Abstract
Rationale: Stress-elicited disruption of immunity begins in utero.
Objectives: Associations among prenatal maternal stress and cord blood mononuclear cell (CBMC) cytokine responses were prospectively examined in the Urban Environment and Childhood Asthma Study (n = 557 families).
Methods: Prenatal maternal stress included financial hardship, difficult life circumstances, community violence, and neighborhood/block and housing conditions. Factor analysis produced latent variables representing three contexts: individual stressors and ecological-level strains (housing problems and neighborhood problems), which were combined to create a composite cumulative stress indicator. CBMCs were incubated with innate (lipopolysaccharide, polyinosinic-polycytidylic acid, cytosine-phosphate-guanine dinucleotides, peptidoglycan) and adaptive (tetanus, dust mite, cockroach) stimuli, respiratory syncytial virus, phytohemagglutinin, or medium alone. Cytokines were measured using multiplex ELISAs. Using linear regression, associations among increasing cumulative stress and cytokine responses were examined, adjusting for sociodemographic factors, parity, season of birth, maternal asthma and steroid use, and potential pathway variables (prenatal smoking, birth weight for gestational age).
Measurements and Main Results: Mothers were primarily minorities (Black [71%], Latino [19%]) with an income less than $15,000 (69%). Mothers with the highest cumulative stress were older and more likely to have asthma and deliver lower birth weight infants. Higher prenatal stress was related to increased IL-8 production after microbial (CpG, PIC, peptidoglycan) stimuli and increased tumor necrosis factor-α to microbial stimuli (CpG, PIC). In the adaptive panel, higher stress was associated with increased IL-13 after dust mite stimulation and reduced phytohemagglutinin-induced IFN-γ.
Conclusions: Prenatal stress was associated with altered innate and adaptive immune responses in CBMCs. Stress-induced perinatal immunomodulation may impact the expression of allergic disease in these children.
Keywords: psychological stress, cord blood, cytokines, innate, adaptive
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Although there are animal data demonstrating the influence of maternal stress on fetal immune development, little is known about this relationship in humans. Still less is known about this relationship in high-risk, inner-city, ethnic minority populations.
What This Study Adds to the Field
We demonstrate that prenatal psychological stress is independently associated with alterations in both innate and adaptive immune responses as indexed by stimulated cord blood cytokine responses.
The increased prevalence and enormous costs in the management of atopic disorders motivate efforts to identify early risk factors (1). Moreover, atopy is associated with asthma, the most costly pediatric disease in the United States. The functional differentiation of immune cells plays a central role with immunological abnormalities preceding development of atopic disorders, even being evident at birth (2, 3). Early life environmental factors may organize physiological systems, including immune function. Although diverse mechanisms are likely involved, focus has been on the systemic propensity for the Th2 allergic response relative to Th1 pathways (4–7). Although useful in understanding a large fraction of atopic subjects, Th2-biased polarization of adaptive immunity may be only one of several axes that alter susceptibility (8). Non-Th2 factors, (e.g., IFN-γ, tumor necrosis factor [TNF]-α, and regulatory T cells) play a role in allergic and nonallergic inflammation (9, 10). Specifically, antigen-independent innate responses may also modify early risk (11, 12).
Additionally, asthma remains a leading cause of health disparities not completely explained by physical factors. Ethnic minorities in urban disadvantaged communities are disproportionately burdened (13). This has led to the reconsideration of social determinants (e.g., psychological stress) in asthma epidemiology (14).
The role of stress in the ontogeny of atopic disorders remains poorly understood (15). Evidence from animal and human studies suggests that stress-elicited disruption of interrelated systems—autonomic, neuroendocrine, and immune systems—may increase vulnerability to atopy even beginning in utero (15). For example, stress-induced alterations in maternal cortisol may influence fetal immunomodulation and Th2 cell predominance through direct influence on cytokine production (16) and prevent the development of regulatory T cells (17). This may induce selective suppression of Th1-mediated cellular immunity and potentiate Th2-mediated humoral immunity (18). In other studies, psychological stress is associated with increased proportions of natural killer T cells as well as altering their functional mechanisms (19, 20). Stress may also impact the maturation process of dendritic cells, further predisposing to a Th2 phenotype (21). Moreover, in animal studies, gestational exposure to maternal stress alters the development of immunocompetence in offspring. Evidence in rhesus monkeys links prenatal stress to antigen-induced responses at birth (22). Dysregulated immune response to antigen challenge has also been demonstrated in prenatally stressed adult mice, reflected by an enhanced Th2 adaptive response (23).
Although behavioral and neuroendocrine effects of prenatal stress have been more extensively studied (24), no human studies have examined effects of prenatal stress on immune responses as indexed by cytokine profiles at birth (25). We examined cord blood mononuclear cell (CBMC) cytokine profiles after stimulation of both innate and adaptive/mitogen activation related to differential stress exposure in an urban birth cohort. We specifically examined whether increased prenatal stress may be associated with distinct patterns of immune modulation assessed at birth.
METHODS
Study Population
The Urban Environment and Childhood Asthma prospective birth cohort examines environmental and genetic factors that influence immunologic development and asthma risk in early childhood. The study was approved by institutional review boards at participating institutions. Expectant families were recruited during the prenatal period in Baltimore, Boston, New York, and St. Louis; written informed consent was obtained. Selection criteria included living in an area with more than 20% of residents below the poverty level, mother or father of the index child with a history of allergic rhinitis, eczema, and/or asthma, and gestational age 34 weeks or more. Between February 2005 and March 2007, 1,853 families were screened, 779 met eligibility criteria, and 557 were enrolled (26). For further detail, see the online supplement.
Prenatal Maternal Stress Assessment
At the baseline prenatal visit, mothers completed the following measures.
Difficult life circumstances.
The 26-item Difficult Life Circumstances scale assesses life events occurring in the past 6 months with partner (including domestic violence), household members, substance abuse, and child rearing (27), tapping into interpersonal difficulties likely to be experienced by low-income women (28). Respondents answer yes or no to items; scores were summed across items. Acceptable reliability and construct validity have been reported (29).
Economic strain.
