Abstract
A personal account is given of the development in my laboratory of designed molecular rearrangements and their application in target-directed synthesis.
1. Introduction
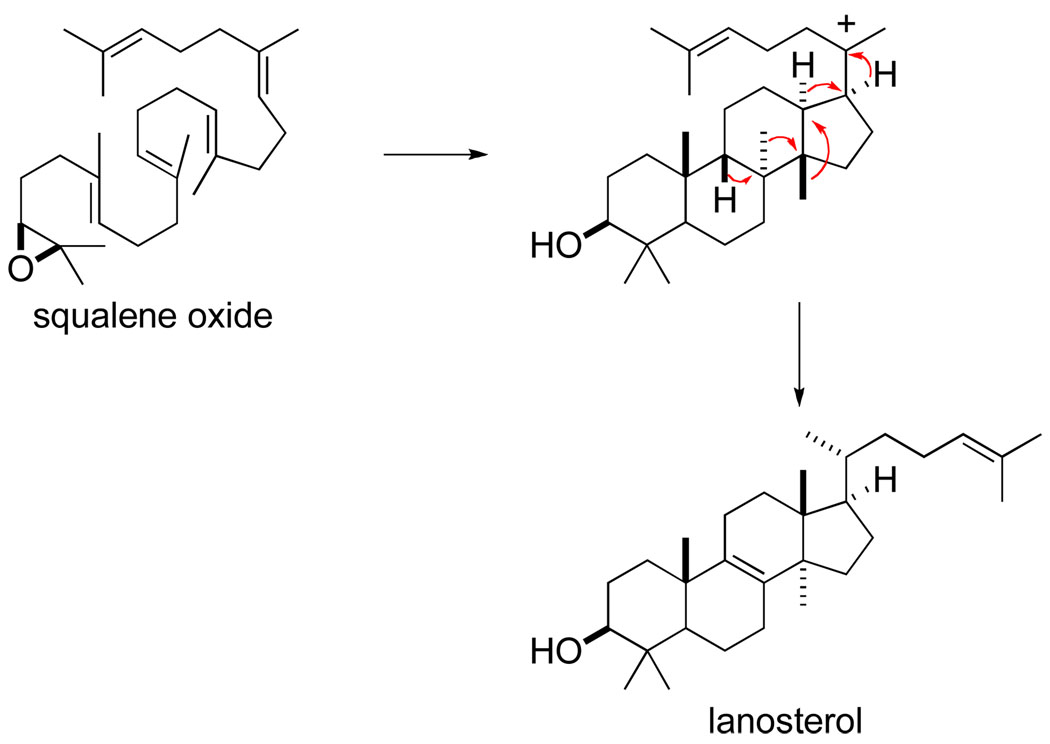
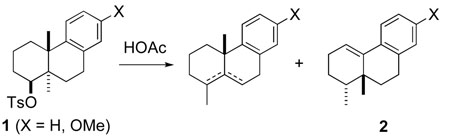
My fascination with rearrangement reactions is easily traced to the fall of 1965, just after I had begun graduate school in the Chemistry Department at the University of Wisconsin, Madison. I was in the office of Howard Whitlock, Jr. to discuss potential research opportunities in his laboratory. He outlined, gloriously as I remember with a fountain pen on white paper, the polyene cyclization and backbone rearrangement steps of the postulated biosynthesis of lanosterol from squalene oxide (Scheme 1). As a potential dissertation project, Whitlock suggested examining in model systems whether or not backbone rearrangements take place in a concerted fashion. I was fascinated. The bulk of my subsequent dissertation focused on solvolytic rearrangements of tosylate 1, with these studies indicating that the backbone rearrangement leading to 2 proceeded by sequential 1,2-methyl shifts and not in a concerted fashion (eq 1).2 Synthesizing such substrates was not a simple matter in the late 1960’s; so besides mechanistic organic chemistry, I was introduced, fortunately as it turned out, to quite a bit of organic synthesis during my Ph.D. studies.2,3
 |
(1) |
Scheme 1.
