Abstract
The signal transducer and activator of transcription STAT5 plays a major role in cytokine-induced expression of genes involved in cell proliferation and survival. Although several STAT5 partners have been identified, the molecular events taking place at the promoter level upon STAT5 recruitment have not yet been characterized in great detail. Using chromatin immunoprecipitation and accessibility assays, we characterized histone acetylation and chromatin remodeling events occurring during transcriptional activation of the endogenous murine Cis gene, a STAT5 target gene, in response to IL-3. We found that STAT5 binding in vivo is associated with low histone H3 and H4 acetylation levels in the proximity of the STAT5 binding sites. STAT5 recruitment also results in chromatin reorganization over that promoter region. These events (STAT5 binding, histone acetylation and chromatin remodeling) are not sufficient for transcriptional activation, which requires a non-histone protein deacetylase. These data reveal novel implications of STAT5 in chromatin regulation during cytokine-induced transcription, thus contributing to a better understanding of the mechanism of transcriptional activation by STAT5.
INTRODUCTION
Transcription in eukaryotes is a multi-step process involving distinct chromatin modifying and remodeling complexes that control the proper recruitment of transcription factors and assembly of the pre-initiation complex (1). Orchestration and coordination of these events are promoter-specific (2–5). Chromatin modification by histone acetyltransferase (HAT) and deacetylase (HDAC) complexes plays a major role in transcriptional regulation (6,7). Transcriptional activation is generally correlated with histone acetylation and repression with histone deacetylation (6,8–11), although several studies have now well established that this correlation is not exclusive (7,12–19). In addition, acetyltransferases and deacetylases can regulate transcription by targeting non-histone proteins (12,20–22), adding another layer of complexity to the process.
The signal transducer and activator of transcription STAT5 functions as an important downstream effector of cytokine signaling. STAT5 proteins are present in a latent form in the cytoplasm of unstimulated cells. Following cytokine stimulation, STAT5 is phosphorylated by the JAK kinases, triggering its dimerization and translocation into the nucleus where it can bind to specific DNA binding sites and activate transcription of target genes (23). STAT5 function is regulated through interaction with a variety of cofactors (24,25), and through functional cooperation with other transcription factors bound on adjacent binding sites and STAT5 tetramerization (23,25). Despite those extensive studies, the molecular mechanism of transcriptional activation by STAT5 remains poorly understood.
We recently showed that transcriptional activation by STAT5 requires a deacetylase activity that targets a yet unknown factor necessary for proper recruitment of the basal transcription machinery (22). In an extension of this study, we report here a detailed analysis of the molecular events occurring at the level of chromatin along the STAT5-dependent Cis gene (26), during IL-3-induced transcription. We present evidence that binding of STAT5 to its target sites in vivo modulates the level of histone H3 and H4 acetylation and chromatin accessibility over the Cis promoter region. Most importantly, these chromatin changes occur even in the absence of transcription, thus supporting the assumption that the deacetylase activity required for transactivation by STAT5 is targeting non-histone proteins.
MATERIALS AND METHODS
Cells
The IL-3-dependent murine pro-B cell line Ba/F3-β has been previously described (22). The Ba/F3-WT and Ba/F3-1*6 cell lines stably expressing STAT5A-Flag wild-type and the 1*6 constitutively active mutant have been kindly provided by Dr Toshio Kitamura. Cells were grown, cytokine stimulated and TSA treated as previously described (22).
STAT5 knock-down
SiRNA duplexes specific for STAT5A (AAGTGTTGTATGGGCAGCATT and AAGGACGAGGTCTTTGCCAAG), STAT5B (AAGCCTGGGACTCAATAGATC and AAGTACTACACACCGGTCCCC) and ScI (Scramble-I) non-specific control (AACAGTCGCGTTTGCGACTGG) were synthesized using the Silencer siRNA Construction Kit (Ambion 1620), according to the manufacturer’s recommendations with the following exception. SiRNA duplexes were eluted with 80 µl nuclease-free water to which was added 40 µl (10×) annealing buffer (1 M KAc, 300 mM HEPES pH 7.4, 20 mM MgAc). To achieve high efficiency (80%) transfection, 3 × 106 Ba/F3-β cells were resuspended in 100 µl PB-sucrose buffer (272 mM sucrose, 7 mM sodium phosphate buffer pH 7.4, 1 mM MgCl2) in the presence of 10 µg siRNA (ScI or 2.5 µg each STAT5 siRNA) and 50 U of a FITC-labeled uptake control (Sequitur 3013), and transferred into a 0.1 cm electroporation cuvette (BioRad). Electroporation was performed using a Gene Pulser II RF Module (BioRad) at 100 V, 0% modulation, 25 kHz, 10× 2 ms burst duration and 0.1 s intervals. Following electroporation, cells were resuspended in RPMI 1640 containing 10% FBS and 0.2 ng/ml IL-3. Four hours following transfection, FITC-positive cells were sorted by FACS to select for 100% transfected cells, and maintained in culture as above for 26 h. Cells were washed in RPMI 1640 and rested for 5 h in RPMI 1640 containing 10% FBS before addition of IL-3 (10 ng/ml). Samples were taken at the time of IL-3 stimulation (0 h) for protein analysis, and at 0, 0.5 and 2 h for RNA analysis.
Protein analysis
Cells were lysed and analyzed by western blot as previously described (22), using antibodies specific for STAT5A, STAT5B and STAT3 (Santa Cruz Biotechnology, sc-1081, sc-835 and sc-483 respectively).
mRNA analysis
RNA isolation, cDNAs synthesis and real-time PCR expression analysis were performed as previously described (22). Data were normalized to S9 ribosomal RNAs and expressed as relative RNA levels. Forward and reverse primers used to amplify mouse cDNAs for S9, Cis and c-Fos have been described (22).
