Summary
The eggs of insects are unusual in that they often have clear bilateral symmetry when they are laid, indicating that both anterior-posterior (AP) and dorsal-ventral (DV) symmetry is broken during oogenesis[1]. The molecular basis of this process is well understood in the fly Drosophila melanogaster, where symmetry breaking events for both axes depend on the asymmetric position of the oocyte nucleus and on germline-soma signaling mediated by the Tgfα-like EGF ligand Gurken [2, 3]. Germline-soma signaling interactions centered around the oocyte nucleus have been proposed in other insect species[4, 5], but the molecular nature of these interactions had not been elucidated. We have examined the behavior of the oocyte nucleus, and the expression, activity, and function of EGF signaling components in the ovaries of the wasp Nasonia vitripennis, the beetle Tribolium castaneum, and the cricket Gryllus bimaculatus. We have found that EGF signaling has broadly conserved roles in mediating the encapsulation of oocytes by the somatic follicle cell layer, in establishing polarity of the egg chambers, and, at least in the Holometabola, in setting up the DV axis of the embryo. These results provide insights into the evolutionary origins of the unique strategy employed by insects to establish embryonic axial polarity during oogenesis.
Results/Discussion
In D. melanogaster, establishment of both the anterior-posterior (AP) and the dorsal-ventral (DV) axes of the embryo depends on signaling provided by the Tgfα-like ligand Gurken (Grk), whose mRNA is localized around the oocyte nucleus, and whose protein product activates the EGF-receptor (EGFR) in the overlying somatic follicle cells. AP symmetry is broken when the oocyte nucleus lies close to the posterior pole of the oocyte, where EGF signaling to the overlying posterior follicle cells, and subsequent back signaling leads to the repolarization of the oocyte cytoskeleton, and the localization of patterning mRNAs at the poles. Concurrently, the oocyte nucleus migrates to an anterior, cortical location that is asymmetric in regard to the short axis of the oocyte. DV polarity is established when a second EGF signal leads to the differentiation of the follicle cells overlying the oocyte nucleus at this position [2, 3].
To address whether signaling events between the germline and somatic cells in the ovaries of non-dipteran insects contribute to the polarization of the embryonic axes, we have observed the behavior of the oocyte nucleus, and have examined the role of EGF signaling components in three insect species that employ the three major modes of insect oogenesis: polytrophic meroistic (N. vitripennis), telotrophic meroistic (T. castaneum) and panoistic (G. bimaculatus) [6] (see Fig. S1 for details).
Asymmetric positioning of the oocyte nucleus
In all three species, the oocyte nucleus moves to an asymmetric position with respect to the short (DV) axis of the oocyte in the late stages of oogenesis, while its final position with respect to the long (AP) axis varies across species (Fig. S1B, D, F). In addition, a clear morphological asymmetry in the follicle cell layer overlying the cortically localized oocyte nucleus becomes apparent during the late stages of oogenesis in G. bimaculatus and T. castaneum (Fig. S1D, F), suggesting that communication between the oocyte (germline) and follicle cells (soma) takes place in these species.
The expression of EGF signaling components during oogenesis
Since germline-soma communication is mediated by EGF signaling in D. melanogaster, we hypothesized that the apparent germline-soma communication seen in the ovaries of the species studied here is also mediated by EGF signaling, and thus searched for orthologs of EGF components in these organisms.
grk is a rapidly evolving gene, and clear orthologs are difficult to find outside of the Diptera. However, single tgfα-like genes were found in each of the N. vitripennis [7] and T. castaneum [8] genomes, and by PCR in G. bimaculatus. These putative EGF ligands are highly similar to the D. melanogaster genes spitz and keren (Fig S2), which are likely paralogous to grk [9, 10].
In N. vitripennis, Nv-tgfα mRNA is localized within the oocyte near the asymmetrically positioned oocyte nucleus, similar to grk in flies. However this domain of localization extends toward the posterior pole (Fig. 1A, G, H), a pattern not seen for D. melanogaster mRNAs, but which has been seen for some honeybee genes [11, 12] and may be related to the structure of the microtubule cytoskeleton of the N. vitripennis oocyte at this stage [13]. The T. castaneum and G. bimaculatus tgfα orthologs (Tc-tgfα, and Gb-tgfα, respectively) are strongly expressed in oocytes starting from early stages, but their mRNAs show no clear localization to the oocyte nucleus (Fig. 1B and 1C, respectively).