Respondents were asked: “How difficult is it for you to live on your total household income right now?”, “In the next two months, how likely is it that you and your family will experience actual hardships, such as inadequate housing, food, or medical attention?”, and “How likely is it that you and your family will have to reduce your standard of living to the bare necessities in life?” Items are scored on a 5-point scale and summed.
Neighborhood/block conditions.
Using the Community Survey Questionnaire, a measure with previously demonstrated validity and reliability (30), subjects were asked to rate 12 urban problems as 0 = “no problem,” 1 = “a minor problem,” or 2 = “a serious problem,” and items were summed: property damage, drugs, groups of young people hanging around, physical assaults on the street, organized gangs, gunshots, lack of supervised activities for youth, feeling unsafe while out alone, inadequate recreational facilities, and poor city services. Respondents were also asked, “On a scale of 1 to 10, how would you rate your neighborhood as a place to live?” (10 is best, 1 is worst, reverse scored).
Perceived community violence.
Subjects reported whether five events had occurred in their neighborhood in the past 6 months (not including self-victimization) and items were summed: (1) fight in which a weapon was used, (2) violent argument between neighbors, (3) gang fight, (4) sexual assault or rape, and (5) robbery or mugging. This measure relates directly to police-reported homicides, which are less vulnerable to reporting limitations (30), and has been linked to urban asthma morbidity (31).
Housing worries.
Adapting previous work linking worry about housing conditions to psychological distress (32, 33), subjects were asked “How much do the following things bother you about the home where you live: hard to heat, high rent, worry about eviction, rodents or cockroaches, water leaks, mold, street noise, others in the home, and landlord difficulties.” These were scored on a 5-point scale from “never” to “all the time,” summing across items. Respondents were also asked, “On a scale of 1 to 10, how would you rate your (house/apartment) as a place to live?” (10 is best, 1 is worst, reverse scored).
Covariates
Information on maternal asthma as well as inhaled corticosteroid use, prenatal smoking, and sociodemographics was collected during a standardized interview. Gestational age and birth weight were abstracted from the medical record. The percentile (z-value) of birth weight adjusted for gestational age was calculated using national reference data (34).
Cord Blood Cytokine Response Outcomes
Procedures are detailed elsewhere (35). Briefly, cord blood was collected using sterile procedures. Blood was transferred from syringes to sterile 50-ml tubes, diluted 1:1 with RPMI 1640 heparinized medium, and kept at room temperature pending cell separation. Mononuclear cells were separated by density gradient using Accuspin tubes (Sigma, St. Louis, MO) and incubated in the presence of medium and specific immune stimulants or medium alone (35). After incubation for 24 hours (innate and polyclonal stimuli) or 5 days (antigens), supernatants were collected, divided into aliquots, frozen at −80°C, and shipped to a central laboratory. Supernatants were analyzed for cytokines with a bead-based multiplex assay (Beadlyte; Upstate Biotechnology, Lake Placid, NY). Cytokines (Table 1) were selected based on specific innate and adaptive immune responses previously related to allergic inflammation and viral immune responses.
TABLE 1.
STIMULANTS USED AND CYTOKINES MEASURED IN THE CYTOKINE SECRETION ASSAYS
| Innate Responses |
Adaptive and Polyclonal Responses |
||||
|---|---|---|---|---|---|
| Stimulants | Final Concentration | Cytokines Measured with All Stimulants | Stimulants | Final Concentration | Cytokines Measured with All Stimulants |
| Lipopolysaccharide* | 0.1 μg/ml | IFN-α | Phytohemagglutinin† | 15 μg/ml | IFN-γ |
| Polyinosinic-polycytidylic acid‡ | 25 μg/ml | IFN-γ | Cockroach extract § | 10 μg/ml | IL-10 |
| Peptidoglycan‖ | 1.25 μg/ml | IL-10 | Dust mite (Dermatophagiodes pteronyssinus) extract§ | 10 μg/ml | IL-13 |
| CpG-C ISS-ODNs¶ | 1 μg/ml | IL-12p40 | Tetanus toxoid** | 10 μg/ml | IL-4 |
| Respiratory syncytial virus†† | 500 sfu/ml | TNF-α | Medium alone | Not applicable | |
| Medium alone | Not applicable | IL-8 | |||
Definition of abbreviations: CpG-C ISS-ODNs = cytosine-phosphate-guanine containing immunostimulatory oligodeoxyribonucleotides TNF = tumor necrosis factor.
Sources for reagents:
Associates of Cape Cod (Falmouth, MA).
Sigma (St. Louis, MO).
Amersham Biosciences (Piscataway, NJ).
Greer Inc. (Lenoir NC).
InvivoGen (San Diego, CA).
Coley Pharmaceuticals (Wellesley, MA).
Massachusetts Biologics (Jamaica Plain, MA).
Ann Mosser (University of Wisconsin-Madison, Madison, WI).
Statistical Analysis
As stress research suggests that individuals may be increasingly vulnerable when adverse events are experienced across multiple domains (i.e., cumulative stress [36, 37]), we used data reduction steps to derive a composite measure of cumulative stress. Principal components factor analysis (38) was used to combine the items on the individual scales in the stress battery and extract a reduced number of factors interpreted to represent different domains of stress. The analysis produced factor loadings that are essentially correlation coefficients between the scales and the unmeasured underlying factor. Scores were derived by multiplying the factor loadings by the values for the variables included in the factor and summing those values. Resultant factors represented three contexts (Table 2): individual-level stressors and ecological-level strains related to housing problems and neighborhood problems. Each subscale was then divided into tertiles and given values of 1 = low, 2 = medium, and 3 = high exposure; an overall cumulative stress score was derived by summing the tertile values across the three factors. For example, if a subject was in tertile 1 for individual-level stressors, tertile 2 for housing problems, and tertile 3 for neighborhood problems, the composite score would equal 6 (range 3 [low on all domains] to 9 [high on all domains]).
TABLE 2.