Chromatin immunoprecipitation (ChIP) assay
ChIPs were performed and analyzed by real-time PCR as previously described (22). Data are expressed as a percentage of input DNA. Antibodies used for immunoprecipitation of STAT5, Acetylated Histone H3 and H4, and RNA polymerase II have been described (22). Antibodies for non-acetylated H4 were from Serotec (AHP413; 7 µl). Forward and reverse primers used to amplify mouse Cis genomic DNA are as follows. Amplicon (–826/–749): AGGGCTGTCTGGGAGCTGA and TCTCTGAGTGGACCGACAGTTG; amplicon (–256/–195): CAACTCTAGGAGCTCCCGCC and AACACCTTTGACAGATTTCCAAGAAC; amplicon (+878/+944): TACCCCTTCCAACTCTGACTGAGC and TTCCCTCCAGGATGTGACTGTG. Primers used to amplify c-Fos genomic DNA are as follows. Amplicon (–246/–187): GAC CATCTCCGAAATCCTACACGC and CACATTTGGGATCTTAGGGGGTCTC; amplicon (–56/+13): GGAAGTCCATCCATTCACAGCG and CAGTCGCGGTTGGAGTAGT AGGC. Primers specific for amplicons (–184/–102) and (–17/+55) of Cis, and (+1273/+1325) of c-Fos have been described (22).
Chromatin accessibility by real-time PCR (CHART-PCR)
CHART-PCR assays were performed and analyzed by real-time PCR as previously described (22). Data are expressed as a percentage of non-digested DNA (% protection). A positive control corresponding to purified genomic (naked) DNA was included in the assay. Primers used for real-time PCR have been described (22).
RESULTS
Validation of c-Fos as a STAT5-independent gene in the IL-3-stimulated murine pro-B cell line Ba/F3
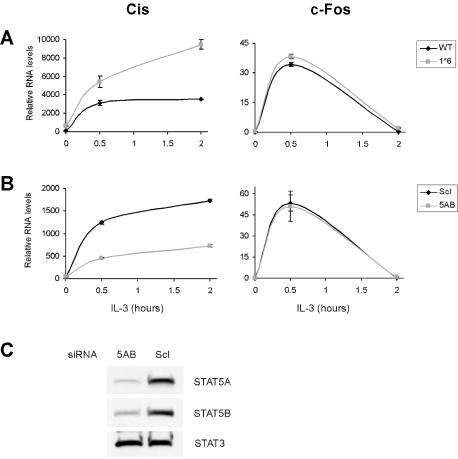
To study the mechanism of STAT5 transactivation on gene promoters, we selected the well established STAT5-regulated gene Cis (26). For comparison, we used a STAT5-independent cytokine inducible gene, c-Fos. The murine c-Fos promoter contains several regulatory elements (see Fig. 2B). Among them is a serum inducible element (SIE) shown to mediate transcriptional activation by various STAT family members in different cell lines (27–32). To validate Cis and c-Fos genes within one chosen cell system, the murine pro-B IL-3-dependent cell line Ba/F3, a number of expression analysis experiments were performed (Fig. 1). First, we used Ba/F3 cells stably expressing a constitutively active mutant form (1*6) of STAT5A (33). Second, we developed an siRNA-mediated approach to specifically knock down expression of both STAT5A and STAT5B endogenous genes in Ba/F3-β cells (Fig. 1C). RNAs were isolated at various times upon IL-3 stimulation. Following a reverse transcription reaction, the cDNAs for Cis and c-Fos were analyzed by real-time PCR (Fig. 1A and B).
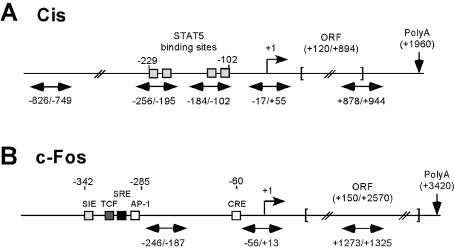
Figure 2.
Schematic representation of murine Cis (A) and c-Fos (B) genes. Amplicons analyzed by ChIP are indicated as horizontal double-headed arrows. Putative transcription factor binding sites are represented as boxes. Positions are relative to the transcription start (or CAP) site (+1). ORF, open reading frame. Cis gene contains no intron and c-Fos gene three introns.
Figure 1.
STAT5-dependent and -independent expression of Cis and c-Fos in Ba/F3 cells stimulated with IL-3. (A) Cis but not c-Fos is up-regulated in cells expressing a constitutively active form of STAT5A. Ba/F3-WT and Ba/F3-1*6 cells were stimulated with IL-3, and mRNAs levels for Cis and c-Fos analyzed by real-time PCR, as described in Materials and Methods. (B) Cis but not c-Fos induction is abrogated in STAT5 knock-down cells. Ba/F3-β cells were transfected with siRNA duplexes specific for both STAT5A and STAT5B isoforms (5AB) or with a non-specific control (ScI), as described in Materials and Methods. Thirty-one hours post-transfection, cells were stimulated with IL-3 and mRNAs levels analyzed as above. (C) STAT5 protein levels were monitored by western blot from STAT5 knock-down (5AB) and control (ScI) cells 31 h following siRNA transfection. As a control for specificity, STAT3 expression was shown to be unaffected following STAT5 knock-down at both the protein (this figure) and RNA (not shown) level.
As expected, expression of Cis was strongly upregulated in IL-3-stimulated Ba/F3-1*6 cells compared to Ba/F3-WT cells stably expressing a wild-type form of STAT5A (Fig. 1A), while it was inhibited in IL-3-stimulated Ba/F3-β cells following STAT5 knock-down (Fig. 1B). By contrast, expression of c-Fos remained unaffected both in Ba/F3-1*6 cells (Fig. 1A) and in STAT5 knock-down Ba/F3-β cells (Fig. 1B). Together with our previous observation that the deacetylase inhibitor trichostatin A (TSA), a specific repressor of STAT5-mediated transcription, abrogates induction of Cis while upregulating expression of c-Fos (22), these experiments clearly confirm Cis as a STAT5-target gene and establish c-Fos as a STAT5-independent gene in IL-3-stimulated Ba/F3 cells, which can be used as a control in our comparative study.
