Abstract
Nicotinic acetylcholine receptors (nAchR) are key receptors in the autonomic nervous system, but also are present on immune cells. The alpha seven subunit of nAchR (α7nAchR) suppresses pro-inflammation in peripheral monocytes by decreasing proinflammatory cytokine production. In spinal cord, α7nAchR are found on microglia, which are known to induce and maintain pain. We predicted that α7nAchR agonists might attenuate intrathecal HIV-1 gp120-induced, proinflammatory cytokine- and microglia-dependent mechanical allodynia. Choline, a precursor for acetylcholine and selective agonist for α7nAchR, was administered intrathecally either with, or 30 min after, intrathecal gp120. Choline significantly blocked and reversed gp120 induced mechanical allodynia for at least 4 hr after drug administration. In addition, intrathecal choline, delivered either with or 30 min after gp120, reduced gp120-induced IL-1β protein and pro-inflammatory cytokine mRNAs within the lumbar spinal cord. A second α7nAchR agonist, GTS-21, also significantly reversed gp120-induced mechanical allodynia and lumbar spinal cord levels of proinflammatory cytokine mRNAs and IL-1β protein. A role of microglia is suggested by the observation that intrathecal choline suppressed the gp120-induced expression of, cd11b, a macrophage/microglial activation marker. Taken together, the data support that α7nAchR may be a novel target for treating pain where microglia maintain the proinflammatory state within the spinal cord.
Keywords: pain, choline, intrathecal, rats, tumor necrosis factor
1. Introduction
Nicotinic acetylcholine receptors (nAchR) are ligand-gated ion channels with 16 known subunits (α1–7, 9 and 10, β1–4, δ, ε and γ) (Conejero-Goldberg et al., 2008). Nicotinic receptors are stimulated by acetylcholine and nicotine and are key receptors in neuromuscular junctions and in the peripheral and central nervous systems (Wang et al., 2003). In addition to neural tissue, nAchR are found on peripheral monocytes, in particular the α7nAchR, which when activated suppress the release of the proinflammatory cytokines, tumor necrosis factor alpha (TNFα), interleukin-1β (IL-1β) (Rosas-Ballina et al., 2009) and interleukin-6 (IL-6) (Waldburger et al., 2008), at least in part via an inhibition of NFκB (Borovikova et al., 2000a; Borovikova et al., 2000b; Yoshikawa et al., 2006). A growing body of literature documents that activation of α7nAchR on peripheral immune cells suppresses peripheral inflammation, a phenomenon integral to what has come to be referred to as the cholinergic anti-inflammatory pathway (Borovikova et al., 2000b).
The present series of studies explores the potential relevance of α7nAchR in pain control. Activation of glial cells (microglia and astrocytes) and the consequent release of glial proinflammatory cytokines are now recognized as being fundamentally important in the induction and maintenance of pathological pain states in animal models (Fields, 2009; Watkins et al., 2007). As pathological pain in humans remains largely unresolved by currently available therapeutics, novel targets for pain control need to be explored. The clinical potential of targeting activated glia and their proinflammatory products is becoming increasingly recognized but is still in its infancy regarding the identification of optimal drug targets for controlling glially-dependent pain. Intriguingly, while microglia, the resident macrophages of the central nervous system are known to express α7nAchR (Shytle et al., 2004), the potential of α7nAchR agonists for controlling glially dependent pain has not previously been explored. Given the known anti-inflammatory effects of α7nAchR agonists on peripheral macrophages noted above, α7nAchR agonists may exert similar effects on spinal cord microglia. Were this to prove true, it would predict that α7nAchR might powerfully suppress glially dependent pain via suppression of glially derived proinflammatory cytokines implicated in pain enhancement.
Therefore, the aim of the present study was to explore whether intrathecally-administered α7nAchR agonists may be able to attenuate glially dependent pain enhancement and suppress proinflammatory cytokine induction associated with enhanced pain. Intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein gp120 was used in these studies as it is well documented to induce pain enhancement (mechanical allodynia) that is dependent upon the activation of microglia (Milligan et al., 2000), the activation of NFκB (Ledeboer et al., 2005a), and the induction and release of proinflammatory cytokines (both TNFα and IL1β, protein and mRNA) (Holguin et al., 2004; Ledeboer et al., 2005b; Milligan et al., 2001), features predicted to be appropriate for analyzing the known effects of α7nAchR agonists on peripheral monocytes/macrophages, as noted above. In vivo studies were performed including behavioral, mRNA, and protein assessments to explore the potential of α7nAchR agonists for pain control.
2. Material and methods
2.1 Animals
Pathogen-free adult male Sprague-Dawley rats (300–350g, Harlan Laboratories, Madison, WI) were used for all experiments. Rats were housed two per cage in a temperature (23±0.3°C) and light (12:12 light:dark cycle; lights on at 07:00) controlled environment. Rats had free access to tap water and standard rat chow. All behavioral testing was conducted within lights on. All animals were allowed one week of acclimation to the colony rooms before experimentation. The Institutional Animal Care and Use Committee of the University of Colorado at Boulder approved all procedures.
2.2 Drugs
Sterile aliquots of endotoxin-free recombinant HIV gp120 were stored at −80°C (1 µg/µl; product #1021; Immunodiagnostics, Bedford, MA). At the time of testing, the gp120 aliquots were slowly thawed and diluted to a concentration of 0.5 µg/µl in 0.1% rat serum albumin (RSA; Accurate Chemical and Scientific Corporation, Westbury, NY). The rat serum was diluted in sterile, endotoxin-free Dulbecco’s phosphate buffered saline (DPBS; Invitrogen, Grand Island, NY). Choline (St Louis, MO, USA) and GTS-21 (gifted by Drs. Kevin Tracey & Yusef Al-Abed) were reconstituted in sterile endotoxin-free isotonic saline (Abbot Laboratories, North Chicago, IL, USA) to a concentration of 10 mM. Aliquots were stored at −20°C. Fresh aliquots were diluted to the appropriate concentration in sterile endotoxin-free isotonic saline. GTS-21 is a partial agonist of α7nAchR, but also binds to α4β2-nAchRs but with very low affinity, resulting in predominantly α7nAchR activity (Briggs et al., 1997; Meyer et al., 1997). Choline is a constituent of acetylcholine and selective for α7nAchR (Alkondon et al., 1997).