INDIVIDUAL- AND ECOLOGICAL-LEVEL STRESSOR DOMAINS DERIVED FROM FACTOR ANALYSIS
| Stressor Domains (Factor Loadings) |
|||
|---|---|---|---|
| Stress Scales | Interpersonal Problems Cronbach α = 0.85 | Housing Problems Cronbach α = 0.90 | Neighborhood Problems Cronbach α = 0.90 |
| Difficult life circumstances | 0.46* | 0.08 | 0.25 |
| Neighborhood/block conditions | 0.20 | 0.32 | 0.72* |
| Perceived community violence | 0.25 | 0.14 | 0.65* |
| Rating of home | 0.26 | 0.65* | 0.18 |
| Rating of neighborhood | 0.13 | 0.55 | 0.49* |
| Housing conditions | 0.46 | 0.48* | 0.33 |
| Economic strain | 0.48* | 0.24 | 0.08 |
Denotes scales included in each factor (within each column) based on factor loadings. A scale was included in a particular factor if the loading was ≥0.45. Two scales loaded highly on more than one factor (i.e., Rating of neighborhood loading with both the Neighborhood problems factor and Housing problems factor; Housing score loading with the Interpersonal problems and Housing problems factors) in which case scales were kept in the factor with which they were most conceptually consistent. A standard extraction strategy supported a three-factor solution accounting for more than 75% of the total variance.
Logarithmic transformation of the cytokine outcomes was used given skewness of the data to symmetrize the residuals. Linear regression was used to model the relationship between cytokine responses and cumulative stress. All models were adjusted for media control background cytokine production. Variables previously identified as being related to psychological stress and cord blood immunomodulation were examined as potential confounders. Stepwise modeling was done initially adjusting for parity (primiparous versus multiparous) (6), sex of the child, and race, next adding income and maternal education. We next adjusted for season of birth, maternal history of asthma, and inhaled corticosteroid use. Finally, we considered variables that are potentially in the pathway through which stress might contribute to altered immune function: birth weight for gestational age and maternal smoking (39). Statistical analyses were performed on SAS Version 9.1.3 (SAS Institute, Cary, NC) and the R system for statistical computing (version 2.9.1) (40). A test for linear trend was used to determine the relationships among the cumulative stress score on mean cytokine outcomes (41). Significance was set at P less than 0.05.
RESULTS
Data from 560 newborns (n = 272 girls, n = 288 boys, three sets of twins) and their mothers were analyzed. The distribution of covariates (Table 3) and cumulative stress scores across covariates (Table 4) are summarized. The majority of mothers self-identify as ethnic minorities (Black [71%], Latino [19%]), with a high school education or less (75%) and report an annual income less than $15,000 (69%). Mothers in the highest cumulative stress group were older, more likely to have a history of asthma, and delivered babies with lower birth weight for gestational age.
TABLE 3.
DEMOGRAPHIC CHARACTERISTICS AND DISTRIBUTION OF OTHER COVARIATES
| Characteristic | ||
|---|---|---|
| Total mothers (No., %) | 557 | 100 |
| Mother's age in years at child's birth (median, range) | 23 | 13–42 |
| Race or ethnicity of mother (No., %) | ||
| Hispanic of any race | 107 | 19 |
| Black alone | 390 | 71 |
| White alone | 22 | 4 |
| More than one race | 20 | 4 |
| All others | 11 | 2 |
| Missing | 7 | — |
| Household income < $15,000 per year (No., %) | 355 | 69 |
| Mother's education (No., %) | ||
| <High school | 231 | 42 |
| High school or GED | 183 | 33 |
| >High school | 136 | 25 |
| Smoked during pregnancy (No., %) | 97 | 18 |
| Maternal asthma (No., %) | 307 | 55 |
| Inhaled corticosteroid use during pregnancy (No., %) | 54 | 10 |
| Total babies (No., %) | 560 | 100 |
| Sex (No., %) | ||
| Male | 288 | 51 |
| Female | 272 | 49 |
| Firstborn (No., %) | 221 | 39 |
| Season of birth (No., %) | ||
| January-March | 142 | 25 |
| April-June | 139 | 25 |
| July-September | 160 | 29 |
| October-December | 121 | 22 |
| Birthweight, g (median, range) | 3,220 | 1,815–4,850 |
| Gestational age, wk (median, range) | 39 | 34–42 |
TABLE 4.
RELATIONSHIP OF COMPOSITE STRESS SCORE TO SOCIODEMOGRAPHIC CHARACTERISTICS
| Composite Stress Score |
||||
|---|---|---|---|---|
| 3-5 | 6-7 | 8-9 | P Value | |
| No. (%) | 222 (41) | 191 (35) | 126 (23) | |
| Mother's age in years at child's birth, median | 22 | 23 | 25 | 0.002 |
| Primiparous, % | 45 | 38 | 31 | 0.04 |
| African American, % | 80 | 75 | 75 | 0.38 |
| Household income < $15,000 per year, % | 68 | 65 | 75 | 0.15 |
| Mother's education, % | ||||
| <High school | 46 | 41 | 36 | 0.11 |
| High school or GED | 35 | 31 | 36 | |
| >High school | 19 | 28 | 29 | |
| Smoked during pregnancy, % | 15 | 18 | 22 | 0.22 |
| Maternal asthma, % | 50 | 56 | 65 | 0.022 |
| Inhaled steroid use during pregnancy, % | 8 | 9 | 14 | 0.12 |
| Season of birth, % | ||||
| January-March | 29 | 24 | 19 | 0.30 |
| April-June | 25 | 24 | 25 | |
| July-September | 26 | 28 | 35 | |
| October-December | 20 | 25 | 21 | |
| Male child, % | 50 | 53 | 48 | 0.65 |