STAT5 binding to the Cis promoter in vivo locally modulates histone H3 and H4 acetylation levels
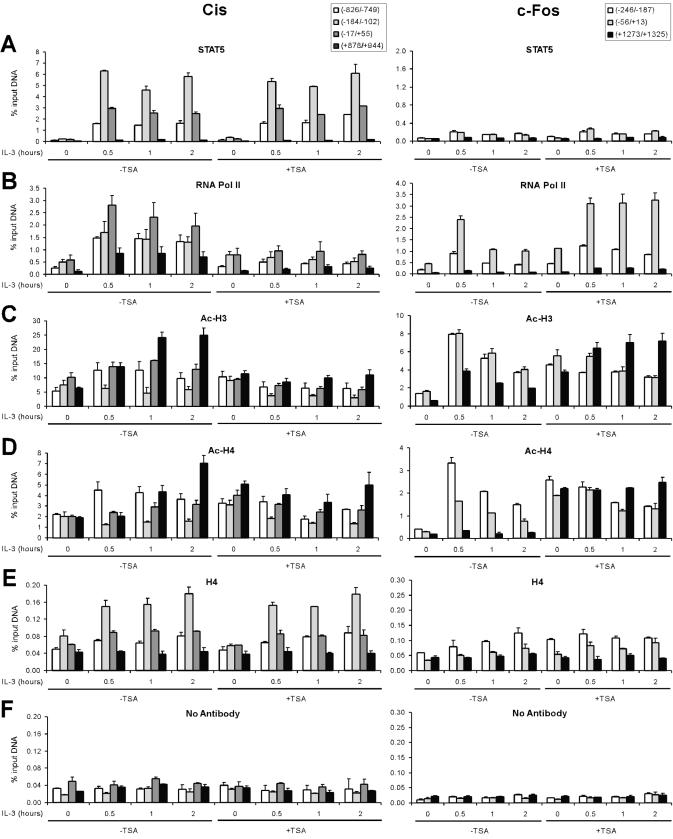
It was previously shown that STAT5 binds to the endogenous Cis promoter within minutes of IL-3 stimulation, closely followed by TBP and RNA polymerase II recruitment. In addition, TSA treatment abrogates TBP and RNA polymerase II recruitment, resulting in transcriptional inhibition, while STAT5 binding remains unaffected (22). In the present study, we investigated how these molecular events correlate with, and possibly regulate, chromatin modifications along the Cis gene. To investigate chromatin acetylation along the STAT5-dependent Cis gene in vivo, chromatin immunoprecipitation (ChIP) assays were conducted, using c-Fos as a control gene. ChIP assays were performed in Ba/F3-β cells stimulated with IL-3 for 0–2 h, in the absence or presence of TSA. Cross-linked chromatin was immunoprecipitated with antibodies specific for acetylated and non-acetylated forms of histone H3 and H4, as well as for STAT5 and RNA polymerase II for comparison. Purified genomic DNA was then subjected to real-time PCR using primers amplifying various regions along both genes. Regions overlapping the STAT5 binding sites, the transcription start (or CAP) site, and upstream and downstream regions of the Cis gene were investigated (Fig. 2A). Identical results were obtained with amplicons –256/–195 and –184/–102 overlapping the two clusters of STAT5 binding sites, and therefore only the data obtained with amplicon –184/–102 are shown. Promoter, CAP site and downstream regions of the c-Fos gene were analyzed in parallel (Fig. 2B).
Prior to characterizing the acetylation status along the Cis gene, a control experiment was carried out to assess the recruitment of STAT5 and RNA polymerase II on Cis and c-Fos genes (Fig. 3A and B). Upon IL-3 stimulation, STAT5 was efficiently and specifically recruited to its binding sites (–184/–102) on the Cis promoter (Fig. 3A). As expected, STAT5 recruitment was not affected by TSA. Gene expression closely correlated with the presence of RNA polymerase II at the CAP site of Cis (–17/+55) and c-Fos (–56/+13) (Fig. 3B). Accordingly, RNA polymerase II was not detected at the Cis promoter upon transcriptional inhibition by TSA. In contrast, in the presence of TSA, the enzyme was found at the c-Fos CAP site during the entire time course of induction, in agreement with the prolonged expression of the gene (Fig. 3B) (22). In the same experiment, the no-antibody control yielded no enrichment (Fig. 3F).
Figure 3.
Regulation of histone H3 and H4 acetylation along the Cis and c-Fos genes upon IL-3 stimulation. Ba/F3-β cells were stimulated with IL-3 in the absence or presence of TSA, and ChIP assays performed as described in Materials and Methods, using antibodies specific for STAT5A+B (STAT5) (A), RNA polymerase II (RNA Pol II) (B), acetylated histone H3 (Ac-H3) (C), acetylated histone H4 (Ac-H4) (D), non-acetylated histone H4 (H4) (E) or no antibody as a control (F). Amplicons indicated in Figure 2 specific for Cis and c-Fos genes were analyzed by real-time PCR (Materials and Methods).