Figure 1. Expression of EGF components in insect ovaries.
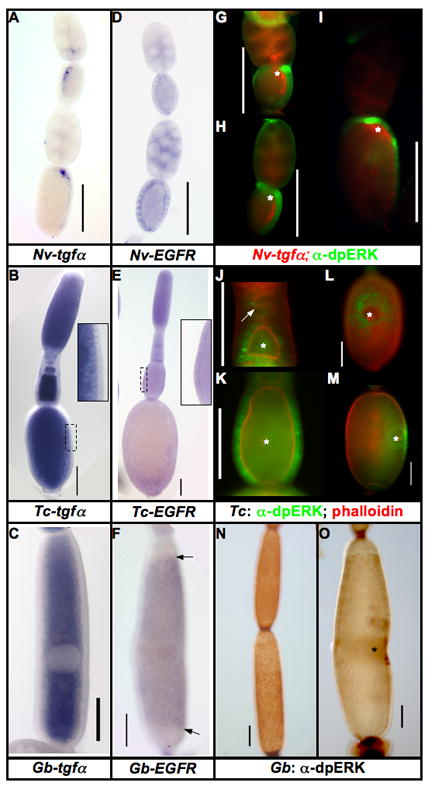
A–C: Expression of tgfα mRNA in the oocytes of N. vitripennis (A), T. castaneum (B), and G. bimaculatus (C). This mRNA is localized only in N. vitripennis, while it is ubiquitous in the oocytes of T. castaneum and G. bimaculatus.
D–F: Expression of EGFR mRNA in the follicular epithelium of N. vitripennis (D), T. castaneum (E), and G. bimaculatus (F) ovarioles. Arrows in F mark the borders of Gb-EGFR expression along the AP axis of the egg chamber.
G–I: Expression of Nv-tgfα mRNA (red) and dpERK (activated MAPK) (green) progressively older N. vitripennis ovarioles.
J–M: Expression of dpERK in T. castaneum ovarioles over the course of oogenesis (dpERK in green, phalloidin in red). dpERK expression is first detected in somatic cells lying just posterior to the very early oocytes (J, white arrow). In slightly older egg chambers, all follicle cells contacting the oocyte express dpERK (J, bottom egg chamber). As the follicle matures (K) dpERK remains strong in all lateral follicle cells, but is downregulated in the termini. During vitellogenesis (L (dorsal view), M (lateral view)), dpERK is found in the nuclei of the follicle cells surrounding the position of the oocyte nucleus, but not in the cells directly overlying it.
N–O: Expression of dpERK in G. bimaculatus ovarioles. G. bimaculatus shows a similar pattern to that of T. castaneum: in early egg chambers (N), dpERK is expressed in most of the follicle cells. In vitellogenic ovarioles, dpERK is markedly reduced in most of the follicular epithelium, except in the follicle cells in the vicinity of the oocyte nucleus (O). Scale bars represent 100μm, asterisks mark the position of the oocyte nucleus.
The EGF receptor (EGFR) is highly conserved in sequence among the insects, and its expression in the somatic follicle cells is also conserved among the three species examined here (Fig. 1D–F). In G. bimaculatus, the expression is downregulated in the anterior and posterior follicle cells (Fig. 1F), possibly due to communication between the juxtaposed ovarian follicles along the AP axis of the ovariole. Some heterogeneity in Nv-EGFR expression within the follicular epithelium is observed, but its significance is not clear.
We used a cross-reactive antibody against dpERK, which recognizes activated MAP kinase (MAPK), to detect activation of the EGF signaling cascade in the ovaries of the three species analyzed here.
In N. vitripennis MAPK activation is observed in follicle cells overlying the localized Nv-tgfα mRNA, which corresponds to a domain extending from the anterior to posterior pole of the oocyte (Fig. 1G–I). The activation of MAPK in N. vitripennis is dynamic during oogenesis, especially in the follicle cells directly over the oocyte nucleus.
In T. castaneum, activated MAPK is initially observed as the oocyte exits the germarium, and begins to associate with the somatic cells (Fig. 1J, arrow). Later, when the nucleus is centrally located within the oocyte, MAPK is activated in most follicle cells associated with the oocyte (Fig. 1J, lower oocyte). As the egg chamber grows, and while the oocyte nucleus remains central, activated MAPK remains highly expressed in all follicle cells along the lateral sides of the oocyte, but appears to be downregulated at the termini (Fig 1K). Once the oocyte nucleus moves to the cortex, activated MAPK staining clears from most follicle cells, except from a roughly circular, graded domain emanating from, but excluding, the region of the epithelium directly overlying the point of contact of the oocyte nucleus and lateral cortex (Fig. 1L, M).
