Abstract
Cancer, in addition to many other chronic diseases, is associated with serious and problematic behavioral symptoms, including cognitive impairments. In humans, various factors likely contribute to cancer-associated cognitive deficits including disease awareness and chemotherapy; however, the endogenous biological factors arising from tumor development may also play a causal role. In the present study, rats with mammary tumors exhibited impaired spatial reference memory on a radial arm maze and amnesia for familiar objects in an object recognition memory test. In contrast, their performance in the Morris water maze and in fear conditioning tests was comparable to that of controls. These select cognitive impairments were accompanied by elevations in hippocampal interleukin-1β mRNA expression, but were not associated with decreases in hippocampal brain-derived neurotrophic factor gene expression. Together the results indicate that peripheral tumors alone are sufficient to induce increases in hippocampal cytokine expression and select deficits in hippocampal-dependent memory tasks.
Keywords: cognition, learning and memory, cancer, BDNF, cytokines, IL-1β, hippocampus, tumor
Introduction
Cancer patients commonly experience emotional distress and cognitive impairments (Wefel et al., 2008), which reduce their quality of life and negatively impact treatment compliance and survival (Brown et al., 2003). Cognitive impairments associated with cancer include deficits in memory, attention, concentration, and problem solving skills (Wefel et al., 2008). Prior research has emphasized deleterious effects of chemotherapy on cognition (Miller et al., 2008); however, mechanisms by which tumors alone affect cognition remain largely unexamined.
Peripheral tumors are sufficient to induce a negative emotional state and trigger parallel changes in the immune and endocrine systems. Rats with mammary tumors exhibit elevated hippocampal proinflammatory cytokines (including interleukin-1β [IL-1 β]), decreased glucocorticoid production, and increased hippocampal glucocorticoid receptor gene expression (Pyter et al., 2009). In light of the potent negative effects of proinflammatory cytokines (Banks et al., 2002), and modulatory effects of glucocorticoids (Wolf, 2003), on cognition, the present work considered whether tumors alone are likewise sufficient to disrupt learning and memory.
To examine this, we assessed several modalities of learning and memory in tumor-bearing and tumor-free rats. We also examined hippocampal proinflammatory cytokine production and growth factor gene expression as possible mediators of tumor-induced changes in hippocampal-dependent memory processes.
Methods
Animals
Female, nulliparous Wistar rats (Harlan, Indianapolis, IN) were used in these experiments. Siblings were pseudo-randomly distributed among treatments and housed 2-3/cage in polypropylene cages (25.9 × 47.6 × 20.9 cm) at temperature and humidity of 22 ± 1 °C and 50 ± 5%, respectively, and ad libitum access to food (Harlan 2018) and filtered tap water. Rats were housed in 16 h light/day (lights on: 20:00 CST) and all behavioral tests were performed between 7.00-13.00 h CST. Two treatment-balanced cohorts of rats were tested using the Morris water maze and fear conditioning paradigms (n=14 tumors; n=12 saline). Separate cohorts were used for radial arm maze (n=4 tumors; n=5 saline), novel object recognition test/BDNF gene expression (n=20 tumors; n=13 saline), and hippocampal IL-1β gene expression (n=3 tumors; n=3 saline).
Tumor induction
Between 30-42 d of age (week 0), rats received an injection (i.p., 50 mg/kg) of the carcinogen (N-nitroso-N-methylurea [NMU]; Sigma, St. Louis, MO) or saline according to the methods described elsewhere (Pyter et al., 2009). All cagemates received the same treatment. NMU administered at this age induces consistent (>90%) malignant mammary tumors (ductal carcinomas) in rats (≥5 weeks post-treatment); comparable to the most common adult human breast tumor (Thompson et al., 2000). Mammary gland palpation performed by an experienced researcher began at week 4; only tumors >3mm in diameter detected by week 10 were included in this study. At week 7, all rats were housed 1/cage.