| Birthweight, z-value for gestational age, median | −0.22 | −0.29 | −0.55 | 0.05 |
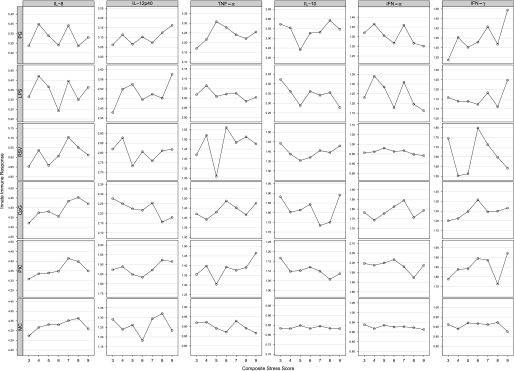
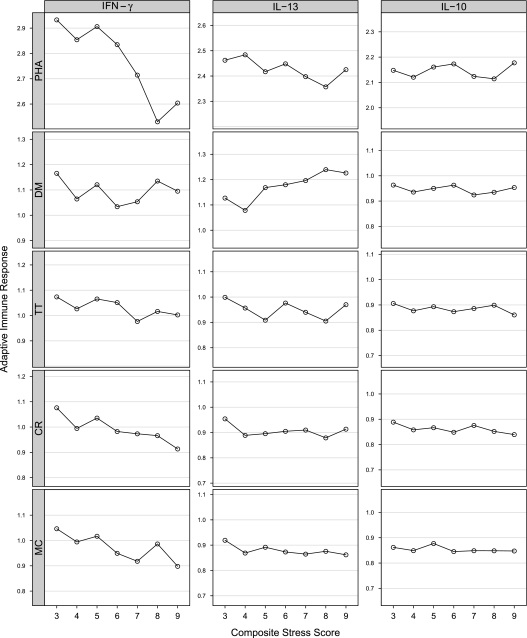
We graphically examined associations among the cumulative stress indicator and the cytokine by stimulant outcomes. Increasing cumulative prenatal stress was related to patterns that were consistent across a number of stimuli on the innate panel (Figure 1): increased IFN-γ after incubation with peptidoglycan (PG) and cytosine-phosphate-guanine dinucelotides (CpG); reduced IL-10 after incubation with polyinosinic-polycytidylic acid (PIC) and lipopolysaccharide; higher levels of TNF-α after incubation with PIC and CpG; and higher levels of IL-8 after incubation with respiratory syncytial virus (RSV), PIC, and CpG. In addition, there was some suggestion for the increased production of IL-8 with increasing levels of cumulative stress in the media control assay. In the adaptive panel (Figure 2), increased cumulative prenatal maternal stress was related to lower levels of IFN-γ when stimulated with phytohemagglutinin, cockroach, and media control, and increased IL-13 when stimulated with dust mite antigen.
Figure 1.
Relationship of cytokine responses (average log-transformed values) with composite stress score: innate panel.
Figure 2.
Relationship of cytokine responses (average log-transformed values) composite stress score: adaptive panel.
Linear regression analyses were run for cumulative stress predicting the cytokine outcomes. Table 5 shows the geometric mean cytokine values (pg/ml) across levels of the composite cumulative stress score (P for trend). For the innate panel, increasing stress was significantly related to increased CpG-induced IL-8 production, even in the fully adjusted model (P = 0.02). A significant association between higher stress and increased TNF-α production after incubation with PIC was also evident.
TABLE 5.
SELECTED GEOMETRIC MEAN CORD BLOOD CYTOKINE LEVELS (PG/ML) BY COMPOSITE STRESS SCORE
| Composite Stress Score |
P Value* Model 1† | P Value Model 2‡ | P Value Model 3§ | P Value Model 4‖ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stimulant | Cytokine | n | % Detectable | 3–5 | 6–7 | 8–9 | ||||
| Innate Panel | ||||||||||
| CpG | IFN-γ | 500 | 64.0 | 16.6 | 18.9 | 18.1 | 0.33 | 0.33 | 0.44 | 0.60 |
| IL-10 | 500 | 93.4 | 68.1 | 61.4 | 66.2 | 0.61 | 0.58 | 0.61 | 0.65 | |
| TNF-α | 500 | 82.0 | 25.9 | 29.5 | 27.9 | 0.12 | 0.09 | 0.10 | 0.10 | |
| IL-8 | 505 | 41.0 | 22,817.9 | 24,800.9 | 27,323.9 | 0.05 | 0.03 | 0.02 | 0.02 | |
| LPS | IFN-γ | 508 | 45.5 | 14 | 14.2 | 15.1 | 0.30 | 0.38 | 0.37 | 0.41 |
| IL-10 | 508 | 99.0 | 227.7 | 224.4 | 207.2 | 0.16 | 0.25 | 0.27 | 0.36 | |
| TNF-α | 508 | 99.6 | 1,072.3 | 1,053.8 | 985.8 | 0.70 | 0.86 | 0.87 | 0.97 | |
| IL-8 | 513 | 96.7 | 232,968.5 | 208,460.8 | 214,626.2 | 0.63 | 0.77 | 0.98 | 0.61 | |
| PG | IFN-γ | 509 | 61.9 | 19.8 | 23.3 | 25.5 | 0.03 | 0.04 | 0.06 | 0.11 |
| IL-10 | 509 | 98.6 | 333 | 338.2 | 371.2 | 0.77 | 0.66 | 0.98 | 0.93 | |
| TNF-α | 508 | 98.6 | 1,704 | 1,817.6 | 1,729 | 0.42 | 0.34 | 0.35 | 0.35 | |
| IL-8 | 512 | 96.7 | 195,505.4 | 195,303 | 181,181 | 0.70 | 0.82 | 0.94 | 0.64 | |
| PIC | IFN-γ | 510 | 82.4 | 66.5 | 77.7 | 69.5 | 0.39 | 0.39 | 0.63 | 0.75 |
| IL-10 | 510 | 56.7 | 13.3 | 12.9 | 11.8 | 0.17 | 0.17 | 0.28 | 0.26 | |
| TNF-α | 510 | 78.2 | 22.5 | 24.2 | 26.8 | 0.03 | 0.02 | 0.02 | 0.02 | |
| IL-8 | 513 | 34.3 | 21,270.9 | 24,075.6 | 23,661.7 | 0.16 | 0.12 | 0.12 | 0.08 | |
| RSV | IFN-γ | 482 | 70.7 | 41.7 | 57 | 41.6 | 0.99 | 1.00 | 0.63 | 0.96 |
| IL-10 | 482 | 78.2 | 24.8 | 24.4 | 25.8 | 0.61 | 0.47 | 0.52 | 0.47 | |
| TNF-α | 482 | 74.9 | 26.1 | 33.3 | 31.3 | 0.26 | 0.33 | 0.12 | 0.11 | |
| IL-8 | 466 | 92.9 | 96,067.1 | 11,3518.8 | 10,7420.6 | 0.30 | 0.24 | 0.21 | 0.13 | |
| Adaptive Panel | ||||||||||
| CR | IL-10 | 440 | 8.0 | 7.4 | 7.3 | 7 | 0.36 | 0.30 | 0.35 | 0.43 |
| IL-13 | 440 | 15.2 | 8.2 | 8.1 | 7.9 | 0.80 | 0.78 | 0.52 | 0.61 | |
| IFN-γ | 440 | 25.5 | 10.8 | 9.5 | 8.7 | 0.46 | 0.47 | 0.53 | 0.53 | |
| DM | IL-10 | 488 | 33.8 | 8.9 | 8.8 | 8.8 | 0.83 | 0.96 | 0.79 | 0.66 |
| IL-13 | 488 | 44.7 | 13.3 | 15.4 | 17.1 | 0.03 | 0.03 | 0.01 | 0.03 | |
| IFN-γ | 488 | 35.0 | 13.1 | 11.1 | 13 | 0.11 | 0.12 | 0.05 | 0.09 | |
| PHA | IL-10 | 503 | 97.6 | 139.1 | 140.8 | 139.9 | 0.93 | 1.00 | 1.00 | 0.80 |
| IL-13 | 503 | 98.4 | 284.8 | 264.8 | 246.2 | 0.13 | 0.12 | 0.16 | 0.11 | |
| IFN-γ | 503 | 97.2 | 790.1 | 595.2 | 369 | 0.004 | 0.002 | 0.006 | 0.004 | |
Definition of abbreviations: CpG = cytosine-phosphate-guanine dinucleotides; CR = cockroach; DM = dust mite; LPS = lipopolysaccharide; PG = peptidoglycan; PHA = phytohemagglutinin; PIC = polyinosinic-polycytidylic acid; RSV = respiratory syncytial virus; TNF = tumor necrosis factor.
Each line represents a separate linear regression model.
Test for linear trend.
Adjusted for media control value and race, sex, parity, and birth order of child.
Adjusted for media control, race, sex, parity, and birth order of child, household income, and education of mother.
