drives retrograde transport leads to motor neuron loss and muscle denervation in a transgenic mouse model [6]. Characterization of multiple lines of mice with either point mutations or a small deletion in dynein heavy chain support the hypothesis that neurons are preferentially susceptible to defects in dynein function [7,8]. Legs at odd angles (Loa), Cramping 1 (Cra1), and Sprawling (Swl) mice express mutant forms of the cytoplasmic dynein heavy chain (Fig. 1). Heterozygous Loa mice exhibit impaired muscle function and motor coordination, with defects in retrograde axonal transport observed in live cell imaging of cultured dorsal root ganglion (DRG) neurons in vitro and sciatic nerve ligation assays in vivo [3]. Embryonic motor neurons cultured from Loa/Loa mice also exhibit a significant retrograde transport defect [7]. While the Loa and Cra1 phenotypes were initially attributed to a loss of alpha motor neurons [7], more recent evidence suggests that mutations in dynein also induce sensory neuropathy [8]. The relative contributions of sensory neuropathy and motor neuron cell death to the Loa, Cra1, and Swl phenotypes are still under debate [7-9].
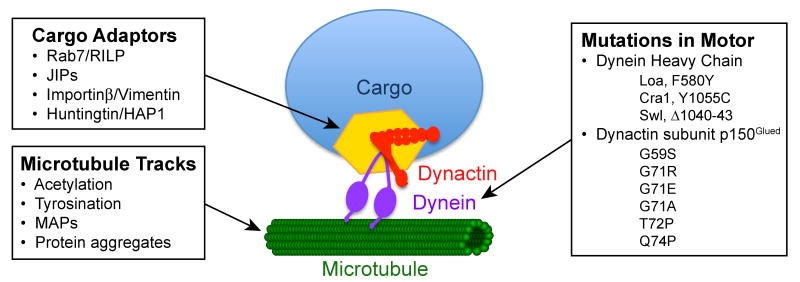
Fig. 1. Retrograde axonal transport may be altered at multiple levels.
Impairment of transport may arise from direct mutations in microtubule motors or their activators and adaptors; mutations in cytoplasmic dynein heavy chain have been identified in the mouse (Loa, Cra1, and Swl), and point mutations in the p150Glued subunit of dynactin have been identified in humans (G59S, G71R, G71E, G71A, T72P, and Q74P). Changes in the velocity or efficiency of retrograde transport may be induced by changes in cargo adaptors or effectors, such as Rab7/RILP or Huntingtin/HAP1, that coordinate cargo-bound motors. Transport along the axon may also be affected by changes in post-translational modifications of the microtubule track, such as changes in acetylation, tyrosination, or the complement of bound microtubule-associate proteins (MAPs). Finally, pathological changes along the axon, such as remodeling of the cellular cytoskeleton or the development of protein aggregates, may deleteriously affect retrograde axonal transport.
Human genetic studies also demonstrate that neurons are uniquely vulnerable to disruptions in the retrograde motor complex. Mutations in the dynein co-factor dynactin cause two distinct forms of neurodegeneration. A G59S point mutation in the DCTN1 gene encoding the p150Glued subunit of dynactin results in a late-onset, slowly progressive form of motor neuron disease termed distal Hereditary Motor Neuropathy, type VIIB (HMN7B; also known as distal Spinal and Bulbar Muscular Atrophy or dSBMA) [10]. HMN7B is an autosomal dominant lower motor neuron disease with prominent bulbar symptoms but no clinically apparent sensory involvement [11]. The G59S mutation in the highly conserved CAP-Gly domain inhibits the interaction of dynactin with microtubules and the microtubule plus-end protein EB1 [12]. The mutation also disrupts folding, favoring aggregation [12]; pathological inclusions of both dynactin and dynein are present in motor neurons of affected individuals [11].
These observations suggest that both loss of normal dynactin function and enhanced protein aggregation contribute to pathogenesis [12]. This hypothesis has been tested in three lines of mice modeling the G59S mutation, all of which show motor neuron degeneration reminiscent of the human disease [13-15]. The most consistent abnormalities among the three models are motor neuron loss, decreased axon caliber, and disruption of the neuromuscular junction (NMJ). An increase in the number of degradative organelles is also observed [13-15], but it remains unclear whether this increase results directly from the accumulation of misfolded dynactin or instead from dysfunction in cellular degradative pathways, which are dependent on normal dynein-dynactin function (discussed below).
More recently, distinct point mutations within the CAP-Gly domain of p150Glued were found to cause Perry syndrome [16]. This autosomal dominant disorder presents as a constellation of symptoms including Parkinsonism, hypoventilation, depression and weight loss. Multiple point mutations identified in 8 families with Perry syndrome localize to the same CAP-Gly domain as the previously characterized G59S mutation (Fig. 1). However, the Perry mutations cluster in or near the surface-exposed microtubule-binding motif while the HMN7B-associated G59S mutation is buried within the folded domain. These mutations may differentially affect p150Glued structure and/or function, potentially accounting for the selective vulnerability of distinct neuronal populations. While the mechanistic basis for selective cell death remains to be investigated, cumulative observations indicate that neurons are uniquely vulnerable to defects in the dynein-dynactin complex.
Axonal transport defects in neurodegenerative disease
The identification of mutations in either dynein or dynactin provides strong support for the hypothesis that defects in axonal transport are sufficient to cause neuronal degeneration. More broadly, a growing body of evidence suggests that slowing of axonal transport is an early event in the pathogenesis of a number of neurodegenerative diseases such as ALS and HD. For example, in the well-characterized SOD1G93A (mSOD1) transgenic mouse model for familial ALS, a significant defect in retrograde transport is observed that is similar in magnitude to that observed in mice with specific disruption in dynein function, such as the Loa mouse [3]. Time course studies indicate that this inhibition is observed well before disease onset [17], consistent with a causative or contributory role in motor neuron cell death.
Similarly, careful analysis of fast axonal transport in neurons isolated from a mouse model of HD (Hdh150Q) indicates significant impairment of both anterograde and retrograde transport [18]. Intriguingly, this impairment is cell-type specific: transport was found to be impaired in both striatal and hippocampal neurons but not in cortical neurons [18], consistent with the differential susceptibility of these neurons to cell death in HD patients.
