Abstract
The relationship between body composition and function in spinal muscular atrophy (SMA) is poorly understood. 53 subjects with SMA were stratified by type and Hammersmith Functional Motor Scale, Expanded score into three cohorts: Low-Functioning Non-Ambulatory (type 2 with Hammersmith score <12, n=19), High-Functioning Non-Ambulatory (type 2 with Hammersmith Score ≥ 12 or non-ambulatory type 3, n=17), and Ambulatory (n=17). Lean and fat mass was estimated using dual-energy x-ray absorptiometry. Anthropometric data was incorporated to measure fat-free (lean mass in kg /stature in m2) and fat (fat mass in kg /stature in m2) mass indices, the latter compared to published age and sex norms. Feeding dysfunction among type 2 subjects was assessed by questionnaire. Fat mass index was increased in the High-Functioning Non-Ambulatory cohort (10.4 ± 4.5) compared with both the ambulatory (7.2 ± 2.1, p = 0.013) and Low-Functioning Non-Ambulatory (7.6 ± 3.1, p = 0.040) cohorts. 12 of 17 subjects (71%) in the High-Functioning Non-Ambulatory cohort had fat mass index >85th percentile for age and gender (connoting “at risk of overweight”) versus 9 of 19 subjects (47%) in the Low-Functioning Non-Ambulatory cohort and 8 of 17 ambulatory subjects (47%). Despite differences in clinical function, a similar proportion of low functioning (7/18, 39%) and high functioning (2/7, 29%) type 2 subjects reported swallowing or feeding dysfunction. Non-ambulatory patients with relatively high clinical function may be at particular risk of excess adiposity, perhaps reflecting access to excess calories despite relative immobility, emphasizing the importance of individualized nutritional management in SMA.
Introduction
Spinal Muscular Atrophy (SMA) is a hereditary neuromuscular disease marked by the loss of anterior horn cells of the spinal cord and the development of progressive weakness and muscle loss, with significant resulting morbidity and mortality[1]. Although there is severe potential morbidity associated with obesity as well as with malnutrition, little is known about optimal body composition and its effect on outcome and function in SMA[2]. Recently a high frequency of swallowing dysfunction and subnormal weight in a large, Italian cohort of patients with SMA type 2 was reported[3]. The observed frequency of extremely low weight was attributed to malnutrition secondary to poor feeding[3]. The optimal body weight of patients with SMA, however, has not been well clarified. Management practice is based loosely on experience with spinal cord injury patients[2] that has shown lower lean tissue and higher body fat percentage in these patients when compared to control subjects[4-7]. Similar findings have been noted among boys with Duchenne Muscular Dystrophy(DMD)[8-10].
Fat mass estimation using dual energy x-ray absorptiometry (DXA) has been used to directly assess body composition in the pediatric population[11-14]. Recently investigators with the New York Pediatric Rosetta Body Project used DXA to describe fat mass index (FMI, kg of fat mass/height in m2) [15-17] percentiles in large sample of healthy American children stratified by age, race and gender. Degree of obesity was described based on percentile among healthy children (above 85th percentile for “at risk for overweight”, above 95th for “overweight”)[15-17]. Using a similar approach we recently reported a high frequency of overweight and at risk for overweight among a cohort of children with SMA[18], despite low weight compared to healthy children.
From this observation we have asserted that the low body mass of these children reflects loss of muscle mass and that adiposity is often greater than that seen in healthy children, in some cases markedly[18]. Increased adiposity may worsen gross motor and pulmonary function from the mechanical effect of increased mass demands placed on weak, tenuously functional muscles. Conversely, sufficient adipose stores may protect against significant muscle catabolism in response to metabolic stress; more severely affected patients (type 1 and low-functioning type 2) may be at significant risk of malnutrition. In this study we sought to assess the relationship between motor function, looking specifically at SMA types 2 and 3 patients, and adiposity.