Adjusted for media control, race, sex, parity, and birth order of child, household income, education of mother, season of birth, whether the mother has asthma, and inhaled corticosteroid use during pregnancy.
Adjusted for media control, race, sex, parity, and birth order of child, household income, education of mother, season of birth, whether the mother has asthma, inhaled corticosteroid use during pregnancy, maternal smoking, and birthweight for gestational age.
In the adaptive panel (Table 5), increased stress was significantly associated with greater dust mite–induced IL-13 when adjusted for potential confounders and pathway variables (P = 0.03). The association between higher cumulative stress and reduced IFN-γ production to stimulation with phytohemagglutinin (a nonspecific mitogen) was robust even in fully adjusted models (P = 0.004).
DISCUSSION
Research continues to delineate the relationships among early environmental influences, immune system alterations, and allergy and asthma (42–44). Children at risk for atopy, including asthma, are born with impairments of both adaptive and innate immune responses. Regulatory T cells have been found to be less effective in neonates born to atopic mothers as compared with children of nonatopic mothers (12). Also, neonates at risk for asthma later in life have been found to have impaired Th1-mediated responses at birth (4). We extend this literature with the first prospective data examining associations between prenatal maternal stress and cytokine profiles in cord blood mononuclear cells in urban infants at high risk for atopic diseases based on family history. These data suggest that prenatal psychological stress may alter both adaptive and innate immune axes assessed at birth.
When examining findings across innate stimuli, notable patterns emerged that were consistent across stimuli likely to operate through similar pathways. For example, higher stress was associated with increased IL-8 production after microbial (CpG, PIC) stimuli, albeit the association was significant only for CpG. We also observed stress-induced increased TNF-α production to microbial stimuli (CpG, PIC); although notably in the same direction, only PIC was significantly related. These findings suggest that stress may modify the neonatal immune response through Toll-like receptor (TLR)-dependent pathways. TLRs, implicated in allergy and asthma, are an essential part of the innate and adaptive immune response (45). They operate through detection of a wide range of microbial- and viral-associated molecular patterns (46). TLRs 7 and 9 recognize pathogen-derived nucleic acid molecular patterns, specifically RNA or DNA, respectively. Bacterial DNA containing unmethylated CpG motifs are ligands for TLR9 (47). TNF-α is an important regulator of TLR2 expression in response to diverse microbial stimuli. Consistent with these findings, evidence in murine models suggests that stress modulates the immune response in a TLR4-dependent manner. Powell and colleagues demonstrated that stress modulates TLR-dependent cytokine secretion in response to CpG DNA and PIC in splenic dendritic cells (48). Zhang and colleagues have linked stress and TLR4-mediated P13K/Akt signaling in mice (49). A number of TLRs have also been identified as candidates for playing a key role in the immune response to RSV, including TLR2, TLR4, TLR6, and TLR7 (50, 51). We did not find a relationship between increasing stress and RSV-induced IL-8 or TNF-α.
In addition, the increased production of IL-8 with increasing levels of cumulative stress in the nonspecific media control assay may point to stress effects on still another immune axis. Certain pollutants, such as ozone, a potent oxidant, particulates, and endotoxin, are believed to induce asthma through nonallergic mechanisms, perhaps related to nuclear factor-κB activation and IL-8 secretion (52). Notably, psychological stress is also an oxidant and may operate through these same pathways (14).
Risk of Th2 skewing may occur through pathways related to the interaction between stress, innate immune cells, adaptive immune cells, and their cytokine and chemokine mediators (4). For adaptive responses, there was evidence that increased stress was associated with lower levels of IFN-γ production, which has previously been linked to increased risk for later atopic disease (53). Macaubas and colleagues found that detectable levels of IFN-γ (constitutively) were associated with lower risk of asthma at age 6 years (44). Mitogen-induced IFN-γ tended to be lower in newborns whose mothers reported more stress. This may suggest that the immunomodulatory effect of higher stress as reflected by reduced expression of IFN-γ by stimulated CBMCs is a more generalized, nonspecific response rather than being allergen specific. A delayed maturation of Th1 immune responses may increase the risk of sensitization to aeroallergens (4) and susceptibility to viral illnesses (54) as these children grow older. Continued follow-up in this cohort with serial assessments of these cytokine responses will allow us to examine whether prenatal stress differentially influences the rate of maturation of the Th1 response and subsequent risk for asthma and allergies.