The role that defective axonal transport may play in AD and PD is less clear. While impaired axonal transport has been shown in a number of AD models, it remains unclear whether this impairment has a causative role in triggering AD, or is a secondary defect resulting from other changes to the cellular environment [19]. As such, defects in transport may still contribute to pathology, for example by inhibiting the localization of mitochondria to the synapse [20]. The possible role for axonal transport impairment in PD has been less well-studied. While mutations in α-synuclein affect the slow axonal transport of this protein in a heterologous expression system [21], current research is focusing on defects in mitochondrial dynamics and function (discussed below).
What is the mechanistic basis for transport inhibition?
One mechanism that might account for the observed transport defects in multiple models is that the well-characterized formation of protein aggregates seen in models of ALS, HD, or AD may lead to a sequestration of active motors; this depletion of motors from the axon could then result in a functional inhibition of transport. There is some evidence that both dynein and kinesin motors are recruited to aggregates of mutant SOD1 or Huntingtin protein [17,22-24]. However, these interactions appear to be generally low-affinity and occur relatively late in the course of the disease, and thus do not explain the observed early inhibition of transport [3,17].
While it remains a possibility that transport impairment reflects direct inhibition of motor function, it is more likely that the defects arise from misregulation of trafficking along the axon. Regulation of axonal transport occurs at multiple levels including modulation of the microtubule track, cargo-specific adaptors and scaffolding proteins that coordinate cargo-bound motors (Fig. 1).
Direct modification of the microtubule cytoskeleton, for example by acetylation [25] or tyrosination [26] of tubulin subunits, has been shown to affect intracellular transport. Experimental modulation of tubulin acetylation was shown to rescue some of the transport deficits seen in a cellular model of HD [25]. Other modifications to the cytoskeletal track involve alterations in MAPs (microtubule-associated proteins), which can compete with motors for binding to the microtubule surface [27,28]. Hence, regulation of MAPs allows spatiotemporal control of transport direction. Consequently, misregulation of MAPs such as the changes in tau expression and localization seen in AD has the potential to alter the steady-state distribution of organelles.
Regulation of axonal transport also involves cargo-specific adaptors, such as Rabs, small membrane-bound GTPases that actively recruit motors to organelles such as endosomes and lysosomes [29]. For example, Rab7-associated late endosomes move long distances in the cell [30], due to the interaction of Rab7 with its effector RILP (Rab-interacting lysosomal protein), which in turn recruits the dynein/dynactin motor complex [31]. Misregulation of adaptor function is associated with neurological disease. Mutant forms of Rab7 cause axonal neuropathy in Charcot-Marie Tooth Disease Type 2B (CMT2B) [32,33]. These mutant forms of Rab7 exhibit aberrant GTP hydrolysis activity [34,35], supporting the hypothesis that misregulation of axonal trafficking may lead to neurodegeneration.
Similarly, disruption in early endosome trafficking may also induce disease, as mutations in the Rab5 GEF (guanine exchange factor), alsin, which facilitates the conversion of inactive Rab-GDP to active Rab-GTP, have been identified in juvenile-onset familial ALS [36,37]. These mutations may potentially affect motor recruitment or function, although this has not been demonstrated. Analysis of Rab5-positive endosomes in neurons cultured from alsin-null mice indicates defects in both endosome morphology and motility, resembling those seen in neurons expressing constitutively active Rab5 [38]. Alsin may function as a negative regulator of Rab5, and mutations in alsin, similar to the defects seen with mutant Rab7, may lead to a misregulation of intracellular trafficking.
Scaffold proteins represent another level of regulation of transport along the axon. Scaffolding proteins bind to both anterograde and retrograde motors, as well as to adaptors and signaling proteins [39]. Thus, scaffolding proteins have the potential to integrate signaling components with motor proteins, allowing localized regulation in response to a changing environment. For example, upon axonal injury, local activation of JNK (c-Jun N-terminal kinase) enhances the interaction of its scaffolding protein JIP (JNK-interacting protein) with dynactin, resulting in retrograde transport of the activated JNK-JIP complex [40]. Interestingly, JIP also binds kinesin [41] and associates with vesicular cargo via interactions with membrane proteins, such as APP (amyloid precursor protein) [42]. JIPs may not be limited to a role in injury signaling, but may actively coordinate bidirectional transport along the axon [43]. Similarly, importinβ and vimentin have an important role in dynein-mediated retrograde injury signaling [44-46]; these proteins may also function in a parallel role in retrograde signaling during neurodegeneration, although this requires further exploration.
Post-translational modifications of another scaffolding protein, Huntingtin (Htt), has been proposed to regulate recruitment of motors to cargo, potentially affecting directionality of transport along the axon. Htt, which is mutated by expansion of polyglutamine repeats in HD, binds directly to dynein, and also associates with kinesin via the adaptor HAP1 (Huntingtin-Associated Protein 1) (reviewed in [39]). Phosphorylation of Htt at Ser421 enhances kinesin recruitment and promotes anterograde transport [47], whereas depletion of Htt inhibits dynein-mediated motility [48]. Htt may function to integrate vesicular transport along the cellular cytoskeleton [39]; it is not clear to what extent disruption of this function through expansion of polyglutamine repeats contributes to pathogenesis in HD.
As axonal transport is a tightly regulated process, deregulation through alterations in Rab activity or post-translational modifications of key adaptors and scaffolds has the potential to significantly disrupt transport. While these disruptions may be the result of specific mutations, such as the Rab7 mutations associated with CMT2B, they are more likely to result from perturbations in the regulatory environment of the cell. While the overall regulation of bidirectional transport along the axon is still poorly understood, further progress should enhance our understanding of disease-associated transport impairment.
