Abstract
Rationale: Endothelial dysfunction is a potential complication of obstructive sleep apnea syndrome (OSAS) in children ascribed to systemic inflammatory changes. However, not all children with OSAS will manifest endothelial dysfunction.
Objectives: The variability in endothelial function in pediatric OSAS may be related to the ability to recruit repair mechanisms such as endothelial progenitor cells (EPCs).
Methods: Prepubertal nonhypertensive children with or without polysomonographically confirmed OSAS had endothelial function assessed in a morning fasted state using a modified hyperemic test involving cuff-induced occlusion of the radial and ulnar arteries. Blood was drawn and EPCs were assessed by flow cytometry and triple staining using antibodies against CD133, CD34, and vascular endothelial growth factor receptor-2 after isolation of peripheral blood mononuclear cells. SDF-1 levels were measured by ELISA.
Measurements and Main Results: Eighty children with OSAS (mean age 8.2 ± 1.4 yr, mean body mass index [BMI] z-score, 1.43 ± 0.3) and 20 controls (CO) matched for BMI, age, sex, and ethnicity were studied. Significant delays to peak capillary reperfusion after occlusion release (Tmax) occurred in OSAS children, but substantial variability was present. Despite similar OSAS severity, EPC counts, and stromal cell–derived factor-1 (SDF-1) levels were significantly lower among the 20 OSAS children with the longest Tmax, when compared with either the 20 children with normal Tmax values or to CO ( P < 0.01). Furthermore, Tmax was significantly and inversely correlated with EPCs (r2, 0.51; P < 0.01), but neither EPCs nor Tmax were associated with apnea-hyponea index (AHI).
Conclusions: Endothelial dysfunction is frequently present in OSAS. Variance in endothelial functional phenotype may not only reside in the individual susceptibility but also in the ability to recruit endothelial repair mechanisms.
Keywords: sleep apnea, endothelial progenitor cells, endothelial function, serum lipids, atherosclerosis
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Obstructive sleep apnea leads to abnormal endothelial function in some but not all children afflicted with this condition. The reasons underlying such differences are unclear.
What This Study Adds to the Field
The number of circulating endothelial progenitor cells in children with sleep apnea may be an important determinant of the magnitude of endothelial dysfunction.
Obstructive sleep apnea syndrome (OSAS) in children is a frequent condition in which intermittent collapse of the upper airway during sleep leads to recurrent oxyhemoglobin desaturations, elevated carbon dioxide levels, sleep fragmentation, and reduced sleep efficiency (1). Among other significant morbidities, OSAS independently elevates the risk for systemic hypertension and cardiovascular disease (2), and also alters lipid homeostasis (3). In fact, endothelial dysfunction, an early risk marker of cardiovascular disease, is frequently present in adults with OSAS (4–7). Recent work from our laboratory has illustrated that endothelial dysfunction, as assessed by a modified hyperemic test after cuff-induced occlusion of the brachial artery, is more likely to be present among nonobese 6- to 9-year-old children diagnosed with OSAS when compared with matched controls (8). In addition, the abnormalities in postocclusive reperfusion responses were reversed upon adequate and effective treatment of the underlying OSAS (8).
It should be emphasized that not all children with OSAS will develop evidence of endothelial dysfunction, and that multiple factors could be playing a role in the endothelial phenotype in the context of pediatric OSA (2, 9). Mechanisms leading to increased sympathetic activity, systemic inflammation and oxidative stress, and ultimately underlying endothelial injury and dysfunction have been extensively examined (4, 9). However, less attention has been paid to repair mechanisms, even though the magnitude of recruitment of such repair mechanisms could account for the substantial variability in endothelial function among patients with OSAS. Three main inducible processes are responsible for maintaining cardiovascular homeostasis, namely, the regenerative production of endothelial progenitor cells, angiogenesis, and macrophage-mediated reverse cholesterol transport (10). Among these, endothelial progenitor cells (EPCs) have generated interest for their potential to reverse vascular injury (11–14). In the context of OSAS, an initial report suggested that adult patients with OSAS who were free of any other known cardiovascular risk factors had reduced numbers of circulating EPCs (15). Such findings were not confirmed in another small group of adult patients with OSAS who seemed to be free of any cardiovascular risk factors (16). Furthermore, EPCs and flow-mediated vascular dilation were reduced in 32 adult patients with OSAS compared with 15 controls, and adherent treatment with CPAP led to improvements in both of these outcomes (17). In contrast, mesenchymal stem cells are increasingly released to the peripheral circulation in a rodent model of OSAS (18). The number of circulating EPCs is greater in children than in adults (19) such that variances in the recruitment potential of EPCs could underlie the differences in the magnitude of endothelial dysfunction in children with OSAS. However, we are unaware of any similar studies in children. Therefore, the purpose of the current study was to investigate whether circulating EPCs are altered by the presence OSAS in children and whether there are any associations between EPCs and endothelial function.