After receiving my Ph.D. in 1969, I went on to spend two stimulating years at Columbia University as a postdoctoral fellow with Ronald Breslow. I became involved with projects in the bioorganic chemistry area, initially using the non-covalent binding properties of cyclodextrin to fashion a model of an enzyme active site. Our paper reporting these studies is the first, I believe, to use the term artificial enzyme.4
I left Columbia in June 1971 to begin my academic career at the Irvine campus of the University of California, which had been founded only six years earlier. I was fortunate to have a wonderfully supportive group of senior organic colleagues – Marjorie Caserio, Harold Moore and Robert Taft – who shared with me needed wisdom about teaching and mounting a research program. The Irvine graduate program at the time was small, and my research group certainly so. Thus, any time that I was not teaching, I could be found in the lab. Like many assistant professors, I initiated studies in several diverse areas. One project, inspired I am sure by Breslow’s remote-functionalization studies,5 employed hemiacetals generated in situ to selectively deliver oxygen nucleophiles to nearby double bonds (Scheme 2).6,7
Scheme 2.
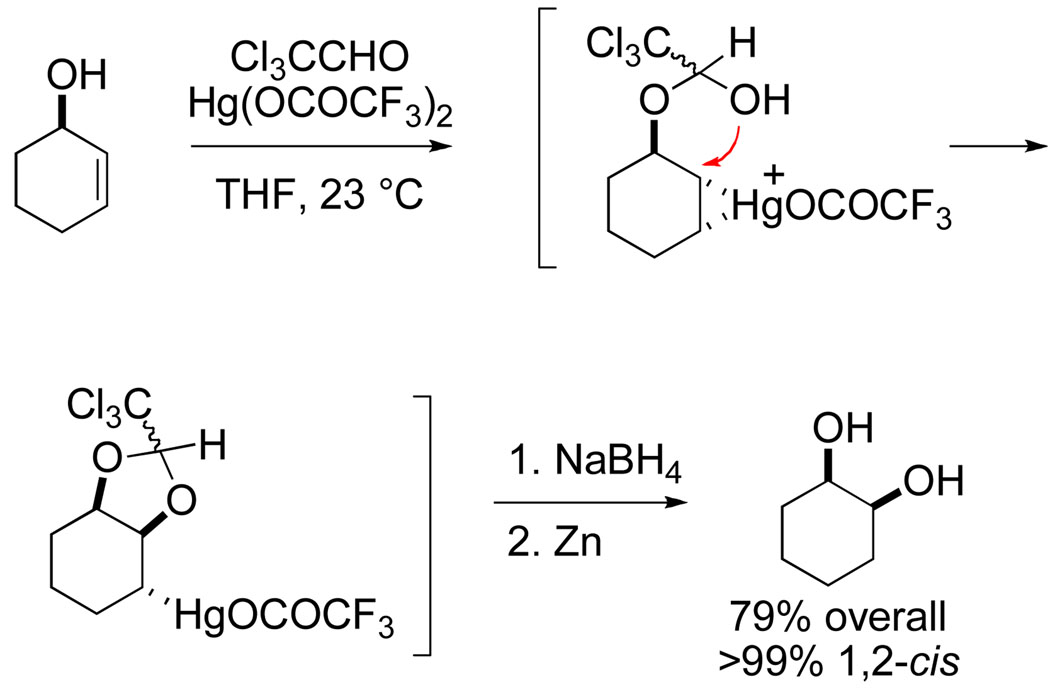
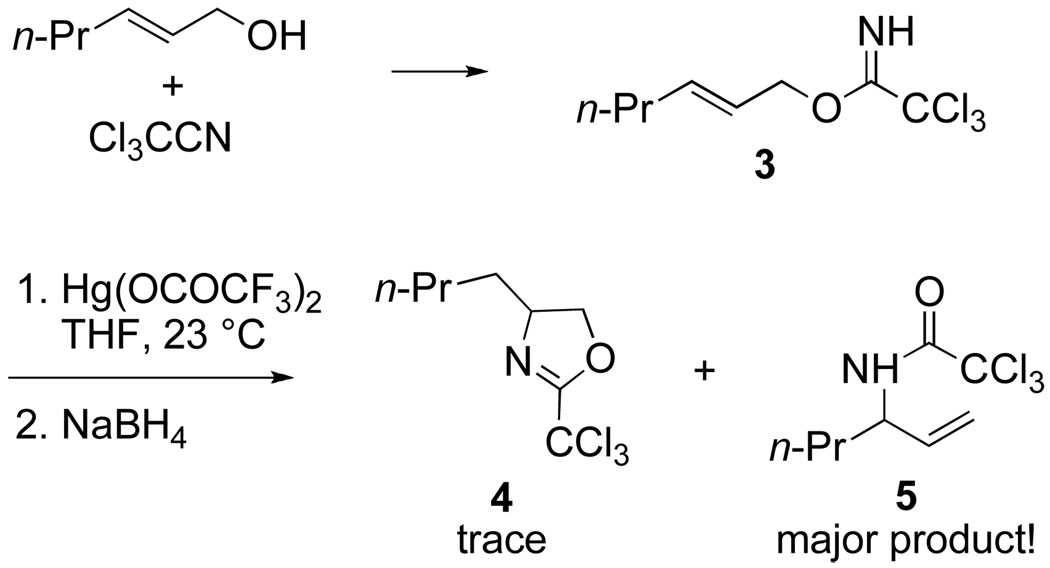
It was in attempting to extend this intramolecular delivery concept to nitrogen nucleophiles that I became reacquainted with rearrangement reactions. Because there is no direct nitrogen equivalent of a hemiacetal, I turned to examine intramolecular aminomercuration reactions of trichloroacetimidate derivatives of allylic alcohols, Cramer having earlier shown that these intermediates could be prepared under mild conditions by base-catalyzed condensation of alcohols with trichloroacetonitrile.8 I anticipated that imidate derivative 3 upon exposure to mercuric trifluoroacetate would produce oxazoline 4, which upon hydrolysis would accomplish the selective conversion of trans-2-hexen-1-ol to 2-aminohexan-1-ol (Scheme 3). However, the major product was not oxazoline 4, but allylic trichloroacetamide 5, the product of formal [3,3]-sigmatropic rearrangement.9
