1. Introduction
Small ring compounds have continually captivated physical and synthetic organic chemists because of their remarkable structures and the reactions that are feasible due to the inherent ring strain of these systems. Isolable, small, strained carbocyclic and heterocyclic rings have been known for over a century, but recently there have been a number of new spiroheterocycles described. Specifically, the utility of oxa- and dioxaspiropentanes as well as oxa- and dioxaspirohexanes has been demonstrated in the synthesis of natural and unnatural products. The ring systems investigated to date have shown interesting and varied reactivity patterns due to the unique physical properties of these small rings. The aim of this review is to provide an overview of the synthesis and reactivity of small strained spiroheterocycles and to illustrate their applications in synthetic endeavors. This review will be restricted to spiro heterocyclic pentane and hexane systems, as other types of strained systems have been recently reviewed.1
An initial, general overview of reactivity patterns for these systems identified by various researchers will hopefully enable the reader to put the following discussion of reactions and applications in perspective. Oxaspiro[2.2]pentanes and 1,4-dioxaspiro[2.2]pentanes I exhibit similar reactivity patterns.2 The C-O bond of the epoxide is cleaved upon either acid-mediated rearrangement or nucleophile addition. 1-Oxaspiro[2.3]hexanes II, 1,4-dioxaspiro[2.3]hexanes III, and 4-oxaspiro[2.3]hexanes IV offer two primary modes of reactivity. The predominant Lewis acid promoted rearrangement through a stabilized carbocation provides cyclopentanone products, yet nucleophilic addition favors the more hindered epoxide C-O bond. However, dioxaspirohexanes III tend to undergo nucleophilic substitution primarily at either of the epoxide C-O bonds. Of the four possible reactive centers in 1,4-dioxaspiro[2.3]hexan-5-ones IV only substitution at the less hindered epoxide C-O bond or the C=O bond has been observed under various reaction conditions.
2. Oxaspiro[2.2]pentanes
The first confirmed example of small simple spirocyclic ring systems, oxaspiro[2.2]pentanes, appeared in 1968.3 This spiro system was initially synthesized by reaction of peracetic acid and an unsaturated cyclopropane derivative 1 to give oxaspiro[2.2]pentane 2 (Scheme 1). This method proved to be quite general, as the substrate could be made by olefination of the corresponding carbonyl compounds with cyclopropyl phosphonium ylides. However, an appropriate directing group (i.e. ether) was required because of the modest diastereoselectivity observed.4 After this initial report, several other methods were devised for the synthesis of oxaspiro[2.2]pentanes, which include addition of lithiated bromocyclopropanes5 or diazocompounds to ketones,6 reaction of singlet oxygen with bicyclopropylidene,7,8 selenone additions to ketones,9 and the addition of cyclopropyl sulfur ylides to carbonyl compounds.10,11
Scheme 1.
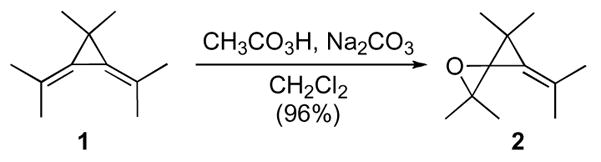
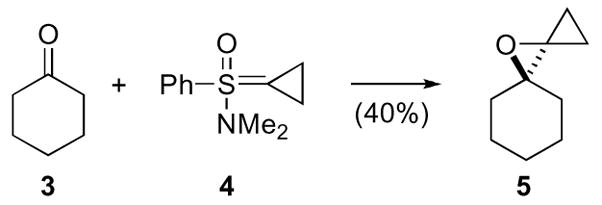
By far the most widely used and arguably the most diastereoselective synthesis of oxaspiro[2.2]pentanes remains the addition of cyclopropyl sulfoxonium ylides to carbonyl compounds.12,13 A series of elegant studies revealed that ylide addition proceeds via equatorial attack at the carbonyl carbon in cyclic compounds. While this process is highly diastereoselective, an enantioselective process has not been developed. Although Johnson reported chiral sulfoximine ylide additions to α,β-unsaturated carbonyl compounds, the racemic counterpart performed poorly with simple carbonyl compounds as in the reaction of cyclohexanone 3 with sulfoximine ylide 4, thus the chiral series was not attempted (Scheme 2).14
Scheme 2.
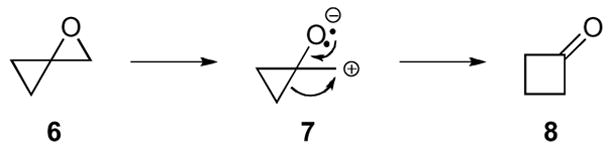
Oxaspiro[2.2]pentanes display two primary modes of reactivity. One mode, leading to a cyclobutanone, appears to be driven by the release of the strain energy of the ring system (Scheme 3).12 However, as cyclobutanes possesses essentially the same amount of strain energy as cyclopropanes (26 kcal/mol and 27 kcal/mol, respectively) due to the eclipsing interactions of the substituents on a cyclobutane, the rearrangement is driven not only by the release of ring strain of both the starting epoxide and the cyclopropane but also by the formation of a C=O bond.15 The ring expansion of a cyclopropane to a cyclobutane proceeds with oxaspiro[2.2]pentane 6 when appropriate stabilizing groups are present. Ionization of the epoxide, either by addition of a Lewis acid or thermal induction, yields a highly stabilized intermediate cyclopropyl carbinyl cation 7. This type of cation is uniquely stabilized due to the enhanced π-character of the σ-bonds in cyclopropanes, which consequently permits a pinacol like rearrangement to cyclobutanone 8. This process is favorable due to the release of ring strain of the starting epoxide and cyclopropane in addition to the formation of a C=O bond.
Scheme 3.
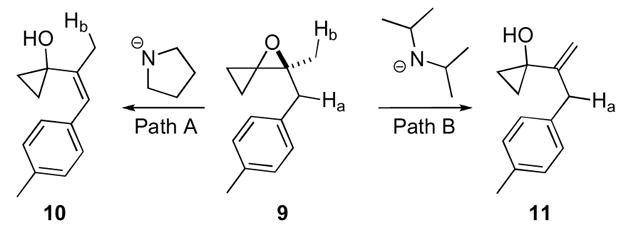
The second major mode of reactivity of oxaspiro[2.2]pentanes is the base induced elimination to form vinyl cyclopropanes. The relief of ring strain upon opening of the epoxide drives this particular transformation. Trost developed conditions allowing access to both regioisomeric products 10 and 11 from spiroepoxide 9 (Scheme 4).16,17 Use of the less sterically demanding lithium amide of pyrrolidine led to deprotonation of less sterically accessible Ha (Path A) which gave the more substituted conjugated olefin 10. Use of the more hindered base, lithium diisopropylamide led to deprotonation of oxaspiro[2.2]pentane 9 at the more accessible Hb (Path B) to yield olefin 11.
Scheme 4.
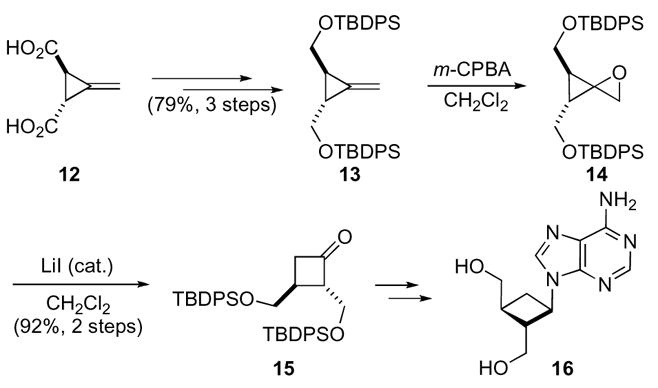
Since their last review,14 oxaspiro[2.2]pentanes, have continued to be valuable in the synthesis of other complex architectures. This ring system has proven to be advantageous for the synthesis of new ligands as well as natural and unnatural products. The initial application of oxaspiro[2.2]pentanes was toward the synthesis of carbocyclic analogues of oxetanocin, an antibiotic/antiviral natural product (Scheme 5).18 Hsiao’s synthesis hinges on the construction of an optically active cyclobutanone 15. Their approach to this ring system depended on the preparation of oxaspiro[2.2]pentane 14 and the subsequent rearrangement to cyclobutanone 15. The synthesis began with Feist’s acid 12, which was resolved utilizing quinine. After resolution and a series of standard manipulations, methylene cyclopropane 13 was obtained in good overall yield. The substrate was then subjected to the key oxidation/rearrangement sequence. This was accomplished by exposing methylene cyclopropane 13 to meta-chloroperbenzoic acid and subsequent rearrangement of oxaspiro[2.2]pentane 14 with catalytic lithium iodide to give key cyclobutanone 15. Selectivity issues were avoided in the oxidation step by the use of a C2 symmetric substrate. Cyclobutanone 15 could be further transformed into carbocyclic oxetanocin analogue 16.19
Scheme 5.
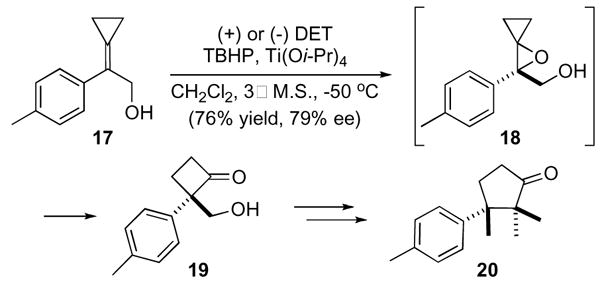
In addition to diastereoselective methods, enantioselective epoxidation methods have also proven to be effective in yielding optically active oxaspiro[2.2]pentanes as in the synthesis of both enantiomers of α-cuparenone (20), a sesquiterpene natural product (Scheme 6).20 The synthesis of either enantiomer of α-cuparenone began with epoxidation of cyclopropene 17 under Sharpless conditions resulting in an aryl substituted oxaspiro[2.2]pentane 18 that rearranged to cyclobutanone 19 in modest enantiomeric excess. Several addition steps, then completed the total synthesis of α-cuparenone 20.21
Scheme 6.
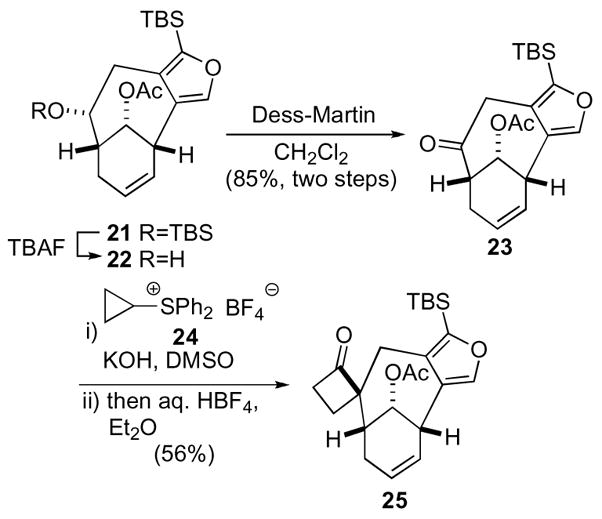
Perhaps one of the more complex settings using an oxaspiro[2.2]pentane, derived from the Trost cyclobutanone procedure, is an elegant model study toward the synthesis of the architecturally intriguing natural products CP-225,917 and CP-263,114.22,23 Advanced furan intermediate 21, derived from cyclohexenone underwent silyl-deprotection and subsequent oxidation to give ketone 23 (Scheme 7). Addition of cyclopropyl sulfonium tetrafluoroborate 24 followed by rearrangement under acidic conditions then delivered cyclobutanone 25.
Scheme 7.
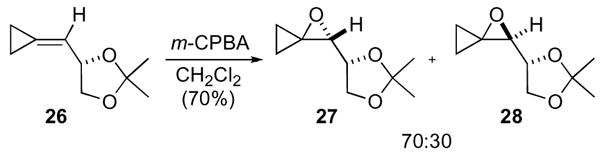
The diastereoselective synthesis of both the acetogenin natural product muricatacin and the pheromone japonilure have also been accomplished by using an oxaspiro[2.2]pentane as an intermediate.24 The first key reaction was the oxidation of methylene cyclopropane 26 to the corresponding oxaspiro[2.2]pentanes 27 and 28 in modest diastereoselectivity (Scheme 8).
Scheme 8.