Subjects and Methods
Subjects
53 consecutive adult and pediatric-aged subjects with SMA types 2 or 3 (26 with SMA type 2 and 27 with SMA type 3, 23 male and 30 female) for whom DXA was performed as part of an ongoing natural history study at the Columbia University Medical Center, the Children's Hospital of Boston and the Children's Hospital of Philadelphia were selected for the study. Diagnostic status (type 2, type 3) is based on classical definitions, with SMA types 2 and 3 defined by a history of ability to sit and walk independently, respectively[19]. This is a well described, genetically characterized cohort of patients for whom a battery of clinical, demographic and anthropometric data has been recorded on an ongoing basis as part of scheduled follow-up assessments and care.
Hammersmith Functional Motor Scale, Expanded (HFMSE)
Motor function was assessed by applying the Hammersmith Functional Motor Scale, Expanded (HFMSE), described previously[20] and designed for sensitive assessment of ambulatory and higher functioning patients with SMA types 2 and 3, to the study cohort. The Hammersmith Functional Motor Scale is a 20 item functional assessment arranged in order of progressive difficulty and designed specifically for use in patients with SMA type 2[21]. This scale has good inter-rater reliability[21, 22], correlates well with biomarkers of disease severity[23], and has been used in single and multi-site clinical trials in SMA[24-26]. The HFMSE augments the original Hammersmith scale by incorporating 13 relevant items from the Gross Motor Function Measure (GMFM) to eliminate the so-called “ceiling” effect of the original scale when applied to ambulant SMA patients. The HFMSE shows good test-retest reliability and correlation with the GMFM and other clinical measures such as forced vital capacity and muscle strength[20].
Anthropometrics
For all subjects who were able to stand erect, height was measured to the nearest centimeter with a wall-mounted stadiometer. For subjects who are unable to stand independently height (length) was measured on the right side of the subject while he or she was positioned supine on the exam table with the spine, hip joint, and knee joint straightened as much as possible. The subject was measured in segments using a straight edge- the top of the head to the right greater trocanter of the hip, hip to the right femoral epicondyle of the knee, and the knee to the distal point of the calcaneus. If the subject had significant joint contractures or scoliosis, measuring the height often required more than one evaluator. In these instances 3 measurements of length were obtained and the mean measurement was recorded. Height was measured to the nearest centimeter or tenth of an inch. All measures were performed by trained evaluators using a standardized protocol across the three clinical sites.
Pulmonary Function Testing: Pulmonary function testing was performed to further characterize differences between the study cohorts. Measurement of FVC has been found reliable, valid and feasible in children with SMA 5 years and older in previous studies[27]. Forced vital capacity (FVC, the volume in liters of gas that can be exhaled by maximum voluntary effort following deep inspiration) was measured as a percentage predicted for gender, height and age was measured in all groups using spirometry and a microprocessor to develop a maximal expiratory flow-volume curve for the evaluation of lower airway function. The best of three trials was recorded.
Dual-Energy X-ray absorptiometry
Whole body DXA scans were performed using Lunar models DPX with pediatric software 3.8G and DPX-L with pediatric software 1.5G (GE Lunar Corporation, General Electric, Madison, WI) in accordance with the manufacturer's instructions[28]. Each scan provided estimates of subjects' fat mass and fat-free mass in kilograms. Technique, coefficient of variation, and quality control procedures (i.e. usage of phantoms to simulate bone, fat and fat-free tissues) has been described previously[17]. FMI and Fat-Free Mass Index (FFMI) was then calculated for each patient by dividing fat and lean mass as estimated using DXA, respectively, by measured height in meters, squared (kg/m2).
Age, gender and race-controlled reference values for FMI were obtained from data published by the Pediatric Rosetta Study at St Luke's-Roosevelt Hospital Center in New York (1995–2000), a cross-sectional study of pediatric body composition in 1208 healthy children and adolescents whose mean height, weight, and BMI were only slightly different from children and adolescents examined in the NHANES data[15, 17, 29]. Adult subjects were referenced to similar previously published datasets obtained using DXA applied to a large cohort of adult Caucasian men and women[30]. “At risk for overweight” status was defined using the modern broadly utilized and accepted [15, 17, 29] standard of FMI above the 85th percentile for age and sex.