2.3 Indwelling intrathecal catheter placement
Each rat was anesthetized with isoflurane (Phoenix Pharmaceuticals, St. Joseph, MO, USA) and its dorsal lumbar (L) region shaved and cleaned. An 18-gauge guide needle, with the hub removed, was percutaneously inserted into the L5/6 intervertebral space. A PE-10 catheter, filled with endotoxin-free physiological saline, was inserted into the guide needle and threaded rostrally until the proximal end of the PE-10 tubing rested over the L4-L6 spinal cord. The exterior length of catheter was then secured to the lumbosacral fascia and underlying muscles and the catheter tip was subcutaneously threaded rostrally where it was externalized through an incision in the nape of the neck. Animals were allowed at least five days recovery before drug administration.
2.4 von Frey testing for mechanical allodynia
The von Frey test was performed on the plantar surface of each hind paw within the region of sciatic nerve innervation, as described previously (Milligan et al., 2000). Before testing, rats are habituated to the testing environment for 4 days, 40 min per day. Rats are placed on wire racks, under clear containers large enough to allow turning and a small amount of walking, elevated above the tester’s eye. The rats are allowed 30 min on the racks before testing is begun. A logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (407 mg - 15.136 g, Stoelting, Wood Dale, IL, USA) was applied randomly to the left and right hind paws, each for 8 s at constant pressure. Each rat is tested three times with the middle filament (2 g). If there are two or three responses the tester drops to the lowest hair (407 mg) and tests incrementally upwards from there until three consecutive positive responses are obtained. If less than two responses are obtained with the 2 g filament, then the tester tests incrementally upwards until three consecutive positive responses are identified. The stimulus intensity threshold is determined by three consecutive responses of the same intensity of filament. The behavioral testing was performed blind with respect to the drug administration. The stimulus intensity threshold to elicit a paw withdrawal response was used to calculate the 50% paw withdrawal threshold (absolute threshold) using the maximum likelihood fit method to fit a Gaussian integral psychometric function (Harvey, 1986) and is described as allodynia or mechanical sensitivity throughout the text. This method normalizes the withdrawal threshold to parametric conditions. For all groups in each experiment, there was no significant difference between the left and the right hind paw values and thus were averaged for each rat.
2.5 Spinal cord tissue collection
The animals were deeply anesthetized with an intraperitoneal injection of sodium pentobarbital, and then transcardially perfused with ice-cold saline for 2 min. The left and right L4-L6 dorsal spinal cord were isolated and the left (for mRNA analysis) and right (for protein analysis) separately flash frozen in liquid nitrogen and stored at −80°C until further analysis.
2.7 RNA isolation and cDNA synthesis
RNA from the lumbar spinal cord was extracted using the standard phenol:chloroform extraction with TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s guidelines. Samples were treated with DNase to remove any contaminating DNA (Ambion, Austin, TX). Total RNA was reverse transcribed into cDNA using Superscript II First-Strand Synthesis System (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized using total RNA, random hexamer primer (5 ng/µl), 1 mM dNTP mix, cDNA synthesis buffer (Invitrogen, Carlsbad, CA) and incubated at 65°C for 5 min. Following 2 min incubation on ice, a cDNA synthesis buffer (1 X RT buffer, Invitrogen, Carlsbad, CA) and dithiothreitol (10 mM) was added and incubated at 25°C for 2 min. Reverse transcriptase (Superscript II, 200 Units, Invitrogen, Carlsbad, CA) was added to a total volume of 20 µl and incubated for 10 min at 25°C, 50 min at 42°C and deactivating the enzyme at 70°C for 15 min. cDNA was diluted 2-fold in nuclease-free water and stored at −80°C. All cDNA was stored at −80°C until PCR was performed.
2.8 Real-time polymerase chain reaction (PCR)
Primer sequences were obtained from the Genbank at the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov) and displayed in Table 1. Amplification of the cDNA was performed using Quantitect SYBR Green PCR kit (Qiagen, Valenica, CA) in iCycler iQ 96-well PCR plates (Bio-Rad, Hercules, CA) on a MyiQ single Color Real-Time PCR Detection System (Bio-Rad). The reaction mixture (26 µl) was composed of QuantiTect SYBR Green (containing fluorescent dye SYBR Green I, 2.5 mM MgCl2, dNTP mix and Hotstart Taq Polymerase), 10 nM fluorescein, 500 nM of each forward and reverse primer (Invitrogen, Carslbad, CA), nuclease-free water and 1 ul of cDNA from each sample. Each sample was measured in duplicate. The reactions were initiated with a hotstart at 95°C for 25 min, followed by 40 cycles of 15 s at 94°C (denaturation), 30 s at 55–60°C (annealing) and 30 s at 72°C (extension). Melt curve analyses were conducted to assess uniformity of product formation, primer-dimer formation and amplification of non-specific products. The PCR product was monitored in real-time, using the SYBR Green I fluorescence, using the MyiQ single Color Real-Time PCR Detection System (Bio-Rad). Threshold for detection of PCR product was set in the log-linear phase of amplification and the threshold cycle (CT) was determined for each reaction. The level of the target mRNA was quantified relative to the housekeeping gene (GAPDH) and presented as percentage of vehicle control. The expression of GAPDH was not significantly different between treatments.