The key challenge was the optimization of the Lewis acid in order to achieve high diastereoselectivity from the rearrangement of oxaspiro[2.2]pentane 27 to cyclobutanone 30 (Scheme 9).25 High selectivity for the desired diastereomer was achieved under the optimized conditions with lithium iodide in dichloromethane. Interestingly, the undesired diastereomer 29 was obtained with good diastereoselectivity by using lithium perchlorate in place of lithium iodide hinting at a change in mechanism to a carbocationic intermediate rather than halohydrin-type intermediates due to the multiple coordination sites available for the lithium atoms.24
Scheme 9.
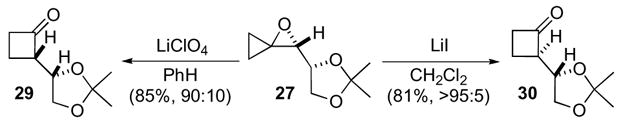
After accomplishing the key oxidation-rearrangement reaction sequence, the subsequent steps toward the formal syntheses of muricatacin and japonilure were rather straightforward (Scheme 10). Baeyer-Villiger oxidation of cyclobutanone 30 to the corresponding γ-lactone 31 followed by deprotection of the acetonide to diol 32, provided a common intermediate in two syntheses of muricatacin.33.26,27 Alternatively, the formal synthesis of japonilure 35 was completed after oxidative cleavage and direct reduction to alcohol 34.28
Scheme 10.
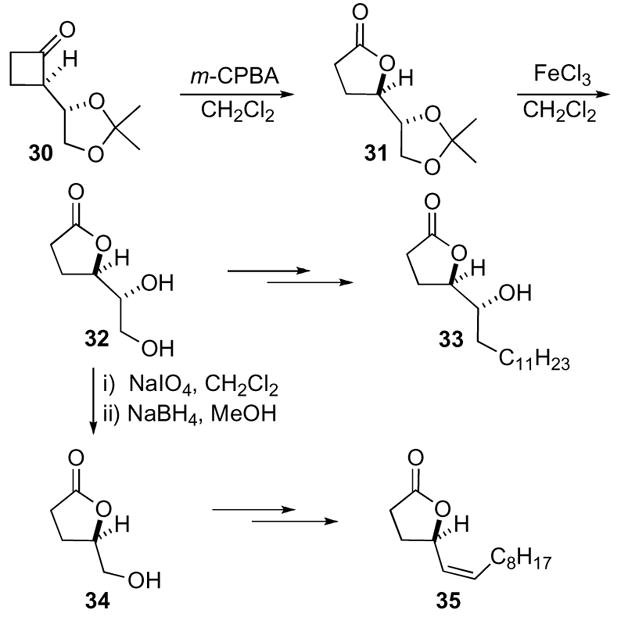
Oxaspiro[2.2]pentanes were also utilized in the total synthesis of the steroid (+)-equilenin 40.29,30 Ihara began with naphthalene derivative 36, which was epoxidized under Jacobsen’s conditions to yield cyclobutanone 38 directly due to instability of the transient oxaspiro[2.2]pentane 37 to the reaction conditions (Scheme 11). The optical purity of the cyclobutanone 38 was not improved with other epoxidation methods. Shi epoxidation resulted in low optical purity, which could not be increased by changing either the solvent or catalyst loading. The reduction in enantioselectivity was presumably due to the stabilization of the developing benzylic cation by the methoxy substituent, which led to an unselective rearrangement to the cyclobutanone. This was supported by the observation that optical purities increased drastically (up to 93% ee) when the methoxy substituent was replaced with hydrogen. Addition of the isoprenyl group to cyclobutanone 38 in the presence of cerium trichloride, followed by treatment with stoichiometric amounts of palladium acetate gave cyclopentanone 39 which was then transformed to (+)-equilenin 40.
Scheme 11.
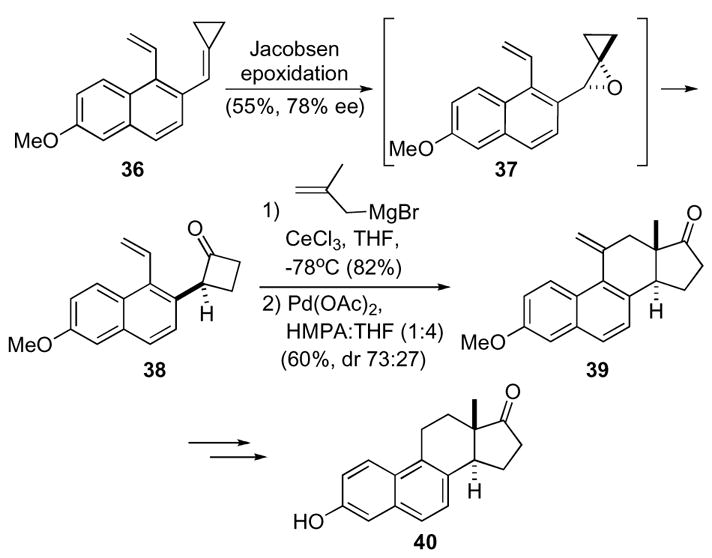
The advantage of small rings in synthesis was illustrated in Paquette’s studies toward the trixikingolides since the application of a Nazarov type cyclization failed.31 Divinyl ketone 41 did not lead to the desired cyclopentenone 42 in sufficient yields to be synthetically useful under a variety of reaction conditions. Assistance of a silicon atom in the β-position did not lead to any improvement and resulted in low yields or mixtures of stereoisomers at the newly formed ring juncture (Scheme 12).
Scheme 12.
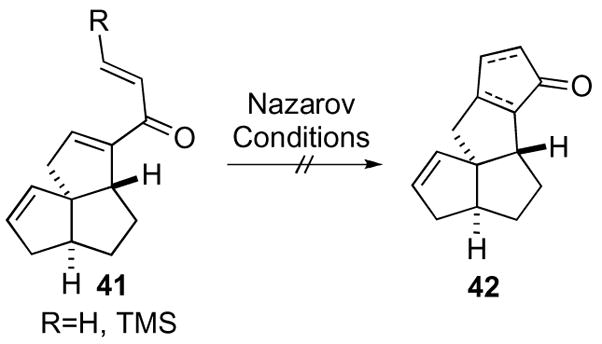
The failures of standard conditions led to the pursuit of cyclopentenone 42 via Trost’s annulation procedure utilizing an oxaspiro[2.2]pentane. The sequence began with condensation of cyclopropyl sulfoxonium ylide 24 and ketone 43 leading to oxaspiro[2.2]pentane derivative 44, which was used without purification in the elimination step to yield cyclopropane 45 (Scheme 13). To complete the annulation cyclopropane 45 was flash-vacuum-pyrolyzed to yield the desired cyclopentanone as its silyl enol ether 46 in excellent yield. Unfortunately, attempts to utilize this strategy toward the trixikingolides 47 did not result in a complete synthesis.
Scheme 13.
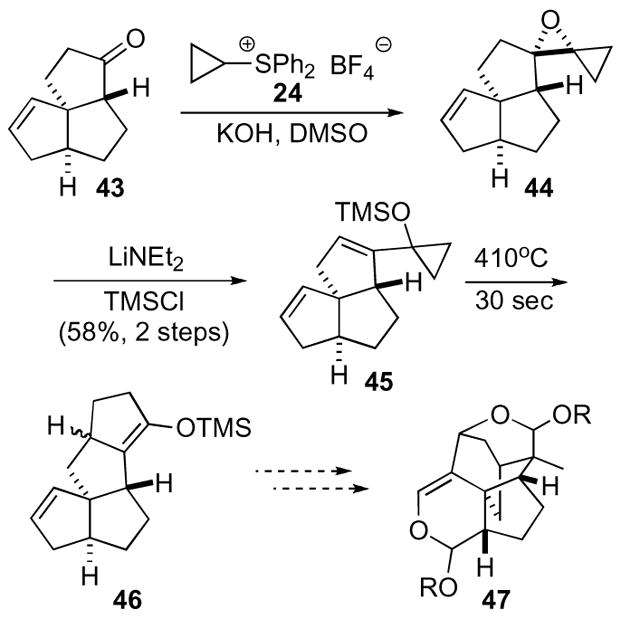
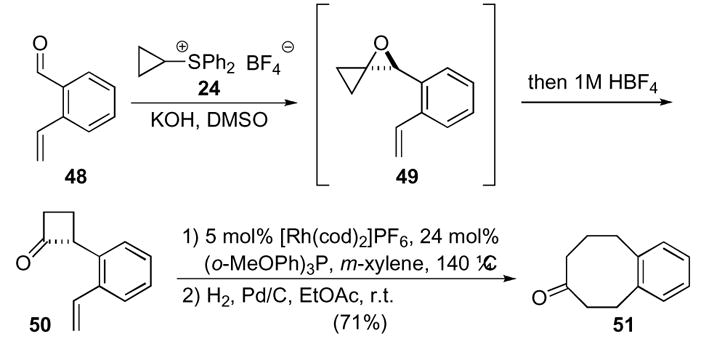
In addition to enabling access to numerous natural products and several new chiral ligands, oxaspiro[2.2]pentanes have also provided access to substrates for interesting transformations. An example is the rhodium-catalyzed synthesis of an eight-membered ring developed by Murakami (Scheme 14).32 The o-styryl-cyclobutanone 50 was synthesized utilizing Trost’s cyclopropyl sulfoxonium ylide 24 procedure to generate oxaspiro[2.2]pentane 49,25 which was subsequently rearranged under protic conditions to yield the desired cyclobutanone 50. Cyclobutenone 50 was transformed to octanone 51 via olefin insertion and subsequent hydrogenation.
Scheme 14.
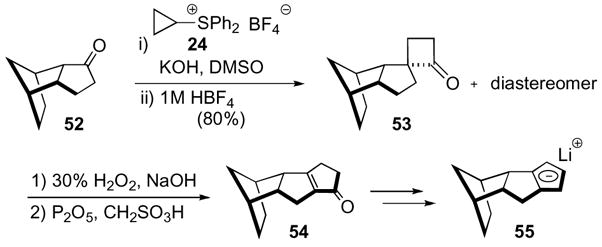
Not only have oxaspiro[2.2]pentanes found application in synthesis of natural and unnatural products, but they have also found application in the synthesis of structurally interesting compounds, such as polyspiranes and ligands for catalysis. The former have been the subject of previous reviews1d and will therefore not be discussed, yet the latter have not been included previously. Paquette showed that oxaspiro[2.2]pentanes could be applied in different arenas other than the total synthesis of natural products, which was exemplified in the synthesis of a new set of chiral cyclopentadienide ligands.33 Ketone 52, derived from cyclopentadiene, was subjected to the Trost cyclobutanone synthesis conditions by exposure to cyclopropyl diphenyl sulfoxonium tetrafluroborate 24 (Scheme 15). Subsequent rearrangement with aqueous acid yielded an inconsequential mixture of cyclobutanones 53 that were oxidized to the γ-lactones and then exposed to phosphorous pentoxide and methane sulfonic acid to yield α,β-unsaturated ketone 54. Completion of the synthesis of the cyclopentadienide ligand 55 was accomplished in a three step sequence, which included reduction of ketone 54 to the alcohol followed by elimination under acidic conditions to yield a cyclopentadiene. Subsequent deprotonation with n-butyl lithium gave the desired cyclopentadienide 55.
Scheme 15.
3. 1, 4-Dioxaspiro[2.2]pentanes
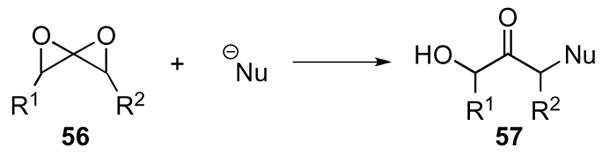
In a similar manner to the oxaspiro[2.2]pentanes, the further oxidized 1,4-dioxaspiro[2.2]pentanes 56 display one primary mode of reactivity. The reactivity of the small spiroacetal, hinges on the relief of strain contained in both epoxide rings to give α-nucleophile substituted α’-hydroxy ketones 57 as products (Scheme 16). These nucleophilic additions are broad in scope and can occur either intra- or intermolecularly.
Scheme 16.