Subject Stratification
Based on performance on the HFMSE, SMA type and clinical state (i.e. whether a type 3 subject remains ambulatory), subjects were stratified into three cohorts: Cohort 1, representing SMA type 2 subjects with low clinical function, included those with HFMSE <12; Cohort 2 comprised relatively high functioning yet non-ambulatory subjects, defined by a clinical diagnosis of either SMA type 2 or 3 and with HFMSE ≥12; and Cohort 3, comprising ambulatory subjects (by definition, exclusively type 3 subjects). Previous studies[31] have used a score of 10 on the standard 40-point Hammersmith scale to divide subjects with SMA type 2 into low and high functioning cohorts. This score is well approximated by a score of 12 on the HFMSE[20], given that a high functioning subject would be expected to achieve an additional 2 points based on successful hip flexion while supine[20, 21]. It is this on this basis that this score was chosen to stratify subjects with SMA type 2.
Feeding/Swallowing dysfunction
For subjects with SMA type 2, the presence of feeding or swallowing dysfunction was assessed by parental questionnaire adapted (translated into English) from a questionnaire designed by a consensus of neurologists, pediatricians and speech therapists and utilized previously in the assessment of swallowing function in SMA[3] and DMD[32]. The questionnaire includes simple specific questions on feeding abilities, including duration to complete a meal and the presence of feeding difficulties subdivided according to whether these were related to the extrabuccal (opening mouth), anterior (keeping food in mouth, chewing) or posterior (choking, repeat attempt to swallow, food aspiration) phases of chewing and swallowing. Dietary modifications and supplement intake, as well as gastrostomy dependence, were also recorded[32].
Statistical Analysis
All results are expressed as mean ± standard deviation unless otherwise specified. Unpaired Student's t-test was used to compare mean differences in FMI between the three cohorts. Fisher's exact test was used to compare incidence of FMI >85th percentile between the three study cohorts. The primary outcome was difference in FMI. Significance was established at P<0.05.
Results
Hammersmith Scale of Motor Function, Expanded
Demographic and clinical characteristics of the total study cohort are presented in Table 1. HFMSE score was increased in Cohort 3 (55.5 ± 8.2) compared to Cohort 2 (19.3 ± 7.5) and Cohort 1 (3.9 ± 3.4).
Table 1. Demographics and Clinical Characteristics of the Study Cohort.
| Classification | Cohort 1 | Cohort 2 | Cohort 3 |
|---|---|---|---|
| SMA Type 2, HFMSE < 12 | Non-Ambulatory SMA Type 2 or 3 with HFMSE ≥ 12 | Ambulatory | |
| n | 19 | 17 | 17 |
| Age | 11.3 ± 6.0 | 11.3 ± 6.8 | 14.9 ± 11.8 |
| Male, % | 8 42% | 8 47% | 7 41% |
| HFMSE | 3.9 ± 3.4 | 19.3 ± 7.5 | 55.5 ± 8.2 |
| FVC | 39.9 ±16.8 | 79.6 ± 12.5 | 104.4 ±15.8 |
| Fat-Free Mass index (Lean mass in Kg/M2) | 8.6 ± 0.9 | 8.9 ± 2.6 | 11.8 ± 1.8 |
Pulmonary Function
Two subjects in Cohort 1 and one subject in Cohort 2 were unable to successfully perform pulmonary function testing and are excluded from the analysis (one child in cohort 1 has a tracheostomy, the other two children were unable to cooperate with testing). As presented in table 1, forced vital capacity (FVC), as percentage of predicted volume for age and gender, was increased in Cohort 3 (104.4 ± 15.8) compared to Cohort 2 (79.6 ± 12.5) and Cohort 1 (39.9 ± 16.8).
Body Composition
Fat-Free Mass
As shown in Table 1, FFMI was also increased in the in Cohort 3 (11.8 ± 1.8) compared to Cohort 2 (8.9 ± 2.6) and Cohort 1 (8.6 ± 0.9).