A similar pattern is observed in G. bimaculatus: before the movement of the oocyte nucleus, dpERK staining is mostly ubiquitous in the follicle cells (Fig. 1N). As the follicles mature, dpERK staining is quite dynamic, but staining is consistently very strong in the follicle cells overlying the oocyte nucleus (Fig 1O). This strong dpERK staining is associated with the formation of the kink in the egg chamber that gives rise to the characteristic “banana” shape of late stage oocytes (Fig. 1O, and see S1F).
Function of EGF signaling in encapsulation and AP axis specification of the oocyte
The potential role of the EGF pathway in establishing the polarity of the egg chamber and embryo was tested by parental RNA interference (pRNAi) against the tgfα genes in the beetle, wasp, and cricket. This treatment caused a severe reduction of fecundity in all three species. This reduced egg production appears to be related to the failure of the somatic follicle cells to properly surround and encapsulate the oocytes (compare wildtype in Fig. 2A, D, G to tgfα pRNAi effects in B, E, H. See also Fig. S3D). A role for the EGF pathway in encapsulation of the oocyte has been detected in D. melanogaster [14, 15], and this function of the EGF pathway appears to be ancestral among insects.
Figure 2. Effects of tgfα pRNAi during oogenesis in T. castaneum, N. vitripennis, and G. bimaculatus.
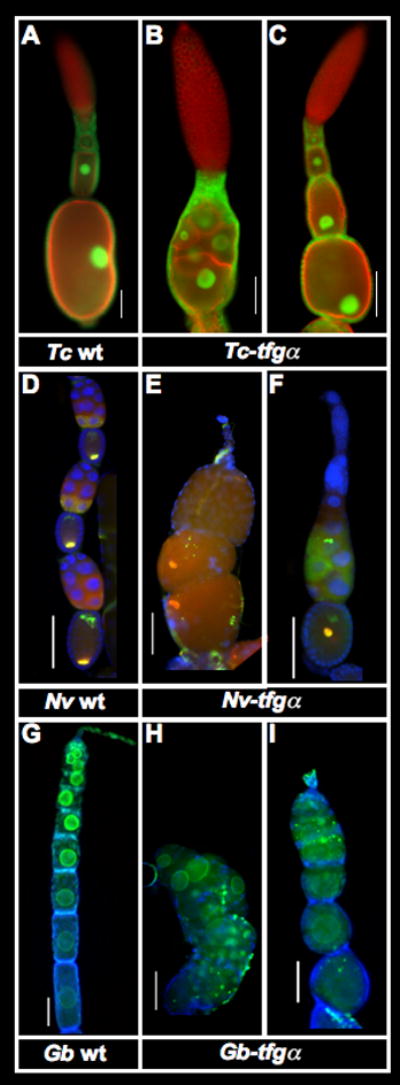
A–C: T. castaneum ovarioles stained with phalloidin (red) and α-nuclear pore antibody (green). In wild type, each oocyte becomes encapsulated by a single layer of follicle cells, and the oocyte nucleus moves to a cortical location about midway along the long axis of the oocyte in late oogenesis (A). In strongly affected Tc-tgfα pRNAi ovarioles, oocytes are not encapsulated, and form a mass of germline cells that are not separated by follicle cells. (B) In presumably weaker cases, and the position of the oocyte nucleus is disrupted by Tc-tgfα pRNAi (C). See also Fig S2.
D–F: Expression of DAPI (blue), Nv-otd1 (green) and Nv-nos (red) in wild type (D) and Nv-tgfα pRNAi (E,F) ovarioles from N. vitripennis. Nv-tgfα pRNAi ovarioles show defects in encapsulation of the oocytes by follicle cells (E), as well as improper localization of normally anteriorly and posteriorly localized mRNAs (E,F). In general there is also a major reduction in production of germline cells.
G–I: Ovarioles of G. bimaculatus stained for α-nuclear pore (green) and DAPI (blue). Egg chambers in wild type ovaraioles show a linear arrangement, and an increasingly elongated cylindrical shape during early oogenesis (G). In strongly affected Gb-tgfα pRNAi ovarioles (H), oocytes are arranged chaotically, and are not encapsulated by a complete layer of follicle cells. In moderately affected ovarioles, oocytes are encapsulated, but take on a more rounded shape (I).