We also observed increasing production of IL-13 to allergen-specific stimulation with dust mite in association with increasing cumulative stress. Notably, the pattern of increased IL-13 response to house dust mite has been associated with allergic sensitization in older children (55). The production of IL-13 by CBMCs stimulated with cockroach extract was unchanged across the cumulative stress levels. These findings may reflect a differential likelihood of prenatal exposure to these inhalant allergens. Prior studies have demonstrated evidence for the transfer of house dust mite through the placenta or in amniotic fluid (56) as well as a dose-related association between prenatal dust mite and cord blood immune response (IgE) (57, 58). Data supporting maternal–fetal transfer of cockroach antigen or the role of cockroach in sensitization at birth are less clear (58). Alternatively, antigenic stimulation can cause low-level activation of recent thymic T-cell emigrants in a nonspecific fashion (59). Although the debate continues as to whether primary sensitization to allergens begins before birth, these findings suggest the possibility that prenatal stress may enhance the neonate's response to inhalant antigens, specifically those antigens that the fetus is likely to encounter more directly in utero (e.g., house dust mite).
These data suggest that psychological stress is involved in perinatal programming (the concept that environmental factors acting early in life may permanently organize or imprint physiological systems) of the infant immune response. Evidence linking stress to asthma and other atopic disorders suggests that early disruption of neuroimmunoregulatory processes are involved (for a detailed discussion see Wright [15] and references therein). Stress experienced in utero begins to shape stress neurobiology, resulting in disturbed regulation of endocrine and autonomic processes (e.g., hypothalamic-pituitary-adrenal [HPA] axis, sympathetic-adrenal-medullary system). It is hypothesized that prenatal stress affects maternal stress physiology, which, in turn, modulates the maternal immune system with enhanced polarization toward a Th2 phenotype. This may expose the fetus to neurohormonal factors (e.g., glucocorticoids, neurotrophins) and an even greater propensity toward a Th2 cytokine/chemokine milieu in utero that then modulates fetal immune development (15). Such changes may set the stage for the altered reactivity characteristic of asthma and related phenotypes. Although these in utero responses may be adaptive in the short term, being geared toward coping with anticipated environmental challenges, they may exact a toll in contributing to an increased risk of atopic disorders as these children get older.
Stress may also influence immunomodulation indirectly through its effects on maternal behaviors, such as smoking, as well as the relationship seen here between increased stress exposure in mothers and lower birth weight adjusted for gestational age. However, including these potential pathway variables in the regression models did not substantially alter our findings.
This study has a number of strengths, including the prospective design, the large sample size, and the broad assessment of immunophenotypes at birth related to both innate and adaptive stimuli in a high-risk urban sample. Another strength is the assessment of psychological stress across a number of domains using standardized measures. Although there is no consensus on how best to conceptualize and measure the role of socioeconomic status–related stressors on health, a number of concepts grounded in stress theory justify the a priori approach taken in the current analyses. Stressors typically predict outcomes similarly across stressor domains rather than specific types of stressors impacting outcomes differently (36). Moreover, beyond experiencing discrete types of stressors, individuals may be increasingly vulnerable when exposed to cumulative effects of multiple stressors (37). This may be particularly relevant in urban poor communities where exposure to multiple stressors is more prevalent. We found qualitatively similar influences of increasing maternal prenatal stress on the cytokine profiles in cord blood mononuclear cells across the independent stress factors (i.e., individual stressors, housing problems, and neighborhood problems) (data not shown) and the composite cumulative stress indicators, which enhances confidence that findings are not spurious. Notably, we found no significant differences in the distribution across cumulative stress categories based on race/ethnicity, income, or mother's educational status. This may reflect that we are considering stress experienced within the context of urban disadvantage where all subjects experience a relatively high “threshold.” Given multiple comparisons, we cannot rule out the possibility that some relationships were observed by chance. However, we also note that despite the reasonably large sample size, the cytokine assays may have enough variability and noise in them to reduce our ability to find a significant association even when one exists. We also note that exploration of these data for nonlinear relationships (e.g., U-shape, threshold effects) between the cumulative stress measure and the stimulant-by-cytokine outcomes did not yield significant relationships (data not shown).
In conclusion, prenatal stress appears to affect immune responses to both innate and adaptive stimuli at the time of birth, effects that may result in enhanced susceptibility to asthma or other atopic disorders. Continued follow-up of this prospective birth cohort will allow us to examine whether the stress-related disruptions in these early innate and adaptive immunophenotypes influence the expression of subsequent allergic sensitization and asthma expression in these children. Moreover, the Urban Environment and Childhood Asthma study design includes repeated measures of stress in these families during critical windows of development (pregnancy and annually during the first 3 years of the child's life), repeated assessment of immune response to the environmental stimuli included here, and ultimately clinical outcomes up to age 3 years. This will allow for a prospective cohort analysis to explore independent effects of prenatal and postnatal stress on infant immune development and ultimately the expression of clinical disease. Exploring the links between maternal prenatal stress and atopic risk may be particularly relevant in urban, high-risk U.S. populations that are disproportionately burdened by both phenomena.