Analysis of the histone acetylation status on Cis and c-Fos genes revealed three main distinctive features (Fig. 3C–E). First, the global level of histone H3 and H4 acetylation in unstimulated cells was on average eight times higher on Cis than on c-Fos (5–10% versus 0.6–1.6% of input DNA for Ac-H3, and 1.9–2.2% versus 0.2–0.4% of input DNA for Ac-H4) (Fig. 3C and D, 0 h IL-3, -TSA). Second, IL-3 stimulation resulted in a different acetylation pattern along both genes. While H3 and H4 acetylation was increased at both loci upon IL-3 stimulation, this increase was transient on c-Fos, whereas it persisted or even increased along the Cis gene (Fig. 3C and D, 0–2 h IL-3, -TSA). Moreover, the increase in histone acetylation was higher within the open reading frame of Cis (4-fold versus 2-fold within the promoter), while it was higher within the promoter region of c-Fos (5- to 8-fold). Third, treatment with the deacetylase inhibitor TSA resulted in histone hyperacetylation (4- to 9-fold) along the c-Fos gene in unstimulated cells, whereas the effect on histone acetylation at the Cis locus was negligible (1.4- to 1.8-fold) (Fig. 3C and D, 0 h IL-3, –/+TSA). This difference might be a consequence of the already high level of histone acetylation along the Cis gene, and suggests that the c-Fos gene but not the Cis gene is targeted by TSA-sensitive histone deacetylases (HDACs). Upon Il-3 stimulation, there was no significant effect of TSA on histone H3 and H4 acetylation on Cis, while the hyperacetylation on c-Fos persisted (Fig. 3C and D, 0–2 h IL-3, +TSA). These experiments thus reveal a general trend in which the acetylation status along the genes reflects quite closely their transcriptional activity. This is particularly clear when looking at H3 acetylation within the open reading frame of Cis and c-Fos in TSA-treated and -untreated IL-3-stimulated cells (Fig. 3C, black bars).
However, the most remarkable feature on the Cis gene was the low level of histone H3 and H4 acetylation (3–6% of input DNA for Ac-H3, and 1.2–1.8% for Ac-H4), within the promoter region bound by STAT5 proteins (–184/–102), which was maintained regardless of IL-3 stimulation or TSA treatment (Fig. 3C and D). The observation that acetylation levels were not increased following TSA treatment demonstrates that they were not the result of deacetylation by TSA-sensitive HDACs at this site. Moreover, the levels of the non-acetylated form of histone H4 increased specifically at the region overlapping the STAT5 binding sites upon IL-3 stimulation (Fig. 3E), suggesting that the decreased acetylation levels were not the consequence of nucleosome loss. Although it remains possible that they were the result of deacetylation by a TSA-insensitive deacetylase, these observations suggest that the region of the Cis promoter bound by STAT5 is not targeted for acetylation, as opposed to adjacent regions of the gene. These data therefore suggest that STAT5 binding can locally influence the level of histone H3 and H4 acetylation on the Cis promoter.
STAT5 binding to the Cis promoter in vivo correlates with chromatin remodeling in the vicinity of the STAT5 binding sites
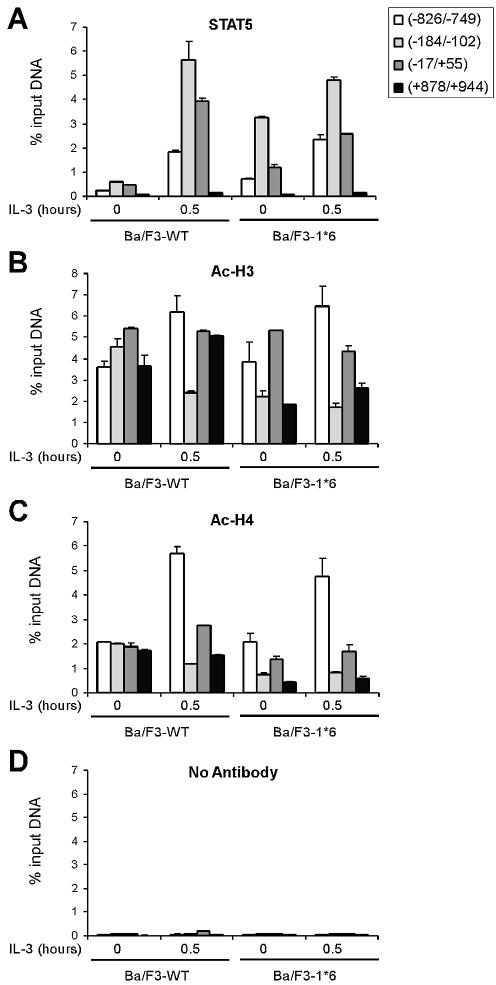
Next, we wished to investigate whether STAT5 binding had an effect on chromatin structure. We showed previously that an AvaII site located between the two clusters of STAT5 binding sites of the Cis promoter (position –184, see Fig. 5A) becomes highly accessible in vivo in response to IL-3 stimulation of Ba/F3-β cells (22). Moreover, this chromatin transition is unaffected by the inhibition of transcription by TSA, as is STAT5 binding (Fig. 3A) (22). To address whether the chromatin transition at position –184 is a consequence of STAT5 recruitment in the vicinity, chromatin accessibility in Ba/F3-1*6 cells expressing a constitutively active form of STAT5A was investigated. In these cells, the mutant STAT5 was shown to be constitutively phosphorylated, to localize in the nucleus and exhibit constitutive DNA binding activity in vitro in the absence of IL-3, although its activity was exacerbated upon IL-3 stimulation (33,34). We made the assumption that if STAT5 binding suffices to induce the chromatin transition, constitutive binding of STAT5A-1*6 in unstimulated cells in vivo should be associated with constitutive chromatin accessibility.
Figure 5.