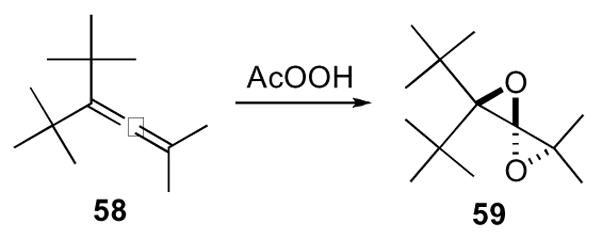
The 1,4-dioxaspiro[2.2]pentane 59 was first synthesized by Crandall in 1966 and has regained the interest of synthetic chemists in recent years.34,35 Crandall found that exposure of tetramethylallene to peracetic acid resulted in the formation of several oxidized products. Although not directly detected, several of the reaction products pointed to the presence of a 1,4-dioxaspiro[2.2]pentane. However, it was not until 1968 that Crandall succeeded in isolating 1,4-dioxaspiro[2.2]pentane 59 by careful distillation of the oxidation products of allene 58 (Scheme 17).36
Scheme 17.
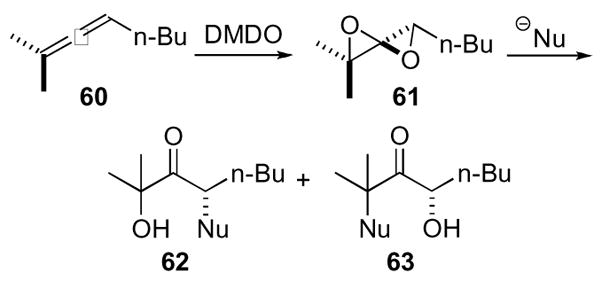
1,4-dioxaspiro[2.2]pentanes did not find utility in more synthetically useful scenarios until two decades later primarily due to the lack of efficient, neutral, and mild oxidizing reagents.37,38 The advent of dimethyl dioxirane by Murray and co-workers provided a more general synthesis of the 1,4-dioxaspiro[2.2]pentanes (Scheme 18).39 Exposure of diepoxide 61, available from allene 60, to various nucleophiles typically yielded substituted ketones of type 62 and 63 depending on the steric demands of the nucleophile and the reaction conditions. More sterically demanding nucleophiles react to form ketone 62 compared to the mixtures of substituted ketones 62 and 63 obtained with less sterically demanding nucleophiles. However, acidic conditions preferred the formation of ketone 63.
Scheme 18.
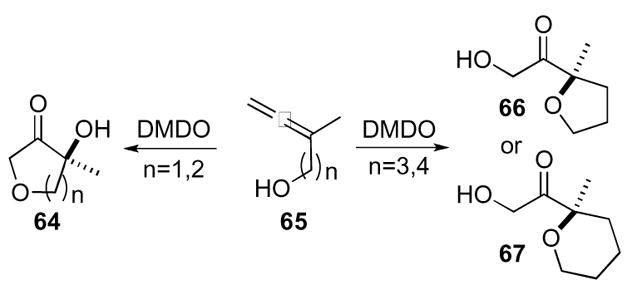
Crandall and co-workers also found that intramolecular cyclizations were possible with an appropriate pendant nucleophile (Scheme 19).40,41 Thus, oxidation of allenic alcohols 65 containing a one or two carbon atom tether yielded furanones 64 that proceeded through attack at the distal epoxide C-O bond by the pendant alcohol. However, when the tether was extended to three or four carbon atoms, nucleophilic addition favored the proximal epoxide C-O bond to form tetrahydrofurans 66 and tetrahydropyrans 67, respectively.
Scheme 19.
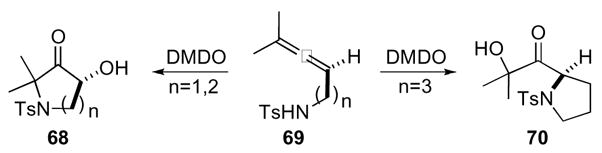
In analogy to allenyl alcohols, protected amines also give heterocycles as products (Scheme 20). Exposure of a tosyl protected allenyl amine 69 with a one or two carbon tether yielded the corresponding pyrrolidinone or piperidinone 68, respectively with nucleophilic attack of the amine occurring at the distal epoxide C-O bond. Conversely, when the tether was lengthened to three carbon atoms, nucleophilic attack occurred at the proximal epoxide C-O bond yielding acylpyrrolidine 70. Amine substrates suffered from lower yields (52% from 69 vs. 87% from 65) due to competing oxidation at the nitrogen atom to give further oxidized by-products.
Scheme 20.
Similarly, when allenic carboxylic acids 71 were employed as substrates the corresponding lactone products were obtained (Scheme 21).42,43 When the tether length was only one carbon atom δ-lactones 72 were obtained as the major product. Interestingly, when the tether had one carbon atom and sodium bicarbonate was added to the reaction both β-lactone 73 and δ-lactone 72 (major product) were observed. The δ-lactone 72 was observed as the major product under acidic conditions.
Scheme 21.
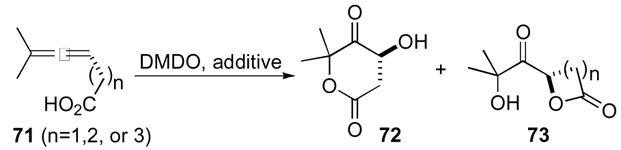
Although it is well known that aldehydes are oxidized to carboxylic acids with DMDO,44 allenyl aldehydes were also explored as substrates (Scheme 22).45 Epoxidation proved to be competitive with oxidation of the aldehyde permitting intramolecular cyclization over intermolecular oxidation of aldehyde 74 resulting in the formation of the unexpected acetal 75 as a 1:1 mixture of diastereomers. The trans-diastereomer 75 can be obtained as a single product by utilizing scrupulously dry DMDO in the presence of a suitable alcohol.
Scheme 22.
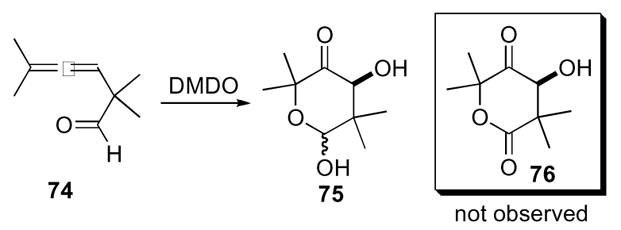
Although the 1,4-dioxaspiro[2.2]pentanes have been known and investigated since 1966, it took thirty years for this ring system to be utilized in the synthesis of a complex molecule. The betamethosone synthesis by Andrews and co-workers demonstrated the practicality of 1,4-dioxaspiro[2.2]pentanes (Scheme 23).46 Starting from allene 77 derived from 9α-hydroxyandrost-4-ene-3,17-dione oxidation with DMDO provided 1,4-dioxaspiro[2.2]pentane derivative 78. Subjection to tetrabutylammonium acetate yielded α-acetate substituted ketone 79, which was then be converted to betamethosone 80 by known reaction conditions.47
Scheme 23.
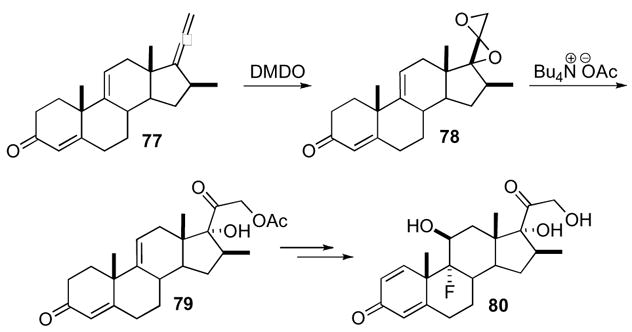
Williams’ modular synthesis of the selective proteasome inhibitor epoxomicin 83 also highlights the utility of this ring system (Scheme 24).48 Allene 81, which was derived from isovaleraldehyde, underwent DMDO oxidation, followed by azide addition, and reduction of the unstable azido-ketone to deliver the stable amino-ketone 82. This route was pursued due to the low yield or instability of other amino-ketones derived from alternate nitrogen sources. Peptide coupling followed by the transformation of the tertiary alcohol into the requisite epoxide produced epoxomicin 83. This sequence proceeded in excellent overall yield from allene 81 (42%, 9 steps).
Scheme 24.
Nucleophilic additions to 1,4-dioxaspiro[2.2]pentanes are not limited to heteroatoms; carbon based nucleophiles can also provide substituted ketone products. The addition of cuprates to this ring system proved difficult at first, but was made effective with the appropriate Gilman reagent (Scheme 25).49 Typical dialkyl cuprates derived from alkyl lithiums provided some of the desired addition product 85 along with undesired ketone 86 as the major product. The production of hydroxyl-ketone 86 was surprising though not unfounded.50
Scheme 25.
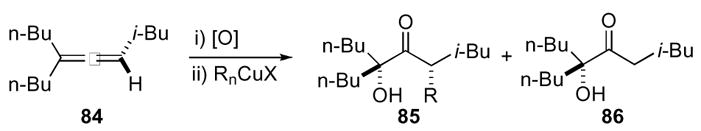
The reaction presumably occurred through oxidative addition of the Cu(I) species to the epoxide 87 to give intermediate α-Cu(III) ketone 88, which provided two divergent reaction pathways (Scheme 26). Expected reductive elimination yielded the desired substituted product 89. In contrast, isomerization to the corresponding enolate 90 led to observed ketone 91. This undesired pathway was avoided by the use of alkylcyano cuprates.
Scheme 26.
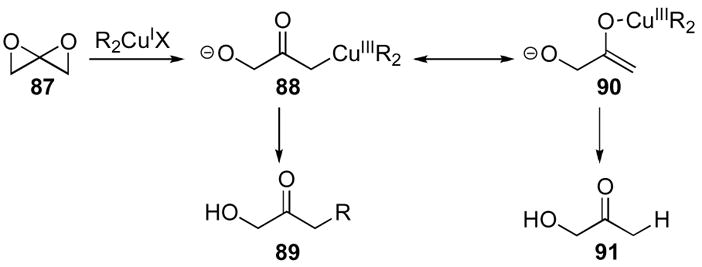
This methodology was applied to the synthesis of a precursor towards the antibiotic erythromycin (Scheme 27). Oxidation of allene 92 and subsequent exposure to methylcyanocuprate yielded ketone 93 with good diastereoselectivity. Ketone 93 was then transformed to the known protected tetrol 94 by standard methods.51
Scheme 27.
The tetrahydropyran moiety of psymberin, a selective anti-tumor agent isolated in 2004 from a sponge off the coast of Papua New Guinea,52 was constructed utilizing Crandall’s methodology (Scheme 28).53 Advanced allene 95 was subjected to DMDO oxidation to give the intermediate 1,4-dioxaspiro[2.2]pentane, which cyclized to dihydropyranone 96 upon the addition of methanol. Dihydropyranone 96 was transformed into a known intermediate in the synthesis of psymberin 97.
Scheme 28.

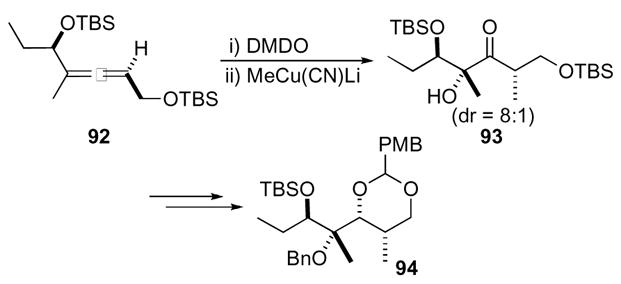
The unusual necessity of methanol to promote cyclization can be explained by the diastereomeric conformations of the 1,4-dioxaspiro[2.2]pentane (Scheme 29). Spontaneous cyclization to give the cis-substituted pyran 100 occurred when the opposite stereochemistry of the alcohol was used in the oxidative cyclization. This is due to the destabilization of the unproductive hydrogen-bonded conformation 98 resulting from steric interactions, which promotes the formation of the productive conformation 99 providing cis-substituted pyran 100. Conversely, the other diastereomer forms a stable hydrogen-bonded complex 101 lacking such destabilizing interactions, although interactions between the alkyne and the epoxide in the productive conformation 102 made the cyclization challenging. However when a protic solvent was added, the stable hydrogen-bonded complex was destroyed and the reaction was driven to the formation of conformer 102 and thus the desired trans-substituted pyran 96.
Scheme 29.