Fat Mass
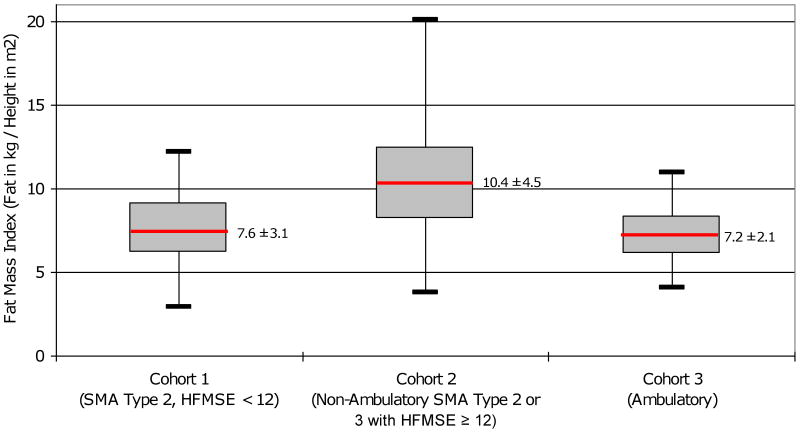
As shown in Table 2 and depicted in Figure 1, FMI was significantly increased in Cohort 2 (10.4 ± 4.5) compared to both Cohort 3 (7.2 ± 2.1, p = 0.013) and Cohort 1 (7.6 ± 3.1, p = 0.040). FMI was not significantly different between Cohorts 1 and 3 (p = 0.633).
Table 2. Comparison of Fat Mass Index and proportion of subjects with Fat Mass Index above 85th percentile across study cohorts.
| Fat Mass Index (Fat mass in Kg/M2) | FMI >85th percentile for age and gender | |||
|---|---|---|---|---|
| Mean ± SD | p-value* | Mean (%) | p-value** | |
| Cohort 1 | 7.6 ±3.1 | p = 0.040 | 9 (47%) | p = 0.192 |
| Cohort 2 | 10.4 ±4.5 | 12 (71%) | ||
| Cohort 2 | 10.4 ±4.5 | p = 0.013 | 12 (71%) | p = 0.296 |
| Cohort 3 | 7.2 ±2.1 | 8 (47%) | ||
| Cohort 1 | 7.6 ±3.1 | p = 0.633 | 9 (47%) | p = 0.999 |
| Cohort 3 | 7.2 ±2.1 | 8 (47%) | ||
Note:
p-values reflect 2-tailed, unpaired Student's T-test
p-values reflect Fisher's Exact Test
Figure 1. Box Plot Distribution of Fat Mass Index by Cohort.
“At risk for overweight” status
Twelve of 17 subjects (71%) in Cohort 2 had FMI >85th percentile for published age and gender norms[15, 17, 29, 30], the level at which patients are considered “at risk for overweight” using conventional definitions [15, 17, 29], compared to 9 of 19 subjects (47%) in Cohort 1 and 8 of 17 in Cohort 3 (47%). No comparison met statistical significance (see Table 2).
Feeding/Swallowing dysfunction
Data regarding swallowing and feeding function was available for 25 of the 26 enrolled subjects with SMA type 2. For three subjects the questionnaire was unable to be completed; data for two of these two individuals was able to be imputed from available clinical records. Nine (36%) of the 25 subjects reported some degree of swallowing or feeding dysfunction (choking on liquids, semi-solids or solids), including 7 of 18 (39%) and 2 of 7 (29%) of the low-functioning (HFMSE < 12) and high functioning (HFMSE ≥ 12) SMA type 2 subjects, respectively. One subject in each group described difficulty “opening mouth”, while 10 of 18 (56%) low-functioning and 2 of 7 (29%) of the high functioning subjects (representing 12/25, or 44%, of the combined cohort) required some form of dietary modification or supplementation. Estimated meal time (excluding the two patients with imputed data) was roughly equal between the two groups (24.3 ± 11.2 minutes in low functioning versus 27.9 ± 15.8 minutes in high functioning cohort).