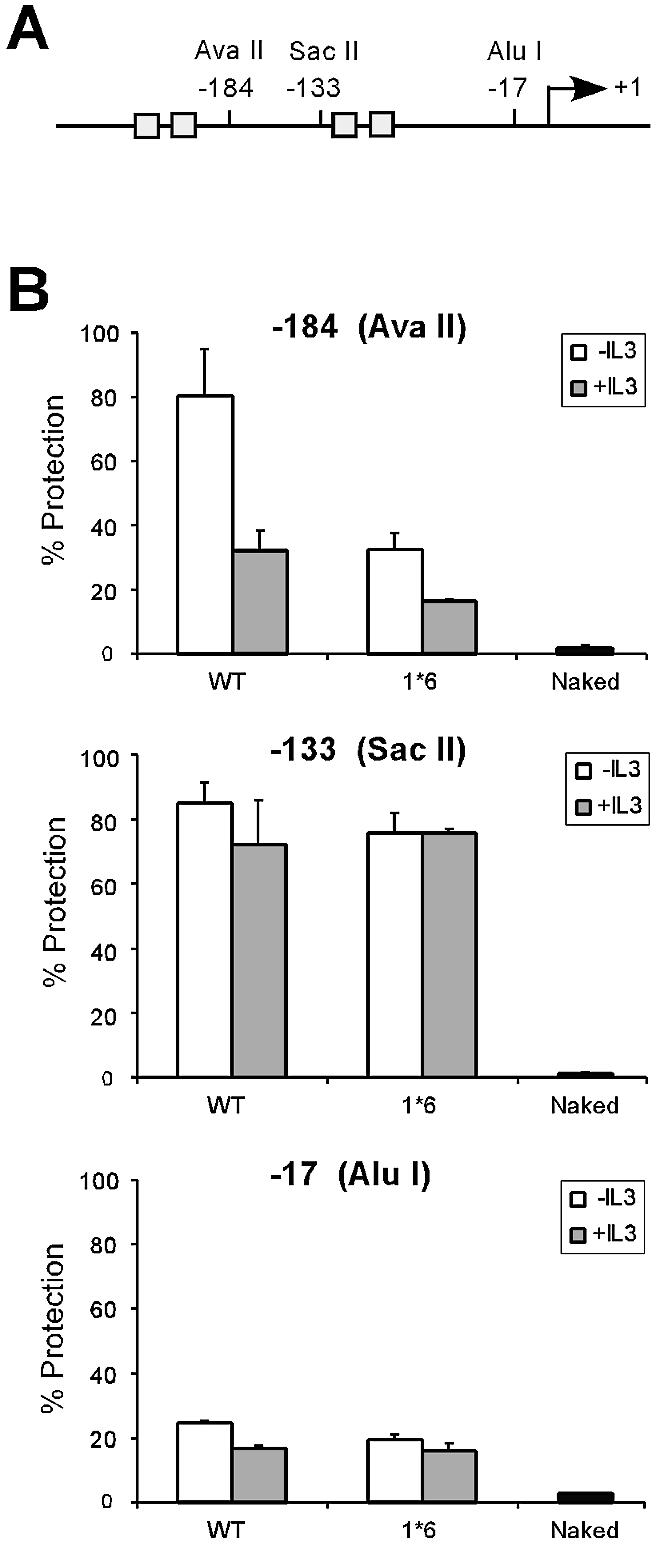
Chromatin is constitutively accessible to restriction enzyme digestion at position –184 of the Cis promoter in cells expressing STAT5A-1*6. (A) Structure of the Cis promoter, indicating the position of the restriction sites analyzed by CHART-PCR. Gray boxes represent STAT5 binding sites. (B) Nuclei from unstimulated and IL-3-stimulated (30 min) Ba/F3-WT and Ba/F3-1*6 cells were analyzed by CHART-PCR, as described in Materials and Methods. Purified genomic (naked) DNA was used as to control the cutting efficiency of the restriction enzyme.
First, we demonstrated that STAT5A-1*6 was constitutively bound to the endogenous Cis promoter in the absence of IL-3. ChIP assays were performed in Ba/F3-1*6 and Ba/F3-WT cells, unstimulated or stimulated with IL-3 for 30 min. Cross-linked chromatin was immunoprecipitated with antibodies specific for STAT5, and purified genomic DNA analyzed by real-time PCR using primers specific for Cis (Fig. 4A). In unstimulated 1*6 cells, a 5-fold enrichment of STAT5 proteins was specifically detected on the Cis promoter (–184/–102) compared to unstimulated WT cells (Fig. 4A). In agreement with in vitro DNA binding data (33,34), STAT5A-1*6 association with the Cis promoter in vivo was further increased upon IL-3 stimulation (Fig. 4A). Thus, the constitutively active form of STAT5A is bound to the endogenous Cis promoter in the absence of IL-3. As expected, histone H3 and H4 acetylation in unstimulated 1*6 cells as determined by ChIP was 2- to 3-fold lower than in WT cells over the STAT5 binding sites (Fig. 4B and C, –184/–102), therefore confirming that binding of STAT5 to DNA in vivo correlates well with a local decrease in histone acetylation (Fig. 3).
Figure 4.
STAT5 is constitutively bound to the Cis promoter in cells expressing STAT5A-1*6, a constitutively active form of STAT5A. Unstimulated and IL-3-stimulated (30 min) Ba/F3-WT and Ba/F3-1*6 cells were analyzed by ChIP, using antibodies specific for STAT5A+B (STAT5) (A), acetylated histone H3 (Ac-H3) (B), acetylated histone H4 (Ac-H4) (C) or no antibody as a control (D), as described above. Amplicons specific for Cis were analyzed by real-time PCR as before.
We next determined chromatin accessibility by real-time PCR (CHART-PCR) in nuclei from Ba/F3-1*6 and Ba/F3-WT cells treated as above. Nuclei were incubated with restriction enzymes cutting in the vicinity of the STAT5 binding sites (–184 and –133) and the transcription start site (–17) of the Cis promoter (Fig. 5A). Genomic DNA was then isolated and analyzed by real-time PCR using primers amplifying the region targeted by the enzyme (Fig. 5B). In Ba/F3-WT cells, position –133 of the Cis promoter was protected from restriction digest, while position –17 was accessible, regardless of IL-3 stimulation. The observed pattern remained the same in Ba/F3-1*6 cells. In contrast, accessibility at position –184 differed in Ba/F3-WT and Ba/F3-1*6 cells (Fig. 5B). While a strong increase in accessibility from 20 to 64% was detected upon IL-3 stimulation in the Ba/F3-WT cells, chromatin from Ba/F3-1*6 was already highly accessible (64%) in the absence of IL-3. In correlation with increased STAT5 DNA binding upon IL-3 stimulation (Fig. 4A), a further increase in chromatin accessibility (83%) was detected in IL-3-stimulated Ba/F3-1*6 cells (Fig. 5B). Altogether, these data show that constitutive binding of STAT5 to DNA is closely coupled to increased chromatin accessibility in vivo, and therefore suggest that STAT5 DNA binding is a prerequisite for chromatin remodeling at position –184 of the Cis promoter.