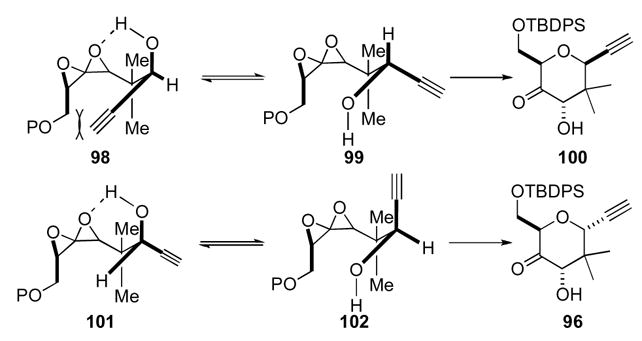
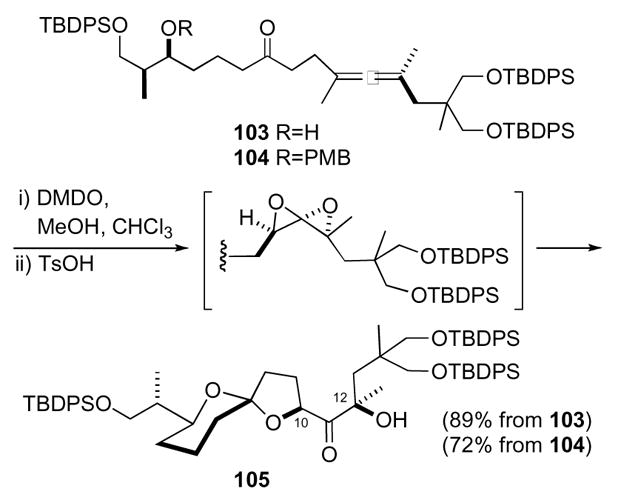
Williams also devised a synthesis of the A–B ring system of pectenotoxin 4 using a 1,4-dioxaspiro[2.2]pentane as a key intermediate (Scheme 30).54 Keto-allene 103 was oxidized by DMDO to the bis-epoxide, which was then captured by the pendant ketone to generate an oxocarbenium ion. The alcohol then captured the cation to give spirocycle 105 featuring the A–B ring system of pectenotoxin 4. Additionally, the diastereoselectivities for both oxidations were good, >20:1 for the C12 alcohol and 7:1 for the C10 center. This cascade reaction sequence was extended by reaction with the PMB protected alcohol 104 wherein DMDO to removed the PMB group.
Scheme 30.
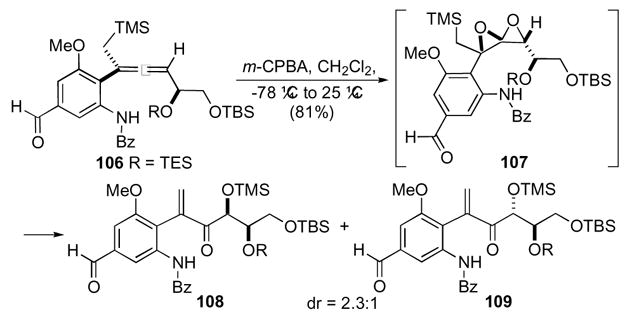
Certain alkaloids possessing a vicinal stereotriad have been envisioned as coming from aryl substituted 1,4-dioxaspiro[2.2]pentanes (Scheme 31).55 Incorporation of aryl substituents particularly electron-rich aromatics, pose several challenges to the preparation of the highly strained spirodiepoxides with regard to stability. After extensive investigation, Williams and co-workers demonstrated a Bronsted acid-induced rearrangement of a presumed densely functionalized 1,4-dioxaspiro[2.2]pentane 107 intermediate to furnish enones 108 and 109 in good yield but low diastereoselectivity. The rearrangement proceeded initially at the more substituted terminus, which led to concomitant elimination of the TMS group. Further elaboration of enones 108 and 109 derived from the Brønsted acid mediated rearrangement can generate precursors applicable towards synthesis of complex natural products.
Scheme 31.
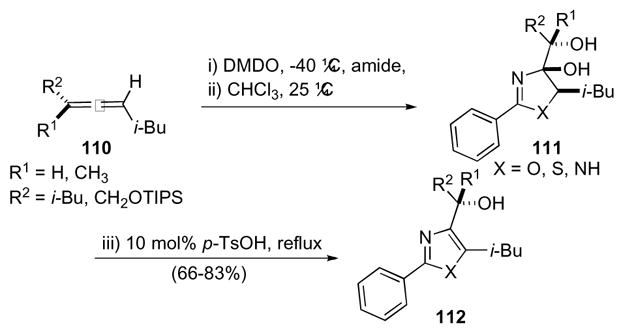
Nitrogen heterocycles have also been accessed via 1,4-dioxaspiro[2.2]pentanes through epoxidation of the corresponding allene (i.e. 110) with DMDO and subsequent ring opening (Scheme 32).56 Benzamides, thiobenzamides, and benzamidine added readily to spirodiepoxides to provide azolines 111 and azoles 112 upon successive treatment with catalytic para-toluene sulfonic acid. Mechanistic studies revealed evidence supporting a concerted asynchronous ring opening of both epoxides rather than a stepwise process. However, a stepwise process cannot be entirely excluded especially when strong alkoxide interactions persist.
Scheme 32.
4. 1-Oxaspiro[2.3]hexanes
Since the introduction of 1-oxaspiro[2.3]hexanes in the 1970’s, the most prevalent method of preparation is the oxidation of methylene cyclobutanes by DMDO or peracids analogously to the previously described ring systems. Alternative methods of preparation include the addition of α-lithio selenocyclobutanes to carbonyl compounds,57,58 addition of sulfur ylides to cyclobutanones,59,60 oxidation of methylene cyclobutanes with N-bromosuccinimide in water,61 transition metal mediated epoxidations of cyclobutenes,62 and oxidation of methylene cyclobutanes with asymmetric versions of DMDO.63,64
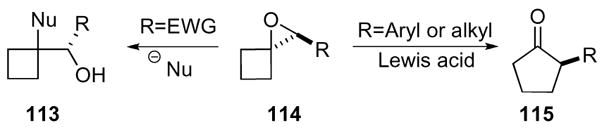
1-Oxaspiro[2.3]hexanes 114 have similar reactivity patterns to oxaspiro[2.2]pentanes upon nucleophilic addition and Lewis acid mediated ring expansion (Scheme 33). When the ring system was functionalized with an aryl or alkyl group, a facile ring expansion under Lewis acidic conditions to cyclopentanone 115 occurred.59,65 The ring expansion was driven by the release of the strain energy of both rings, and the presence of an aryl group likely stabilized the forming carbocation necessary for the rearrangement. Conversely, nucleophilic addition to the ring system functionalized with an electron withdrawing group furnished substituted cyclobutanes 113 due to the destabilization of the cation.
Scheme 33.
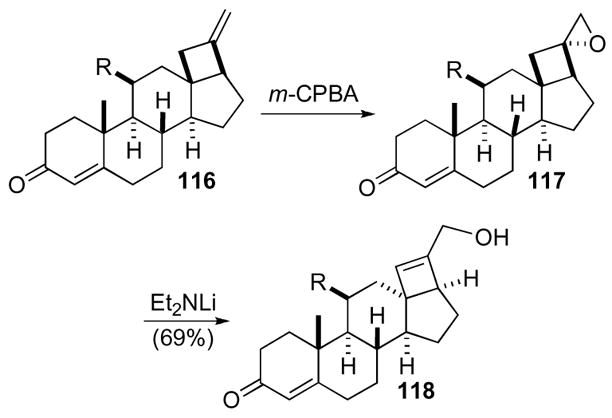
A 1-oxaspiro[2.3]hexane was initially used in synthesis during the attempted construction of an aldosterone derivative (Scheme 34).66 Steroid 116, which was obtained from pregnenolone acetate, was oxidized by m-CPBA to give 1-oxaspiro[2.3]hexane 117. Subsequent elimination with lithium diethylamide gave cyclobutene 118. The reaction sequence only worked well on unsubstituted systems (i.e. R=H) otherwise elimination did not occur. This route was later abandoned for a more productive pathway.
Scheme 34.
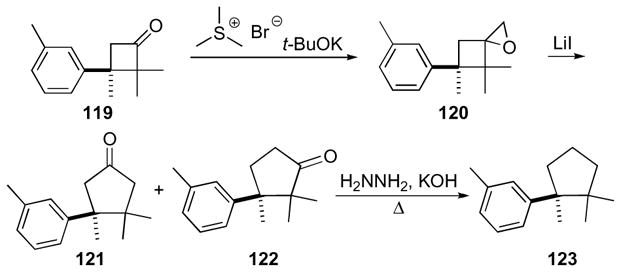
The aromatic sesquiterpene herbertene, isolated from the liverwort Herberta adunca, is another early example of the use of 1-oxaspiro[2.3]hexanes in natural product synthesis (Scheme 35).59,60 The synthesis of the key 1-oxaspiro[2.3]hexane 120 began with cyclobutanone 119, which was made by thermal cyclization of a toluene derivative and dimethyl ketene. Cyclobutanone 119 was then condensed with trimethylsulfonium bromide under basic conditions to yield the desired spirosystem 120. The spirosystem rearranges to an inconsequential mixture of carbonyl isomers 121 and 122 upon exposure to catalytic lithium iodide. Herbertene 123 was then completed (overall yield of ~30%) after reduction of the carbonyl to the corresponding methylene using the Huang-Minlon modification of Wolff-Kischner reduction.67
Scheme 35.
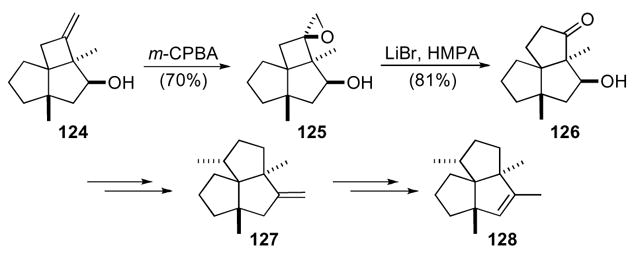
1-oxaspiro[2.3]hexanes have also been envisioned for the preparation of triquinane natural products. A common 1-oxaspiro[2.3]hexane permitted the synthesis of both isocomene and β-isocomene (Scheme 36).68 The key 1-oxaspiro[2.3]hexane 125 was derived from cyclobutene 124 by hydroxyl directed epoxidation with m-CPBA to give a single epoxide 125. Regioselective rearrangement in the presence of lithium bromide and HMPA gave cyclopentanone 126 that was then transformed into β-isocomene 127 and isocomene 128, respectively.
Scheme 36.
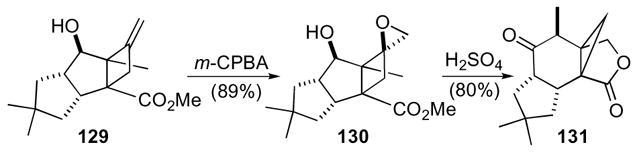
Tobe’s synthesis of marasmic acid is a particularly elegant use of a 1-oxaspiro[2.3]hexane (Scheme 37).69,70 In the synthesis, cyclobutene 129 was oxidized with m-CPBA to give epoxide 130. A cascade rearrangement of epoxide 130 with sulfuric acid gave lactone 131, which is a precursor to marasmic acid.
Scheme 37.
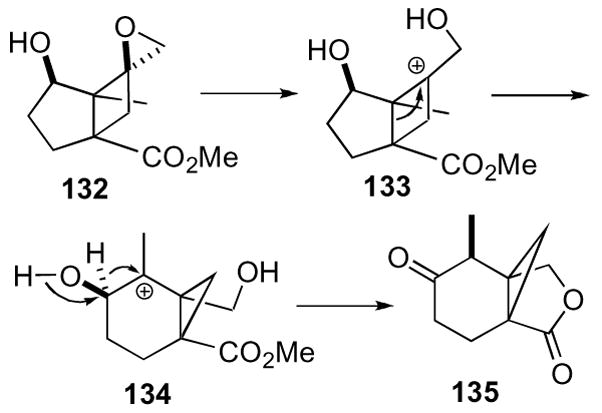
The mechanism of this cascade rearrangement is depicted in simplified version, omitting the cyclopentane for clarity (Scheme 38). Protonation of epoxide 132 led to the formation of cyclobutyl cation 133, which then underwent a bond migration to give cyclopropyl carbinyl cation 134. A 1,2-hydride shift gave the observed product 135 after lactonization.
Scheme 38.