Discussion
Despite the increasing prominence of body composition and obesity in both the scientific and lay press, nutritional goals remain sparsely described and poorly defined for patients with neuromuscular diseases. This is especially true with regards to spinal muscular atrophy[2]. Recently Messina and colleagues described the results of a large survey of Italian SMA type 2 patients, reporting a high incidence of markedly underweight patients (compared to healthy age and gender norms) in this group and a concurrent high incidence of swallowing dysfunction[3]. The authors, in reporting the latter finding, concluded that an integrated, multidisciplinary approach to identify feeding difficulties, malnutrition, and the need for supplementation with enteric feeding should become standard of care in the management of patients with SMA type 2[3]. We recently described increased adiposity and risk of overweight status among a cohort comprised largely of SMA type 2 and 3 children[18], using conventional definitions[17, 29] applied to modern body composition imaging techniques (DXA)[15]. The present study supports the seemingly contradictory findings of these two prior studies, specifically that patients with SMA may be at risk for both complications: malnutrition due to feeding and swallowing dysfunction and obesity due to nutritional inputs markedly greater than the modest energy needs in this largely inactive population; non-ambulatory patients with relatively high levels of clinical function may be at particular risk of one, the other, or both of these nutritional complications depending on the degree of severity of the underlying disease.
In this study, relatively high functioning but non-ambulatory subjects were observed to have significantly higher fat mass index compared to both lower functioning and ambulant peers, with a trend toward higher likelihood of FMI percentile above the 85th, connoting “at risk for overweight” using conventional definitions. This population, comprising both mild type 2 patients as well as type 3 patients who have lost ambulation, may be at especial risk of obesity resulting from a combination of low metabolic demand relative to higher functioning peers and high caloric inputs relative to those with poorer motor function.
Consistent with the report by Messina et al (2008), reported swallowing or feeding dysfunction was widespread among the subjects with SMA type 2 surveyed in this study, with high prevalence among both low and high functioning subjects with SMA type 2[3]. While a survey of the type applied is likely insensitive to subtle differences in swallowing function (particularly in comparison to formalized evaluations of swallowing function such as a video esophagram), this study failed to demonstrate any notable differences between high and low functioning subjects with SMA type 2 despite large differences in clinical function. Importantly, this approach does not address differences in food portions and independent access to calories between the two groups. It may be that relative ease of access to excess calories may in part underlay the increased adiposity seen among high functioning non-ambulatory subjects (Cohort 2) compared to their lower functioning peers. At the same time, fat mass index was also significantly higher among these subjects than among those with retained ambulation, reflecting, perhaps, the effect of diminished metabolic demand among the non-ambulatory. In this context, just as with otherwise healthy individuals, caloric input above metabolic demand will result in excess adiposity.
There are several limitations to this study. The rarity of SMA limits the available size of the study cohort; the age requirement for inclusion (>5 years), a necessity given the ethical (radiation exposure) and technical (the need to remain still) constraints of DXA, further limits the applicability of these observations, particularly to younger children. While body composition and nutrition is neither a primary endpoint nor a significant focus of the natural history study from which subjects were selected, the co-administration of extensive medical care, including ongoing management by a nutritionist, may alter the patient population studied in unexpected ways and confound the reported results. Furthermore, whether the observed incidence of obesity is reflective of societal factors specific to this cohort of American patients with SMA or can be extrapolated to other populations is unknown. As noted above, while the stratification of subjects with SMA type II mirrors the work of other groups[31], the choice of a somewhat arbitrary stratification raises the possibility of observer bias into the reported result. While the survey chosen for this study has been applied previously in studies of SMA[3] and Duchenne Muscular Dystrophy[32], observations using an indirect, survey-based method must be approached with considerable caution. Importantly, body composition and swallowing function are at best secondary markers for dietary imbalance; caloric intake was not addressed in this study. Clarification of the caloric and nutritional requirements of patients with SMA is an important and largely unaddressed area for future study.
In conclusion, despite widespread recognition of the importance of nutritional optimization in the care and management of patients with this disease[2] it is far from clear what comprises optimum nutritional management and body composition in SMA. In this study we describe body composition using DXA and the presence of feeding and swallowing dysfunction, among a well described, genetically confirmed cohort of patients subdivided by clinical status and motor function on a standardized rating scale (the HFMSE). We observe that non-ambulatory SMA subjects with relatively high function have increased fat mass and are likely at particular risk of becoming overweight when compared to their lower functioning or ambulator peers. We speculate that the increased adiposity seen in this particular subset of SMA reflects the specific challenges faced by these patients who may be especially predisposed, despite limitations in mobility and the possible presence of swallowing dysfunction, to excess caloric intake. For this subset of the SMA population, increased adiposity may represent a significant, modifiable factor contributing to the overall morbidity of the disease. This study further emphasizes and elaborates previous recommendations[2, 3, 18] regarding the role of a dedicated and experienced nutritionist in the care of these children and highlights the importance of individualized dietary care and management in SMA.