From slowed transport to neuronal cell death
While defects in either motor proteins or the regulatory pathways modulating intracellular transport can lead to neurodegeneration, what is the proximal cause of cell death in affected neurons? The most obvious possibility is that inhibition of transport leads to defects in the localization or delivery of essential cargos (Fig. 2). For example, failure to deliver mitochondria to areas of need may induce cell death through energy deprivation. Or, disruption of lysosomal/autophagosome motility may lead to the toxic buildup of aggregated proteins or defective organelles. An alternative hypothesis is that the key defect in axonal transport is not a disruption in bulk supply/clearance, but instead is an alteration in cell signaling (Fig. 3). Evidence for each of these possibilities will be reviewed.
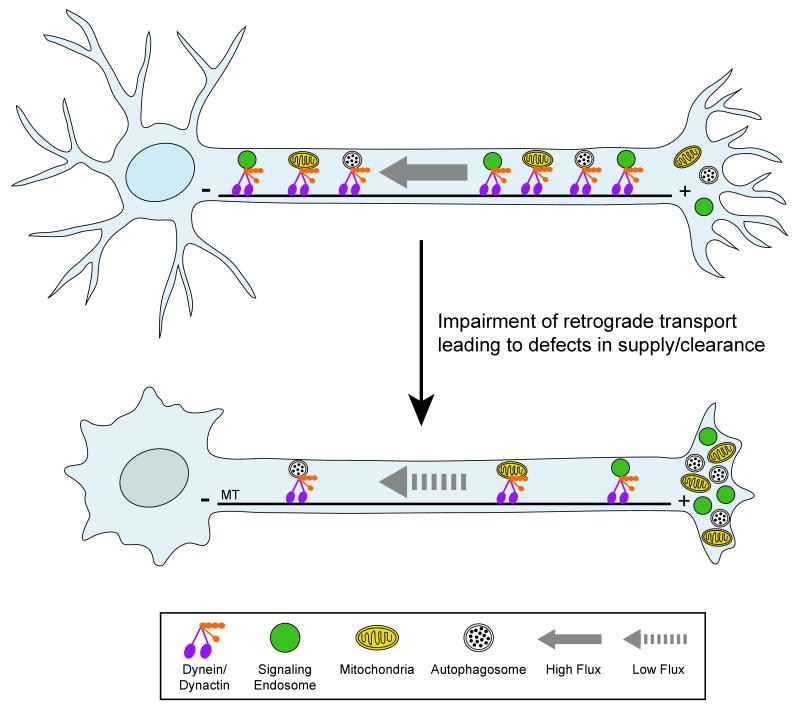
Fig. 2. Changes in retrograde flux along the axon may lead to defects in supply and clearance.
Top, Neuronal function and survival depend on retrograde axonal transport driven by the microtubule motor protein dynein and its activator dynactin. Cargos that are actively transported along the axon include transport vesicles, mitochondria, lysosomes, autophagosomes, and signaling endosomes. Bottom, a significant slowing of retrograde axonal transport is observed at early stages of disease in several neurodegenerative models, consistent with a role in pathogenesis. This slowed transport leads to decreased flux, and potentially to defects in supply and clearance. MT: microtubules, oriented with plus (+) ends distal and minus (-) ends proximal to the cell body.
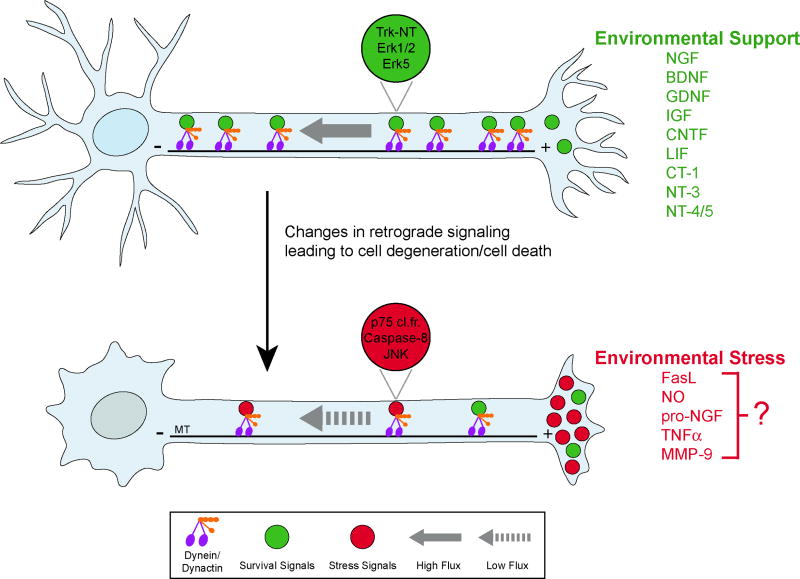
Fig. 3. Changes in retrograde signaling lead to neurodegeneration and cell death.
Top, In a healthy neuron, pro-survival signals (green) associated with signaling endosomes, are actively transported from the cell periphery to the nucleus to promote neuron survival. Neurotrophic factors activate downstream effectors such as Trk receptors, which are transported to the soma via a retrograde signaling endosome. During neurodegeneration, bottom, activation of retrograde death pathways (red) in a non-cell autonomous process may activate cell stress pathways and lead to cell death; decreased retrograde flux may also contribute. For instance, p75 cleavage fragment, activated caspase8 and p-JNK are transported to the cell soma to initiate cell stress/death responses in a mouse model of familial ALS [3]. The specific identity of the environmental stress ligands, as well as the specific combinatorial role of the receptors and co-receptors that regulate survival/death pathways, remain to be determined. Examples of possible pro-survival ligands present in a supportive environment are listed in green. Examples of possible pro-death ligands secreted in a stressful environment are listed in red. NT-neurotrophins; p75 cl.fr.- p75 cleavage fragment; MT-microtubules.
Death by starvation?
Mitochondrial functions, such as aerobic production of ATP and calcium buffering, are vital to the health of the neuron, and therefore neurons must have a proper intracellular distribution of mitochondria. Mitochondria are actively transported to areas of high metabolic demand by the motors kinesin and dynein [2,49-52] in a calcium-regulated process involving the protein Milton and the GTPase Miro [53-55].