Scheme 3.
I still remember the excitement I felt that Saturday afternoon in the lab when the diagnostic 1H NMR signals for the terminal vinyl group of allylic trichloroacetamide 5 suggested what had transpired, and quickly thereafter I surmised how a mercury(II) complex might promote an allylic transposition. This serendipitous discovery initiated my laboratory’s involvement with three research themes that persist to this day: rearrangement reactions, synthesis of nitrogen-containing compounds, and metal-catalyzed transformations.
I published five single-author papers during my early years at Irvine, which reflects both my enjoyment of laboratory research and the small size of my research group at the time. This number was uncommon even in the 1970s, although many of my highly successful contemporaries initiated their independent research programs with at least one single-author paper.
2. Allylic Rearrangements of Imidates and Related Intermediates and Diels–Alder Reactions of N-Acylamino-1,3-dienes
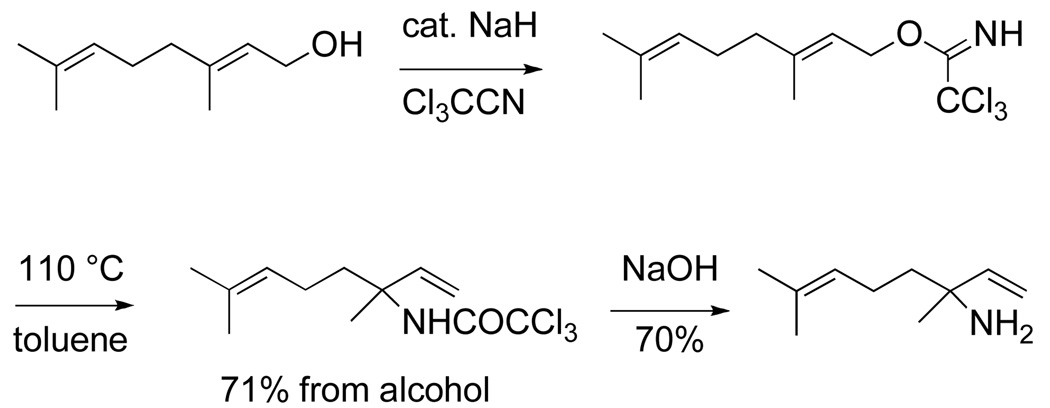
I was quickly able to show that allylic trichloroacetimidate rearrangements could be accomplished at room temperature or below using catalytic amounts of mercuric trifluoroacetate (eq 2), or more generally by simple thermal rearrangement (Scheme 4).9,10,11 This method for 1,3-transposition of alcohol and amine functional groups, first reported in 1974, remains today a widely used method for accomplishing this conversion.12 The mechanism I suggested for the catalytic rearrangement (eq 2) was inspired by a related mechanism suggested by Patrick Henry for Pd(OAc)2-catalysis of allylic ester equilibrations.13 Soon thereafter, we illustrated the generality of this catalysis mechanism, which we termed "cyclization-induced rearrangement atalysis".14,15
 |
(2) |
Scheme 4.