METHODS
Consecutive healthy habitually snoring and nonsnoring prepubertal children (ages 4–12 yr) participating in a study on neurocognitive function and sleep in children at the University of Louisville Pediatric Sleep Medicine Center were recruited to investigate endothelial function in the context of OSAS. All methods outlined in this study were approved by the University of Louisville Human Research Committee. Subjects were recruited from November 2007 through September 2008. All participants underwent baseline overnight polysomnography and a fasting blood draw in the morning.
Anthropometry
Children were weighed in a calibrated scale and their weight was recorded to nearest 0.1 kg. Height (to 0.1 cm) was measured with a stadiometer (Holtain, Crymych, UK). Body mass index (BMI) was calculated and BMI z-score was computed using the Center for Disease Control 2000 growth standards (www.cdc.gov/growthcharts) and online software (www.cdc.gov/epiinfo). A BMI z-score greater than 1.65 (>95th percentile) was considered as fulfilling obese criteria.
Sphygmomanometry
Arterial blood pressure was measured noninvasively in all children using an automated mercury sphygmomanometer (Welch Allyn, Skaneateles Falls, NY) at the brachial artery using a guidelines-defined appropriate cuff size on the nondominant arm (20). Blood pressure measurements were made in the evening before commencement of nocturnal polysomnography and in the morning immediately after awakening. Systolic and diastolic blood pressure indices were calculated by dividing the average systolic and diastolic pressure by the respective 95th percentile for blood pressure (www.nhlbi.nih.gov/guidelines/hypertension/child_tbl.htm) computed for age, sex, and height. Hypertension was defined by a systolic or a diastolic blood pressure index exceeding 1 and led to exclusion from the study.
Overnight Polysomnography
Polysomnographies (PSG) were conducted and scored as previously reported (21–25). The diagnosis of children with OSAS was defined by the presence of an obstructive apnea index of 1 or more per hour of total sleep time and an obstructive apnea-hypopnea index (AHI) of 5 or more per hour of total sleep time, respectively and a nadir oxyhemoglobin saturation less than 92% (23). Control children had AHI of less than 1 per hour of total sleep time and no oxygen desaturations events during sleep.
Endothelial Function
Endothelial function was assessed using a modified hyperemic test after cuff-induced occlusion of the radial and ulnar arteries by placing the cuff over the wrist. All tests were performed at awakening to ensure that children were in a fasted state. A laser Doppler sensor (Periflux 5000 System integrated with the PF 5050 Pressure Unit, Perimed; Järfälla, Sweden) was applied over the volar aspect of the hand at the first finger distal metacarpal surface and the hand was gently immobilized. This site was chosen as an area to minimize the effects of motion artifact and was also found to have a density of skin capillary blood flow that was of appropriate magnitude for detection. Again, children lay supine with the head of the bed elevated 45°. Once cutaneous blood flow over the area became stable, the pressure within an inflatable cuff placed at the forearm and connected to a computer-controlled manometer was raised to 200 mm Hg for 60 seconds during which blood flow was reduced to undetectable levels. An occlusion time of 60 seconds was chosen to minimize discomfort for the child. Using a computer-controlled pressure release to allow for consistent deflation times, the cuff was rapidly deflated and the hyperemic responses were measured by the laser-Doppler device. The maneuver was repeated twice within 10 minutes with at least 2 minutes separating both trials to ensure a return to baseline perfusion. The average of both maneuvers was then computed for subsequent analyses. Laser Doppler determines the magnitude of perfusion at rest, at occlusion, and at post- occlusion. Although detection of microvascular perfusion varies by child according to density of capillary blood vessels, thickness of skin, etc., all measurements are extrapolated to baseline perfusion before cuff occlusion, and analysis of reperfusion kinetics is based on time measurements. Commercially available software (Perimed; Järfälla, Sweden) allowed for unbiased estimates of the time to peak regional blood flow response post- occlusion release, which is considered representative of the postocclusion hyperemic response, an index of endothelial function (26).