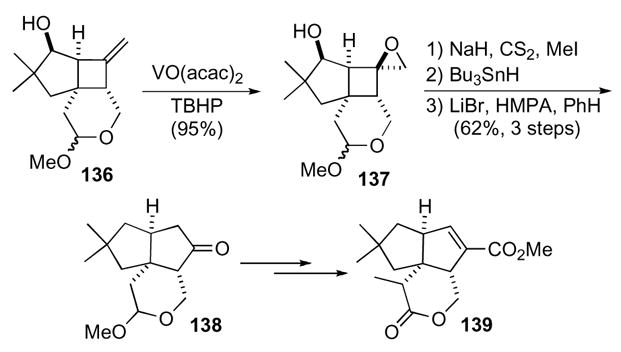
The ring expansion of a 1-oxaspiro[2.3]hexane proved to be particularly effective for the synthesis of pentalenolactone E and G methyl esters (Scheme 39).71 Cyclobutene 136 was constructed by a photochemical [2+2] cycloaddition from the corresponding allene and enone. Hydroxyl directed epoxidation of alkene 136 in the presence of VO(acac)2 and tert-butylhydroperoxide provided epoxide 137 with excellent diastereoselectivity. After Barton-McCombie deoxygenation, ring expansion gave cyclopentanone 138 as a single regioisomer. Pentalenolactone E methyl ester 139 and pentalenolactone G methyl ester were obtained after further manipulations of cyclopentanone 138.
Scheme 39.
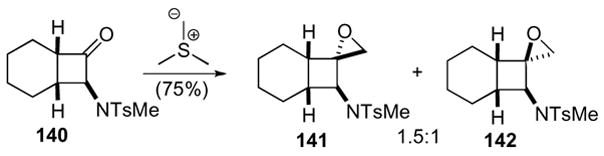
Amino-substituted 1-oxaspiro[2.3]hexanes are also accessible via a two-step process in good optical purity (Scheme 40).72,73 Amino-cyclobutanone 140 was made directly from the [2+2] cycloaddition of the corresponding keteniminium salt and disubstituted olefin. Condensation of cyclobutanone 140 with a sulfur ylide yielded the corresponding epoxides 141 and 142 in good yield but poor diastereoselectivity.
Scheme 40.
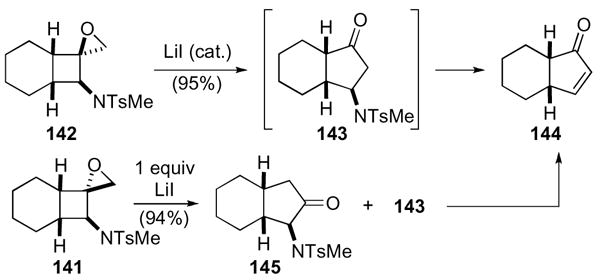
Exposure of these amino-substituted 1-oxaspiro[2.3]hexanes to either a catalytic or stoichiometric amount of lithium iodide facilitated conversion to the subsequent cyclopentenone (Scheme 41). The exo-isomer 142 underwent a more facile rearrangement requiring only a catalytic amount of lithium iodide, while the endo-isomer 141 required a full equivalent of lithium iodide. The same β-aminocyclopentanone intermediate 143 was presumably formed with both isomers, but under the reaction conditions elimination occurred to give cyclopentenone 144. The rearrangement of endo-isomer 141 was not as regioselective as the exo-isomer 142, which resulted in the formation of α-aminocyclopentanone 145 and cyclopentenone 144. The optical purity of the starting epoxide was preserved in the products in several examples.
Scheme 41.
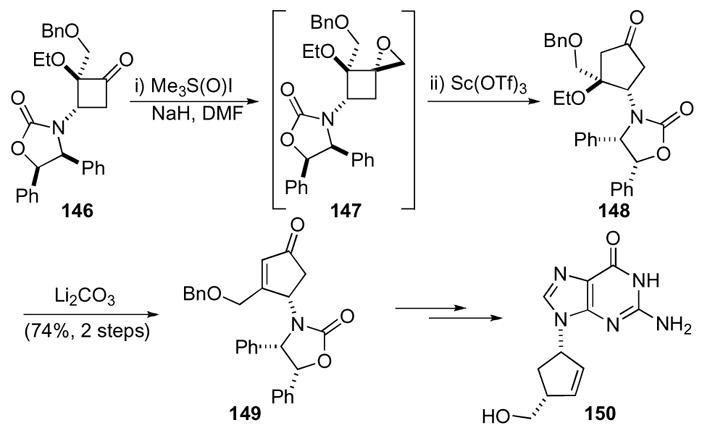
Compared to the regioisomeric mixtures obtained from diazomethane addition to cyclobutanones, 1-oxaspiro[2.3]hexanes offer significant advantages because of their facile and highly regioselective rearrangement to the corresponding cyclopentanones. For instance, Hegedus and co-workers demonstrated increased regioselectivity in the synthesis of carbovir 150 (Scheme 42).74 Photolysis between a chromium carbene and an ene-carbamate constructed cyclobutanone 146. Further reaction with dimethylsulfoxonium methylide delivered 1-oxaspiro[2.3]hexane 147, which was then exposed to scandium triflate to trigger a rearrangement to cyclopentanone 148. Scandium triflate provided complete conversion to the product in higher yield compared to other Lewis acids. The ethoxy group was then eliminated under basic conditions to give cyclopentenone 149, which was then transformed after further manipulations to carbovir 150.
Scheme 42.
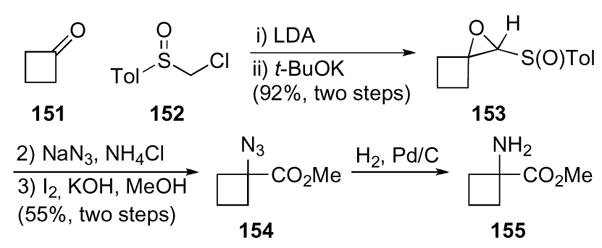
Attachment of an appropriate electron withdrawing group can suppress 1-oxaspiro[2.3]hexanes from opening to cyclobutylcarbinyl cations and subsequently rearranging under Lewis acid conditions. These substrates were synthesized via a two-step protocol to generate a sulfoxyl substituted epoxide (Scheme 43).75,76 Cyclobutanone 151 reacted with chloromethyl substituted sulfoxide 152 in the presence of LDA to give an alcohol that was cyclized to the corresponding epoxide 153. A nucleophile was added to the distal carbon of the epoxide 153 to give an azido-aldehyde, which was directly oxidized to the methyl ester 154 by basic iodine in methanol. The azido ester 154 was then reduced to the corresponding amine 155 by catalytic hydrogenation. This procedure proved effective in the racemic series for a variety of substrates, yet the analogous process with a chiral sulfoxide was limited to a few substrates.
Scheme 43.
5. 4-Oxaspiro[2.3]hexanes
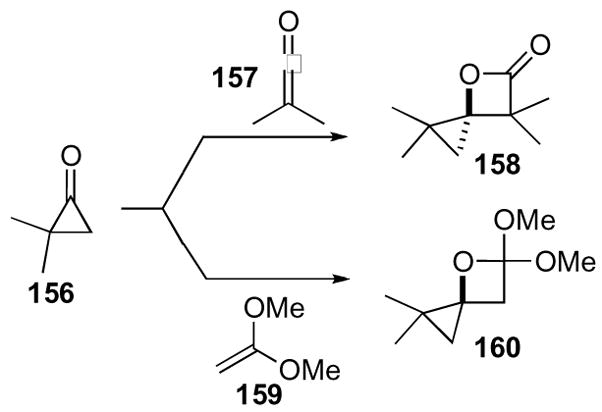
The application of 4-oxaspiro[2.3]hexanes to the synthesis of natural and unnatural products has yet to be reported, presumably due to the limited scope in their syntheses. These systems will therefore only be covered briefly. The first construction of this ring system was described by Turro in the late 1960’s (Scheme 44).77,78 While the cycloaddition between cyclopropanone 156 and ketene 157 proceeded to cyclopropane 158, a higher yield (90%) was obtained with the dimethyl acetal of ketene 159 to give orthoester 160. However, the lack of efficient syntheses of the requisite cyclopropanones hindered broad application.
Scheme 44.
One of the widely applied syntheses of 4-oxaspiro[2.3]hexanes is the decomposition of diazo-compounds in the presence of diketene 161 (Scheme 45).79 A variety of diazocompounds 162 bearing an electron withdrawing group in the form of a phosphonate or ester gave the 4-oxaspiro[2.3]hexanes 163. Although both light mediated and transition metal mediated decomposition of the diazocompounds gave similar results, the identity of the substituent appears to be crucial to the outcome of the reaction.
Scheme 45.

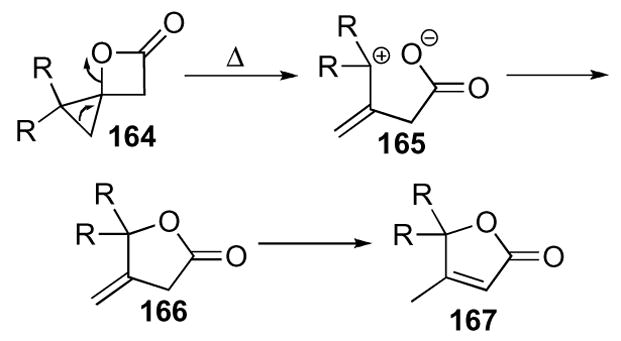
A major reaction pathway of this highly strained system is the thermal rearrangement to furanones and butenolides (Scheme 46). The reaction proceeds via cleavage of a cyclopropyl C-C bond and the C-O bond of the β-lactone to give zwitterionic intermediate 165. Ring closure to give lactone 166 then partially or completely isomerizes to butenolide 167, under the reaction conditions.
Scheme 46.
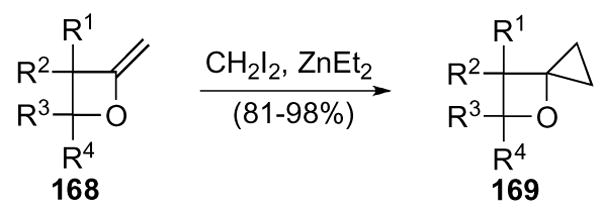
The cyclopropanation of 2-methylene oxetanes, which are available from the corresponding β-lactones by the method developed by Howell,80 is the most general synthesis of 4-diaoxaspiro[2.3]hexanes to date. 2-Methylene oxetanes 168, took part in a Simmons-Smith cyclopropanation to give 4-diaoxaspiro[2.3]hexanes 169 (Scheme 47).81 Structural variation was limited to substrates bearing substituents on the oxetane ring, yet cyclopropanation proceeded in good yields for a variety of substrates despite the limitations.
Scheme 47.
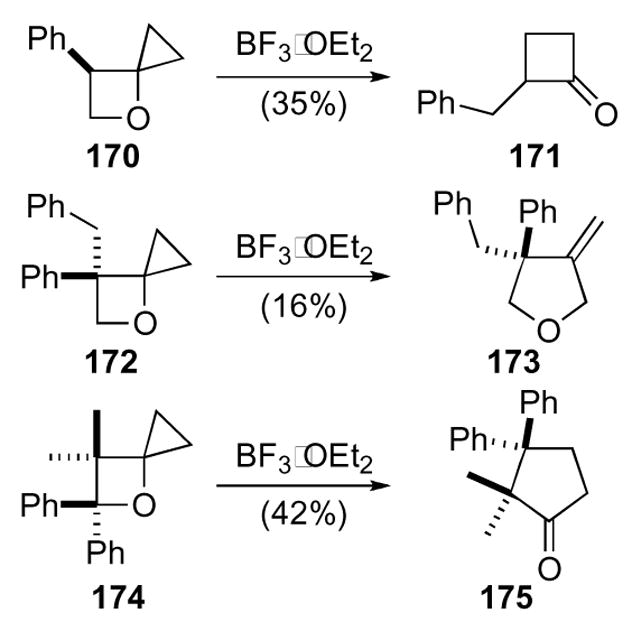
Attention was turned to the reactivity of the ring system since a more general synthesis was in hand (Scheme 48). The 4-oxaspiro[2.3]hexanes gave a variety of different structures upon exposure to Lewis acid. Unfortunately, the yields for these reactions were moderate thus limiting their potential in synthesis.
Scheme 48.