Scale bars represent 100μm.
In some tgfα pRNAi knockdowns, oocytes were encapsulated, but showed AP polarity defects. In T. castaneum, the oocyte nucleus, rather than moving to a cortical location about midway along the AP axis of the oocyte at the beginning of vitellogenesis (Fig 2A), was instead found at the posterior pole after Tc-tgfα RNAi (Fig. 2C). That this mislocalization was the result of a defect in axial polarity is supported by the observation that mRNA for the normally anteriorly localized mRNA of Tc-eagle [16] was often found, in addition, at the posterior pole after Tc-tgfα RNAi (Fig. S3A–C).
In N. vitripennis, Nv-nos mRNA is localized at the posterior pole, while Nv-otd1 mRNA is localized at both the anterior and posterior poles of the oocyte (Fig 2D, [13, 17]). After Nv-tgfα pRNAi, Nv-nos and otd1 are co-localized in the center of the oocyte, rather than at the posterior (Fig. 2F, S3D), which is highly reminiscent of what is seen for oskar mRNA in grk, EGFR, and cornichon mutants of D. melanogaster [2, 3]. In other cases, encapsulation defects are combined with polarity defects in compound egg chambers (Fig S3E).
AP polarity defects in G. bimaculatus ovaries are more difficult to assess, as no localized mRNAs are known, and the oocyte nucleus localization does not appear to be sensitive to reduction of EGF signaling. However, we observe misshapen early egg chambers that are more spherical than cylindrical in shape (Fig 2I), indicating a defect in the polarized growth of the egg chambers along the AP axis after reduction of EGF signaling.
The function of EGF signaling in DV axis specification
Despite the disruption of AP polarity in oocytes observed in both T. castaneum and N. vitripennis after tgfα pRNAi, we do not observe major AP patterning defects in blastoderm stage embryos of either species (Fig S4 A–J). However, in both T. castaneum and N. vitripennis, pRNAi against tgfα transcripts leads to variable defects in DV patterning.
In T. castaneum, the most common (34/71) phenotype can be interpreted as a lateralization of the embryo, as Tc-cact (representing the most ventral embryonic fates (Fig 3A)) is missing from the main body of the embryo, while Tc-sog expression, which normally marks more lateral fates, expands to completely encircle the blastoderm (Fig. 3B). Strikingly, Tc-cact and Tc-sog expression patterns that are perpendicular to their normal domains are also observed (8/71) (Fig. 3C). Occasionally (5/71), duplications of the DV axis occur, where multiple domains of Tc-cact and Tc-sog expression are observed (Fig 3D). We have found that these defects in marker gene expression are correlated with changes in the pattern of Tc-Dorsal nuclear uptake in the blastoderm stage embryos (Fig S4 K–O), indicating that, as in D. melanogaster [18, 19], maternal EGF signaling acts upstream of Dorsal gradient formation in T. castaneum.
Figure 3. EGF signaling is required for proper DV patterning in insects.
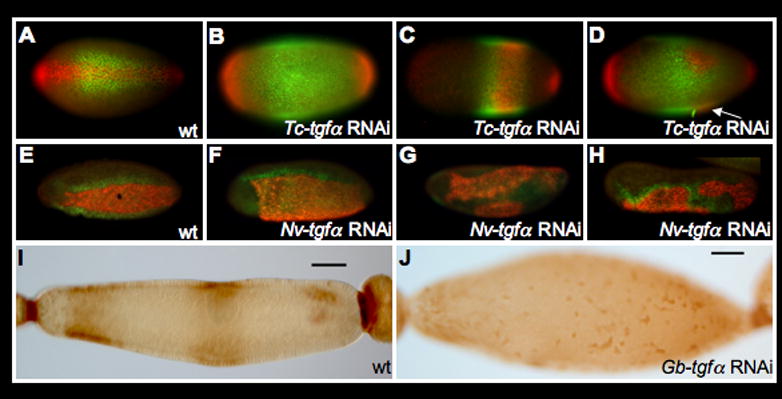
A–D: Disruption of DV polarity in T. castaneum after Tc-tgfα pRNAi as revealed by the expression of Tc-sog (green) and Tc-cact (red). A: Wild-type expression of Tc-sog and Tc-cact (ventral view). B–D: Representatives of different classes of Tc-tgfα pRNAi phenotypes (see text). Arrow in (D) indicates the out of focus additional ectopic spot of Tc-cact expression.