Supplementary Material
Supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contracts NO1-AI-25496 and NO1-AI-25482, and by the National Center for Research Resources, National Institutes of Health grants RR00052, M01RR00533, M01RR00071, and 5 UL1RR024992–02. During preparation of this manuscript R.J.W. was also supported by National Institutes of Health grant R01 HL080674.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200904-0637OC on March 1, 2010
Conflict of Interest Statement: R.J.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.M.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.H.G. received $50,001–$100,000 from Genentech as an investigator-initiated research grant. D.R.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.T.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.L-P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.A.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.R.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.T. is an employee of the National Institutes of Health. F.R.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.E.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Galli E, Gianni S, Auricchi G, Brunetti E, Mancino G, Rossi P. Atopic dermatitis and asthma. Allergy Asthma Proc 2007;28:540–543. [DOI] [PubMed] [Google Scholar]
- 2.Prescott SL, Macaubas C, Holt BJ, Smallacombe TB, Loh R, Sly PD, Holt PG. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol 1998;160:4730–4737. [PubMed] [Google Scholar]
- 3.Piccinni MP, Mecacci F, Sampognaro S, Manetti R, Parronchi P, Maggi E, Romagnani S. Aeroallergen sensitization can occur during fetal life. Int Arch Allergy Immunol 1993;102:301–303. [DOI] [PubMed] [Google Scholar]
- 4.Holt PG, Upham JW, Sly PD. Contemporaneous maturation of immunologic and respiratory functions during early childhood: implications for development of asthma prevention strategies. J Allergy Clin Immunol 2005;116:16–24, quiz 25. [DOI] [PubMed] [Google Scholar]
- 5.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol 2002;3:715–720. [DOI] [PubMed] [Google Scholar]
- 6.Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy 2002;32:43–50. [DOI] [PubMed] [Google Scholar]
- 7.Mossman TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today 1996;17:138–146. [DOI] [PubMed] [Google Scholar]
- 8.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008;372:1107–1119. [DOI] [PubMed] [Google Scholar]
- 9.Wenzel SE, Busse WW. Severe asthma: lessons from the severe asthma research program. J Allergy Clin Immunol 2007;119:14–21. [DOI] [PubMed] [Google Scholar]
- 10.Wright RJ, Finn PW, Contreras JP, Cohen S, Wright RO, Staudenmayer J, Wand M, Perkins DL, Weiss ST, Gold DR. Chronic caregiver stress and IgE expression, allergen-induced proliferation and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol 2004;113:1051–1057. [DOI] [PubMed] [Google Scholar]
- 11.Suarez CJ, Parker NJ, Finn PW. Innate immune mechanism in allergic asthma. Curr Allergy Asthma Rep 2008;8:451–459. [DOI] [PubMed] [Google Scholar]
- 12.Schaub B, Liu JS, Hoppler S, Huag S, Sattler C, Lluis A, Illi S, von Mutius E. Impariment of T-regulatory cells in cord blood of atopic mothers. J Allergy Clin Immunol 2008;121:1491–1499. [DOI] [PubMed] [Google Scholar]
- 13.Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health 2005;26:89–113. [DOI] [PubMed] [Google Scholar]
- 14.Wright RJ. Stress and atopic disorders. J Allergy Clin Immunol 2005;116:1301–1306. [DOI] [PubMed] [Google Scholar]
- 15.Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatr Perinat Epidemiol 2007;21:8–14. [DOI] [PubMed] [Google Scholar]
- 16.von Hertzen LC. Maternal stress and T-cell differentiation of the developing immune system: possible implications for the development of asthma and atopy. J Allergy Clin Immunol 2002;109:923–928. [DOI] [PubMed] [Google Scholar]
- 17.Stock P, Akbari O, Berry G, Freeman GJ, Dekruyff RH, Umetsu DT. Induction of T helper type 1-like regulatory cells that express foxp3 and protect against airway hyper-reactivity. Nat Immunol 2004;5:1149–1156. [DOI] [PubMed] [Google Scholar]
- 18.Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab 1999;10:359–368. [DOI] [PubMed] [Google Scholar]
- 19.Lutgendorf SK, Moore MB, Bradley S, Shelton BJ, Lutz CT. Distress and expression of natural killer receptors on lymphocytes. Brain Behav Immun 2005;19:185–194. [DOI] [PubMed] [Google Scholar]
- 20.Oya H, Kawamura T, Shimizu T, Bannai M, Kawamura H, Minagawa M, Watanabe H, Hatakeyama K, Abo T. The differential effect of stress on natural killer T (NKT) and NK cell function. Clin Exp Immunol 2000;121:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joachim RA, Handjiski B, Blois SM, Hagen E, Paus R, Arck PC. Stress-induced neurogenic inflammation in murine skin skews dendritic cells towards maturation and migration. Key role of intercellular adhesion molecule-1/leukocyte function-associated antigen interactions. Am J Pathol 2008;173:1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coe CL, Lubach GR, Karaszewski JW. Prenatal stress and immune recognition of self and nonself in the primate neonate. Biol Neonate 1999;76:301–310. [DOI] [PubMed] [Google Scholar]
- 23.Pincus-Knackstedt MK, Joachim RA, Blois SM, Douglas AJ, Orsal AS, Klapp BF, Wahn U, Hamelmann E, Arck PC. Prenatal stress enhances susceptibility of murine adult offspring toward airway inflammation. J Immunol 2006;177:8484–8492. [DOI] [PubMed] [Google Scholar]
- 24.de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy – a review. Neurosci Biobehav Rev 2005;29:295–312. [DOI] [PubMed] [Google Scholar]
- 25.Merlot E, Couret D, Otten W. Prenatal stress, fetal imprinting and immunity. Brain Behav Immun 2008;22:42–51. [DOI] [PubMed] [Google Scholar]
- 26.Gern JE, Visness CM, Gergen PJ, Wood RA, Bloomberg GR, O'Connor GT, Kattan M, Sampson HA, Witter FR, Sandel MT, et al. The urban environment and childhood asthma (URECA) birth cohort study: design, methods, and study population. BMC Pulm Med 2009;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booth C, Mitchell S, Barnard K, Spieker S. Development of maternal social skills in multiproblem families: effects on the mother-child relationship. Dev Psychol 1989;25:403–412. [Google Scholar]
- 28.Ritter C, Hobfoll SE, Lavin J, Cameron RP, Hulsizer MR. Stress, psychosocial resources, and depressive symptomatology during pregnancy in low-income, inner-city women. Health Psychol 2000;19:576–585. [DOI] [PubMed] [Google Scholar]
- 29.Pascoe JM, Kokotailo PK, Broekhuizen FF. Correlates of multigravida women's binge drinking during pregnancy: a longitudinal study. Arch Pediatr Adolesc Med 1995;149:1325–1329. [DOI] [PubMed] [Google Scholar]
- 30.Sampson RJ, Raudenbush SW, Earls FJ. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science 1997;277:918–924. [DOI] [PubMed] [Google Scholar]
- 31.Wright RJ, Mitchell H, Visness CM, Cohen S, Stout J, Evan R, Gold DR. Community violence and asthma morbidity in the Inner-City Asthma Study. Am J Public Health 2004;94:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunn JR. Housing and inequalities in health: A study of socioeconomic dimensions of housing and self reported health from a survey of Vancouver residents. J Epidemiol Community Health 2002;56:671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans J, Hyndman S, Stewart-Brown S, Smith DM, Petersen S. An epidemiological study of the relative importance of damp housing in relation to adult health. J Epidemiol Community Health 2000;54:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oken E, Kleinman KP, Rich-Edwards JW, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreffler WG, Visness CM, Burger MS, Cruikshank WW, Lederman HM, de la Morena M, Grindle K, Calatroni A, Sampson HA, Gern JE. Standardization and performance evaluation of mononuclear cell cytokine secretion assays in a multi-center study. BMC Immunol 2006;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morales JR, Guerra NG. Effects of multiple context and cumulative stress on urban children's adjustment in elementary school. Child Dev 2006;77:907–923. [DOI] [PubMed] [Google Scholar]
- 37.Myers HF. Ethnicity- and socio-economic status-related stresses in context: an integrative review and conceptual model. J Behav Med 2009;32:9–19. [DOI] [PubMed] [Google Scholar]
- 38.Jolliffe IT. Principal component analysis. 2nd ed. New York: Springer-Verlag; 2002.