Table 1.
| Gene | Primer sequence (5’ -3’) | GenBank accession No. |
|---|---|---|
| GAPDH | GTTTGTGATGGGTGTGAACC (forward) | M17701 |
| TCTTCTGAGTGGCAGTGATG (reverse) | ||
| TNFα | CTTCAAGGGACAAGGCTG (forward) | D00475 |
| GAGGCTGACTTTCTCCTG (reverse) | ||
| IL-1β | GAAGTCAAGACCAAAGTGG (forward) | M98820 |
| TGAAGTCAACTATGTCCCG (reverse) | ||
| IL-6 | ACTTCACAGAGGATACCAC (forward) | NM_012589 |
| GCATCATCGCTGTTCATAC (reverse) | ||
| IκB | CACCAACTACAACGGCCACA (forward) | NM_001105720.2 |
| GCTCCTGAGCGTTGACATCA | ||
| cd11b | CTGGGAGATGTGAATGGAG (forward) | NM_012711 |
| ACTGATGCTGGCTACTGATG (reverse) |
2.9 Protein quantification
Interleukin (IL)-1β protein in rat dorsal spinal cord was analyzed using a commercially available ELISA kit specific for rat IL-1β (R & D Systems, Minneapolis, MN, USA). The lumbar dorsal spinal cord was sonicated in 500 µl of cold Iscove’s culture medium containing 5% fetal calf serum and a cocktail enzyme inhibitor (100 mM amino-n-caproic acid, 10 mM EDTA, 5 mM benzamidine–HCl, and 0.2 mM phenylmethylsulfonyl fluoride). Sonicated samples were centrifuged at 14,000 rpm at 4°C for 10 min. Supernatants were removed and stored at −80°C until an ELISA was performed. The protein concentration from each sample was determined using a Bradford protein assay as described previously (Bradford, 1976) before each ELISA and used to normalize the results from the ELISAs. The sensitivity for the rat IL-1β assay is 5 pg/ml.
2.10 Statistical analysis
Behavioral measures were normalized as described above and analyzed using repeated measures 2-way ANOVA with time and treatment as main effects. The ELISA and RT-PCR data were analyzed using an ANOVA. Student-Newman Keuls post-hoc tests were used where appropriate and P<0.05 was considered statistically significant.
2.11 Experimental design
Blockade
In order to assess the effect of α7nAchR agonists on gp120-induced mechanical allodynia, spinal cord pro-inflammatory cytokine gene expression and content, we intrathecally administered gp120 (3 ug in 6 ul, 8 ul saline flush) to induce robust mechanical allodynia, known to be associated with an increase in spinal proinflammatory cytokines. In order to determine whether the allodynia could be prevented, we co-administered choline (1 µl of 0.1 µM, 1 µM solution) or vehicle with the gp120 in awake, gently restrained rats. Behavioral responses were assessed before intrathecal catheter surgery, before drug administration and 1 h, 2 h, 3 h and 4 h after drug administration.
Reversal
In order to assess whether the mechanical allodynia could be reversed, we administered choline (1 µl of 0.1 µM, 1 µM solution), GTS-21 (1 µl of 1 µM or 10 µM solution) or vehicle 30 min after gp120 (3 µg in 6 µl with 11 µl saline flush) or equivolume vehicle. A ten fold higher dose of GTS-21 was tested to identify if comparable behavioral results could be obtained with GTS-21 to that of choline. We tested GTS-21 at the 1 µM dose to test for equimolar potency. Since GTS-21 was not as effective as choline at the same dose, but there was some effect noted, we tested the ten fold higher dose of GTS-21 to differentiate between an α7nAchR generality effect or a choline specific effect. All drugs were administered over 30 s (1 µl drug followed by 20 µl sterile saline flush) in awake, gently restrained rats. Behavior was assessed before intrathecal catheter surgery, before and 30 min after gp120/vehicle administration, and then 30 min, 1 h, 2 h, 3 h, and 4 h after α7nAchR agonist or vehicle administration. Immediately upon completion of the last testing time point, rats were deeply anesthetized and lumbar spinal cord tissue was collected for protein and mRNA analysis. The catheter was verified for placement over the lumbar enlargement and patency during each tissue dissection.
3. Results
3.1 Blockade of gp120-induced mechanical allodynia
In order to assess whether the mechanical allodynia induced by gp120 could be prevented by an α7nAchR agonist (choline), we coadministered choline (0.1 µM, 1 µM or vehicle; 1 ul) with the gp120 (3 µg or vehicle; 6 µl). Before injection of gp120, there was no significant difference in the behavioral responses to the calibrated probes between groups (Figure 1). Intrathecal gp120 reliably produced robust mechanical allodynia from 1 h to 4 h compared to vehicle controls (main effect of drug: F4,37 = 10.95, time effect: F5,185 = 84.44, Interaction: F20, 185 = 5.22, all P < 0.0001). There was a dose dependent attenuation of the gp120-induced allodynia with 0.1 µM choline significantly attenuating the allodynia only at 2 h post injection (P < 0.05). 1 µM choline significantly attenuated the gp120 induced allodynia throughout the time course (1–4 h after injection, P < 0.01). There was no significant effect of choline (1 µM) on vehicle-injected rats at any time measured (P >0.05).
Figure 1.
Intrathecal α7nAchR agonist choline (1 µM, 0.1 µM) inhibits gp120-induced mechanical allodynia in a dose-dependent fashion. Mechanical sensitivity was tested using the von Frey test, before (BL) surgery, before (0) and 1–4 h after intrathecal administration of gp120 (3 µg) or vehicle (0.1% RSA). Choline or vehicle was coadministered with the gp120 or vehicle. Solid circle depicts gp120+vehicle (n=6), grey diamond depicts gp120+choline (1 µM) (n=7), grey square depicts gp120+choline (0.1 µM) (n=10), open square depicts vehicle + choline (1 µM) (n=11) and open diamond depicts vehicle +vehicle (n=8). Data are presented as average absolute thresholds of left and right hind paws (mean ± SEM). # P < 0.001, * P < 0.01, $ P < 0.05 against gp120 + vehicle group.
3.2 Reversal of gp120-induced mechanical allodynia
While blocking the action of gp120 induced mechanical allodynia is informative of drug action, therapeutically reversing the mechanical allodynia is clinically more relevant. Therefore, allodynia was induced 30 min before drug administration (1 µM choline or vehicle; 1 µl). Once again, there was no significant difference between groups before gp120 or vehicle injection and the data are presented in Figure 2A. gp120 induced significant allodynia from 30 min to 4.5 h after gp120 administration (SNK, P < 0.001). Choline (1 µM) administered intrathecally 30 min after the intrathecal gp120 administration reversed the mechanical allodynia 2–4 h after drug administration (post hoc P < 0.001, main effect of drug: F3,21 = 69.04, main effect of time: F7,256 = 28.81, Interaction: F21,256 = 5.54, all P < 0.0001). Therefore, choline, an α7nAchR agonist, is able to both reverse, as well as prevent, gp120 induced mechanical allodynia when administered intrathecally.