E–H: Disruption of DV polarity in N. vitripennis after Nv-tgfα pRNAi as revealed by the expression of Nv-twi (red) and Nv-vnd (green). E: Wild type expression pattern of Nv-twi and Nv-vnd (ventral-lateral view). F–H: Expression of Nv-twi and Nv-vnd in Nv-tgfα pRNAi embryos representing different phenotypic classes (see text for details).
I–J: After Gb-tgfα RNAi, the bilaterally symmetric banana shape, and strong activation of MAPK signaling (brown) over the oocyte nucleus of late egg chambers (I) are lost, giving rise to egg chambers with radial symmetry (J)
In N. vitripennis (34/54), a major expansion of ventral fates (marked by Nv-twist) and a shift of lateral fates (marked by Nv-vnd ), is the most common (34/54) phenotype observed (compare Fig. 3E to 3F). Partial axis duplications (7/54) are also seen in these experiments (Fig. 3G), as are major disruptions of ventral patterning, where the borders of Nv-twi and vnd are neither parallel to nor continuous along the long axis of the embryo (6/54) (Fig. 3H). Finally, embryos where Nv-twi is only activated in small, chaotically distributed patches are observed at low frequency (4/54) (not shown).
The observation of partial axis duplications and chaotic induction of ventral fates after tgfα pRNAi indicates that the DV patterning systems of N. vitripennis and T. castaneum have some self-regulatory properties that are normally constrained by maternal EGF signaling to give an single source of ventralizing activity along the AP axis of the embryo. Such a role for EGF signaling is well described in D. melanogaster, where strong grk mutations lead to duplicated peaks of ventralizing activity, rather than a simple expansion of ventral fates [20]. Self regulatory properties, albeit of different nature, have been described for the Dorsal gradient in T. castaneum [21], and a similar system could exist in N. vitripennis.
We were not able to obtain embryos from G. bimaculatus after Gb-tgfα pRNAi, but we were able to detect a role for this gene in generating asymmetry along the short (DV) axis of the egg chamber: Gb-tgfα pRNAi produces radially symmetric late egg chambers (Fig. 3J), rather than the normal banana shape (Fig 3I). The degree to which asymmetry is lost appears to correlate with of EGF signaling knockdown (Fig S4 O–Q).
We propose that the DV patterning role of EGF signaling is related to the activation of this pathway in the follicle cells overlying the asymmetric oocyte nucleus(Fig. 1I, L, M, O), and that this signal acts as a dorsalizing influence to the embryo. This idea is supported by earlier observations in crickets that the position of the oocyte nucleus marks the future dorsal side of the egg [22], as well as our observation that Nv-tgfα mRNA persists on the dorsal side of freshly laid N. vitripennis eggs (not shown). In T. castaneum, we used the position of the polar body, marked with EGFP, as a proxy for the final position of the oocyte nucleus, and found that the position of the polar body is strongly associated with the future dorsal side of the embryo (Figure S5).
The evolution of DV patterning in the insects
In sum, the most parsimonious explanation of our observations is that the EGF pathway had functions in mediating germline-soma communication and in establishing axial polarity in the most recent common ancestor of crickets, wasps and beetles. In addition, since asymmetric activation of the EGF pathway is correlated with the position of the oocyte nucleus in all species examined and the final position of this organelle is strongly associated with the future DV side of the embryo, the migration of oocyte nucleus to the cortex likely represents a symmetry breaking event for DV patterning. How could such a system have originated?
The role of EGF signaling in mediating the encapsulation of the oocyte by the follicle cells probably predated the role of this pathway in DV patterning, since the latter process depends on the former. In addition, the eggshell structures produced by follicle cells were likely a crucial adaptation of insects to a terrestrial lifestyle [23]. Similarly, the migration of the oocyte nucleus to an asymmetric cortical location likely also predated any role in breaking DV symmetry, as the interaction of the egg cortex with the products of meiosis is critical for the formation and regulation of the polar bodies [24]. We propose that mechanisms later evolved to concentrate EGF signaling activity around an already asymmetrically localized oocyte nucleus, and to allow transmission of this asymmetric signal to the overlying follicle cells to be used later during embryogenesis. This hypothesis is summarized in Fig 4.
Figure 4. Model for the evolutionary origin of the insect DV patterning strategy.