- 39.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol 2008;27:604–615. [DOI] [PubMed] [Google Scholar]
- 40.Team RDCR. A language and environment for statistical computing. http://www.R-project.Org. Accessed 2008 May 16.
- 41.Kleinbaum DG, Kupper LL, Muller KE, Nizam A. Applied regression analysis and other multivariate methods. Boston, MA: PWS-KENT Publishing Company; 1998.
- 42.Heaton T, Rowe J, Turner S, Salberse RC, de Klerk N, Suriyaarahchi D, Seralha M, Holt GJ, Holloms E, Yerkovich S, et al. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet 2005;365:142–149. [DOI] [PubMed] [Google Scholar]
- 43.Prescott SL. The development of respiratory inflammation in children. Paediatr Respir Rev 2006;7:89–96. [DOI] [PubMed] [Google Scholar]
- 44.Macaubas C, De Klerk NH, Holt BJ, Wee C, Kendall G, Firth M, Sly PD, Holt PG. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet 2003;362:1192–1197. [DOI] [PubMed] [Google Scholar]
- 45.Iwamura C, Nakayama T. Toll-like receptors in the respiratory system: their roles in inflammation. Curr Allergy Asthma Rep 2008;8:7–13. [DOI] [PubMed] [Google Scholar]
- 46.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell Immunol 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- 47.Hemmi H, Takeuchi O, Dawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A toll-like receptor recognizes bacterial DNA. Nat Immunol 2000;4088:740–745. [DOI] [PubMed] [Google Scholar]
- 48.Powell ND, Bailey MT, Mays JW, Stiner-Jones LM, Hanks ML, Padgett DA, Sheridan JF. Repeated social defeat activates dendritic cells and enhances toll-like receptor dependent cytokine secretion. Brain Behav Immun 2009;23:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Zhang Y, Miao J, Hanley G, Stuart C, Sun X, Chen T, Yin D. Chronic restraint stress promotes immune suppression through Toll-like receptor 4-mediated phosphinostide 3-kinase signaling. J Neuroimmunol 2008;204:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murawski MR, Bowen GN, Cerny AM, Anderson LJ Haynes LM, Tripp RA, Kurt-Jones EA, Finberg RW. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol 2009;83:1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncitial virus. J Virol 2001;75:10730–10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peden DB. Air pollution in asthma: effect of pollutants on airway inflammation. Ann Allergy Asthma Immunol 2001;87:12–17. [DOI] [PubMed] [Google Scholar]
- 53.Martinez FD. Maturation of immune responses at the beginning of asthma. J Allergy Clin Immunol 1999;103:355–361. [DOI] [PubMed] [Google Scholar]
- 54.Friedlander SL, Jackson DJ, Gangnon RE, Evans MD, Li Z, Roberg KA, Anderson EL, Carlson-Dakes KT, Adler KJ, Gilbertson-White S, et al. Viral infections, cytokine dysregulation and the origins of childhood asthma and allergic diseases. Pediatr Infect Dis J 2005;24:S170–S176. [DOI] [PubMed] [Google Scholar]
- 55.Prescott SL, King B. Strong TL, Holt PG. The value of perinatal immune responses in predicting allergic disease at 6 years of age. Allergy 2003;58:1187–1194. [DOI] [PubMed] [Google Scholar]
- 56.Holloway JA, Warner JO, Vance GH, Diaper ND, Warner JA, Jones CA. Detection of house-dust-mite allergen in amniotic fluid and umbilical cord blood. Lancet 2000;356:1900–1902. [DOI] [PubMed] [Google Scholar]
- 57.Schonberger HJ, Dompeling E, Knottnerus JA, Kuiper S, van Weel C, van Schayck CP. Prenatal exposure to mite and pet allergens and total serum IgE at birth in high-risk children. Pediatr Allergy Immunol 2005;16:27–31. [DOI] [PubMed] [Google Scholar]
- 58.Peters J, Franco Suglia S, Platts-Mills TAE, Hosen J, Gold DR, Wright RJ. Relationships among prenatal aeroallergen exposure and maternal and cord blood IgE: Project ACCESS. J Allergy Clin Immunol 2009;123:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thornton CA, Uphan JW, Wilstrom ME, Holt BJ, White GP, Sharp MJ, Sly PD, Holt PG. Functional maturation of CD4+CD25+CTLA4+CD45ra+ T regulatory cells in human neonatal T cell responses to environmental antigens/allergens. J Immunol 2004;173:3084–3092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.