Figure 2.
Intrathecal α7nAchR agonist choline (1 µM) and GTS-21 (10 µM, 1 µM) reverses gp120-induced mechanical allodynia in a dose-dependent fashion. Mechanical sensitivity was tested using the von Frey test, before (BL) surgery, before (0) and 30 min after gp120 (3 µg) or vehicle (0.1% RSA) administration, and 1–4 h after intrathecal administration of choline, GTS-21 or vehicle administration. For panel A: Solid circle depicts gp120+vehicle (n=9), solid square depicts gp120+choline (1 µM) (n=8), open square depicts vehicle + choline (1 µM) (n=9), open circle depicts vehicle +vehicle (n=10). For panel B: Solid circle depicts gp120+vehicle (n=9), solid square depicts gp120+GTS-21 (1 µM) (n=9), open square depicts gp120 + GTS-21 (10 µM) (n=7), open diamond depicts vehicle + GTS-21 (10 µM) (n=5) and open circle depicts vehicle + vehicle (n=10). Data are presented as average absolute thresholds of left and right hind paws (mean ± SEM). * P < 0.01 against gp120 + vehicle group.
3.3 Effect of GTS-21 on gp120-induced mechanical allodynia
In order to assess whether these effects are specific to choline, another α7nAchR agonist, GTS-21, was tested for its ability to reverse gp120 induced mechanical allodynia. GTS-21 (1 µM, 10 µM or vehicle) was administered 30 min after gp120 or vehicle (Figure 2B). There was no significant difference between groups before gp120 or vehicle injection. GTS-21 (1 µM) significantly reversed the mechanical allodynia from 1–3 h after GTS-21 administration (post hoc tests P < 0.05) while the 10 µM dose of GTS-21 reversed the allodynia from 2–4 h after GTS-21 administration (post hoc tests P < 0.01). There was a main effect of drug: F2,7 = 7.775, P <0.001, main effect of time: F7,168 = 59.14, P < 0.0001, Interaction: F14,168 = 5.808, P < 0.0001). Therefore, GTS-21 and choline, both α7nAchR agonists, reverse gp120 induced mechanical allodynia.
3.4 Effect of α7nAchR agonists on gp120 induced IL-1β content
In agreement with prior studies (Holguin et al., 2004; Ledeboer et al., 2005b; Schoeniger-Skinner et al., 2007), dorsal lumbar spinal cord IL-1β protein was significantly elevated 4 hours after intrathecal administration of gp120 compared to vehicle controls (Figure 3, SNK post hoc P < 0.001). Coadministration of choline or vehicle with gp120 or vehicle showed a significant difference in IL-1β between groups (F4,52 = 6.944, P < 0.0001, Figure 3A) with choline (1 µM) significantly attenuating IL-1β compared to gp120 + vehicle at 4h after drug administration (SNK, P < 0.01). Choline at 0.1 µM had no significant effect on IL-1β concentrations (SNK, P >0.05) but does show a trend towards decreasing the IL-1β. In addition, 1 µM choline had no effect on vehicle controls compared to vehicle + vehicle group (SNK, P > 0.05). In addition, gp120+choline (1 µM and 0.1 µM) groups were not significantly different to vehicle + vehicle group (SNK, P > 0.05).
Figure 3.
The effect of α7nAchR agonists on IL-1β protein within the lumbar dorsal spinal cord, 4 h after drug administration. Intrathecal gp120 significantly increased the IL-1β production compared to vehicle controls. In all instances choline had no effect on vehicle controls. Choline (1 µM) coadministered with gp120 significantly attenuated IL-1β production (panel A) but did not significantly affect IL-1β when administered 30 min after gp120 but did show a trend towards significance (panel B, P > 0.05). GTS-21 (1 µM) significantly attenuated IL-1β concentrations when administered 30 min after gp120 (panel B, P < 0.01). Data are presented mean ± SEM. * P < 0.05 against gp120 + vehicle group. For Panel A: gp120+vehicle (n=13), gp120+choline (1 µM) (n=9), gp120+choline (0.1 µM) (n=11), vehicle + choline (1 µM) (n=12) vehicle +vehicle (n=12). For panel B: gp120+vehicle (n=9), gp120+choline (1 µM) (n=8), gp120+GTS-21 (1 µM) (n=8), vehicle + choline (1 µM) (n=10), vehicle +vehicle (n=12).
When choline (1 µM) or GTS-21 (1 µM) was administered 30 min after gp120, there was a significant difference in IL-1β protein between groups (F4,42 = 9.257, P < 0.001, Figure 3B). While choline (1 µM) or GTS-21 (1 µM, data not shown) had no significant effect on IL-1β protein (SNK, P > 0.05) compared to gp120+vehicle, a trend is apparent that choline decreases IL-1β protein. GTS-21 (1 µM) administered 30 min after gp120 significantly attenuated IL-1β production 4 h after drug administration (SNK, P < 0.001). Although the absolute values between the gp120 + vehicle groups in the blockade and reversal data are different, most likely from assay to assay variability, the overall effect of the α7nAchR agonist shows an overall decrease in IL-1β protein compared to gp120+vehicle.