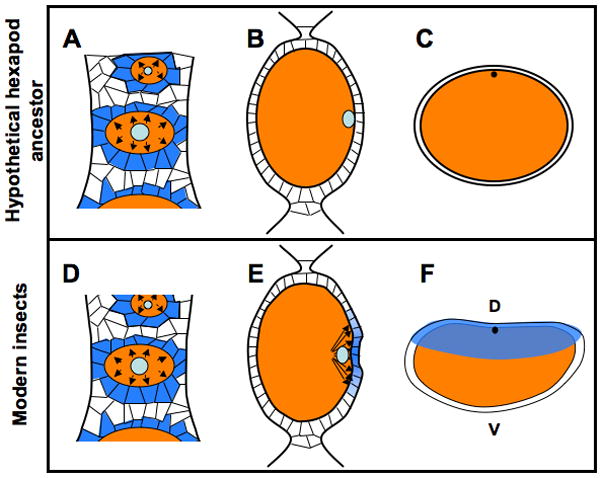
A–C: Schematic representation of features present in a hypothetical insect ancestor that predated the use of germline-soma signaling for DV axis establishment. Since the ovariole structure of ovaries is synapomorphic for insects, a mechanism for germline-soma communication in mediating the encapsulation of oocytes (orange ovals) by somatic cells was present in a common insect ancestor. Those somatic cells (marked in blue in A) receiving the EGF signal emanating from the oocyte (represented by outward pointing arrows) will give rise to the follicle cells, which later give rise to eggshell structures such as the vitelline membrane and chorion, but at this stage have no role in receiving or transmitting patterning information. In this hypothetical ancestor, the oocyte nucleus (light blue) moved to a cortical location (B), to allow the formation of the polar body (black spot) in the egg after laying, but originally had no role in embryonic patterning (C).
D–F: Features present in modern insects, based on results presented in this manuscript. The role in EGF signaling in recruiting follicle cells to the oocyte (A) is maintained (D). The oocyte nucleus still moves to the cortex (E), as this event is still critical for regulation of meiosis. In this ancestor, however, EGF signaling becomes strongly associated with the position of the oocyte nucleus, allowing asymmetric activation of this pathway (blue gradient in E) in the overlying follicle cells. This patterning information is stored in the follicle cells, where it is incorporated into the eggshell (transparent blue in F) and is then later transmitted to the embryo to provide a basis for patterning the now maternally established DV axis.
The recruitment of EGF signaling and the oocyte nucleus for roles in DV patterning likely took place in a common ancestor of the Orthoptera and Holometabola, and analysis of this system in more basally branching insect or hexapod lineages will be required to precisely map its evolutionary origin.
Experimental Procedures
Additional Experimental Procedures can be found in the online supplementary materials.
in situ hybridization and immunohistochemistry
Single color in situ hybridization was performed as described in [25]. Two color in situ hybridizations involved the use of α-biotin or α-fluorescein::AP antibodies, using Fast Red and HNPP (Roche) as substrates to give a red fluorescent signal, and α-digoxigen::POD antibodies using AlexaFluor 488 tyramide (Invitrogen) as a substrate following the method of [26].
Immunohistochemistry was performed as described in [27].
Antibodies were used as follows:
α-Nuclear Pore (Sigma N8786) 1:2000
α-dpERK clone MAPK-YT (Sigma M-8159) 1:1000
α-Nv-Hb [28]1:1000
α-Tc-Dl (Jessica Cande, Mike Levine) 1:50
α-mouse or rabbit::AP 1:2500
α-mouse or rabbit::POD 1:100
Antibodies were detected either with AP conjugated secondary antibodies, using either NBT/BCIP or FastRed TR/HNPP (Roche) as substrates, or with POD conjugates using either DAB or AlexaFluor 488 tyramide (Invitrogen) as substrates. AlexaFluor 555 conjugated phalloidin (Invitrogen) was used at a dilution of 1:100 in PBT (1x PBS, 0.1% Tween-20) for 1 hour to detect F-actin.
RNAi
Young N. vitripennis pupae were injected as in [29]. Adult T. castaneum females were injected as in [30]. G. bimaculatus adult females were injected with 5μL of dsRNA solution using the method in [31]
Concentrations of dsRNA used were:
Nv-tgfα: 0.8μg/μL
Tc-tgfα: 0.6μg/μL
Gb-tgfα: 0.5μg/μL
Embryo and ovary dissection and fixation
All N. vitripennis embryos were collected and hand devitellinized by the method in [29]. Due to the large reduction in fecundity, Tc-tgfα pRNAi embryos were also prepared by this method, to minimize the loss of embryos inherent in the methanol devitellinization protocol used for wild-type eggs.