Morris water maze
Long-term, spatial learning and memory was assessed according to the methods described previously (Morris, 1984). Under dim light, video was obtained from above the maze (1.75 m diameter × 58 cm height; 27 °C opaque water) and analyzed using Ethovision software (Noldus, Wageningen, NL). On day 1, rats were allowed to swim freely for 60 s to acclimate. During training (days 2-5), a platform (18 cm diameter) was placed 1.5 cm below the water surface in one quadrant. Rats were given 2 trial blocks/day consisting of three 60-s trials (15-s ITI). Rats remained on the platform for 10 s following each trial. On day 6, the platform was removed and a 60-s probe trial examined retention. On days 7 and 8, reversal learning was evaluated in 3 trial blocks followed by a probe trial on day 9. On day 10, a 60-s visible (1 cm above the surface) platform trial was run.
Fear conditioning
Fear responses to contextual and auditory stimuli were measured according to established procedures (LeDoux et al., 1990). Four days after the Morris water maze, rats were placed individually in stainless steel/glass modular conditioning chambers with metal grid floors (25 × 31 × 25 cm, ENV-008, Med Associates, St. Albans, VT) enclosed in a sound-dampening box with an overhead camera. On day 1, rats habituated to the chamber for 3 min. On day 2, rats received 3 conditioning trials, each trial consisting of the presentation of a tone (20 s, 5 kHz, 75 dB, 60- or 120-s ITI) accompanied by an electric shock (1 s, 1.0 mA) delivered through the floor during the last 1 s of the tone. On day 3, context conditioning was assessed by measuring freezing behavior during 3-min exposure to the conditioning chamber in the absence of tones/shocks Cued conditioning was assessed 2.5 h after context conditioning by placing rats in a novel chamber (i.e., different color, shape, material, and odor) for 3 min of acclimatization, followed by 3 tone-only (20 s, 5 kHz, 75 dB) trials. Conditioning was assessed by measuring freezing behavior throughout each 20-s tone and for 10 s afterwards. A researcher blind to treatments scored behavioral videos using Etholog software (Sao Paul, Brazil).
Radial arm maze
Spatial working and reference memory was measured according to protocols described elsewhere (Wenk, 2004) using an 8-arm (68.5 × 11 cm each) plexiglass maze. Within 1 week of tumor detection, rats were subjected to food restriction to maintain 80% of ad lib body weight. Rats were progressively acclimated to the maze over 6 days with all arms available and baited (45 mg sugar pellet; Bio-Serv, Frenchtown, NJ) as detailed in Davis et al. (2005). Following acclimation and pre-training, rats received 8 days of training under dim light, which required locating a reward in the cup at the end of a single arm (the goal arm, which did not change) from starting positions on the distal end of one of the other 7 arms (start position) in blocks of 10 trials/day. Trials were scored live by two researchers blind to experimental treatments. Reference memory errors were defined as entries into a non-goal arm. Working memory errors were defined as entries into a previously visited arm within a trial.
Delayed novel object recognition
Rats were allowed to investigate 2 identical objects (7.5 cm diameter plastic pipe caps) for 10 min in a novel cage, after which they were returned to their home-cage. Three hours later, one familiar object was replaced with a novel object (7.5 cm steel pipe) and investigation behavior was scored for 5 min. All objects were rinsed with 70% ethanol between trials. Videos were scored using Etholog. Investigation was defined as object-directed behavior with the nose 1 cm from an object and vibrissae moving. A discrimination index (DI) was calculated as described in (Ruby et al., 2008)
Brain-derived neurotrophic factor (BDNF) gene expression
Within 2 min of novel object recognition testing, rats were rapidly decapitated and hippocampal tissue was collected, frozen at -70 °C, and later processed for BDNF gene expression as described below.
IL-1β gene expression
Hippocampal tissue was collected in rats without prior behavioral testing experience, frozen at -70 °C following rapid decapitation, and processed for IL-1β gene expression as described below.
qPCR
Total RNA was extracted (RNeasy Mini Kit, Qiagen, Valencia, CA), verified by A260/A280 ratios, and reverse transcribed. TaqMan primer and probe sets for BDNF (Rn02531967_s1), IL-1β (Rn00676333_g1), and 18S ribosomal RNA (labeled with VIC) were purchased (Applied Biosystems). Amplification was performed using an ABI-7900HT at the Functional Genomics Center. The universal two-step RT-PCR cycling conditions used were: 50° C (2 min), 95° C (10 min), 40 cycles of 95° C (15 s) and 60° C (1 min). Relative gene expression of individual samples run in duplicate was calculated by the relative standard curve method consisting of serial dilutions of pooled rat hippocampal cDNA (1:1, 1:10, 1:100, 1:1000, 1:10,000) followed by normalization to 18S rRNA gene expression.