DISCUSSION
We present here a detailed mapping of histone acetylation and chromatin transitions taking place on the endogenous Cis gene, a model STAT5 target gene, following IL-3 stimulation of the murine pro-B cells Ba/F3.
We found that the Cis gene is highly acetylated in vivo, suggesting that it is targeted by histone acetyltransferase (HAT) activities. Since histone acetylation levels are already high in unstimulated cells, and increase only moderately upon STAT5 binding, it suggests that these HATs act in a STAT5-independent manner. The acetyltransferase p300/CBP was shown to interact with the C-terminal transactivation domain of STAT5 in vitro and in overexpression systems, and to enhance STAT5 transactivation using reporter systems (24,35,36). Our in vivo data thus suggest that, if it is recruited by STAT5 on the Cis promoter in vivo, p300/CBP might not target histones but rather acetylate another factor in the process of STAT5-mediated transcriptional activation. In contrast, a distinct histone acetylation pattern was observed on our control promoter c-Fos, with a strong and transient increase in H3 and H4 acetylation within the proximal promoter and CAP site regions upon IL-3 stimulation. c-Fos expression was shown to be induced through the MAPK pathway, which activates downstream transcription factors, such as Elk-1 and CREB/ATF1, which bind to the TCF (Elk-1), AP-1 and CRE (CREB/ATF1) elements of the c-Fos promoter (Fig. 2B) (37–39). Interestingly, CREB/ATF1 is known to recruit p300/CBP (39), which might be responsible for the histone acetylation pattern observed over the AP-1 and CRE elements.
While our data suggest that the endogenous Cis promoter is targeted by HATs, the observation that TSA treatment did not result in histone hyperacetylation along the Cis gene also suggests that it is not targeted by histone deacetylase (HDAC) activities. We have shown previously that deacetylase inhibitors (TSA, SAHA, NaB) can prevent induction of STAT5 target genes, including Cis (22), therefore suggesting that a deacetylase activity is required for STAT5-mediated transcription. Our data also suggested that the inhibitory effect of those drugs is not mediated through changes in histone acetylation levels or chromatin accessibilities within the Cis promoter (22). The present study thus confirms that the deacetylase activity required for STAT5 transactivation does not target histone H3 and H4 acetylation, and further support the involvement of a deacetylase specific for non-histone proteins.
We found that STAT5 binding to its target sites in vivo is closely associated with a local decrease in histone H3 and H4 acetylation. A similar decrease was observed on the endogenous Osm gene (data not shown), another STAT5 target gene (40). Our data favor a model in which STAT5 binding limits access of histone acetyltransferases to chromatin, rather than active deacetylation. Although we cannot rule out that the observed acetylation pattern is the result of the action of a TSA-insensitive deacetylase, such as the Sir2-like family of deacetylases (41), this appears unlikely since histone acetylation levels remained significantly high at that site, as compared to those at the c-Fos control gene. Our data suggest that this local acetylation pattern is a consequence of STAT5 binding since it was detected in unstimulated cells in which a mutant STAT5 protein (1*6) was constitutively bound to the Cis promoter, in the absence of other signaling. Whether this decrease in acetylation is a prerequisite for downstream events (such as chromatin remodeling and transcriptional activation) remains to be elucidated.
We also show here that STAT5 DNA binding in vivo resulted in a local increase in chromatin accessibility to restriction endonucleases. Since this chromatin transition was detected in unstimulated 1*6 cells, it suggests that it is a consequence of, rather than a prerequisite for, STAT5 binding. Our data therefore support a model in which STAT5 binding induces chromatin remodeling. This remodeling could be the direct result of STAT5 DNA binding or could involve the recruitment of a chromatin remodeling complex by STAT5. Interestingly, analysis of the levels of acetylated and non-acetylated forms of histone H4 suggested the persistence of a nucleosome within the region overlapping the STAT5 binding sites. This observation therefore makes it unlikely that the increase in chromatin accessibility over that region is the result of a nucleosome loss, and rather suggests that it reflects either a conformational change in the nucleosome architecture, or nucleosome sliding along the Cis promoter. Chromatin reorganization at this site might be involved in the transcriptional activation process, by facilitating binding of STAT5 heterodimers on the adjacent binding sites and/or tetramer formation (23,25), or by exposing a region of DNA previously unaccessible, thus permitting binding of transcriptional coactivators. Our data also confirmed that STAT5 binding and chromatin remodeling are necessary but not sufficient for transcriptional activation, as they occurred in unstimulated Ba/F3-1*6 cells in which Cis is not transcribed (Fig. 1A), and are not affected upon inhibition of transcription by TSA (Fig. 3A) (22).
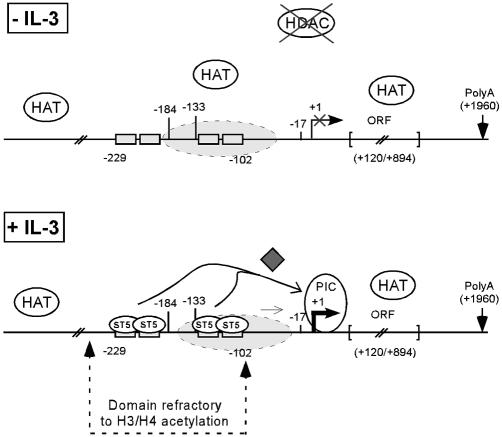
These conclusions are summarized in a model shown in Figure 6. Collectively, these studies reveal characteristic features of transcriptional regulation of the murine Cis gene in response to IL-3. They also highlight novel implications of the transcription factor STAT5, linking it to chromatin modification and remodeling in vivo, thus leading toward a better understanding of the molecular mechanism of transcriptional regulation by STAT5.