Palladium(II) complexes eventually emerged as preferred catalysts for allylic trichloroacetimidate rearrangements,16,17 serving today as the foundation of modern developments of catalytic asymmetric variants of this synthesis of allylic amines.12,18 This finding led to our long-standing interest in organopalladium chemistry, initially to the discovery of the palladium(II)-catalyzed Cope rearrangement,19 and subsequently to our development of intramolecular Heck reactions20,21 and catalytic asymmetric Heck reactions22 as broadly useful transformations for assembling complex polycyclic structures.23,24
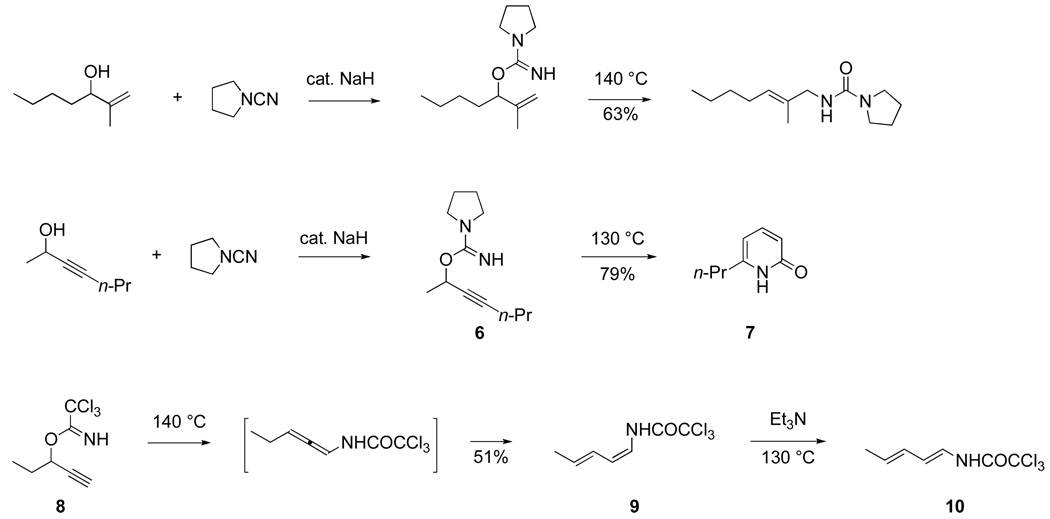
Thermal rearrangements of imidate,25 cyanate26 and pseudourea27,28 derivatives of allylic and propargylic alcohols were studied by several of my earliest coworkers (Scheme 5).16 Sadao Tsuboi, my first postdoctoral fellow, uncovered a new synthesis of 2-pyridones by thermolysis of propargylic pseudourea derivatives (e.g., 6 → 7).28,29 The thermal rearrangement of propargylic trichloroacetimidates to initially generate allenyl trichloroacetamides,30 which after tautomerization and 1,5-sigmatropic hydrogen migration, yield (Z)-1-trichloroacetamido-1,3-dienes (e.g., 8 → 9) was discovered by Lane Clizbe, one of my first Ph.D. students.25,31 This diene synthesis soon led us to become involved in alkaloid total synthesis.
Scheme 5.