Defects in mitochondrial transport would lead to altered distribution of mitochondria along the axon, in turn leading to an inability to meet local ATP demands and/or toxic changes in calcium buffering. Indeed, defects in mitochondrial transport along the axon have been implicated in several disease models. For example, distal depletion of mitochondria may be of significance for the synaptic deficits seen in AD [20].
Recently, Pink1, a mitochondrial protein that is mutated in familial PD, was suggested to play a role in mitochondrial transport when it was found to be complexed with Milton and Miro [56]. Furthermore, in neurons expressing the mutant form of mitofusin found in CMT2A, mitochondrial mobility was decreased dramatically, resulting in a redistribution of mitochondria to the soma and the proximal axon [57]. Primary motor neurons from a mouse model of familial ALS expressing mutant SOD1 also exhibit an aberrant distribution of mitochondria, with a decrease in both the frequency and velocity of mitochondrial transport [58].
Together, these data implicate alterations in mitochondrial localization, mobility, and/or function as critical factors in neuronal degeneration in a number of diseases. What remains unclear is whether these defects are a causal factor in the onset of further pathology, or a downstream response to pathogenic changes in the cellular environment.
Is active transport required for efficient degradation?
Defects in the transport and distribution of degradative organelles have also been observed in neurons from mouse models of neurodegenerative disease [13,15]. These defects are of particular interest due to the frequent correlation between the development of protein aggregates and the onset and/or progression of neurodegeneration, seen in a wide range of diseases including ALS, HD, and AD. This correlation suggests that clearance of aggregates via cellular degradative pathways may be defective in these diseases.
Macroautophagy (hereafter referred to as autophagy) is a lysosomal degradation process that eliminates damaged and long-lived proteins, organelles and protein aggregates. Autophagy is generally considered a non-selective, bulk degradation process, although recent reports have uncovered signals that specifically target mutant proteins and impaired mitochondria for degradation [59,60]. Autophagy initiates when a portion of the cytoplasm is enclosed within a double-membrane organelle, termed an autophagosome [61]. These organelles then fuse with degradative compartments in the endosomal/lysosomal pathway. Autophagosomes are formed throughout the cell, but are transported to the perinuclear region for efficient fusion with lysosomes [62,63]. This centripetal movement is dependent on microtubules and dynein [62,63]. While the precise events underlying autophagosome biogenesis in neurons remain unclear, evidence suggests that autophagosomes can be generated locally within the axon [64,65]. They are then transported in a retrograde direction along the axon towards the cell body for fusion with lysosomes.
Autophagy serves as a quality control to maintain homeostasis and prevent accumulation of toxic material within the cell. Post-mitotic cells, such as neurons, are particularly sensitive to this toxic accumulation. Neuron-specific ablation of Atg5 or Atg7, genes required for autophagosome formation, results in protein aggregation and neuronal cell death [66,67]. Furthermore, autophagy is deregulated in ALS, AD, HD, and PD. An increase in autophagic vacuoles has been observed in patients and/or animal models of AD, HD, PD and ALS [68,69]; reviewed in [70-72].
It remains unclear whether autophagosome accumulation during neurodegeneration is due to an activation of autophagy or rather, due to an inhibition of basal autophagy as a result of defective retrograde transport and/or lysosome fusion. In patients with AD, autophagic vacuoles accumulate within neurites of dystrophic cortical neurons [73]; this distal accumulation may be due to defective retrograde transport. In the mSOD1 model of ALS, autophagosomes accumulate in the soma of motor neurons [68,69]. Autophagic activity within the axon remains to be investigated.
In HD, it has been proposed that autophagy is activated due to sequestration of mTOR (mammalian target of rapamycin), a negative regulator of autophagy, by Htt aggregates [74]. One cannot exclude the possibility, however, that defective transport may also contribute to the buildup of autophagosomes in HD. For example, mutations in dynein lead to an accumulation of autophagosomes and enhanced protein aggregation and toxicity in a model of HD [75].
In PD, autophagy may be activated as a secondary response to a primary block in substrate-specific chaperone-mediated autophagy by mutant alpha-synuclein [76]. More directly, a pathway involving the proteins PINK1 and Parkin has been shown to selectively target impaired mitochondria for degradation by autophagy [77]. As mutations in either PINK1 or Parkin lead to early onset PD, there is now substantial evidence implicating defects in the autophagic clearance of damaged mitochondria to pathogenesis in this disease.
Long-distance signaling along the axon
While the inhibition of organelle transport is a common feature in many models of neurodegenerative disease, it remains unclear if this altered organelle distribution is in itself pathogenic. Alternatively, changes in intracellular signaling may play a more significant role. The soma must receive and integrate precise and timely information from the cell periphery and from neighboring cells in order to maintain neuronal function and viability [78]. The transmittal of vital information along the axon to the soma can signal either neuronal survival or cell death [79]. These signals must be accurate, specific and tightly controlled. Either the loss of a positive signal (such as a neurotrophic factor) or the gain of a negative signal (such as the activation of stress kinases) may lie behind the observed links between alterations in axonal transport and neurodegeneration.
Neuronal viability depends on diverse survival factors whose inhibition may activate apoptosis and cell death. The best-studied survival factors are members of the neurotrophin family. Neurotrophins such as NGF and BDNF bind to members of the Trk receptor family as well as the p75 neurotrophic receptor (p75NTR) [80]. Ligand binding induces Trk dimerization, internalization, autophosphorylation and recruitment of downstream effectors for activation of a signal transduction cascade. The signals are retrogradely transported to the cell body by dynein via signaling endosomes [81] and promote survival of target-dependent neurons [78,82-85].
Impairments in Trk-mediated signaling have been observed in mouse models with impaired dynein function [3], potentially supporting the hypothesis that neurotrophic factor deprivation contributes to neuronal degeneration. However, the observed disruptions in neurotrophic signaling do not correlate with the extent of neuronal loss, as similar levels of disruption were observed in both slowly-progressive and rapidly progressive mouse models (Loa and mSOD1, respectively)[3]. Thus, in the mature CNS, loss of on-going trophic factor support may be less important than the activation of stress factor signaling pathways, as described below.