3.5 Effect of α7nAchR agonist on gp120-induced mRNA expression
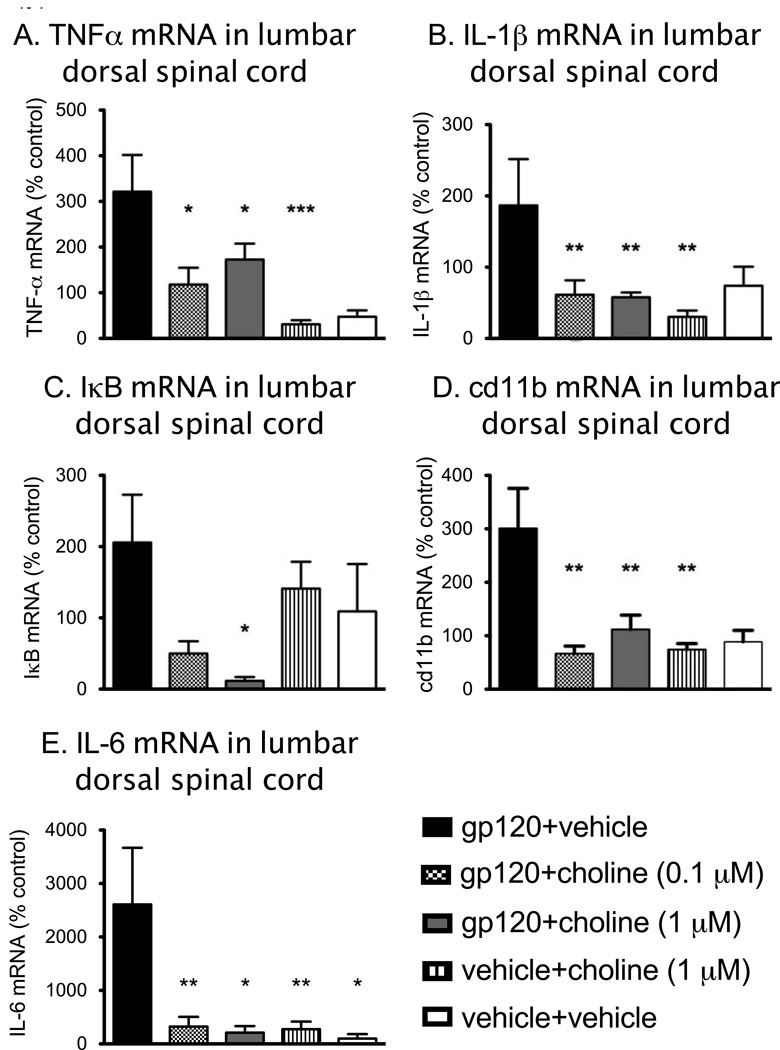
α7nAchR activation decreases some pro-inflammatory cytokine production at a transcriptional level in human monocytes and macrophages (Rosas-Ballina et al., 2009) via interference with IκB phosphorylation (Yoshikawa et al., 2006). However, it is unclear whether transcriptional changes occur in all cell types. Therefore, mRNA levels from the lumbar dorsal spinal cord, collected 4 h after gp120 +/− choline coadministration, were assessed for TNF-α, IL-1β and IL-6 gene expression and presented as percentage of control (vehicle + vehicle, Figure 4A & B). All the mRNA measured showed a significant difference between gp120 + vehicle and vehicle + vehicle groups (SNK post hoc test, P < 0.05). TNFα, IL-1β and IL-6 mRNA showed a significant difference between groups (TNFα: F3,30 = 6.506 P < 0.0025; IL-1β: F3,34 = 5.155, P < 0.005; IL-6: F3,21 = 6.013, P < 0.005). Post hoc tests showed there was a significant decrease in TNFα mRNA following 0.1 µM and 1 µM choline in groups that received gp120 compared to gp120+vehicle rats (P < 0.05). The same significant decrease in IL-1β and IL-6 mRNA was noted with both doses of choline (SNK, P < 0.05) such that gp120+choline was not significantly different to vehicle+choline (1 µM) group (P>0.05).
Figure 4.
Gene expression in dorsal spinal cord tissue collected 4 h after administration of choline (0.1 µM, 1 µM) or vehicle in rats coadministered gp120 (3 µg) or vehicle. TNFα, IL-1β, IL-6, cd11b and IκB mRNAs were elevated significantly in gp120 groups compared to vehicle controls (TNFα: P < 0.005; IL-1β: P < 0.05; cd11b: P < 0.01; IκB: P < 0.05, IL-6: P < 0.05). Choline had a significant effect on TNFα and IL-6 mRNA at both 0.1 µM and 1 µM choline (panel A & E) but at 1 µM only for IL-1β mRNA (panel B, P< 0.05) and IκB mRNA (panel C, P < 0.01) compared to gp120 alone (P < 0.05). Both 0.1 µM and 1 µM choline significantly attenuated cd11b mRNA (panel D, P < 0.01). Data are presented as mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001 compared to gp120+vehicle. gp120+vehicle (n=8–10), gp120+choline (1 µM) (n=9–12), gp120+choline (0.1 µM) (n=6–10), vehicle + choline (1 µM) (n=8–10) vehicle +vehicle (n=9–11).
In order to identify whether NFκB was affected by α7nAchR activation, we measured IκB gene expression, which increases in response to IκB degradation, a required step in NFκB activation (Ledeboer et al., 2005a; Yoshikawa et al., 2006). At the time point we tested, there was a significant difference in IκB mRNA between groups (F3,31 = 4.35, P < 0.025, Figure 4C). Post hoc tests showed there was a significant decrease in IκB mRNA following 1 µM choline in groups that received gp120 compared to gp120 + vehicle rats (P < 0.05) and a comparable trend, though not statistically significant, occurring with the 0.1 µM dose of choline.
We assessed the effect of the α7nAchR agonists on microglial/macrophage activation in vivo, using cd11b gene expression, a marker for microglial/macrophage activation (Figure 4D). There was a significant difference in cd11b mRNA between groups (F3,36 = 7.11, P < 0.001). Post hoc tests showed there was a significant decrease in cd11b mRNA following both 0.1 µM and 1 µM choline in groups that received gp120 compared to gp120+vehicle rats (P < 0.01).
When the drug was administered 30 min after gp120 administration and tissues collected 4 h later, both TNF-α gene expression (Figure 5A) and IL-1β (Figure 5B) were significantly different between groups (TNF-α: F3,28 = 6.049, P =0.003, IL-1β: F3,28 = 8.196, P< 0.0005) but not IL-6 (F3,28 = 1.199, P >0.05). Post hoc tests showed that GTS-21 (1 µM) significantly attenuated TNF-α (P < 0.01) and IL-1β gene expression (P < 0.01). Choline (1 µM) significantly attenuated IL-1β (P < 0.01) compared to gp120+vehicle. While choline (1 µM) showed no significant effect on TNF-α mRNA at this time point, there is a comparable trend in attenuating TNFα mRNA to that seen with GTS-21 and to choline (1 µM) coadministered with gp120, and there was no significant difference between gp120+choline (1 µM) or vehicle+GTS-21 (data not shown) and vehicle+choline (1 µM) groups (P > 0.05).