Statistical Analyses
Statistics were performed using StatView 5.0 (SAS, Cary, NC). Differences between groups over time were assessed using repeated-measures ANOVA and t-tests where warranted by a significant omnibus F-statistic. One-sample t-tests were used to determine if DIs differed from 0. Differences were considered significant if P ≤ 0.05.
Results
Morris water maze
Latencies to reach the hidden platform decreased (p<0.05) over time equally (p>0.9) in tumor-bearing and control rats in both the original (Figure 1A) and reversal (data not shown) learning trials. Additionally, the time spent in the quadrant of the pool in which the platform had been removed for the memory probe tests was comparable between groups (p>0.1 for both; Figure 1A) as was the latency to reach the platform in the visible platform trial (p>0.4; data not shown).
Figure 1. Effects of mammary tumors on cognition.
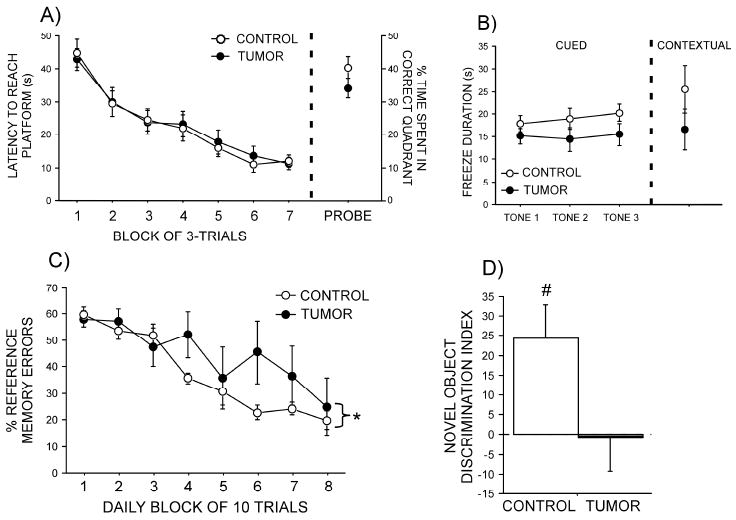
(A) Latency to reach the hidden platform during the Morris water maze acquisition training and percent time spent in the target quadrant in the subsequent probe trial. (B) Freezing duration in the 30 seconds following 3 presentations of the fear-conditioned tone alone and time spent freezing during a 3-min exposure to the conditioned context. (C) Percent reference memory errors in a radial arm maze. (D) Discrimination indices in a novel object recognition test. Data are mean (± SEM). * p<0.05 vs. control (Panel C * identifies interaction effect of treatment over time) and # p<0.05 vs. 0
Fear conditioning
Tumor-bearing rats tended to spend less time freezing following presentation of the auditory cue alone (F1,46=3.44, p=0.08; Figure 1B). Freezing behavior in the contextual fear conditioning trial was comparable in both groups (p>0.2; Figure 1B).
Radial arm maze
The percentage of reference memory errors decreased over blocks of trials in all rats (F7,49=21.7, p<0.0001), but this decrease occurred significantly slower in tumor-bearing relative to control rats (F7,49=2.7, p=0.02; Figure 1C). The percentage of working memory errors likewise decreased over time (F7,49 = 10.5, p<0.0001); however, there were no differences between groups in this measure (p>0.4; data not shown).
Novel object recognition
Control rats readily discriminated the novel object from the familiar object after a 3-h delay and spent significantly more time investigating the novel object (p<0.05; Figure 1D). In contrast, tumor-bearing rats spent comparable amounts of time investigating both objects (p>0.9).
IL-1β gene expression
Hippocampal IL-1β gene expression was elevated 3-4-fold in tumor-bearing relative to control rats (p<0.05; Figure 2A).
Figure 2. Effects of mammary tumors on hippocampal gene expression.