In addition to the above described procedures, 4-oxaspiro[2.3]hexanes have been observed in several other reactions. This ring system was isolated in low yields from the photochemical reaction between carbonyl compounds and olefins,82,83 and by the cyclopropanation of alkylidene oxetanes with dichlorocarbene.84 4-Oxaspiro[2.3]hexanes are intermediates in the synthesis of a polyspirane,85 but the most current example of their synthesis is an isolated report of the fragmentation of a benzylidene acetal.86
6. 1,4-Dioxaspiro[2.3]hexanes
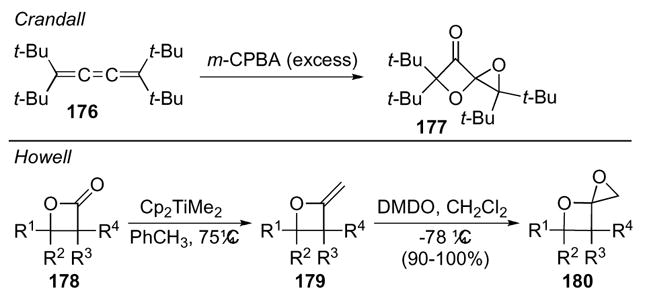
1,4-Dioxaspiro[2.3]hexanes were initially synthesized fortuitously in 1987.87 Crandall found that exposure of cumulene 176 to an excess of m-CPBA yielded several products, one of them being 1,4-dioxaspiro[2.3]hexane 177 (Scheme 49). Although this was not a synthetically practical preparation method given the starting material, a more synthetically viable route was only developed more than a decade later. Howell and co-workers showed that in the presence of DMDO methyleneoxetanes 179 yielded 1,4-dioxaspiro[2.3]hexanes 180 in good yields and modest to good diastereoselectivity.88 Additionally, methyleneoxetanes 179 are readily accessed from β-lactones 178.80 This particular method also tolerates a variety of functional groups, which should prove useful in the synthetic arena.
Scheme 49.
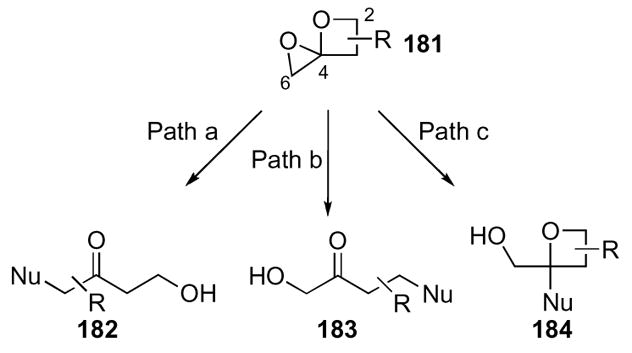
In contrast to the previously described heterocycles, 1,4-dioxaspiro[2.3]hexanes 181 display varied reactivity depending on the reaction conditions employed (Scheme 50). There are three possible modes of reactivity, and the most probable is path ‘a’ because nucleophilic attack occurs at the least hindered C6 completely unraveling the ring system with relief of all ring strain to yield α-substituted ketones 182. The next probable pathway ‘b’ permits attack at C2 to relieve ring strain to yield β-substituted ketones 183. Finally, the least predominant reaction pathway is ‘c’, in which the nucleophile adds to C4, gives substituted oxetanes 184 as products. Pathway ‘a’ seemed most likely due to the sterically unhindered attack of a nucleophile, and pathway ‘c’ was initially precluded due to the increased basicity of the oxetane oxygen over the epoxide oxygen that increases its lability.
Scheme 50.
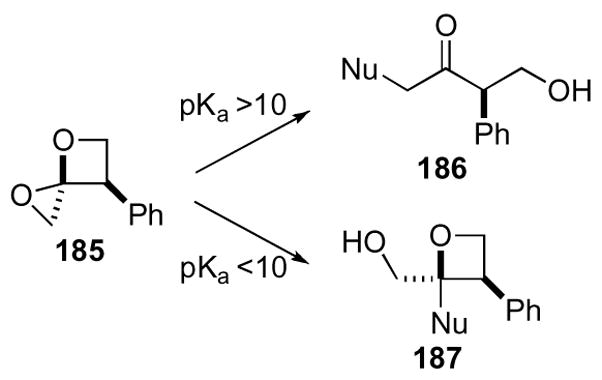
Howell and co-workers discovered interesting reactivities during their early investigations of this ring system.89,90 Initially, most nucleophiles added to the least hindered C6 position following reaction pathway ‘a’ to yield α-substituted ketones. These nucleophiles were quite diverse and included alcohols, acetate, and sodium thiophenoxide. Contrary to these results, when DIBAl-H, Me3Al, or TMSN3 were utilized as nucleophiles the major products observed were substituted oxetane products resulting from addition at C4. The Lewis acidic aluminum or silicon atom may have facilitated the ring opening of the epoxide, but later this was attributed to the pKa. Heterocyclic nucleophiles, with acidic protons that have a pKa > 10 gave α-substituted ketones as products 186, while those below a pKa of <10 yielded substituted oxetanes 187 (Scheme 51).
Scheme 51.
Computational analysis also revealed that with anionic nucleophiles there is a small preference for attack at C6.90 This mode of attack results in a highly exothermic (59.9 kcal/mol) unraveling of both rings to yield the observed product. Conversely, in acidic solution the product is driven by the stabilities of the generated oxocarbenium ions, since there is no partiality for protonation at the oxetane or the epoxide oxygen. Obviously, the preference is for opening of the epoxide ring with generation of an oxetane oxonium, which is more stable than the corresponding epoxide oxocarbenium by 18.8 kcal/mol.
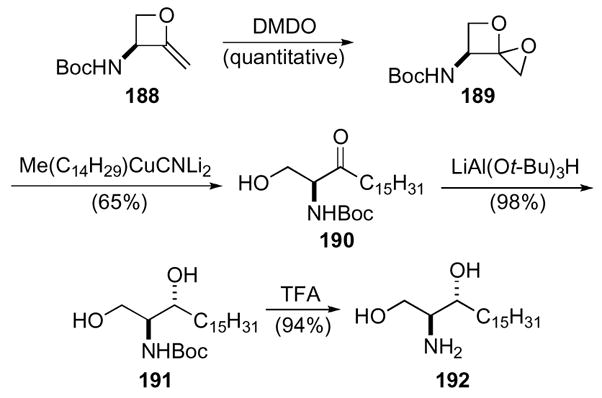
The 1,4-dioxaspiro[2.3]hexane ring system proved to be a versatile building block toward the cell signaling and immunomodulatory molecules dihydrosphingosines, sphingosines, and phytosphingosines.91 Scheme 52 demonstrates this versatility in the synthesis of D-erythro-sphinganine. Methylene oxetane 188, derived from serine, was first oxidized to the 1,4-dioxaspiro[2.3]hexane 189 by DMDO in quantitative yield. Addition of a higher order cuprate yielded ketone 190, which was then selectively reduced with lithium tri-t-butoxy aluminum hydride to give alcohol 191. Deprotection of the Boc-group with trifluoroacetic acid afforded D-erythro-sphinganine 192.
Scheme 52.
7. 1,4-Dioxaspiro[2.3]hexan-5-ones
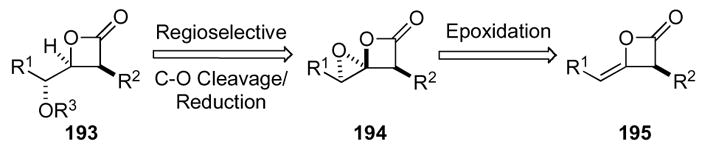
Exploiting the potential of β-lactones as synthetic intermediates and in particular their application to natural product total syntheses92 culminated in the first example of the novel 1,4-dioxaspiro[2.3]hexan-5-one ring system.93 One endeavor required direct access to an anti-cis-γ-hydroxy-β-lactone (e.g. β-lactone 193). However, existing diastereoselective methods for β-lactone synthesis from chiral α-oxy aldehydes provide only syn-cis or syn-trans selectivity.94 This led us to explore the synthesis and reactivity of 1,4-dioxaspiro[2.3]hexan-5-ones (e.g. 194) that were envisioned to be accessible via epoxidation of optically active ketene dimers.95 A subsequent, and likely required in situ regio- and facially selective C-O reductive bond cleavage, would allow access to the desired anti-cis-γ-hydroxy-β-lactone 193 (Scheme 53).
Scheme 53.
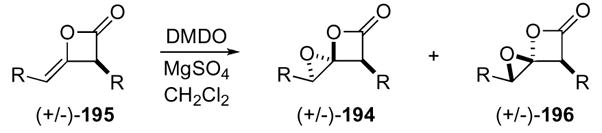
Oxidation of ketene dimers with DMDO39 successfully yielded 1,4-dioxaspiro[2.3]hexan-5-ones analogous with spiroepoxy oxetanes88 and spirocyclopropyl-β-lactones.79b The oxidation was applicable to a number of functionalized ketene dimers 195 to give several 1,4-dioxaspiro[2.3]hexan-5-ones 194 in modest to good yields (Table 1).
Table 1.
Epoxidation of Ketene Dimers Leading to 1,4-Dioxaspiro[2.3]hexan-5-ones 194/196
 | ||||
|---|---|---|---|---|
| entry | R | cmpd. | dr (194:196)b | % yieldc |
| 1 | n-Bu | 194a | 14:1 | 80 |
| 2 | CyCH2 | 194b | 10:1 | 76 |
| 3 | PhCH2 | 194c | 24:1 | 57 |
| 4 | (i-Pr3)SiO(CH2)4 | 194d | 17:1 | 40 |
| 5 | N3(CH2)4 | 194e | 16:1 | 61 |
Epoxidations were performed at ~0.1M concentration using isolated, purified ketene dimers 195a–e prepared by the method of Calter.95
Ratios determined by analysis of crude reaction mixtures by 1H NMR (500 MHz).
Refers to isolated, purified (SiO2) yields.
Four possible modes of reactivity with nucleophiles were envisioned upon consideration of the functionalities present in this ring system (Scheme 54). The first mode is addition to the distal epoxide C-O bond, pathway ‘a’, which would lead to a γ-substituted β-keto-carboxylate. Subsequent facile decarboxylation would yield α-substituted ketones 198 as the final product. The second mode, pathway ‘b’, involves addition to an oxocarbenium derived from cleavage of the epoxide ring at the spirocenter resulting in a γ-hydroxy-β-lactone 199, which was the initially desired reaction manifold to obtain the requisite β-lactone for haterumalide synthesis.96 Alternatively, a nucleophile could add to an oxocarbenium derived from cleavage of the β-lactone ring at the spirocenter instead of the epoxide (pathway ‘c’), which would result in the formation of an epoxy-acid 200. The final mode, pathway ‘d’, involves nucleophilic addition at the β-lactone carbonyl leading to acylation and cleavage of both rings to initially yield a γ-hydroxy-β-keto-acid 201.
Scheme 54.