Acknowledgments
This research was supported by funding from the SMA Foundation and a NINDS, Neurological Sciences Academic Development Award (K12 NS01698), and was performed with the support of the Pediatric Neuromuscular Clinical Research Network (PNCR). We thank Jonathan Marra for his assistance in data processing. We would like to thank patients who participated in the study, and their parents, for their generosity of time and commitment to furthering clinical knowledge and research in SMA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oskoui M, Levy G, Garland CJ, et al. The changing natural history of spinal muscular atrophy type 1. Neurology. 2007;69(20):1931–6. doi: 10.1212/01.wnl.0000290830.40544.b9. [DOI] [PubMed] [Google Scholar]
- 2.Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22(8):1027–49. doi: 10.1177/0883073807305788. [DOI] [PubMed] [Google Scholar]
- 3.Messina S, Pane M, De Rose P, et al. Feeding problems and malnutrition in spinal muscular atrophy type II. Neuromuscul Disord. 2008;18(5):389–393. doi: 10.1016/j.nmd.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 4.McDonald C, Abresch-Meyer A, Nelson M, et al. Body mass index and body composition measures by dual x-ray absorptiometry in patients aged 10 to 21 years with spinal cord injury. J Spinal Cord Med. 2007;30(Suppl 1):S 97–104. doi: 10.1080/10790268.2007.11754612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liusuwan A, Abresch R, McDonald C. Altered body composition affects resting energy expenditure and interpretation of body mass index in children with spinal cord injury. J Spinal Cord Med. 2004;27(Suppl 11):S24–S28. doi: 10.1080/10790268.2004.11753781. [DOI] [PubMed] [Google Scholar]
- 6.Dopler-Nelson M, Widman L, Abresch R, et al. Metabolic syndrome in adolescents with spinal cord dysfunction. J Spinal Cord Med. 2007;30:S 130–142. doi: 10.1080/10790268.2007.11754591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abresch R, McDonald D, Widman L, et al. Impact of spinal cord dysfunction and obesity on the health-related quality of life of children and adolescents. J Spinal Cord Med. 2007;30(Suppl 1):S 112–118. doi: 10.1080/10790268.2007.11754614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willig T, Carlier L, Legrand M, et al. Nutritional assessment in Duchenne muscular dystrophy. Dev Med Child Neurol. 1993 Dec;35(12):1074–1082. doi: 10.1111/j.1469-8749.1993.tb07925.x. [DOI] [PubMed] [Google Scholar]
- 9.Pichiecchio A, Uggetti C, Egitto M, et al. Quantitative MR evaluation of body composition in patients with Duchenne muscular dystrophy. Eur Radiol. 2002 Nov;12(11):2704–2709. doi: 10.1007/s00330-002-1392-4. [DOI] [PubMed] [Google Scholar]
- 10.Zanardi M, Tagliabue A, Orcesi S, et al. Body composition and energy expenditure in Duchenne muscular dystrophy. Eur J Clin Nutr. 2003 Feb;57(2):273–278. doi: 10.1038/sj.ejcn.1601524. [DOI] [PubMed] [Google Scholar]
- 11.Chan G. Performance of dual-energy x-ray absorptiometry in evaluating bone, lean body mass, and fat in pediatric subjects. J Bone Miner Res. 1992;7:369–374. doi: 10.1002/jbmr.5650070403. [DOI] [PubMed] [Google Scholar]
- 12.Leroy-Willig A, Willig T, Henry-Feugeas M, et al. Body composition determined with MR in patients with Duchenne muscular dystrophy, spinal muscular atrophy, and normal subjects. Magn Reson Imaging. 1997;15(7):737–744. doi: 10.1016/s0730-725x(97)00046-5. [DOI] [PubMed] [Google Scholar]
- 13.Margulies L, Horlick M, Thornton J. Reproducibility of whole body bone and body composition measures by dual energy X-ray absorptiometry using GE Lunar Prodigy. J Clin Densitometry. 2005;8:298–304. doi: 10.1385/jcd:8:3:298. [DOI] [PubMed] [Google Scholar]
- 14.Schmelzle H, Fusch C. Body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry. Am J Clin Nutr. 2002 Nov;76(5):1096–1100. doi: 10.1093/ajcn/76.5.1096. [DOI] [PubMed] [Google Scholar]