Figure 5.
Gene expression mRNA in lumbar dorsal horn tissue collected 4 h after choline, GTS-21 or vehicle administration +/− gp120. The α7nAchR agonists were administered 30 min after gp120 or vehicle administration to assess the ability of the drug to reverse the pro-inflammatory effect of gp120. TNF-α gene expression (panel A) and IL-1β (panel B) were elevated significantly after gp120 + vehicle administration compared to vehicle + vehicle controls and compared to vehicle + choline group (TNF-α: P =0.021, IL-1β: P< 0.0002). GTS-21 (1 µM) significantly attenuated TNF-α and IL-1β gene expression (each, P < 0.05), but choline (1 µM) only had an effect on IL-1β (P < 0.05) but not TNF-α (P >0.05). There was no significant effect of either choline (1 µM) or GTS-21 (1 µM) on IκB mRNA (panel C). cd11b mRNA (panel D) was significantly decreased following GTS-21 (1 µM) administration after gp120 compared to gp120 + vehicle (P = 0.0041). There was no significant treatment effect on IL-6 mRNA (panel E). Data are presented as mean ± SEM. * P < 0.05, ** P < 0.01 compared to gp120+vehicle. gp120+vehicle (n=7–10), gp120+choline (1 µM) (n=6–8), gp120+GTS-21 (1 µM) (n=6–7), vehicle + choline (1 µM) (n=7–8), vehicle +vehicle (n=5–9).
IκB gene expression when drugs were given 30 min after gp120 and tissues collected 4 hr later showed a significant difference between groups (F3,30 = 5.508, P < 0.005; Figure 5C). Post hoc tests showed that GTS-21 (1 µM) significantly attenuated IκB mRNA compared to gp120+vehicle group (P < 0.05). In addition, cd11b mRNA was significantly different between groups (F3,27 = 5.12, P < 0.01; Figure 5D). Post hoc tests showed that GTS-21 (1 µM), but not choline, significantly attenuated cd11b mRNA compared to gp120+vehicle group (P < 0.05).
4. Discussion
The present studies demonstrate that intrathecal administration of a selective α7nAchR agonist, choline, dose-dependently blocks and reverses centrally mediated mechanical allodynia induced by intrathecal administration of gp120. An additional α7nAchR agonist, GTS-21, also was able to reverse the mechanical allodynia induced by intrathecal gp120 (prevention was not tested). Interestingly, the higher dose of GTS-21 took longer to induce a reverse of allodynia; the reason for which is unclear. There was no effect of choline in non-allodynic rats suggestive of an anti-allodynic action rather than an analgesic mechanism of action. It is likely that activation of α7nAchR within the spinal cord attenuates the gp120 induced pain behavior via a suppression of spinally produced pro-inflammatory cytokines since dorsal lumbar spinal tissue IL-1β gene expression was reduced and IL-1β protein and IL-6 and TNFα mRNA was attenuated, at the time point measured, such that they were not significantly different to vehicle+vehicle or vehicle+choline (1 µM) groups. While the choline did not significantly attenuate IL-1β protein (0.1 µM choline in blocking paradigm; 1 µM choline in reversal paradigm), the data show a clear trend towards attenuating protein levels and that choline is effective in attenuating IL-1β mRNA when administered with gp120 or after. Whether the IL-1β protein levels reflect intracellular content versus released protein present in the tissue samples is at present unknown and may explain inconsistencies between protein and mRNA data. However, with all proinflammatory mediators and behavioral responses, choline was less effective when administered after induction of gp120 allodynia than before. IκB gene expression also was significantly attenuated with 1 µM choline administered before gp120 and 1 µM GTS-21 delivered afterwards. Therefore, α7nAchR agonism attenuated the pro-inflammatory cytokine cascade, possibly via prevention of NFκB activation. It is possible that a key effect of α7nAchR activation within the spinal cord is on microglia, as cd11b gene expression (microglial/macrophage activation marker) was significantly attenuated within the spinal cord following either choline + gp120 or GTS-21 administered after gp120, compared to gp120 positive controls. However, cd11b mRNA was not, at the time point analyzed, affected by choline administered after gp120. Notably, this correlates with the lack of effect on IL-1β protein levels also observed in this group. The reversal of allodynia occurred later after post-gp120 treatment with choline compared to GTS-21, which correlates with trends toward the reduction in IL-1β protein and TNFα mRNA. It is intriguing that an equimolar dose of GTS-21 compared to choline produced a less robust effect on behavior, but showed a significant attenuation of pro-inflammatory markers within the spinal cord. However, we have measured the pro-inflammatory mediators at one time point only. It is difficult to draw conclusion and direct correlations between the pro-inflammatory cytokine and the behavioral measures when both the behavior and cytokine levels are dynamic and we have measured at a single time point.
Intrathecal administration of gp120 induces mechanical allodynia in rats, which is associated with an increase in pro-inflammatory cytokine mRNA, protein and release (Holguin et al., 2004; Ledeboer et al., 2005b; Milligan et al., 2000; Milligan et al., 2001; Schoeniger-Skinner et al., 2007). Intrathecal gp120-induced proinflammatory cytokines are likely glial in origin as the production of the pro-inflammatory cytokines are attenuated by intrathecal delivery of the glial activation inhibitors, fluorocitrate and minocycline (Ledeboer et al., 2005b; Milligan et al., 2001). The increase in proinflammatory cytokine production and release in response to gp120 is causal to pain enhancement since gp120-induced allodynia is suppressed by antagonists or inhibitors of IL-1β, TNFα and IL-6 (Milligan et al., 2001; Schoeniger-Skinner et al., 2007). Previous studies have shown nicotine and acetylcholine to attenuate TNFα release from microglial cells in vitro (De Simone et al., 2005; Giunta et al., 2004; Shytle et al., 2004; Suzuki et al., 2006).
Intrathecal administration of gp120 has previously been shown to increase the activation of NFκB within the spinal cord, and that the activation is associated with enhanced pain behaviors (Ledeboer et al., 2005a). However, we are the first to show that choline significantly attenuates IκB mRNA in vivo within immunologically challenged spinal cord tissue.