Figure 6.
Model of STAT5-regulated transcription of Cis in response to IL-3. In unstimulated Ba/F3 cells, the level of histone H3 and H4 acetylation along the Cis gene is high, probably through the action of HAT complexes. The absence of effect of TSA suggests that the gene is not targeted by HDACs. Upon IL-3 stimulation, STAT5 (ST5) is recruited to its binding sites (gray boxes). STAT5 binding results in a local decrease in histone H3/H4 acetylation, and in chromatin remodeling at position –184, possibly through nucleosome sliding (dashed oval). STAT5 also allows, directly or indirectly, an as yet uncharacterized TSA-sensitive factor (gray diamond) to participate in the recruitment of the basal transcription machinery (PIC, pre-initiation complex), in turn permitting transcriptional initiation to occur (22).
Acknowledgments
ACKNOWLEDGEMENTS
We are very grateful to Dr Joachim Griesenbeck for thoughtful discussions and critical reading of the manuscript, and to Dr Toshio Kitamura for providing us with the Ba/F3-WT and Ba/F3-1*6 cell lines. We also thank Drs Jim Johnston and Bruno Amati for their support at different stages along this work. DNAX Research Inc. is fully supported by Schering-Plough Corporation.
REFERENCES
- 1.Narlikar G.J., Fan,H.Y. and Kingston,R.E. (2002) Cooperation between complexes that regulate chromatin structure and transcription. Cell, 108, 475–487. [DOI] [PubMed] [Google Scholar]
- 2.Agalioti T., Lomvardas,S., Parekh,B., Yie,J., Maniatis,T. and Thanos,D. (2000) Ordered recruitment of chromatin modifying and general transcription factors to the IFN-beta promoter. Cell, 103, 667–678. [DOI] [PubMed] [Google Scholar]
- 3.Cosma M.P., Tanaka,T. and Nasmyth,K. (1999) Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell, 97, 299–311. [DOI] [PubMed] [Google Scholar]
- 4.Cosma M. (2002) Ordered recruitment. Gene-specific mechanism of transcription activation. Mol. Cell, 10, 227. [DOI] [PubMed] [Google Scholar]
- 5.Soutoglou E. and Talianidis,I. (2002) Coordination of PIC assembly and chromatin remodeling during differentiation-induced gene activation. Science, 295, 1901–1904. [DOI] [PubMed] [Google Scholar]
- 6.Struhl K. (1998) Histone acetylation and transcriptional regulatory mechanisms. Genes Dev., 12, 599–606. [DOI] [PubMed] [Google Scholar]
- 7.Deckert J. and Struhl,K. (2001) Histone acetylation at promoters is differentially affected by specific activators and repressors. Mol. Cell. Biol., 21, 2726–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadosh D. and Struhl,K. (1998) Targeted recruitment of the Sin3-Rpd3 histone deacetylase complex generates a highly localized domain of repressed chromatin in vivo. Mol. Cell. Biol., 18, 5121–5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadosh D. and Struhl,K. (1998) Histone deacetylase activity of Rpd3 is important for transcriptional repression in vivo. Genes Dev., 12, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Balbas M.A., Bannister,A.J., Martin,K., Haus-Seuffert,P., Meisterernst,M. and Kouzarides,T. (1998) The acetyltransferase activity of CBP stimulates transcription. EMBO J., 17, 2886–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang Y., Hu,X., DiRenzo,J., Lazar,M.A. and Brown,M. (2000) Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell, 103, 843–852. [DOI] [PubMed] [Google Scholar]
- 12.Mulholland N.M., Soeth,E. and Smith,C.L. (2003) Inhibition of MMTV transcription by HDAC inhibitors occurs independent of changes in chromatin remodeling and increased histone acetylation. Oncogene, 22, 4807–4818. [DOI] [PubMed] [Google Scholar]
- 13.Wang A., Kurdistani,S.K. and Grunstein,M. (2002) Requirement of Hos2 histone deacetylase for gene activity in yeast. Science, 298, 1412–1414. [DOI] [PubMed] [Google Scholar]
- 14.Grienenberger A., Miotto,B., Sagnier,T., Cavalli,G., Schramke,V., Geli,V., Mariol,M.C., Berenger,H., Graba,Y. and Pradel,J. (2002) The MYST domain acetyltransferase chameau functions in epigenetic mechanisms of transcriptional repression. Curr. Biol., 12, 762–766. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein B.E., Tong,J.K. and Schreiber,S.L. (2000) Genomewide studies of histone deacetylase function in yeast. Proc. Natl Acad. Sci. USA, 97, 13708–13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nawaz Z., Baniahmad,C., Burris,T.P., Stillman,D.J., O‘Malley,B.W. and Tsai,M.J. (1994) The yeast SIN3 gene product negatively regulates the activity of the human progesterone receptor and positively regulates the activities of GAL4 and the HAP1 activator. Mol. Gen. Genet., 245, 724–733. [DOI] [PubMed] [Google Scholar]
- 17.Vidal M. and Gaber,R.F. (1991) RPD3 encodes a second factor required to achieve maximum positive and negative transcriptional states in Saccharomyces cerevisiae. Mol. Cell. Biol., 11, 6317–6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidal M., Strich,R., Esposito,R.E. and Gaber,R.F. (1991) RPD1 (SIN3/UME4) is required for maximal activation and repression of diverse yeast genes. Mol. Cell. Biol., 11, 6306–6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimoto H., Ohmae,M. and Yamashita,I. (1992) The Saccharomyces cerevisiae GAM2/SIN3 protein plays a role in both activation and repression of transcription. Mol. Gen. Genet., 233, 327–330. [DOI] [PubMed] [Google Scholar]