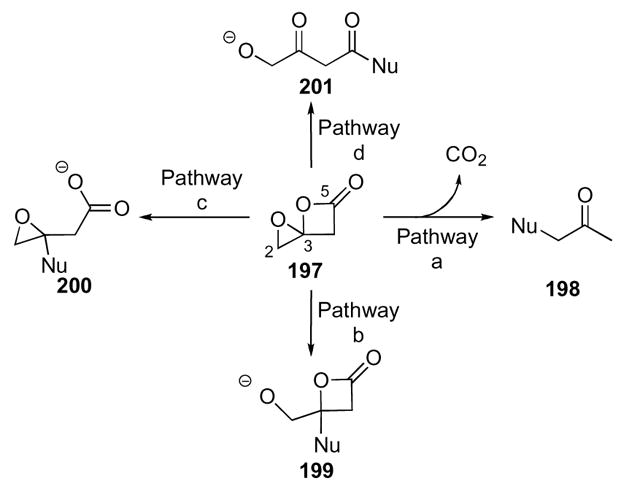
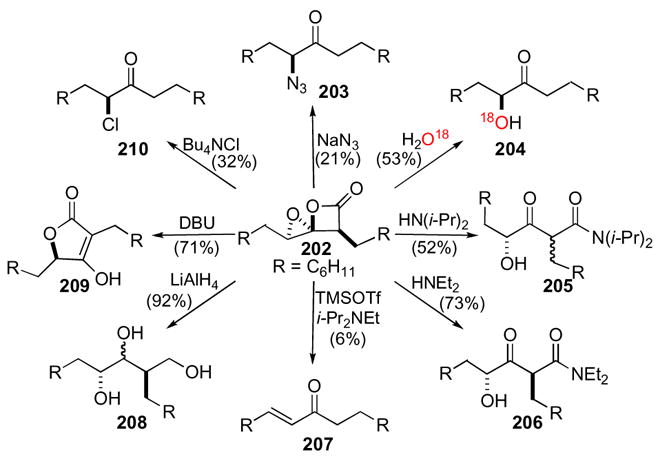
Though four possible modes of reactivity potentially exist for 1,4-dioxaspiro[2.3]hexan-5-ones, the predominant pathways are ‘a’ and ‘d’; whereas reactions proceeding through pathway ‘b’ or ‘c’ have not been observed to date. Nucleophilic addition by pathway ‘a’ was demonstrated by opening spiroepoxy-β-lactone 202 to α-azidoketone 203 and α-chloroketone 210, which were obtained upon exposure to tetrabutylammonium chloride and sodium azide, respectively (Scheme 55). Two different reaction manifolds were observed by reaction of spiroepoxy-β-lactone 202 with two different amines. Reaction with the less sterically hindered diethylamine resulted in a single diastereomer of a β-keto-amide 206 arising from simple nucleophilic addition through pathway ‘d’. However, when spiroepoxy-β-lactone 202 was exposed to the more sterically hindered amine diisopropylamine, it surprisingly resulted in essentially a 1:1 mixture of diastereomeric keto-amides 205. While this initial result was intriguing, our only viable initial hypothesis was that the reaction had to proceed through an intermediate in which the α-stereocenter was lost prior to or as a result of nucleophilic addition of the amine since A1,3 strain severely retards the epimerization of α-substituted β-ketoamides.97 In fact, exposure of a single diastereomer of diethyl amide 206 to deuterated diisopropylamine led to no deuterium incorporation, which is suggestive of no epimerization of that center over 4 h. The reaction of spiroepoxy-β-lactone 202 with water over prolonged periods of time led to α-hydroxy ketone 204. Mechanistically, there are two routes that could provide the same ketone product and in this instance, use of heavy water, H2O18, served to unequivocally infer the mechanism followed. The results indicate, not unexpectedly, that nucleophilic attack of water occurs exclusively at C2 leading to ketone 204 in analogy to several related systems described above. Spiroepoxy-β-lactone 202 also reacts with base in the presence of Lewis acid to provide a low yield of α,β-unsaturated ketone 207 (Scheme 55). Reduction of the spiroepoxy-β-lactone 202 with lithium aluminum hydride gave the corresponding triol 208 in excellent yield, which is a result of initial addition to the carbonyl carbon, (pathway ‘d’). No deoxygenation is observed at C3 or C5, which is also consistent with reduction of β-lactones with metal hydrides leading to acyl C-O versus C-O alkyl cleavage. Also, the product obtained from the reduction of the β-lactone carbonyl suggests existence of an α-hydroxy ketone that is then unselectively reduced to the observed triol 208. Interestingly, when spiroepoxy-β-lactone 202 was exposed to the tertiary base, DBU, the tetronic acid derivative 209 was obtained as the sole product.
Scheme 55.
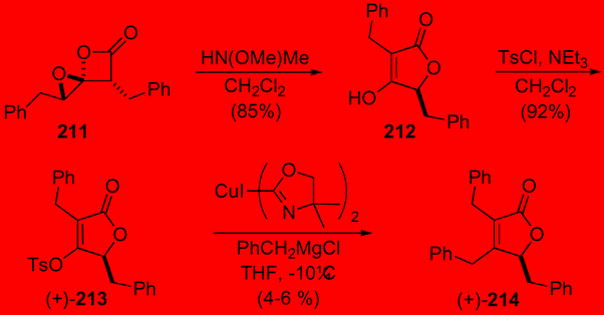
The utility of 1,4-dioxaspiro[2.3]hexan-5-ones was demonstrated by an enantioselective total synthesis of maculalactone A 214 (Scheme 56). Maculalactone A belongs to a family of γ-lactone containing natural products isolated from the marine cyanobacterium Kyrtuthrix maculans, where it inhibits growth of marine bivalves thus possessing potential use as an anti-fouling agent.98 1,4-dioxaspiro[2.3]hexan-5-one 211 was prepared from the optically active ketene dimer99 with subsequent epoxidation with DMDO.93 Rearrangement to the tetronic acid 212 (>95% ee as determined by 19F NMR of derived Mosher ester) with N-methyl-N-methoxy amine followed by tosylation afforded the tetronate 213 readied for coupling in good yield. The synthesis was completed following a challenging coupling to introduce the final benzyl group in this natural product. The only conditions found involved the use of a benzyl cuprate reagent bearing an oxazoline ligand providing (+)-maculalactone A (214) in only poor yield.100
Scheme 56.
8. Conclusions
Recent advances in the synthesis of small heterocyclic rings including asymmetric routes have enabled access to several novel spiroheterocycles that serve as useful chiral synthons in organic synthesis including natural and unnatural product targets. The synthesis of these ring systems also provided access to molecules of theoretical interest for unprecedented studies of ring strain, anomeric effects, and reactivity patterns. These novel ring systems undergo various rearrangements with high stereoselectivity and thus provide efficient chirality transfer, while more traditional methods towards similar products are often less stereoselective. The combination of novel reactivity and high stereochemical control provide certainty that there will be continued interest in development of innovative transformations and continued exploitation in synthetic endeavors of spiroheterocycles.
Figure 1.
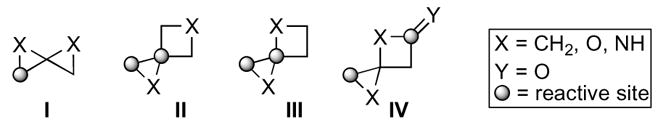
Structures of known small, spirocyclic ring systems.
Acknowledgments
We gratefully acknowledge NSF (CHE-0416260), NIH (GM069874), and the Welch Foundation (A-1280) for support of our work in the area of β-lactones and spiroepoxy-β-lactones. K.A. Morris gratefully acknowledges an American Chemical Society Division of Organic Chemistry Graduate Fellowship sponsored by Eli Lilly.
Abbreviations
- acac
acetylacetonate
- Bz
benzoyl
- cod
1,5-cyclooctadiene
- DBU
1,8-diazabicyclo[5.4.0]undec-7-ene
- DET
diethyl tartrate
- DMDO
dimethyl dioxirane
- DMSO
dimethylsulfoxide
- EtOAc
ethyl acetate
- EWG
electron-withdrawing group
- HMPA
hexamethylphosphoric acid triamide
- LDA
lithium diisopropylamide
- M.S
molecular sieves
- m-CPBA
meta chloroperbenzoic acid
- Nu
nucleophile
- PMB
p-methoxybenzyl
- r.t
room temperature
- TBHP
tert-butyl hydroperoxide
- TBDPS
t-butyldiphenylsilyl
- TBS
t-butyldimethylsilyl
- TBAN3
tetra-n-butylammonium azide
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- T.M
transition metal
- TMS
trimethylsilyl
- Ts
tosyl or 4-toluenesulfonyl
- TsOH
p-toluenesulfonic acid
Biographies

Richard J. Duffy was born in Wilmington, Delaware. He received a B.S. degree in Chemistry and B.S. degree in Botany in 2001 from the University of Oklahoma. In December of 2007, he received his Ph.D. degree in Chemistry under Professor Daniel Romo at Texas A&M University. His doctoral research focused on the synthesis and application of spiroepoxy-β-lactones to natural product synthesis and trans-selective cross couplings of 1,1-dichloroolefins. He began his postdoctoral studies with Professor Michael P. Doyle at the University of Maryland in November of 2007.

Kay A. Morris was born in Oklahoma City, Oklahoma. She received her B.S. degree in Chemistry in 2004 from Cameron University. In August 2004, she began her Ph.D. studies in the Romo laboratories at Texas A&M University and she is currently an ACS Graduate Fellow (Division of Organic Chemistry) sponsored by Eli Lilly. Her current research focuses on the development of a double asymmetric, nucleophile catalyzed aldol-lactonization process with applications to bicyclic tetrahydrofurans and bridged carbocycle systems.

Daniel Romo received his B.A. in chemistry/biology from Texas A&M and a Ph.D. in Chemistry from Colorado State University as a NSF Minority Graduate Fellow under the tutelage of the late Prof. Albert I. Meyers. Following postdoctoral studies at Harvard as an American Cancer Society Fellow, with Prof. Stuart L. Schreiber he began his independent career at Texas A&M in 1993 and is currently Professor of Chemistry. Research interests in the Romo Group are at the interface of chemistry and biology focused on total synthesis and biomechanistic studies of natural products and the asymmetric synthesis and application of β-lactones in organic synthesis and as potential drug candidates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.For selected recent reviews, see: Rubin M, Rubina M, Gevorgyan V. Chem Rev. 2007;107:3117. doi: 10.1021/cr050988l.Zimmer R. Chem Rev. 2003;103:1151. doi: 10.1021/cr010016n.De Meijere A, Kozhushkov SI. Chem Rev. 2000;100:93. doi: 10.1021/cr960153y.Marek I, Simaan S, Masarwa A. Angew Chem Int Ed. 2007;46:7364. doi: 10.1002/anie.200604774.Lee-Ruff E, Madenova G. Chem Rev. 2003;103:1449. doi: 10.1021/cr010013a.Namslo JC, Kaufmann DE. Chem Rev. 2003;103:1485. doi: 10.1021/cr010010y.Xia QH, Ge HQ, Ye C-P, Liu ZM, Su KX. Chem Rev. 2005;105:1603. doi: 10.1021/cr0406458.Yang HW, Romo D. Tetrahedron. 1999;55:6403.Dejaegher Y, Kuz’menck NM, Zvonok AM, De Kimpe N. Chem Rev. 2002;102:29. doi: 10.1021/cr990134z.Hu XE. Tetrahedron. 2004;60:2701.Singh GS, D’hooghe M, De Kimpe N. Chem Rev. 2007;107:2080. doi: 10.1021/cr0680033.Singh GS. Tetrahedron. 2003;59:7613.Alcaide B, Almendros P, Aragoncillo C. Chem Rev. 2007;107:4437. doi: 10.1021/cr0307300.
- 2.The nomenclature used for the ring systems discussed in this report is in accordance with IUPAC rules.