Activation of retrograde death signals
While neurotrophins are positive factors that are transported along the axon, there is also evidence for the active transport of negative factors by dynein (Figure 3). Activation of axonal signaling factors such as p75NTR can initiate cell death [86]. Retrograde transport of growth-inhibitory signals may be part of the normal neuronal maturation pathway during development. For example, the retrograde transport of Nogo-A endosomes initiates growth cone collapse and inhibits neurite outgrowth [87]; this signaling may be essential for blocking unwanted outgrowth and branching during myelination. Similarly, retrograde signaling, both positive and negative, has been found to play a profound role in neuronal injury response [88].
Several stress/death signaling pathways that lead to neurodegeneration have been identified. For example, NGF deprivation induces a retrograde signal that activates the pro-apoptotic transcription factor, c-Jun [89]. In another neuronal self-destruction pathway, binding of a cleaved fragment of APP to death receptor 6 (DR6) triggers a caspase-dependent degeneration process [90]. Furthermore, the Fas/p38 ER stress-signaling pathway activates caspase-8 leading to the degeneration of motor neuron subpopulations in mSOD1 mice at an early stage of disease [91]. Caspase-8 has been shown to interact directly with the p150Glued subunit of dynactin [92], potentially linking this process to retrograde axonal signaling. In an unbiased proteomic screen for dynein-mediated retrograde signaling in mSOD1 mice, p-JNK, p75NTR cleavage fragments, and activated caspase-8 were all shown to be retrogradely transported along the axon, leading to activation of c-Jun and subsequent cell death [3]. Inhibition of this signaling is sufficient to rescue cell death [3].
Neurotrophin signaling is subject to combinatorial regulation. For example, differential signaling cascades involving the Trk and p75NTR receptors can lead to either survival or death [79]. Proteolytic cleavage of pro-neurotrophins may also be a mechanism to regulate cell fate; while mature neurotrophins bind with high affinity to Trk receptors (and with low affinity to p75NTR) and signal for survival, proneurotrophins bind with high affinity to p75NTR and induce cell death [93]. Finally, alterations in the cellular environment may change prosurvival signals to proapoptotic signals [94,95], so it is clear that context matters.
It is also likely that a set of signaling factors rather than a single pathway may be altered in neurodegenerative diseases leading to cell death. This may explain the limited effects observed when crossing mSOD1 mice with mice null for genes implicated in cell death-inducing pathways, including p75NTR [96], FasL-/- [97] or Bax-/- [98]. In contrast, crossing mSOD1 mice with Loa mice led to an extension in lifespan [99], which could be attributed to the delayed transport of stress/death signals to the soma by mutant dynein.
Changes in the balance of retrograde signaling, from neuroprotective/survival signals to apoptotic/stress signaling may be a critical switch in pathogenesis [3]. Many neurodegenerative diseases are not cell autonomous, but instead involve interactive signaling with neighboring non-neuronal cells [100]. Further studies on retrograde axonal signaling of neurons in context is needed to develop a more comprehensive understanding of how the physiological environment contributes to neuronal cell death [4]
Conclusions: How do we get there from here?
Neurons are uniquely dependent on active intracellular transport, so it is not surprising that defects in transport lead to neuronal stress and cell death. Multiple mechanisms are likely to be involved, including disruptions in energy metabolism, degradative pathways, trophic factor signaling, and stress signaling. Studies across disease models suggest that differing aspects of axonal transport may be more critically involved in one disease process or another, and that axonal transport defects may sometimes be a proximal cause and, in other cases, a downstream consequence of disease progression. Causality will need to be assessed for each case.
Further, our current level of understanding does not provide insight into the basis for the cell type-specificity characteristic of many neurodegenerative diseases. ALS, HD, PD, and AD all preferentially affect one or a few neuronal subclasses, at least initially. Of the mechanisms discussed here, perhaps the possibility that disease-induced changes in the cellular signaling program lead to cell death has the highest potential to explain why one type of neuron, for example motor or striatal, might be selectively affected during disease progression. One can easily envision that changes in the full complement of positive and negative signals actively transported along the axon might selectively affect neurons in a cell-type specific and context-specific manner, but this needs to be demonstrated more directly.
Together, these observations suggest that a multi-pronged approach will be needed to effectively intervene in diseases in which axonal transport is affected. For example, strategies that include compensation for decreased neurotrophic factor transport as well as inhibition of stress signaling pathways are likely to be more successful than approaches directed toward a single target. Further research is required to actively transport us to a more thorough understanding of the biology involved and will allow more thoughtfully designed therapeutic interventions in future.