Circulating EPCs Assay
Fasting blood samples were drawn by venipuncture in the morning immediately after the endothelial function testing and the nocturnal polysomnogram. After the initial 5 ml of blood was drawn by venipuncture and used for standard lipid measurements, high sensitivity C-reactive protein (CRP), and fasting insulin and glucose levels, using methods previously described (3), the subsequent 5 ml were obtained and immediately transferred into ethylenediaminetetraacetic acid collection tubes. Blood specimens were immediately transferred on wet ice to the laboratory for EPCs assay. Circulating EPCs were quantitated using the method described by Duda and colleagues (27) with some modifications. In brief, peripheral blood mononuclear cells were prepared by gradient centrifugation using Ficoll-Hypaque (Amersham Biosciences; Piscataway, NJ). The expression of cell-surface antigens was determined by 4-color immunofluorescence-staining. One hundred μl of peripheral blood mononuclear cell (containing 1 × 106 cells) were incubated with 10 μl of Fc receptor–blocking reagent (Miltenyi Biotec; Bergisch Gladbach, Germany) for 10 minutes to inhibit nonspecific binding. Cells were then incubated at 4°C for 30 minutes with 10 μl phycoerythrinconjugated antihuman CD133 mAb (Miltenyi Biotec), 10 μl peridinin chlorophyll protein–conjugated anti-human CD34 mAb (BD Biosciences; San Jose, CA), 10 μl allophycocyanin-conjugated VEGF R2 mAb (R&D Systems; Minneapolis, MN), and 10 μl fluorescein isothiocyanate–conjugated Annexin V mAb (BD Biosciences). Phycoerythrin-, peridinin chlorophyll protein-,allophycocyanin-, and fluorescein isothiocyanate–conjugated isotypematched immunoglobulin G1 and immunoglobulin G2a antibodies (DakoCytomation; Glostrup, Denmark) were used for each subject and each measurement, and served as negative controls. The cells were washed three times to remove unbound antibodies and finally resuspended in 400 μl of fluorescence-activated cell sorting (FACS) solution (BD Biosciences). FACS analysis was performed on a FACS ARIA flow cytometer (BD Biosciences). A minimum of 500, 000 events were collected. FACS analysis of each probe was performed in triplicate. The frequency of EPCs in peripheral blood was determined by a 2-dimensional side-scatter/fluorescence dot-plot analysis of the samples after exclusion of Annexin V-positive cells and appropriate gating. The exclusion of Annexin V-positive cells was performed to rule out contamination with apoptotic cells. EPC counts were expressed as percentage of total peripheral blood mononuclear cells in each subject.
Exclusion Criteria
All children found hypertensive (with either a systolic or diastolic blood pressure index >1) or using anti-hypertensive therapies were excluded. Furthermore, children with diabetes (fasting serum glucose ≥120 mg/dl), with a craniofacial, neuromuscular or defined genetic syndrome, and children on chronic anti-inflammatory therapy, or with any known acute or chronic illness were excluded. In addition, children placed on sympathomometic agents such as psychostimulants were not tested.
Stromal cell–derived factor-1 (SDF-1) levels were measured using a commercially available ELISA kit according to the manufacturer's instructions (Human SDF-1 ELISA Kit; R&D Systems; cat # DSA00). The assay has a sensitivity of 20 and was linear between 100 and 20,000 pg/ml. The inter-assay and intra-assay of coefficients of variability were 3.8 and 7.3%, respectively.
Data Analysis
Results are presented as means ± SD, unless stated otherwise. All numerical data were subjected to statistical analysis using independent Student t tests or analysis of variance followed by post-hoc tests as appropriate using Statistica (StatSoft, Inc.; 2008; version 8.0. www.statsoft.com). No variance stabilizing transformations were undertaken. A two-tailed P < 0.05 was considered to define significance.
RESULTS
In total, 147 children fitting initial inclusion criteria were recruited into the study. Of these, 80 children fulfilled criteria for OSAS and were included, and 20 were age-, sex-, ethnicity-, and proportion of BMI z score-defined obese and overweight matched nonsnoring controls (Table 1). The remaining 47 children with primary snoring were not included in this study. Twenty-seven children were obese in the OSAS group, and six obese children were included in the control group. The mean age of nonobese and obese groups was similar (7.2 ± 1.4 yr compared with 7.1 ± 1.6 yr, respectively; P value was not significant). Sex and ethnic distribution were also similar across the two groups (Table 1). Eighteen children in the OSAS group and five children in the control group had parents with systemic essential hypertension or with early onset cardiovascular disease (before age 50). Overnight polysomnographic findings revealed the anticipated differences in respiratory and sleep measures (Table 2). The main differences consisted in reductions in delta sleep in OSA along with increased respiratory events, desaturations, and arousals due to either snoring or after a respiratory event (Table 2). Although blood pressure measurements did not fulfill any of the criteria for hypertension in the 100 children included in the present study, they revealed that OSAS children had significantly higher mean systolic blood pressures (114.2 ± 7.7 vs. 96.9 ± 6.8 mm Hg ; P < 0.01) and systolic blood pressure indices (0.95 ± 0.04 vs. 0.83 ± 0.07 ; P < 0.01) compared with control children. There were no differences in either diastolic blood pressure or diastolic blood pressure indices between the two groups.