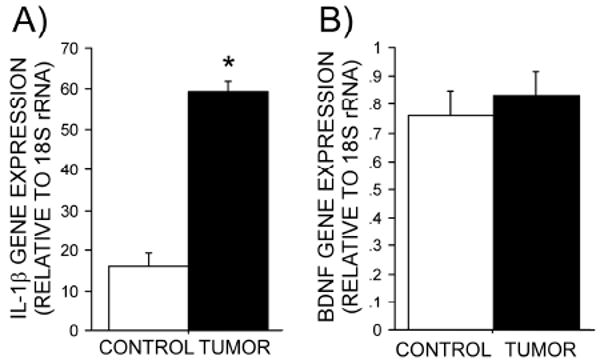
Mean (± SEM) of hippocampal (A) IL-1β and (B) BDNF gene expression relative to 18S rRNA in tumor-bearing and control rats.
BDNF gene expression
Hippocampal BDNF gene expression did not differ between groups (p>0.3; Figure 2B).
Discussion
Previous work indicated that mammary tumors alone are sufficient to induce depressive-like behaviors, increase hippocampal cytokine production, and reduce glucocorticoid responses to a stressor (Pyter et al., 2009). In light of the effects of hippocampal glucocorticoid and cytokine signaling on learning and memory (Banks et al., 2002), we examined whether tumors also impair cognitive function. To our knowledge, this is the first study to report significant cognitive deficits in tumor-bearing rats. Modest impairments were evident in spatial reference memory using the radial arm maze and marked memory impairment was evident in a novel object recognition test in rats with tumors. In contrast, neither fear conditioning nor long-term spatial memory performance was affected by tumor formation. Elevated IL-1β gene expression was detected in the hippocampus of tumor-bearing rats, but post-behavior hippocampal BDNF gene expression, which has been reported to facilitate learning and memory (Tyler et al., 2002), remained comparable to controls. The present data suggest that tumors provoke select cognitive deficiencies. These changes in cognition are associated with elevations in hippocampal IL-1β gene expression, but not with changes in hippocampal BDNF gene expression.
Drugs commonly used as chemotherapeutic agents in clinical settings impair learning in rodent models (Konat et al., 2008). However, studies examining the effects of tumors alone on cognitive function are not evident. In the present study, two tests measuring spatial learning and memory were used, but reference memory deficits were detected in tumor-bearing rats only in a radial arm maze, not in the Morris water maze. Differences in the motivational demands of the tests (appetitive versus aversive learning) may contribute to the differences in performance (Dudchenko et al., 1997). If so, the outcomes suggest that changes in cognition caused by tumor development are modest and only evident when motivation is reward-based. Inferences made regarding the selective basis of reference versus working memory impairment from the radial arm maze data are preliminary, however, because of the low sample sizes in the present work.
Of the four tests of learning and memory examined, only the cued test of fear conditioning is hippocampal-independent (Rudy et al., 2004). The absence of significant differences in both context and cued fear conditioning do not permit clear inferences about the relative effects of tumors on hippocampal-dependent versus -independent systems. However, potential contextual conditioning differences between tumor-bearing and control rats may have been masked given the relatively low freezing responses. Potential differences in cued conditioning may be further delineated using a continuous tone rather than the intermittent tone presentation used in the present paradigm (i.e., Barrientos et al., 2009). In contrast, the robust impairment in novel object recognition in the tumor-bearing rats corroborates the effects on spatial reference memory in the radial arm maze, and suggests that the hippocampus may be a target of tumor-derived factors. Further investigation of the effects of tumors on cognitive processes (e.g., attention, impulsivity) is relevant to performance in these tasks, and might bear clinical relevance as well.
The increase in IL-1β gene expression in the hippocampus of tumor-bearing rats supports earlier findings of increased hippocampal IL-1β protein expression (Pyter et al., 2009). Both humoral and neural routes remain viable candidate mechanisms by which tumors induce brain IL-1β production (Dantzer et al., 2000). Previous studies comparing cued (hippocampus-independent) versus context (hippocampus-dependent) learning have demonstrated that learning deficits due to elevated IL-1β are confined to hippocampal-based learning (Pugh et al., 2001). No such clear distinctions can be made from the present results, therefore, IL-1β is unlikely to be the sole mediator of cancer-associated cognitive deficits. Indeed, more complex interactions among elevated hippocampal cytokines and diminished glucocorticoid signaling in tumor-bearing rats may underlie these behavioral changes.