Glossary
- Microtubules
Microtubules are cytoskeletal filaments formed from the head-to-tail assembly of α- and β-tubulin dimers that serve as tracks for the motors that drive fast axonal transport. Microtubules are oriented in a polarized array in the axon, with their plus, or fast growing ends, directed outward, and their minus, or slow growing ends, directed toward the cell center. Microtubule organization is more complex in dendrites, where the mixed polarity seen in mammalian neurons may contribute to axonal-dendritic sorting and specification
- Kinesins
Kinesins are an extended superfamily of proteins that share homology in a conserved motor domain. Kinesin tail domains are more divergent, which allows coupling to a diverse array of cargos. Many kinesins are microtubule-based motors, some are microtubule depolymerizers, while the function of others has yet to be explored. Kinesins known to drive anterograde axonal transport include kinesin-1 (aka KHC or KIF5), kinesin-2 (aka KIF3), and kinesin-3 (aka KIF1)
- Dynein
Cytoplasmic dynein is the major minus end-directed microtubule motor in the neuron. Dynein is a large protein complex with two heavy chains that form the two motor domains, as well as associated intermediate, light intermediate, and light chains that are involved in cargo recognition and binding specificity
- Dynactin
Dynactin is a large, multi-subunit complex required as an activator for most dynein functions in the cell, including retrograde axonal transport. The largest subunit of dynactin is p150Glued, which binds directly to dynein and the microtubule. Multiple mutations in the DCTN1 gene encoding p150Glued cause neurodegeneration
- Vesicle-Associated Motors and Adaptors
Vesicular cargos are transported along the axon by both kinesin and dynein motors. The activity of these cargo-bound motors may be co-regulated by scaffolding proteins such as JIPs and Htt/HAP1 that can interact with either kinesin or dynein motors, or potentially with both
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chevalier-Larsen E, Holzbaur EL. Axonal transport and neurodegenerative disease. Biochim Biophys Acta. 2006;1762:1094–1108. doi: 10.1016/j.bbadis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perlson E, Jeong GB, Ross JL, Dixit R, Wallace KE, Kalb RG, Holzbaur ELF. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. Journal of Neuroscience. 2009;29:9903–9917. doi: 10.1523/JNEUROSCI.0813-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 6.LaMonte BH, Wallace KE, Holloway BA, Shelly SS, Ascano J, Tokito M, Van Winkle T, Howland DS, Holzbaur EL. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 7.Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 8.Chen XJ, Levedakou EN, Millen KJ, Wollmann RL, Soliven B, Popko B. Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic Dynein heavy chain 1 gene. J Neurosci. 2007;27:14515–14524. doi: 10.1523/JNEUROSCI.4338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilieva HS, Yamanaka K, Malkmus S, Kakinohana O, Yaksh T, Marsala M, Cleveland DW. Mutant dynein (Loa) triggers proprioceptive axon loss that extends survival only in the SOD1 ALS model with highest motor neuron death. Proc Natl Acad Sci U S A. 2008;105:12599–12604. doi: 10.1073/pnas.0805422105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, et al. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 11.Puls I, Oh SJ, Sumner CJ, Wallace KE, Floeter MK, Mann EA, Kennedy WR, Wendelschafer-Crabb G, Vortmeyer A, Powers R, et al. Distal spinal and bulbar muscular atrophy caused by dynactin mutation. Ann Neurol. 2005;57:687–694. doi: 10.1002/ana.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy JR, Sumner CJ, Caviston JP, Tokito MK, Ranganathan S, Ligon LA, Wallace KE, LaMonte BH, Harmison GG, Puls I, et al. A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation. J Cell Biol. 2006;172:733–745. doi: 10.1083/jcb.200511068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chevalier-Larsen ES, Wallace KE, Pennise CR, Holzbaur EL. Lysosomal proliferation and distal degeneration in motor neurons expressing the G59S mutation in the p150Glued subunit of dynactin. Hum Mol Genet. 2008;17:1946–1955. doi: 10.1093/hmg/ddn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai C, Lin X, Chandran J, Shim H, Yang WJ, Cai H. The G59S mutation in p150(glued) causes dysfunction of dynactin in mice. J Neurosci. 2007;27:13982–13990. doi: 10.1523/JNEUROSCI.4226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laird FM, Farah MH, Ackerley S, Hoke A, Maragakis N, Rothstein JD, Griffin J, Price DL, Martin LJ, Wong PC. Motor neuron disease occurring in a mutant dynactin mouse model is characterized by defects in vesicular trafficking. J Neurosci. 2008;28:1997–2005. doi: 10.1523/JNEUROSCI.4231-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrer MJ, Hulihan MM, Kachergus JM, Dachsel JC, Stoessl AJ, Grantier LL, Calne S, Calne DB, Lechevalier B, Chapon F, et al. DCTN1 mutations in Perry syndrome. Nat Genet. 2009;41:163–165. doi: 10.1038/ng.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ligon LA, LaMonte BH, Wallace KE, Weber N, Kalb RG, Holzbaur EL. Mutant superoxide dismutase disrupts cytoplasmic dynein in motor neurons. Neuroreport. 2005;16:533–536. doi: 10.1097/00001756-200504250-00002. [DOI] [PubMed] [Google Scholar]
- 18.Her LS, Goldstein LS. Enhanced sensitivity of striatal neurons to axonal transport defects induced by mutant huntingtin. J Neurosci. 2008;28:13662–13672. doi: 10.1523/JNEUROSCI.4144-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muresan V, Muresan Z. Is abnormal axonal transport a cause, a contributing factor or a consequence of the neuronal pathology in Alzheimer's disease? Future Neurol. 2009;4:761–773. doi: 10.2217/fnl.09.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao XL, Wang WA, Tan JX, Huang JK, Zhang X, Zhang BZ, Wang YH, YangCheng HY, Zhu HL, Sun XJ, et al. Expression of beta-amyloid Induced age-dependent presynaptic and axonal changes in Drosophila. J Neurosci. 30:1512–1522. doi: 10.1523/JNEUROSCI.3699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saha AR, Hill J, Utton MA, Asuni AA, Ackerley S, Grierson AJ, Miller CC, Davies AM, Buchman VL, Anderton BH, et al. Parkinson's disease alpha-synuclein mutations exhibit defective axonal transport in cultured neurons. J Cell Sci. 2004;117:1017–1024. doi: 10.1242/jcs.00967. [DOI] [PubMed] [Google Scholar]
- 22.Zhang F, Strom AL, Fukada K, Lee S, Hayward LJ, Zhu H. Interaction between familial amyotrophic lateral sclerosis (ALS)-linked SOD1 mutants and the dynein complex. J Biol Chem. 2007;282:16691–16699. doi: 10.1074/jbc.M609743200. [DOI] [PubMed] [Google Scholar]