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS, BLOOD PRESSURE, AND BLOOD METABOLIC PROFILES IN 80 CHILDREN WITH OSAS AND 20 CONTROL SUBJECTS
| Characteristics | OSAS | Controls | P value |
|---|---|---|---|
| Number of Subjects | 80 | 20 | |
| Age, years | 7.2 ± 1.4 | 7.1 ± 1.6 | NS |
| Sex, males | 48 | 11 | NS |
| Race | |||
| White non-Hispanic | 47 | 12 | NS |
| African American | 26 | 5 | NS |
| Hispanic | 3 | 1 | NS |
| Biracial | 2 | 2 | NS |
| Other | 2 | 0 | NS |
| Family history of cardiovascular disease | 18 | 5 | NS |
| BMI z score | 0.96 ± 0.3 | 0.56 ± 0.2 | <0.04 |
| Obese BMI z score > 1.65 | 27 (33.7%) | 6 (30%) | NS |
| Triglycerides, mg/dl | 83.2 ± 21.5 | 77.2 ± 30.8 | NS |
| Cholesterol, mg/dl | 169.5 ± 24.2 | 154.0 ± 18.5 | <0.04 |
| HDL, mg/dl | 46.8 ± 9.7 | 61.0 ± 8.4 | <0.02 |
| LDL, mg/dl | 98.1 ± 14.2 | 89.6 ± 17.1 | <0.02 |
| Glucose, mg/dl | 79.5 ± 15.8 | 77.2 ± 8.4 | NS |
| Insulin, mIU/ml | 12.9 ± 7.6 | 7.5 ± 6.2 | <0.05 |
| hsCRP, mg/L | 2.9 ± 1.7 | 0.4 ± 0.7 | <0.01 |
| Mean systolic blood pressure, mm Hg | 114.2 ± 7.7 | 96.9 ± 6.8 | <0.01 |
| Systolic blood pressure index | 0.95 ± 0.04 | 0.83 ± 0.07 | <0.01 |
| Mean diastolic blood pressure, mm Hg | 67.4 ± 5.4 | 62.5 ± 5.8 | NS |
| Diastolic blood pressure index | 0.81 ± 0.12 | 0.77 ± 0.10 | NS |
Definition of abbreviations: HDL = high density lipoprotein ; hsCRP = high sensitivity C-reactive protein; LDL = low density lipoprotein; NS = not significant; OSAS = obstructive sleep apnea syndrome.
TABLE 2.
POLYSOMNOGRAPHIC CHARACTERISTICS OF 80 CHILDREN WITH OSAS AND 20 CONTROL SUBJECTS
| OSAS (n = 80) | Controls (n = 20) | P value | |
|---|---|---|---|
| Total sleep time, min | 478.1 ± 47.4 | 468.3 ± 48.3 | NS |
| Sleep efficiency, % | 86.9 ± 8.2 | 89.8 ± 8.3 | NS |
| Sleep onset latency, min | 23.7 ± 23.2 | 32.2 ± 25.7 | NS |
| REM onset latency, min | 143.7 ± 59.2 | 153.7 ± 60.3 | NS |
| Awakenings, n | 14.3 ± 5.5 | 11.6 ± 5.8 | <0.05 |
| WASO, %TST | 4.7 ± 5.1 | 4.1 ± 4.9 | <0.05 |
| Stage 1 | 6.1 ± 3.2 | 4.5 ± 3.4 | NS |
| Stage 2 | 47.9 ± 7.6 | 41.3 ± 7.4 | 0.05 |
| Stage 3 | 7.2 ± 7.4 | 12.1 ± 7.3 | <0.05 |
| Stage 4 | 18.4 ± 7.3 | 23.1 ± 9.0 | <0.05 |
| Stage REM, %TST | 14.2 ± 6.1 | 17.1 ± 5.2 | NS |
| SAI/hr TST | 8.3 ± 6.7 | 12.4 ± 7.7 | <0.05 |
| RAI/hr TST | 6.5 ± 1.9 | 0.2 ± 0.6 | <0.01 |
| PLMI/hr TST | 7.8 ± 8.5 | 4.7 ± 2.4 | NS |
| PLMAI/hr TST | 0.2 ± 0.7 | 0.1 ± 0.5 | NS |
| OAHI/hr TST | 12.9 ± 8.5 | 0.4 ± 0.3 | <0.0001 |
| O2 saturation nadir, % | 86.9 ± 5.8 | 93.3 ± 2.3 | <0.0001 |
| Mean ETco2, mm Hg | 45.2 ± 3.1 | 42.7 ± 2.9 | NS |
| Peak ETco2, mm HG | 55.9 ± 2.8 | 50.1 ± 1.4 | <0.01 |
Definition of abbreviations: ETco2 = end tidal carbon dioxide; NS = not significant; OAHI = obstructive apnea hypopnea index; OSAS = obstructive sleep apnea syndrome; PLMAI = periodic limb movement with arousal index; PLMI = periodic limb movement index; RAI = respiratory arousal index; REM = rapid eye movement, SAI = spontaneous arousal index; TST = total sleep time; WASO = wake after sleep onset.
Metabolic profiles revealed small, albeit significant differences in fasting serum cholesterol levels but not triglyceride levels (Table 1 ). Fasting glucose levels were similar in the two groups, but insulin levels were higher in OSAS (Table 1). Serum levels of high sensitivity CRP were significantly elevated in the OSAS group (Table 1).