Among its many functions, BDNF regulates neuronal factors linked to learning including neuronal survival, dendritic structure, and neuronal function in the adult hippocampus (Binder and Scharfman, 2004). Decreased hippocampal BDNF is often associated with impaired learning or memory consolidation (Heldt et al., 2007), whereas increased hippocampal BDNF reported in response to a central immune challenge may be neuroprotective (de Pablos et al., 2006). These conflicting roles for BDNF could, in part, explain the absence of changes in BDNF mRNA levels following a novel object recognition test in tumor-bearing rats. Changes in BDNF also occur in a regionally-specific manner during learning (e.g., Bilbo et al., 2008) and the present approach, using a whole hippocampal analysis, may have failed to identify such sub-region-specific changes. In current form, these results do not indicate that changes in BDNF gene expression mediate tumor-induced cognitive deficits, although interpretation of these data compared with behaviorally-naïve controls may be more informative.
Taken together, the data suggest that tumors alone are sufficient to impair some aspects of declarative memory and to elevate hippocampal IL-1β mRNA in the absence of changes in whole hippocampal BDNF gene expression. These experiments extend previous findings that tumors induce a negative emotional state and thereby identify a broader role for the biological consequences of chronic disease on brain function. Although other potential causes likely contribute to cognitive deficits associated with cancer, these data indicate that physiological consequences of the disease itself may contribute to the comorbidity between chronic, peripheral disease and altered brain function.
Acknowledgments
We thank Jason Yee, Jerome Galang, Stephanie Tang, Jennifer Wei, Mike McCarthy, Curtis Wilson, Nicole Sikora, and Dr. Betty Theriault for technical advice and assistance. This project was supported by an American Cancer Society fellowship (PF-08-086-TBE), NIH Grant AI-67406, and a grant from the Brain Research Foundation.
Footnotes
Conflict of Interest Statement: All authors declare that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banks WA, Farr SA, Morley JE. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation. 2002;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Barrientos RM, Eads AS, Northcutt A, Watkins LR, Rudy JW, Maier SF. Early-life infection leads to altered BDNF and IL-1beta mRNA expression in rat hippocampus following learning in adulthood. Brain Behav Immun. 2008;22:451–455. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Levy AR, Rosberger Z, Edgar L. Psychological distress and cancer survival: a follow-up 10 years after diagnosis. Psychosom Med. 2003;65:636–643. doi: 10.1097/01.psy.0000077503.96903.a6. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Konsman JP, Bluthe RM, Kelley KW. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton Neurosci. 2000;85:60–65. doi: 10.1016/S1566-0702(00)00220-4. [DOI] [PubMed] [Google Scholar]
- de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA, Goodridge JP, Seiterle DA, Taube JS. Effects of repeated disorientation on the acquisition of spatial tasks in rats: dissociation between the appetitive radial arm maze and aversive water maze. J Exp Psychol Anim Behav Process. 1997;23:194–210. doi: 10.1037//0097-7403.23.2.194. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konat GW, Kraszpulski M, James I, Zhang HT, Abraham J. Cognitive dysfunction induced by chronic administration of common cancer chemotherapeutics in rats. Metab Brain Dis. 2008;23:325–333. doi: 10.1007/s11011-008-9100-y. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Pineros V, Galang JA, McClintock MK, Prendergast BJ. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc Natl Acad Sci U S A. 2009;106:9069–9074. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, Heller HC. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci U S A. 2008;105:15593–15598. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Singh M, McGinley J. Classification of premalignant and malignant lesions developing in the rat mammary gland after injection of sexually immature rats with 1-methyl-1-nitrosourea. J Mammary Gland Biol Neoplasia. 2000;5:201–210. doi: 10.1023/a:1026495322596. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel JS, Witgert ME, Meyers CA. Neuropsychological sequelae of non-central nervous system cancer and cancer therapy. Neuropsychol Rev. 2008;18:121–131. doi: 10.1007/s11065-008-9058-x. [DOI] [PubMed] [Google Scholar]
- Wenk GL. Assessment of spatial memory using the radial arm maze and Morris water maze. Curr Protoc Neurosci. 2004;Chapter 8(Unit 8 5A) doi: 10.1002/0471142301.ns0805as26. [DOI] [PubMed] [Google Scholar]
- Wolf OT. HPA axis and memory. Best Pract Res Clin Endocrinol Metab. 2003;17:287–299. doi: 10.1016/s1521-690x(02)00101-x. [DOI] [PubMed] [Google Scholar]