In stimulated microglial cell cultures, similar mechanisms have been identified in reducing TNFα following activation of nicotinic receptors via mitogen-activated kinases (p38 and p42/44). Regardless of the immunocompetent cells or their location, including microglial cells, it does appear that activation of α7nAchR is able to attenuate inflammation via a suppression of TNFα (Giunta et al., 2004; Hamano et al., 2006; Shytle et al., 2004; Wang et al., 2003; Yoshikawa et al., 2006). We have presented, for the first time, that IL-1β mRNA, an additional, prominent proinflammatory cytokine, and IκB mRNA are both significantly attenuated in vivo within the spinal cord following choline (a selective α7nAchR agonist) plus gp120 compared to gp 120 alone.
Both α7nAchR and α4β2nAchR are found on neuronal cells (Conejero-Goldberg et al., 2008). Therefore, effects on neuronal cells cannot be excluded. However, the reductions in mRNA of both the proinflammatory cytokines as well as cd11b (a microglial/macrophage activation marker) in vivo following coadministration of intrathecal gp120 and choline is suggestive of α7nAchR activation on microglia and resultant reduction in the pain-enhancing pro-inflammatory response. Whether these receptors are found on other glial cells within the spinal cord is yet to be determined.
It has been proposed that normal tonic activity of cholinergic neurons is reduced in animals with neuropathic pain, possibly contributing to the pain, and that administration of a nicotinic receptor agonist restores the cholinergic tone thus alleviating pain (Matsumoto et al., 2007; Young et al., 2008). General nicotinic receptor agonists, such as nicotine, bind to both the α4β2nAchR and α7nAchR and have previously been shown to be effective for attenuating neuropathic pain from a tibial nerve transection following intrathecal administration (Abdin et al., 2006). Intrathecal nicotine administration and (+) epibatidine (a potent agonist of α4β2nAchR) (Badio and Daly, 1994; Dukat and Glennon, 2003) were both effective in reducing partial sciatic nerve induced neuropathic pain (Rashid and Ueda, 2002) and spinal nerve ligation (Young et al., 2008), but had no effect on sham-operated mice. However, these authors reported no effect of choline when administered intrathecally at 0.1uM, comparable to the present results with gp120 induced allodynia. Also, using various antagonists, they identified that the mechanism of action of these drugs is possibly restoring GABAergic inhibitory tone contributing to the reversal of the allodynia. All previous studies of nicotinic regulation of pain have assumed that the action of such drugs was via their direct effects on neurons. However, we are the first study to explore the effect of nAchR agonists on pain known to involve glial activation.
Interestingly, α7nAchR agonists administered intrathecally had no effect on sham-operated animals compared to neuropathic animals with both thermal and mechanical hind paw behavioral testing (Medhurst et al., 2008). In contrast, in the tail flick test to radiant heat, intrathecal administration of choline (90 µg) was antinociceptive (Damaj et al., 2000). However, here the intrathecal injections were given in unanesthetized mice, possibly causing some injury at the injection site (Damaj et al., 2000). In addition, the dose used for antinociceptive testing was equivalently 0.64 M compared to our study where we used 1 µM. Therefore, it is unclear whether such experimental issues, species, drug dose, specific nociceptive test, or other variable may account for the differences observed. Beyond selective α7nAchR agonists, numerous studies have investigated the effects of intrathecal administration of various nicotinic agonists and found some to be pronociceptive (Khan et al., 1994; Rueter et al., 2000) and some antinociceptive (Damaj et al., 2000). There is evidence that cholinergic tone within the spinal cord is altered in pain states which may contribute to the changes occurring between nociceptive testing and testing drug effect in pain models (Matsumoto et al., 2007; Young et al., 2008). In addition, the doses administered in prior publications are significantly higher (by 4 to 6 fold) than used here. Our studies contribute to the extent of knowledge on α7nAchR agonists administered intrathecally, at the doses tested, in that they are effective anti-allodynic agents mediating the reduction in IL-1β mRNA and other pro-inflammatory cytokines within the spinal cord.
Pain is not the only outcome where selective α7nAchR agonists appear to attenuate neuroinflammation. Cognitive impairments associated with Alzheimer’s disease are caused, in part, from a loss of cholinergic neurons (Conejero-Goldberg et al., 2008). In addition, activation of α7nAchR found on primary neonatal astrocytes cultures modulate calcium release from intracellular calcium stores resulting in activation of astrocytes, as measured by calcium waves (Sharma and Vijayaraghavan, 2001).