Endothelial Function Testing
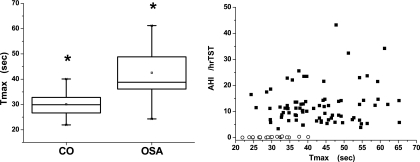
Laser-Doppler analysis of cutaneous blood flow did not reveal significant differences in resting blood flow between both groups (Table 3). Release of occlusion did not reveal significant differences in the magnitude of peak flow after release of occlusion. However, the time to attain peak reperfusion flow (Tmax) in children with OSAS was significantly delayed compared with nonobese children (Figure 1A). However, there was significant variability in Tmax among the 80 children with OSAS (Figure 1B) and no significant associations were identified between Tmax and AHI, nadir SaO2, or any of the other sleep-related measures. Similarly, there were no significant correlations between lipid profile or fasting insulin and Tmax. However, high-sensitivity CRP (hsCRP) was significantly correlated with vascular function even after controlling for potential confounders (r2: 0.16; P < 0.04).
TABLE 3.
PERFUSION KINETIC MEASURES OF ENDOTHELIAL FUNCTION IN 80 CHILDREN WITH OSAS AND 20 CONTROL SUBJECTS
| OSAS (n = 80) | Controls (n = 20) | P value | |
|---|---|---|---|
| Rest flow, PU | 21.2 ± 10.6 | 21.8 ± 8.6 | NS |
| Biological zero, PU | 3.3 ± 2.1 | 3.1 ± 1.7 | NS |
| Peak flow, PU | 46.7 ± 19.8 | 49.2 ± 22.3 | NS |
| Time to peak flow, s | 42.9 ± 9.7 | 30.1 ± 4.7 | P < 0.001 |
Definition of abbreviations: NS = not significant; OSAS = obstructive sleep apnea syndrome; PU = perfusion units.
Figure 1.
(Left panel) Boxplots of postocclusive maximal reperfusion times (Tmax) in 80 children with OSAS and 20 control subjects. (Right panel) Scatterplot of Tmax against corresponding obstructive apnea-hypopnea index (AHI) in 80 children with OSAS (closed squares) and 20 control subjects (open circles). TST = total sleep time.
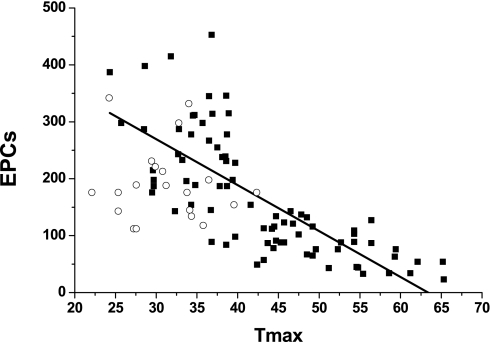
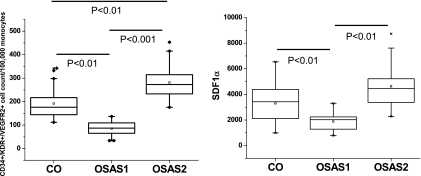
Although mean EPCs counts were lower in the OSAS group, these differences did not reach significance. Mean SDF-1 levels were similar in OSAS and controls. However, a highly significant inverse correlation emerged between EPC counts and Tmax (r2: 0.55; P < 0.000001; Figure 2). Accordingly, and based on the variance found in Tmax in controls, we partitioned the OSAS group into two subgroups, namely, those with abnormal endothelial function (i.e., Tmax > 45 s; OSAS1) and those with endothelial function within two standard deviations of the range found in control subjects (Tmax ≤ 45 s; OSAS2). EPCs were significantly lower in OSAS1 compared with control subjects ( P < 0.01; Figure 3A) and significantly higher in OSAS2 ( P < 0.01; Figure 3A). Of note, the severity of OSAS in the two subgroups was similar based on their mean AHI and nadir SaO2. In addition, BMI z scores and the proportion of obese children in these two OSA subgroups were similar, and the proportion of obese children was also similar to control subjects. Similarly, SDF-1 levels showed a similar pattern, with concentrations being significantly higher in OSAS2 compared with OSAS1 ( P < 0.01), and similar to control subjects (Figure 3B).
Figure 2.
Scatterplot showing the relationship between time to reperfusion peak flow (Tmax) and circulating endothelial progenitor cells (EPCs; r2 = 0.55; P < 0.000001).
Figure 3.
(Left panel) Boxplots of circulating EPCs in control children and in children with OSAS with (OSAS1) and without (OSAS2) endothelial dysfunction. (Right panel) Boxplots of plasma SDF-1 concentrations in control children and in children with OSAS with (OSAS1) and without (OSAS2) endothelial dysfunction.