Cuticle preparations were performed as described in [29]
Ovaries from all species were dissected from adult females quickly in PBS using fine forceps. The peritoneal sheath was then removed using 27 gauge hypodermic needles. The ovaries were then fixed in 5% EM grade formaldehyde in PBT (1x PBS, 0.1% Tween-20) for 30 minutes, and either used immediately, or dehydrated in methanol and stored at −20° until needed.
Highlights.
Early EGF signaling is required for encapsulation of the oocyte by follicle cells in three phylogenetically diverse insect species
Asymmetric positioning of the oocyte nucleus along the short axis of the egg leads to localized EGF signaling to the overlying follicle cells in these species.
Localized EGF signaling is the symmetry-breaking event that establishes embryonic DV polarity in insects.
Supplementary Material
Acknowledgments
This work was supported by Ruth L. Kirschstein fellowship F32 GM078832 to JAL, SFB 680 to SR, and the Marie Curie programme ‘ZOONET’ (Framework Programme 6, European Union) for supporting the work of AP and MA. We thank Mike Levine and Jessica Cande for providing the α-Tc-Dorsal antiserum. We would like to thank Kristen Panfilio, Claude Desplan, Ava Brent, Eugenia Olesnicky, and Orhan Özüak for helpful comments on the manuscript.
References
- 1.Gutzeit HO, Sander K. Establishment of polarity in the insect egg. Vol. 1. San Diego: Academic Press; 1985. [Google Scholar]
- 2.Gonzalez-Reyes A, Elliott H, St Johnston D. Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature. 1995;375:654–658. doi: 10.1038/375654a0. [DOI] [PubMed] [Google Scholar]
- 3.Roth S, Neuman-Silberberg FS, Barcelo G, Schupbach T. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–978. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 4.Netzel H. Über die Übereinstimmung von Polarität und Bilateralsymmetrie in Embryo, Ei, Oocyte, und Oocytenkern bei Gryllus domesticus L. Roux’ Archiv für Entwicklungsmechanik. 1965;156:88–95. doi: 10.1007/BF00576720. [DOI] [PubMed] [Google Scholar]
- 5.Ogorzalek A. Structural and functional diversification of follicular epithelium in Coreus marginatus (Coreidae: Heteroptera) Arthropod Struct Dev. 2007;36:209–219. doi: 10.1016/j.asd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Büning J. The insect ovary. London: Chapman & Hall; 1994. [Google Scholar]
- 7.Werren JH, Richards S, Desjardins CA, Niehuis O, Gadau J, Colbourne JK, Beukeboom LW, Desplan C, Elsik CG, Grimmelikhuijzen CJP, et al. Functional and Evolutionary Insights from the Genomes of Three Parasitoid Nasonia Species. Science. 2010;327:343–348. doi: 10.1126/science.1178028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R, Beeman RW, Gibbs R, Bucher G, Friedrich M, Grimmelikhuijzen CJP, et al. The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452:949–955. doi: 10.1038/nature06784. [DOI] [PubMed] [Google Scholar]
- 9.Urban S, Lee JR, Freeman M. A family of Rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands. Embo Journal. 2002;21:4277–4286. doi: 10.1093/emboj/cdf434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reich A, Shilo BZ. Keren, a new ligand of the Drosophila epidermal growth factor receptor, undergoes two modes of cleavage. Embo Journal. 2002;21:4287–4296. doi: 10.1093/emboj/cdf439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson MJ, Dearden PK. Evolution of the insect Sox genes. Bmc Evolutionary Biology. 2008;8 doi: 10.1186/1471-2148-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson MJ, Dearden PK. Tailless patterning functions are conserved in the honeybee even in the absence of Torso signaling. Developmental Biology. 2009;335:276–287. doi: 10.1016/j.ydbio.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Olesnicky EC, Desplan C. Distinct mechanisms for mRNA localization during embryonic axis specification in the wasp Nasonia. Dev Biol. 2007;306:134–142. doi: 10.1016/j.ydbio.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz C, Wood CG, Jones DL, Tazuke SI, Fuller MT. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development. 2002;129:4523–4534. doi: 10.1242/dev.129.19.4523. [DOI] [PubMed] [Google Scholar]