- 23.Tateno M, Kato S, Sakurai T, Nukina N, Takahashi R, Araki T. Mutant SOD1 impairs axonal transport of choline acetyltransferase and acetylcholine release by sequestering KAP3. Hum Mol Genet. 2009;18:942–955. doi: 10.1093/hmg/ddn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trushina E, Dyer RB, Badger JD, 2nd, Ure D, Eide L, Tran DD, Vrieze BT, Legendre-Guillemin V, McPherson PS, Mandavilli BS, et al. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol Cell Biol. 2004;24:8195–8209. doi: 10.1128/MCB.24.18.8195-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci. 2009;12:559–567. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- 27.Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP. Multiple-motor based transport and its regulation by Tau. Proc Natl Acad Sci U S A. 2007;104:87–92. doi: 10.1073/pnas.0607919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caviston JP, Holzbaur EL. Microtubule motors at the intersection of trafficking and transport. Trends Cell Biol. 2006;16:530–537. doi: 10.1016/j.tcb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verhoeven K, De Jonghe P, Coen K, Verpoorten N, Auer-Grumbach M, Kwon JM, FitzPatrick D, Schmedding E, De Vriendt E, Jacobs A, et al. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am J Hum Genet. 2003;72:722–727. doi: 10.1086/367847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meggouh F, Bienfait HM, Weterman MA, de Visser M, Baas F. Charcot-Marie-Tooth disease due to a de novo mutation of the RAB7 gene. Neurology. 2006;67:1476–1478. doi: 10.1212/01.wnl.0000240068.21499.f5. [DOI] [PubMed] [Google Scholar]
- 34.Spinosa MR, Progida C, De Luca A, Colucci AM, Alifano P, Bucci C. Functional characterization of Rab7 mutant proteins associated with Charcot-Marie-Tooth type 2B disease. J Neurosci. 2008;28:1640–1648. doi: 10.1523/JNEUROSCI.3677-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCray BA, Skordalakes E, Taylor JP. Disease mutations in Rab7 result in unregulated nucleotide exchange and inappropriate activation. Hum Mol Genet. 19:1033–1047. doi: 10.1093/hmg/ddp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hadano S, Hand CK, Osuga H, Yanagisawa Y, Otomo A, Devon RS, Miyamoto N, Showguchi-Miyata J, Okada Y, Singaraja R, et al. A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat Genet. 2001;29:166–173. doi: 10.1038/ng1001-166. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Hentati A, Deng HX, Dabbagh O, Sasaki T, Hirano M, Hung WY, Ouahchi K, Yan J, Azim AC, et al. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet. 2001;29:160–165. doi: 10.1038/ng1001-160. [DOI] [PubMed] [Google Scholar]
- 38.Lai C, Xie C, Shim H, Chandran J, Howell BW, Cai H. Regulation of endosomal motility and degradation by amyotrophic lateral sclerosis 2/alsin. Mol Brain. 2009;2:23. doi: 10.1186/1756-6606-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caviston JP, Holzbaur EL. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol. 2009;19:147–155. doi: 10.1016/j.tcb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavalli V, Kujala P, Klumperman J, Goldstein LS. Sunday Driver links axonal transport to damage signaling. J Cell Biol. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verhey KJ, Meyer D, Deehan R, Blenis J, Schnapp BJ, Rapoport TA, Margolis B. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J Cell Biol. 2001;152:959–970. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuda S, Yasukawa T, Homma Y, Ito Y, Niikura T, Hiraki T, Hirai S, Ohno S, Kita Y, Kawasumi M, et al. c-Jun N-terminal kinase (JNK)-interacting protein-1b/islet-brain-1 scaffolds Alzheimer's amyloid precursor protein with JNK. J Neurosci. 2001;21:6597–6607. doi: 10.1523/JNEUROSCI.21-17-06597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horiuchi D, Barkus RV, Pilling AD, Gassman A, Saxton WM. APLIP1, a kinesin binding JIP-1/JNK scaffold protein, influences the axonal transport of both vesicles and mitochondria in Drosophila. Curr Biol. 2005;15:2137–2141. doi: 10.1016/j.cub.2005.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perlson E, Michaelevski I, Kowalsman N, Ben-Yaakov K, Shaked M, Seger R, Eisenstein M, Fainzilber M. Vimentin binding to phosphorylated Erk sterically hinders enzymatic dephosphorylation of the kinase. J Mol Biol. 2006;364:938–944. doi: 10.1016/j.jmb.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 45.Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 46.Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 47.Colin E, Zala D, Liot G, Rangone H, Borrell-Pages M, Li XJ, Saudou F, Humbert S. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caviston JP, Ross JL, Antony SM, Tokito M, Holzbaur EL. Huntingtin facilitates dynein/dynactin-mediated vesicle transport. Proc Natl Acad Sci U S A. 2007;104:10045–10050. doi: 10.1073/pnas.0610628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ligon LA, Steward O. Movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:340–350. doi: 10.1002/1096-9861(20001120)427:3<340::aid-cne2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 50.Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- 52.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacAskill AF, Brickley K, Stephenson FA, Kittler JT. GTPase dependent recruitment of Grif-1 by Miro1 regulates mitochondrial trafficking in hippocampal neurons. Mol Cell Neurosci. 2009;40:301–312. doi: 10.1016/j.mcn.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 56.Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ. Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry. 2009;48:2045–2052. doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baloh RH, Schmidt RE, Pestronk A, Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci. 2007;27:422–430. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF, Brownlees J, Ackerley S, Shaw PJ, McLoughlin DM, et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet. 2007;16:2720–2728. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeong H, Then F, Melia TJ, Jr, Mazzulli JR, Cui L, Savas JN, Voisine C, Paganetti P, Tanese N, Hart AC, et al. Acetylation targets mutant huntingtin to autophagosomes for degradation. Cell. 2009;137:60–72. doi: 10.1016/j.cell.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 62.Kimura S, Noda T, Yoshimori T. Dynein-dependent movement of autophagosomes mediates efficient encounters with lysosomes. Cell Struct Funct. 2008;33:109–122. doi: 10.1247/csf.08005. [DOI] [PubMed] [Google Scholar]