DISCUSSION
The major findings of this study are that OSAS in children leads to altered endothelial function in some but not all patients and that the variance in vascular function can be accounted for, at least in part, by differential induction of SDF-1 concentration gradients and corresponding changes in recruitment of EPCs to the peripheral circulation. Thus, delays in vascular reperfusion kinetics do occur in some of the children with OSA and are indicative of compromised integrity of the vasculature that is critical for adequate responses to vasodilatory stimuli. However, although we found that such impairments were not correlated to the degree of OSAS severity, dyslipidemia, or insulin resistance, a significant, but weak association emerged between Tmax and hsCRP. More importantly, the density of EPCs in patients with OSAS seemed to account for a substantial proportion of the variance in vascular function.
Before discussing the potential implications of our findings, several methodological issues deserve comment. First, the present study confirms and expands upon our previous findings in a smaller cohort of nonobese children with severe OSAS (8). However, the large variability in vascular function, independent of the spectrum of OSAS severity, points toward the presence of substantial phenotypic variation in the vasculature that appears to be partially related to inflammatory processes as evidence by the association with hsCRP. Of note, this observation is reminiscent of the previously reported association between neurocognitive morbidity and hsCRP in the context of pediatric OSAS (28). Second, it is highly unlikely that differences in the presence of significant family history of early onset hypertension or premature cardiovascular disease may have accounted for the large differences in vascular function among the children with OSAS. Third, only nonhypertensive children were included in the study, further restricting the potential confounder effects of hypertension on the impact of OSAS on vascular function (29). However, and worthy of comment, is the observation that the children with OSAS studied herein were not hypertensive, yet increases in systolic blood pressures and systolic blood pressure indices were identified in the absence of similar changes in diastolic measures. Fourth, we should emphasize that our approach to delineation of endothelial dysfunction using a cutaneous vascular reperfusion test differs from the more widely use of flow- mediated reperfusion tests using Doppler technology assessments of a muscular artery (e.g., brachial artery), and that therefore, the present findings should not be assumed as identical to those obtained with other measurement techniques.
Postnatal vasculogenesis is increasingly being identified in a variety of conditions characterized by vascular growth and vascular repair. As initially described by Asahara and colleagues, new blood vessels arise from circulating bone marrow–derived EPCs in postnatal vasculogenesis rather than from local endothelial cells (30). Evidence to the presence of heightened ability to recruit EPCs in childhood has begun to emerge and seem to be responsive to the beneficial effects of exercise on the vasculature (19, 31, 32). To the best of our knowledge, this study is the first to document not only that the number of circulating EPCs is adversely affected by the presence of OSAS in some children, but also that such changes are strongly associated with the vascular functional phenotype. Based on the association between inflammatory markers such as hsCRP along with that of EPCs and endothelial function, it is reasonable to assume that the balance between OSAS-activated injury-mediating processes and repair processes such as those associated with recruitment and homing of EPCs will ultimately dictate the phenotypic presentation of vascular function in any given pediatric patient with OSAS.
The mechanisms leading to endothelial injury are only now being elucidated. Nevertheless, substantial evidence would support an important role by intermittent hypoxia during sleep that most likely would involve activation of systemic low-grade inflammatory processes and increased oxidative stress (33–35) leading to a conglomerate interaction of pathways affecting endothelial, platelet, and inflammatory cells that are orchestrated into an enhanced proatherogenic state (9). Indeed, in children with OSAS up-regulation of adhesion molecules and other markers of inflammation have been previously reported (36–40), and the present study further reinforces this concept. However, we also present evidence to suggest that reparative mechanisms are also activated in a substantial proportion of children and may effectively palliate any adverse consequence on endothelial function.
During hypoxia, the increased expression and stabilization of the transcription factor, hypoxia inducible factor-1 (HIF-1), promotes the local production of SDF-1 and vascular endothelial growth factor (VEGF) hypoxic endothelial cells (41). Although we now report on the increased plasma concentrations of SDF-1 in the very same children in whom circulating EPCs are increased, we have previously shown that serum VEGF concentrations are increased in children with OSAS in a severity-dependent fashion (42). Both SDF-1 and VEGF are capable of enhancing the mobilization and recruitment of EPCs to damaged endothelium during postnatal vasculogenesis (43, 44).
The endothelial surface of the vascular wall constitutes the frontline of vascular functional integrity and is instrumental in the early formation of the atherogenic plaques. Whereas paracrine functioning of healthy vascular endothelium contributes to protection against such plaque formation, the identification of abnormal endothelial function in some but not all children with OSAS would suggest that these homeostatic processes have failed to preserve normal function in those with prolonged postocclusive reperfusion times, thereby posing the risk of early onset atherosclerosis in prepubertal children. Based on our findings, it would seem that these children are either unable to mount a chemokine recruitment response (as evidenced by the reduced SDF-1 plasma levels among those children with abnormal vascular responses), or that despite mounting of the appropriate initial response of the SDF-1–EPCs axis, these children were unable to sustain the repair-targeted response. These possibilities would require future assessment of changes in vasculature and EPCs after treatment of OSAS and also genotyping studies of children with and without vascular dysfunction and enhanced or reduced SDF-1–EPCs responses. A recent study assessing SDF-1 single nucleotide polymorphisms has indeed identified an association between specific SDF-1 genotypes and the number of circulating EPCs (45).