- 20.Kouzarides T. (2000) Acetylation: a regulatory modification to rival phosphorylation? EMBO J., 19, 1176–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soutoglou E., Katrakili,N. and Talianidis,I. (2000) Acetylation regulates transcription factor activity at multiple levels. Mol. Cell., 5, 745–751. [DOI] [PubMed] [Google Scholar]
- 22.Rascle A., Johnston,J.A. and Amati,B. (2003) Deacetylase activity is required for recruitment of the basal transcription machinery and transactivation by STAT5. Mol. Cell. Biol., 23, 4162–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J.X. and Leonard,W.J. (2000) The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene, 19, 2566–2576. [DOI] [PubMed] [Google Scholar]
- 24.Shuai K. (2000) Modulation of STAT signaling by STAT-interacting proteins. Oncogene, 19, 2638–2644. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima H., Brindle,P.K., Handa,M. and Ihle,J.N. (2001) Functional interaction of STAT5 and nuclear receptor co-repressor SMRT: implications in negative regulation of STAT5-dependent transcription. EMBO J., 20, 6836–6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto A., Masuhara,M., Mitsui,K., Yokouchi,M., Ohtsubo,M., Misawa,H., Miyajima,A. and Yoshimura,A. (1997) CIS, a cytokine inducible SH2 protein, is a target of the JAK-STAT5 pathway and modulates STAT5 activation. Blood, 89, 3148–3154. [PubMed] [Google Scholar]
- 27.Mui A.L., Wakao,H., Kinoshita,T., Kitamura,T. and Miyajima,A. (1996) Suppression of interleukin-3-induced gene expression by a C-terminal truncated Stat5: role of Stat5 in proliferation. EMBO J., 15, 2425–2433. [PMC free article] [PubMed] [Google Scholar]
- 28.Brizzi M.F., Defilippi,P., Rosso,A., Venturino,M., Garbarino,G., Miyajima,A., Silengo,L., Tarone,G. and Pegoraro,L. (1999) Integrin-mediated adhesion of endothelial cells induces JAK2 and STAT5A activation: role in the control of c-fos gene expression. Mol. Biol. Cell., 10, 3463–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajotte D., Sadowski,H.B., Haman,A., Gopalbhai,K., Meloche,S., Liu,L., Krystal,G. and Hoang,T. (1996) Contribution of both STAT and SRF/TCF to c-fos promoter activation by granulocyte-macrophage colony-stimulating factor. Blood, 88, 2906–2916. [PubMed] [Google Scholar]
- 30.Leaman D.W., Pisharody,S., Flickinger,T.W., Commane,M.A., Schlessinger,J., Kerr,I.M., Levy,D.E. and Stark,G.R. (1996) Roles of JAKs in activation of STATs and stimulation of c-fos gene expression by epidermal growth factor. Mol. Cell. Biol., 16, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian S.S., Tapley,P., Sincich,C., Stein,R.B., Rosen,J. and Lamb,P. (1996) Multiple signaling pathways induced by granulocyte colony-stimulating factor involving activation of JAKs, STAT5 and/or STAT3 are required for regulation of three distinct classes of immediate early genes. Blood, 88, 4435–4444. [PubMed] [Google Scholar]
- 32.Wehinger J., Gouilleux,F., Groner,B., Finke,J., Mertelsmann,R. and Weber-Nordt,R.M. (1996) IL-10 induces DNA binding activity of three STAT proteins (Stat1, Stat3 and Stat5) and their distinct combinatorial assembly in the promoters of selected genes. FEBS Lett., 394, 365–370. [DOI] [PubMed] [Google Scholar]
- 33.Nosaka T., Kawashima,T., Misawa,K., Ikuta,K., Mui,A.L. and Kitamura,T. (1999) STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J., 18, 4754–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onishi M., Nosaka,T., Misawa,K., Mui,A.L., Gorman,D., McMahon,M., Miyajima,A. and Kitamura,T. (1998) Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol. Cell. Biol., 18, 3871–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfitzner E., Jahne,R., Wissler,M., Stoecklin,E. and Groner,B. (1998) p300/CREB-binding protein enhances the prolactin-mediated transcriptional induction through direct interaction with the transactivation domain of Stat5, but does not participate in the Stat5-mediated suppression of the glucocorticoid response. Mol. Endocrinol., 12, 1582–1593. [DOI] [PubMed] [Google Scholar]
- 36.Zhu M., John,S., Berg,M. and Leonard,W.J. (1999) Functional association of Nmi with Stat5 and Stat1 in IL-2- and IFNgamma-mediated signaling. Cell, 96, 121–130. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y. and Prywes,R. (2000) Activation of the c-fos enhancer by the erk MAP kinase pathway through two sequence elements: the c-fos AP-1 and p62TCF sites. Oncogene, 19, 1379–1385. [DOI] [PubMed] [Google Scholar]
- 38.Liao J., Hodge,C., Meyer,D., Ho,P.S., Rosenspire,K. and Schwartz,J. (1997) Growth hormone regulates ternary complex factors and serum response factor associated with the c-fos serum response element. J. Biol. Chem., 272, 25951–25958. [DOI] [PubMed] [Google Scholar]
- 39.Montminy M. (1997) Transcriptional regulation by cyclic AMP. Annu. Rev. Biochem., 66, 807–822. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimura A., Ichihara,M., Kinjyo,I., Moriyama,M., Copeland,N.G., Gilbert,D.J., Jenkins,N.A., Hara,T. and Miyajima,A. (1996) Mouse oncostatin M: an immediate early gene induced by multiple cytokines through the JAK-STAT5 pathway. EMBO J., 15, 1055–1063. [PMC free article] [PubMed] [Google Scholar]
- 41.Gray S.G. and Ekstrom,T.J. (2001) The human histone deacetylase family. Exp. Cell Res., 262, 75–83. [DOI] [PubMed] [Google Scholar]