- 15.Freedman D, Wang J, Maynard L, et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond) 2005;29(1):1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 16.Freedman D, Wang J, Ogden C, et al. The prediction of body fatness by BMI and skinfold thicknesses among children and adolescents. Ann Hum Biol. 2007 Mar-Apr;34(2):183–194. doi: 10.1080/03014460601116860. [DOI] [PubMed] [Google Scholar]
- 17.Mei Z, Grummer-Strawn LM, Wang J, et al. Do skinfold measurements provide additional information to body mass index in the assessment of body fatness among children and adolescents? Pediatrics. 2007;119(6):e1306–13. doi: 10.1542/peds.2006-2546. [DOI] [PubMed] [Google Scholar]
- 18.Sproule DM, Montes J, Montgomery M, et al. Increased fat mass and high incidence of overweight despite low body mass index in patients with spinal muscular atrophy. Neuromuscul Disord. 2009;19(6):391–396. doi: 10.1016/j.nmd.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russman BS. Spinal muscular atrophy: clinical classification and disease heterogeneity. J Child Neurol. 2007;22(8):946–951. doi: 10.1177/0883073807305673. [DOI] [PubMed] [Google Scholar]
- 20.O'Hagen JM, Glanzman AM, McDermott MP, et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord. 2007;17(9-10):693–7. doi: 10.1016/j.nmd.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Main M, Kairon H, Mercuri E, et al. The Hammersmith functional motor scale for children with spinal muscular atrophy: a scale to test ability and monitor progress in children with limited ambulation. Eur J Paediatr Neurol. 2003;7(4):155–9. doi: 10.1016/s1090-3798(03)00060-6. [DOI] [PubMed] [Google Scholar]
- 22.Mercuri E, Messina S, Battini R, et al. Reliability of the Hammersmith functional motor scale for spinal muscular atrophy in a multicentric study. Neuromuscul Disord. 2006;16(2):93–8. doi: 10.1016/j.nmd.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Tiziano FD, Bertini E, Messina S, et al. The Hammersmith functional score correlates with the SMN2 copy number: a multicentric study. Neuromuscul Disord. 2007;17(5):400–3. doi: 10.1016/j.nmd.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Mercuri E, Bertini E, Messina S, et al. Pilot trial of phenylbutyrate in spinal muscular atrophy. Neuromuscul Disord. 2004;14(2):130–5. doi: 10.1016/j.nmd.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Mercuri E, Bertini E, Messina S, et al. Randomized, double-blind, placebo-controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology. 2007;68(1):51–5. doi: 10.1212/01.wnl.0000249142.82285.d6. [DOI] [PubMed] [Google Scholar]
- 26.Pane M, Staccioli S, Messina S, et al. Daily salbutamol in young patients with SMA type II. Neuromuscul Disord. 2008;18(7):536–40. doi: 10.1016/j.nmd.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Iannaccone ST. Outcome measures for pediatric spinal muscular atrophy. Arch Neurol. 2002;59(9):1445–50. doi: 10.1001/archneur.59.9.1445. [DOI] [PubMed] [Google Scholar]
- 28.Mazess R, Barden H, Bisek J, et al. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 29.Kuczmarski R, Ogden C, Guo S. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002 May;(246):1–190. [PubMed] [Google Scholar]
- 30.Schutz Y, Kyle UUG, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18 – 98 y. International Journal of Obesity. 2002;26:953–960. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 31.Tiziano F, Bertini E, Messina S, et al. The Hammersmith functional score correlates with the SMN2 copy number: a multicentric study. Neuromuscul Disord. 2007;17(5):400–403. doi: 10.1016/j.nmd.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Pane M, Vasta I, Messina S, et al. Feeding problems and weight gain in Duchenne muscular dystrophy. Eur J Paediatr Neurol. 2006;10:231–236. doi: 10.1016/j.ejpn.2006.08.008. [DOI] [PubMed] [Google Scholar]