Activation of nicotinic receptors on neurons and astrocytes appears to improve cognition and clinical symptoms in Alzheimers disease and Parkinson’s disease. However, it is now thought that nicotine is neuroprotective in neuroinflammatory diseases such as Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, amyotrophic lateral sclerosis and even frontal cortical changes in aging, via a suppression of pro-inflammatory cytokines released by microglia (Conejero-Goldberg et al., 2008; de Jonge and Ulloa, 2007; Streit, 2002, 2005; Wang et al., 2000). Phase II clinical trials (Roche Pharmaceutical) are currently underway to investigate the impact of α7nAchR activation in conjunction with 5-HT3 receptor antagonism in Alzheimer’s disease and schizophrenia (Conejero-Goldberg et al., 2008). Therefore, α7nAchR agonists may be an effective target for the treatment of neuroinflammatory diseases including pain, especially those pains known to be driven or maintained by microglial activation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdin MJ, Morioka N, Morita K, Kitayama T, Kitayama S, Nakashima T, Dohi T. Analgesic action of nicotine on tibial nerve transection (TNT)-induced mechanical allodynia through enhancement of the glycinergic inhibitory system in spinal cord. Life Sci. 2006;80:9–16. doi: 10.1016/j.lfs.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Badio B, Daly JW. Epibatidine, a potent analgetic and nicotinic agonist. Mol Pharmacol. 1994;45:563–569. [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M, Tracey KJ. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000a;85:141–147. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000b;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Briggs CA, Anderson DJ, Brioni JD, Buccafusco JJ, Buckley MJ, Campbell JE, Decker MW, Donnelly-Roberts D, Elliott RL, Gopalakrishnan M, Holladay MW, Hui YH, Jackson WJ, Kim DJ, Marsh KC, O'Neill A, Prendergast MA, Ryther KB, Sullivan JP, Arneric SP. Functional characterization of the novel neuronal nicotinic acetylcholine receptor ligand GTS-21 in vitro and in vivo. Pharmacol Biochem Behav. 1997;57:231–241. doi: 10.1016/s0091-3057(96)00354-1. [DOI] [PubMed] [Google Scholar]
- Conejero-Goldberg C, Davies P, Ulloa L. Alpha7 nicotinic acetylcholine receptor: a link between inflammation and neurodegeneration. Neurosci Biobehav Rev. 2008;32:693–706. doi: 10.1016/j.neubiorev.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Meyer EM, Martin BR. The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785–2791. doi: 10.1016/s0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone R, Ajmone-Cat MA, Carnevale D, Minghetti L. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation. 2005;2:4. doi: 10.1186/1742-2094-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukat M, Glennon RA. Epibatidine: impact on nicotinic receptor research. Cell Mol Neurobiol. 2003;23:365–378. doi: 10.1023/a:1023692705700. [DOI] [PubMed] [Google Scholar]
- Fields R. New culprits in chronic pain. Sci Am. 2009:50–57. doi: 10.1038/scientificamerican1109-50. [DOI] [PubMed] [Google Scholar]
- Giunta B, Ehrhart J, Townsend K, Sun N, Vendrame M, Shytle D, Tan J, Fernandez F. Galantamine and nicotine have a synergistic effect on inhibition of microglial activation induced by HIV-1 gp120. Brain Res Bull. 2004;64:165–170. doi: 10.1016/j.brainresbull.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Hamano R, Takahashi HK, Iwagaki H, Yoshino T, Nishibori M, Tanaka N. Stimulation of alpha7 nicotinic acetylcholine receptor inhibits CD14 and the toll-like receptor 4 expression in human monocytes. Shock. 2006;26:358–364. doi: 10.1097/01.shk.0000228168.86845.60. [DOI] [PubMed] [Google Scholar]
- Harvey LO. Efficient estimation of sensory thresholds. Behav. Res. Methods Instrum. Comput. 1986;18:623–632. [Google Scholar]
- Holguin A, O'Connor KA, Biedenkapp J, Campisi J, Wieseler-Frank J, Milligan ED, Hansen MK, Spataro L, Maksimova E, Bravmann C, Martin D, Fleshner M, Maier SF, Watkins LR. HIV-1 gp120 stimulates proinflammatory cytokine-mediated pain facilitation via activation of nitric oxide synthase-I (nNOS) Pain. 2004;110:517–530. doi: 10.1016/j.pain.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Khan IM, Taylor P, Yaksh TL. Stimulatory pathways and sites of action of intrathecally administered nicotinic agents. J Pharmacol Exp Ther. 1994;271:1550–1557. [PubMed] [Google Scholar]
- Ledeboer A, Gamanos M, Lai W, Martin D, Maier SF, Watkins LR, Quan N. Involvement of spinal cord nuclear factor kappaB activation in rat models of proinflammatory cytokine-mediated pain facilitation. Eur J Neurosci. 2005a;22:1977–1986. doi: 10.1111/j.1460-9568.2005.04379.x. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005b;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Xie W, Inoue M, Ueda H. Evidence for the tonic inhibition of spinal pain by nicotinic cholinergic transmission through primary afferents. Mol Pain. 2007;3:41. doi: 10.1186/1744-8069-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhurst SJ, Hatcher JP, Hille CJ, Bingham S, Clayton NM, Billinton A, Chessell IP. Activation of the alpha7-nicotinic acetylcholine receptor reverses complete freund adjuvant-induced mechanical hyperalgesia in the rat via a central site of action. J Pain. 2008;9:580–587. doi: 10.1016/j.jpain.2008.01.336. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Tay ET, Papke RL, Meyers C, Huang GL, de Fiebre CM. 3-[2,4-Dimethoxybenzylidene]anabaseine (DMXB) selectively activates rat alpha7 receptors and improves memory-related behaviors in a mecamylamine-sensitive manner. Brain Res. 1997;768:49–56. doi: 10.1016/s0006-8993(97)00536-2. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid MH, Ueda H. Neuropathy-specific analgesic action of intrathecal nicotinic agonists and its spinal GABA-mediated mechanism. Brain Res. 2002;953:53–62. doi: 10.1016/s0006-8993(02)03270-5. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15:195–202. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter LE, Meyer MD, Decker MW. Spinal mechanisms underlying A-85380-induced effects on acute thermal pain. Brain Res. 2000;872:93–101. doi: 10.1016/s0006-8993(00)02472-0. [DOI] [PubMed] [Google Scholar]
- Schoeniger-Skinner DK, Ledeboer A, Frank MG, Milligan ED, Poole S, Martin D, Maier SF, Watkins LR. Interleukin-6 mediates low-threshold mechanical allodynia induced by intrathecal HIV-1 envelope glycoprotein gp120. Brain Behav Immun. 2007;21:660–667. doi: 10.1016/j.bbi.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci U S A. 2001;98:4148–4153. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and neuroprotection: implications for Alzheimer's disease. Brain Res Brain Res Rev. 2005;48:234–239. doi: 10.1016/j.brainresrev.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Matsubara A, Hama C, Harada K, Miyano K, Andra M, Matsubayashi H, Sakai N, Kohsaka S, Inoue K, Nakata Y. Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res. 2006;83:1461–1470. doi: 10.1002/jnr.20850. [DOI] [PubMed] [Google Scholar]
- Waldburger JM, Boyle DL, Pavlov VA, Tracey KJ, Firestein GS. Acetylcholine regulation of synoviocyte cytokine expression by the alpha7 nicotinic receptor. Arthritis Rheum. 2008;58:3439–3449. doi: 10.1002/art.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wang HY, Lee DH, D'Andrea MR, Peterson PA, Shank RP, Reitz AB. beta-Amyloid(1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer's disease pathology. J Biol Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the "bad guys": implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E, Kamochi H, Suzuki N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol. 2006;146:116–123. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T, Wittenauer S, Parker R, Vincler M. Peripheral nerve injury alters spinal nicotinic acetylcholine receptor pharmacology. Eur J Pharmacol. 2008;590:163–169. doi: 10.1016/j.ejphar.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]