Conclusions
Cardiovascular morbidity is emerging as a relatively frequent complication of OSAS in children. In this article, we show that abnormal endothelial function is significantly associated with OSAS in otherwise healthy, nonhypertensive, prepubertal children, and that the presence of endothelial dysfunction appears to be associated not only with the magnitude of the systemic inflammatory response to OSAS as measured by hsCRP, but also with reduced SDF-1 plasma concentrations and a lower number of circulating EPCs. Further studies are warranted to assess the role of specific genotypes and the effect of OSAS treatment on these parameters.
Supported by a Sleep Fellowship Grant from Jazz Pharmaceuticals (R.B.) and a National Institutes of Health grant HL65270 (D.G.).
Originally Published in Press as DOI: 10.1164/rccm.200912-1845OC on May 6, 2010
Conflict of Interest Statement: L.K.G. is the recipient of an investigator-initiated grant on montelukast in treatment of pediatric sleep apnea by Merck Co.; she received more than $100,001 from Merck & Co. as an investigator-initiated grant. R.B. received $10,001–$50,000 from Jazz Pharmaceuticals in industry-sponsored grants (1-year research fellowship). J.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.B.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.G. received $10,001–$50,000 from Galleon Pharmaceuticals for serving as a consultant on respiratory control and $5,001–$10,000 from Merck for lectures on pediatric asthma.
References
- 1.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharjee R, Kheirandish-Gozal L, Pillar G, Gozal D. Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children. Prog Cardiovasc Dis 2009;51:416–433. [DOI] [PubMed] [Google Scholar]
- 3.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med 2008;177:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Circulation 2008;118:1080–1111. [DOI] [PubMed] [Google Scholar]
- 5.Itzhaki S, Lavie L, Pillar G, Tal G, Lavie P. Endothelial dysfunction in obstructive sleep apnea measured by peripheral arterial tone response in the finger to reactive hyperemia. Sleep 2005;28:594–600. [DOI] [PubMed] [Google Scholar]
- 6.Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 2000;102:2607–2610. [DOI] [PubMed] [Google Scholar]
- 7.Oflaz H, Cuhadaroglu C, Pamukcu B, Meric M, Ece T, Kasikcioglu E, Koylan N. Endothelial function in patients with obstructive sleep apnea syndrome but without hypertension. Respiration 2006;73:751–756. [DOI] [PubMed] [Google Scholar]
- 8.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation 2007;116:2307–2314. [DOI] [PubMed] [Google Scholar]
- 9.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med 2008;177:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno PR, Sanz J, Fuster V. Promoting mechanisms of vascular health: circulating progenitor cells, angiogenesis, and reverse cholesterol transport. J Am Coll Cardiol 2009;53:2315–2323. [DOI] [PubMed] [Google Scholar]
- 11.Zenovich AG, Taylor DA. Atherosclerosis as a disease of failed endogenous repair. Front Biosci 2008;13:3621–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sirker AA, Astroulakis ZM, Hill JM. Vascular progenitor cells and translational research: the role of endothelial and smooth muscle progenitor cells in endogenous arterial remodelling in the adult. Clin Sci (Lond) 2009;116:283–299. [DOI] [PubMed] [Google Scholar]
- 13.Besler C, Doerries C, Giannotti G, Lüscher TF, Landmesser U. Pharmacological approaches to improve endothelial repair mechanisms. Expert Rev Cardiovasc Ther 2008;6:1071–1082. [DOI] [PubMed] [Google Scholar]
- 14.Umemura T, Higashi Y. Endothelial progenitor cells: therapeutic target for cardiovascular diseases. J Pharmacol Sci 2008;108:1–6. [DOI] [PubMed] [Google Scholar]
- 15.de la Peña M, Barceló A, Barbe F, Piérola J, Pons J, Rimbau E, Ayllón O, Agustí AG. Endothelial function and circulating endothelial progenitor cells in patients with sleep apnea syndrome. Respiration. 2008;76:28–32. [DOI] [PubMed] [Google Scholar]
- 16.Martin K, Stanchina M, Kouttab N, Harrington EO, Rounds S. Circulating endothelial cells and endothelial progenitor cells in obstructive sleep apnea. Lung 2008;186:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 2008;117:2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carreras A, Almendros I, Acerbi I, Montserrat JM, Navajas D, Farré R. Obstructive apneas induce early release of mesenchymal stem cells into circulating blood. Sleep 2009;32:117–119. [PMC free article] [PubMed] [Google Scholar]
- 19.Jie KE, Goossens MH, van Oostrom O, Lilien MR, Verhaar MC. Circulating endothelial progenitor cell levels are higher during childhood than in adult life. Atherosclerosis 2009;202:345–347. [DOI] [PubMed] [Google Scholar]
- 20.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004; 114(2 Suppl ):555–576. [PubMed] [Google Scholar]
- 21.Rechstschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. University of California, Los Angeles: Brain Information Services/Brain Research Institute, 1968.