- 63.Jahreiss L, Menzies FM, Rubinsztein DC. The itinerary of autophagosomes: from peripheral formation to kiss-and-run fusion with lysosomes. Traffic. 2008;9:574–587. doi: 10.1111/j.1600-0854.2008.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hollenbeck PJ. Products of endocytosis and autophagy are retrieved from axons by regulated retrograde organelle transport. J Cell Biol. 1993;121:305–315. doi: 10.1083/jcb.121.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yue Z. Regulation of neuronal autophagy in axon: implication of autophagy in axonal function and dysfunction/degeneration. Autophagy. 2007;3:139–141. doi: 10.4161/auto.3602. [DOI] [PubMed] [Google Scholar]
- 66.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 67.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 68.Morimoto N, Nagai M, Ohta Y, Miyazaki K, Kurata T, Morimoto M, Murakami T, Takehisa Y, Ikeda Y, Kamiya T, et al. Increased autophagy in transgenic mice with a G93A mutant SOD1 gene. Brain Res. 2007;1167:112–117. doi: 10.1016/j.brainres.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 69.Li L, Zhang X, Le W. Altered macroautophagy in the spinal cord of SOD1 mutant mice. Autophagy. 2008;4:290–293. doi: 10.4161/auto.5524. [DOI] [PubMed] [Google Scholar]
- 70.Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- 71.Ventruti A, Cuervo AM. Autophagy and neurodegeneration. Curr Neurol Neurosci Rep. 2007;7:443–451. doi: 10.1007/s11910-007-0068-5. [DOI] [PubMed] [Google Scholar]
- 72.McCray BA, Taylor JP. The role of autophagy in age-related neurodegeneration. Neurosignals. 2008;16:75–84. doi: 10.1159/000109761. [DOI] [PubMed] [Google Scholar]
- 73.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 74.Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 75.Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, O'Kane CJ, Brown SD, Rubinsztein DC. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet. 2005;37:771–776. doi: 10.1038/ng1591. [DOI] [PubMed] [Google Scholar]
- 76.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 77.Narendra D, Jin S, Tanaka A, Suen D, Gautier C, Shen J, Cookson M, Youle R. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biology. 2010;8 doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 79.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 80.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 81.Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 82.Ibanez CF. Message in a bottle: long-range retrograde signaling in the nervous system. Trends Cell Biol. 2007;17:519–528. doi: 10.1016/j.tcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 83.Salinas S, Bilsland LG, Schiavo G. Molecular landmarks along the axonal route: axonal transport in health and disease. Curr Opin Cell Biol. 2008;20:445–453. doi: 10.1016/j.ceb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 84.Wu C, Cui B, He L, Chen L, Mobley WC. The coming of age of axonal neurotrophin signaling endosomes. J Proteomics. 2009;72:46–55. doi: 10.1016/j.jprot.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cosker KE, Courchesne SL, Segal RA. Action in the axon: generation and transport of signaling endosomes. Curr Opin Neurobiol. 2008;18:270–275. doi: 10.1016/j.conb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haase G, Pettmann B, Raoul C, Henderson CE. Signaling by death receptors in the nervous system. Curr Opin Neurobiol. 2008;18:284–291. doi: 10.1016/j.conb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joset A, Dodd DA, Halegoua S, Schwab ME. Pincher-generated Nogo-A endosomes mediate growth cone collapse and retrograde signaling. J Cell Biol. 188:271–285. doi: 10.1083/jcb.200906089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abe N, Cavalli V. Nerve injury signaling. Curr Opin Neurobiol. 2008;18:276–283. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mok SA, Lund K, Campenot RB. A retrograde apoptotic signal originating in NGF-deprived distal axons of rat sympathetic neurons in compartmented cultures. Cell Res. 2009;19:546–560. doi: 10.1038/cr.2009.11. [DOI] [PubMed] [Google Scholar]
- 90.Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, Haase G, Pettmann B. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 92.Carson C, Saleh M, Fung FW, Nicholson DW, Roskams AJ. Axonal dynactin p150Glued transports caspase-8 to drive retrograde olfactory receptor neuron apoptosis. J Neurosci. 2005;25:6092–6104. doi: 10.1523/JNEUROSCI.0707-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Teng KK, Felice S, Kim T, Hempstead BL. Understanding proneurotrophin actions: Recent advances and challenges. Dev Neurobiol. 2010;70:350–359. doi: 10.1002/dneu.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matrone C, Marolda R, Ciafre S, Ciotti MT, Mercanti D, Calissano P. Tyrosine kinase nerve growth factor receptor switches from prosurvival to proapoptotic activity via Abeta-mediated phosphorylation. Proc Natl Acad Sci U S A. 2009;106:11358–11363. doi: 10.1073/pnas.0904998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harel L, Costa B, Tcherpakov M, Zapatka M, Oberthuer A, Hansford LM, Vojvodic M, Levy Z, Chen ZY, Lee FS, et al. CCM2 mediates death signaling by the TrkA receptor tyrosine kinase. Neuron. 2009;63:585–591. doi: 10.1016/j.neuron.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 96.Kust BM, Brouwer N, Mantingh IJ, Boddeke HW, Copray JC. Reduced p75NTR expression delays disease onset only in female mice of a transgenic model of familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:100–105. [PubMed] [Google Scholar]
- 97.Petri S, Kiaei M, Wille E, Calingasan NY, Flint Beal M. Loss of Fas ligand-function improves survival in G93A-transgenic ALS mice. J Neurol Sci. 2006;251:44–49. doi: 10.1016/j.jns.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 98.Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, Milligan CE, Oppenheim RW. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26:8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kieran D, Hafezparast M, Bohnert S, Dick JR, Martin J, Schiavo G, Fisher EM, Greensmith L. A mutation in dynein rescues axonal transport defects and extends the life span of ALS mice. J Cell Biol. 2005;169:561–567. doi: 10.1083/jcb.200501085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gunawardena S, Her LS, Brusch RG, Laymon RA, Niesman IR, Gordesky-Gold B, Sintasath L, Bonini NM, Goldstein LS. Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron. 2003;40:25–40. doi: 10.1016/s0896-6273(03)00594-4. [DOI] [PubMed] [Google Scholar]
- 102.Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 103.Zhao C, Takita J, Tanaka Y, Setou M, Nakagawa T, Takeda S, Yang HW, Terada S, Nakata T, Takei Y, et al. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 2001;105:587–597. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]
- 104.Ebbing B, Mann K, Starosta A, Jaud J, Schols L, Schule R, Woehlke G. Effect of spastic paraplegia mutations in KIF5A kinesin on transport activity. Hum Mol Genet. 2008;17:1245–1252. doi: 10.1093/hmg/ddn014. [DOI] [PubMed] [Google Scholar]