- 15.Goode S, Morgan M, Liang YP, Mahowald AP. Brainiac encodes a novel, putative secreted protein that cooperates with Grk TGF alpha in the genesis of the follicular epithelium. Dev Biol. 1996;178:35–50. doi: 10.1006/dbio.1996.0196. [DOI] [PubMed] [Google Scholar]
- 16.Bucher G, Farzana L, Brown SJ, Klingler M. Anterior localization of maternal mRNAs in a short germ insect lacking bicoid. Evolution & Development. 2005;7:142–149. doi: 10.1111/j.1525-142X.2005.05016.x. [DOI] [PubMed] [Google Scholar]
- 17.Lynch JA, Brent AE, Leaf DS, Pultz MA, Desplan C. Localized maternal orthodenticle patterns anterior and posterior in the long germ wasp Nasonia. Nature. 2006;439:728–732. doi: 10.1038/nature04445. [DOI] [PubMed] [Google Scholar]
- 18.Peri F, Technau M, Roth S. Mechanisms of Gurken-dependent pipe regulation and the robustness of dorsoventral patterning in Drosophila. Development. 2002;129:2965–2975. doi: 10.1242/dev.129.12.2965. [DOI] [PubMed] [Google Scholar]
- 19.Sen J, Goltz JS, Stevens L, Stein D. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell. 1998;95:471–481. doi: 10.1016/s0092-8674(00)81615-3. [DOI] [PubMed] [Google Scholar]
- 20.Roth S. Mechanisms of dorsal-ventral axis determination in Drosophila embryos revealed by cytoplasmic transplantations. Development. 1993;117:1385–1396. doi: 10.1242/dev.117.4.1385. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca RN, Lynch JA, Roth S. Evolution of axis formation: mRNA localization, regulatory circuits and posterior specification in non-model arthropods. Curr Opin Genet Dev. 2009 doi: 10.1016/j.gde.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Netzel H. Die ausprägung von Polarität und Bilateralsymmetrie in den Oocyten von Gryllus domesticus L. Roux’ Archiv für Entwicklungsmechanik. 1968;160:119–166. doi: 10.1007/BF00573649. [DOI] [PubMed] [Google Scholar]
- 23.Zeh DW, Zeh JA, Smith RL. Ovipositors, Amnions and Eggshell Architecture in the Diversification of Terrestrial Arthropods. Quarterly Review of Biology. 1989;64:147–168. [Google Scholar]
- 24.Counce SJ. Analysis of Insect Embryogenesis. Annual Review of Entomology. 1961;6:295. [Google Scholar]
- 25.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 26.Mazzoni EO, Celik A, Mathias FWC, Vasiliauskas D, Johnston RJ, Cook TA, Pichaud F, Desplan C. Iroquois complex genes induce co-expression of rhodopsins in Drosophila. Plos Biology. 2008;6:825–835. doi: 10.1371/journal.pbio.0060097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel NH. Imaging neuronal subsets and other cell types in whole mount Drosophila embryos and larvae using antibody probes. In: Goldstein LSB, Fyrberg E, editors. Methods in Cell Biology. Vol. 44. New York: Academic Press; 1994. [DOI] [PubMed] [Google Scholar]
- 28.Pultz MA, Westendorf L, Gale SD, Hawkins K, Lynch J, Pitt JN, Reeves NL, Yao JCY, Small S, Desplan C, et al. A major role for zygotic hunchback in patterning the Nasonia embryo. Development. 2005;132:3705–3715. doi: 10.1242/dev.01939. [DOI] [PubMed] [Google Scholar]
- 29.Lynch JA, Desplan C. A method for parental RNA interference in the wasp Nasonia vitripennis. Nat Protoc. 2006;1:486–494. doi: 10.1038/nprot.2006.70. [DOI] [PubMed] [Google Scholar]
- 30.van der Zee M, Stockhammer O, von Levetzow C, da Fonseca RN, Roth S. Sog/Chordin is required for ventral-to-dorsal Dpp/BMP transport and head formation in a short germ insect. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16307–16312. doi: 10.1073/pnas.0605154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronco M, Uda T, Mito T, Minelli A, Noji S, Klingler M. Antenna and all gnathal appendages are similarly transformed by homothorax knock-down in the cricket Gryllus bimaculatus. Developmental Biology. 2008;313:80–92. doi: 10.1016/j.ydbio.2007.09.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