- 3.Crandall JK, Paulson DR. J Org Chem. 1968;33:991. [Google Scholar]
- 4.Bernard AM, Floris C, Frongia A, Piras PP. Tetrahedron. 2000;56:4555. [Google Scholar]
- 5.Hiyama T, Takehara S, Kitatani K, Nozaki H. Tetrahedron Lett. 1974;15:3295. [Google Scholar]
- 6.Wiseman JR, Chan HF. J Am Chem Soc. 1970;92:4749. [Google Scholar]
- 7.Erden I, De Meijere A, Rousseau G, Conia JM. Tetrahedron Lett. 1980;21:2501. [Google Scholar]
- 8.De Meijere A, Erden I, Weber W, Kaufmann D. J Org Chem. 1988;53:152. [Google Scholar]
- 9.Krief A, Dumont W, Laboureur JL. Tetrahedron Lett. 1988;29:3265. [Google Scholar]
- 10.Trost BM, LaRochelle R, Bogdanowicz MJ. Tetrahedron Lett. 1970;11:3449. [Google Scholar]
- 11.Trost BM, Bogdanowicz MJ. J Am Chem Soc. 1971;93:3773. [Google Scholar]
- 12.Trost BM. Top Curr Chem. 1986;133:3. [Google Scholar]
- 13.Trost BM, Bogdanowicz MJ. J Am Chem Soc. 1973;95:5311. [Google Scholar]
- 14.Johnson CR, Katekar GF, Huxol RF, Janiga ER. J Am Chem Soc. 1971;93:3771. [Google Scholar]
- 15.Pell AS, Pilcher G. Trans Faraday Soc. 1965;61:71. [Google Scholar]
- 16.Trost BM, Kurozumi S. Tetrahedron Lett. 1974;15:1929. [Google Scholar]
- 17.Trost BM, Bogdanowicz MJ. J Am Chem Soc. 1973;95:289. [Google Scholar]
- 18.Hsiao CN, Hannick SM. Tetrahedron Lett. 1990;31:6609. [Google Scholar]
- 19.Ichikawa Y, Narita A, Shiozawa A, Hayashi Y, Narasaka K. J Chem Soc, Chem Comm. 1989:1919. [Google Scholar]
- 20.Nemoto H, Ishibashi H, Nagamochi M, Fukumoto K. J Org Chem. 1992;57:1707. [Google Scholar]
- 21.Gadwood RC. J Org Chem. 1983;48:2098. [Google Scholar]
- 22.Kwon O, Su DS, Meng D, Deng W, D’Amico DC, Danishefsky SJ. Angew Chem Int Ed. 1998;37:1877. [Google Scholar]
- 23.Kwon O, Su DS, Meng D, Deng W, D’Amico DC, Danishefsky SJ. Angew Chem Int Ed. 1998;37:1880. [Google Scholar]
- 24.Bernard AM, Frongia A, Piras PP, Secci F. Org Lett. 2003;5:2923. doi: 10.1021/ol035061r. [DOI] [PubMed] [Google Scholar]
- 25.Trost BM, Bogdanowicz MJ. J Am Chem Soc. 1973;95:5321. [Google Scholar]
- 26.Saniere M, Charvet I, Le Merrer Y, Depezay JC. Tetrahedron. 1995;51:1653. [Google Scholar]
- 27.Baylon C, Prestat G, Heck MP, Mioskowski C. Tetrahedron Lett. 2000;41:3833. [Google Scholar]
- 28.Chattopadhyay S, Mamdapur VR, Chadha MS. Synth Commun. 1990;20:1299. [Google Scholar]
- 29.Nemoto H, Yoshida M, Fukumoto K, Ihara M. Tetrahedron Lett. 1999;40:907. [Google Scholar]
- 30.Yoshida M, Ismail MAH, Nemoto H, Ihara M. J Chem Soc, Perkin Trans. 2000;1:2629. [Google Scholar]
- 31.Cheney DL, Paquette LA. J Org Chem. 1989;54:3334. [Google Scholar]
- 32.Matsuda T, Fujimoto A, Ishibashi M, Murakami M. Chem Lett. 2004;33:876. [Google Scholar]
- 33.Sivik MR, Bauer W, Schleyer PvR, Paquette LA. Organometallics. 1996;15:5202. [Google Scholar]
- 34.Crandall JK, Machleder WH. Tetrahedron Lett. 1966;7:6037. [Google Scholar]
- 35.Crandall JK, Machleder WH. J Am Chem Soc. 1968;90:7292. [Google Scholar]
- 36.Crandall JK, Machleder WH, Thomas MJ. J Am Chem Soc. 1968;90:7346. [Google Scholar]
- 37.Crandall JK, Batal DJ, Sebesta DP, Lin F. J Org Chem. 1991;56:1153. [Google Scholar]
- 38.Crandall JK, Batal DJ. J Org Chem. 1988;53:1338. [Google Scholar]
- 39.Murray RW, Jeyaraman R. J Org Chem. 1985;50:2847. [Google Scholar]
- 40.Crandall JK, Batal DJ. Tetrahedron Lett. 1988;29:4791. [Google Scholar]
- 41.Crandall JK, Batal DJ, Lin F, Reix T, Nadol GS, Ng RA. Tetrahedron. 1992;48:1427. [Google Scholar]
- 42.Crandall JK, Rambo E. J Org Chem. 1990;55:5929. [Google Scholar]
- 43.Crandall JK, Rambo E. Tetrahedron. 2002;58:7027. [Google Scholar]
- 44.Murray RW, Jeyaraman R. J Org Chem. 1985;50:2847. [Google Scholar]
- 45.Crandall JK, Rambo E. Tetrahedron Lett. 1994;35:1489. [Google Scholar]
- 46.Andrews DR, Giusto RA, Sudhakar AR. Tetrahedron Lett. 1996;37:3417. [Google Scholar]
- 47.Charney W, Herzog HL. Microbial Transformations of Steroids: A Handbook. 1967:728. [Google Scholar]
- 48.Katukojvala S, Barlett KN, Lotesta SD, Williams LJ. J Am Chem Soc. 2004;126:15348. doi: 10.1021/ja044563c. [DOI] [PubMed] [Google Scholar]
- 49.Ghosh P, Lotesta SD, Williams LJ. J Am Chem Soc. 2007;129:2438. doi: 10.1021/ja068813w. [DOI] [PubMed] [Google Scholar]
- 50.Mitani M, Matsumoto H, Gouda N, Koyama K. J Am Chem Soc. 1990;112:1286. [Google Scholar]
- 51.Peng ZH, Woerpel KA. J Am Chem Soc. 2003;125:6018. doi: 10.1021/ja034865z. [DOI] [PubMed] [Google Scholar]
- 52.Cichewicz RH, Valeriote FA, Crews P. Org Lett. 2004;6:1951. doi: 10.1021/ol049503q. [DOI] [PubMed] [Google Scholar]
- 53.Shangguan N, Kiren S, Williams LJ. Org Lett. 2007;9:1093. doi: 10.1021/ol063143k. [DOI] [PubMed] [Google Scholar]
- 54.Lotesta SD, Hou Y, Williams LJ. Org Lett. 2007;9:869. doi: 10.1021/ol063087n. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Shangguan N, Cussick JR, Williams LJ. Synlett. 2008:213. [Google Scholar]
- 56.Lotesta SD, Kiren S, Sauers RR, Williams LJ. Angew Chem Int Ed. 2007;46:7108. doi: 10.1002/anie.200701401. [DOI] [PubMed] [Google Scholar]
- 57.Halazy S, Krief A. Tetrahedron Lett. 1980;21:1997. [Google Scholar]
- 58.Halazy S, Krief A. J Chem Soc, Chem Commun. 1982:1200. [Google Scholar]
- 59.Leriverend ML, Leriverend P. C R Acad Sci Ser C. 1975;280:791. [Google Scholar]
- 60.Leriverend ML, Vazeux M. J Chem Soc, Chem Commun. 1982:866. [Google Scholar]
- 61.Razin VV, Ulin NV. Russ J Org Chem. 2003;39:33. [Google Scholar]
- 62.Pirrung MC, Thomson SA. J Org Chem. 1988;53:227. [Google Scholar]
- 63.Shen YM, Wang B, Shi Y. Angew Chem Int Ed. 2006;45:1429. doi: 10.1002/anie.200501520. [DOI] [PubMed] [Google Scholar]
- 64.Shen YM, Wang B, Shi Y. Tetrahedron Lett. 2006;47:5455. [Google Scholar]
- 65.Leriverend ML. C R Acad Sci Ser C. 1974;279:755. [Google Scholar]
- 66.Miyano M. J Org Chem. 1981;46:1846. [Google Scholar]
- 67.Huang M. J Am Chem Soc. 1946;68:2487. [Google Scholar]
- 68.Tobe Y, Yamashita T, Kakiuchi K, Odaira Y. J Chem Soc, Chem Commun. 1985:898. [Google Scholar]
- 69.Tobe Y, Sato J, Sorori T, Kakiuchi K, Odaira Y. Tetrahedron Lett. 1986;27:2905. [Google Scholar]
- 70.Tobe Y, Yamashita D, Takahashi T, Inata M, Sato J, Kakiuchi K, Kobiro K, Odaira Y. J Am Chem Soc. 1990;112:775. [Google Scholar]
- 71.Pirrung MC, Thomson SA. J Org Chem. 1988;53:227. [Google Scholar]
- 72.Mahuteau-Betzer F, Ghosez L. Tetrahedron Lett. 1999;40:5183. [Google Scholar]
- 73.Mahuteau-Betzer F, Ghosez L. Tetrahedron. 2002;58:6991. [Google Scholar]
- 74.Brown B, Hegedus LS. J Org Chem. 2000;65:1865. doi: 10.1021/jo9919759. [DOI] [PubMed] [Google Scholar]
- 75.Satoh T, Hirano M, Kuroiwa A. Tetrahedron Lett. 2005;46:2659. [Google Scholar]
- 76.Satoh T, Hirano M, Kuroiwa A, Kaneko Y. Tetrahedron. 2006;62:9268. [Google Scholar]
- 77.Turro NJ, Williams JR. Tetrahedron Lett. 1969;10:321. [Google Scholar]
- 78.Turro NJ, Edelson SS, Williams JR, Darling TR, Hammond WB. J Am Chem Soc. 1969;91:2283. [Google Scholar]
- 79.(a) Kato T, Katagiri N, Sato R. J Org Chem. 1980;45:2587. [Google Scholar]; (b) Geraghty NWA, Murphy PA. Tetrahedron Lett. 1994;35:6737. [Google Scholar]; (c) Murphy PV, O’Sullivan TJ, Geraghty NWA. J Chem Soc, Perkin Trans. 2000;1:2109. [Google Scholar]; (d) Kato T, Katagiri N, Sato R. Chem Pharm Bull. 1981;29:2361. [Google Scholar]
- 80.Dollinger LM, Howell AR. J Org Chem. 1996;61:7248. doi: 10.1021/jo9611733. [DOI] [PubMed] [Google Scholar]
- 81.Bekolo H, Howell AR. New J Chem. 2001;25:673. [Google Scholar]
- 82.Shimizu N, Ishikawa M, Ishikura K, Nishida S. J Am Chem Soc. 1974;96:6456. [Google Scholar]
- 83.Funke CW, Cerfontain H. J Chem Soc, Perkin Trans. 1976;2:1902. [Google Scholar]
- 84.Marschall H, Muehlenkamp WB. Chem Ber. 1976;109:2785. [Google Scholar]
- 85.Fitjer L, Rissom B, Kanschik A, Egert E. Tetrahedron. 1994;50:10879. [Google Scholar]
- 86.O’Neil KE, Kingree SV, Minbiole KPC. Org Lett. 2005;7:515. doi: 10.1021/ol047426t. [DOI] [PubMed] [Google Scholar]
- 87.Crandall JK, Salazar GE, Watkins RJ. J Am Chem Soc. 1987;109:4338. [Google Scholar]
- 88.Ndakala AJ, Howell AR. J Org Chem. 1998;63:6098. doi: 10.1021/jo981309s. [DOI] [PubMed] [Google Scholar]
- 89.Howell AR, Ndakala AJ. Org Lett. 1999;1:825. [Google Scholar]
- 90.Taboada R, Ordonio GG, Ndakala AJ, Howell AR, Rablen PR. J Org Chem. 2003;68:1480. doi: 10.1021/jo0206465. [DOI] [PubMed] [Google Scholar]
- 91.(a) Ndakala AJ, Hashemzadeh M, So RC, Howell AR. Org Lett. 2002;4:1719. doi: 10.1021/ol0200448. [DOI] [PubMed] [Google Scholar]; (b) So RC, Ndonye R, Izmirian DP, Richardson SK, Guerrera RL, Howell AR. J Org Chem. 2004;69:3233. doi: 10.1021/jo030355b. [DOI] [PubMed] [Google Scholar]; (c) Howell AR, So RC, Richardson SK. Tetrahedron. 2004;60:11327. [Google Scholar]
- 92.Wang Y, Tennyson RL, Romo D. Heterocycles. 2004;64:605. [Google Scholar]
- 93.Duffy RJ, Morris KA, Romo D. J Am Chem Soc. 2005;127:16754. doi: 10.1021/ja053478h. [DOI] [PubMed] [Google Scholar]
- 94.Known, direct methods for β-lactone synthesis from chiral α-oxy aldehydes are either unsuccessful or provide only syn selectivity (see ref. 1h).
- 95.Calter MA, Orr RK, Song W. Org Lett. 2003;5:4745. doi: 10.1021/ol0359517. and references cited within. [DOI] [PubMed] [Google Scholar]
- 96.(a) Takada N, Sato H, Suenaga K, Arimoto H, Yamada K, Ueda K, Uemura D. Tetrahedron Lett. 1999;40:6309. [Google Scholar]; (b) Kigoshi H, Hayakawa I. Chem Rec. 2007;7:254. doi: 10.1002/tcr.20119. [DOI] [PubMed] [Google Scholar]
- 97.Evans DA, Ennis MD, Le T, Mandel N, Mandel G. J Am Chem Soc. 1984;106:1154. [Google Scholar]
- 98.(a) Lee SC, Williams GA, Brown GD. J Nat Prod. 1998;61:29. doi: 10.1021/np970322p. [DOI] [PubMed] [Google Scholar]; (b) Lee SC, Williams GA, Brown GD. Phytochemistry. 1999;52:537. [Google Scholar]; (c) Wong HF, Williams GA, Brown GD. Phytochemistry. 2002;60:425. doi: 10.1016/s0031-9422(02)00138-3. [DOI] [PubMed] [Google Scholar]; (d) Brown GD, Wong HF, Hutchinson N, Lee SC, Chan BKK, Williams GA. Phytochem Rev. 2005;3:381. [Google Scholar]
- 99.(a) Calter MA, Guo X, Liao W. Org Lett. 2001;3:1499. doi: 10.1021/ol015814e. [DOI] [PubMed] [Google Scholar]; (b) Purohit VC, Richardson RD, Smith JW, Romo D. J Org Chem. 2006;71:4549. doi: 10.1021/jo060392d. [DOI] [PubMed] [Google Scholar]
- 100.Duffy RJ, Morris KA, Vallakati R, Zhang W, Romo D. doi: 10.1021/jo900499e. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]