- 22.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med 1996;153:866–878. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics 2006;117:741–753. [DOI] [PubMed] [Google Scholar]
- 24.Iber C, Ancoli-Israel S, Chesson AL Jr., Quan SF. The AASM manual for the scoring of sleep and associated events. American Academy of Sleep Medicine 2007.
- 25.Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report. Sleep 1992;15:173–184. [PubMed] [Google Scholar]
- 26.Wahlberg E, Olofsson P, Swendenborg J, Fagrell B. Changes in postocclusive reactive hyperaemic values as measured with laser Doppler fluxmetry after infrainguinal arterial reconstructions. Eur J Vasc Endovasc Surg 1995;9:197–203. [DOI] [PubMed] [Google Scholar]
- 27.Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc 2007;2:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med 2007;176:188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aggoun Y, Farpour-Lambert NJ, Marchand LM, Golay E, Maggio AB, Beghetti M. Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur Heart J 2008;29:792–799. [DOI] [PubMed] [Google Scholar]
- 30.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Yi D, Wang Y, Zheng R, Sun G, Wang J, Liu Y, Ren J, Wang Y, Zhang S, et al. Age-dependent mobilization of circulating endothelial progenitor cells in infants and young children undergoing cardiac surgery with cardiopulmonary bypass. Cytokine 2009;47:206–213. [DOI] [PubMed] [Google Scholar]
- 32.Walther C, Adams V, Bothur I, Drechsler K, Fikenzer S, Sonnabend M, Bublitz B, Körner A, Erbs S, Busse M, et al. Increasing physical education in high school students: effects on concentration of circulating endothelial progenitor cells. Eur J Cardiovasc Prev Rehabil 2008;15:416–422. [DOI] [PubMed] [Google Scholar]
- 33.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 2004;169:348–353. [DOI] [PubMed] [Google Scholar]
- 34.Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med 2004;169:354–360. [DOI] [PubMed] [Google Scholar]
- 35.Kraiczi H, Caidahl K, Samuelsson A, Peker Y, Hedner J. Impairment of vascular endothelial function and left ventricular filling: association with the severity of apnea-induced hypoxemia during sleep. Chest 2001;119:1085–1091. [DOI] [PubMed] [Google Scholar]
- 36.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med 2008;9:254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kheirandish-Gozal L, Capdevila OS, Tauman R, Gozal D. Plasma C-reactive protein in nonobese children with obstructive sleep apnea before and after adenotonsillectomy. J Clin Sleep Med 2006;2:301–304. [PMC free article] [PubMed] [Google Scholar]
- 38.Khalyfa A, Capdevila OS, Buazza MO, Serpero LD, Kheirandish-Gozal L, Gozal D. Genome-wide gene expression profiling in children with non-obese obstructive sleep apnea. Sleep Med 2009;10:75–86. [DOI] [PubMed] [Google Scholar]
- 39.Tauman R, O'Brien LM, Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing. Sleep Breath 2007;11:77–84. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien LM, Serpero LD, Tauman R, Gozal D. Plasma adhesion molecules in children with sleep-disordered breathing. Chest 2006;129:947–953. [DOI] [PubMed] [Google Scholar]
- 41.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004;10:858–864. [DOI] [PubMed] [Google Scholar]
- 42.Gozal D, Lipton AJ, Jones KL. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep 2002;25:59–65. [DOI] [PubMed] [Google Scholar]
- 43.Kalka C, Tehrani H, Laudenberg B, Vale PR, Isner JM, Asahara T, Symes JF. VEGF gene transfer mobilizes endothelial progenitor cells in patients with inoperable coronary disease. Ann Thorac Surg 2000;70:829–834. [DOI] [PubMed] [Google Scholar]
- 44.Garg R, Tellez A, Alviar C, Granada J, Kleiman NS, Lev EI. The effect of percutaneous coronary intervention on inflammatory response and endothelial progenitor cell recruitment. Catheter Cardiovasc Interv 2008;72:205–209. [DOI] [PubMed] [Google Scholar]
- 45.Xiao Q, Ye S, Oberhollenzer F, Mayr A, Jahangiri M, Willeit J, Kiechl S, Xu Q. SDF1 gene variation is associated with circulating SDF1alpha level and endothelial progenitor cell number: the Bruneck Study. PLoS One 2008;3:e4061. [DOI] [PMC free article] [PubMed] [Google Scholar]