Application of hetero-substituted 1,3-dienes, such as the Danishefsky diene,32 in Diels– Alder constructions was an area of active study at this time. Wolfgang Oppolzer had just described the use of 1-N-acyl-1-alkyl-1-amino-1,3-dienes in intramolecular Diels–Alder reactions,33 and we began to develop the bimolecular Diels–Alder chemistry of 1-N-trichloroacetyl-1-amino-1,3-dienes.34–36 The Z stereoisomer of such dienes is the product of the rearrangement of progargylic trichloroacetimidates, which necessitates subsequent base-promoted isomerization (9 → 10) to access the more useful 1E stereoisomers. Moreover, this route did not allow for much variation in the nature of the nitrogen substituent. As a result, we developed a straightforward Curtius rearrangement route to more useful (E)-1-JV-acyloxy-1-amino-1,3-dienes (eq 3) 34,37
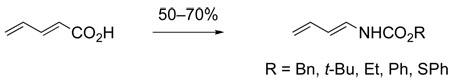 |
(3) |
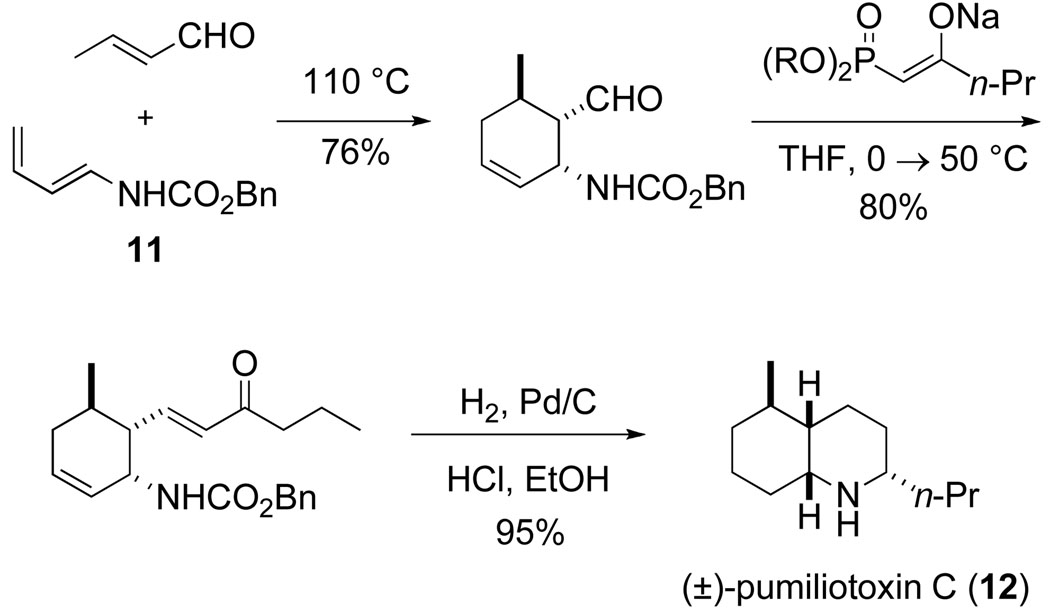
The first natural product total synthesis achieved in my laboratory, that of (±)-pumiliotoxin C (12), was accomplished by postdoctoral fellow Peter Jessup during late 1976 and early 1977 38 The most direct variant of this synthesis is depicted in Scheme 6.39 Although pumiliotoxin C is a simple molecule by contemporary standards, I believe that the conciseness of this synthesis is notable even today.
Scheme 6.
During the 1970s natural products total synthesis was a rapidly growing focus of organic chemistry, as increasingly complex target structures were being prepared by imaginative synthetic sequences.40 The accomplishments of R. B. Woodward, E. J. Corey, Gilbert Stork, W. S. Johnson, Albert Eschenmoser, Yoshito Kishi and many others created enormous excitement in the field of synthesis at the time. My decision to get involved, even though an outsider, in natural products total synthesis was a product of this remarkable environment. I was also fortunate early in my career that Samuel Danishefsky spent a brief sabbatical period at Irvine, spring quarter of 1977 as I remember. I am sure that my decision to have target-directed synthesis play an increasingly important role in my research program was influenced by the many stimulating discussions Sam and I had during that period.
3. The Aza-Cope–Mannich Reaction
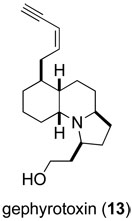
In contrast to the accidental discovery of the rearrangement of allylic trichloroacetimidates to allylic trichloroacetamides, the aza-Cope–Mannich reaction was invented to solve a stereoelectronic problem in a total synthesis endeavor. The context was gephyrotoxin (13), a more elaborate poison-dart alkaloid that, like pumiliotoxin C, had been isolated by the pioneering studies of amphibian natural products by the late John Daly.41
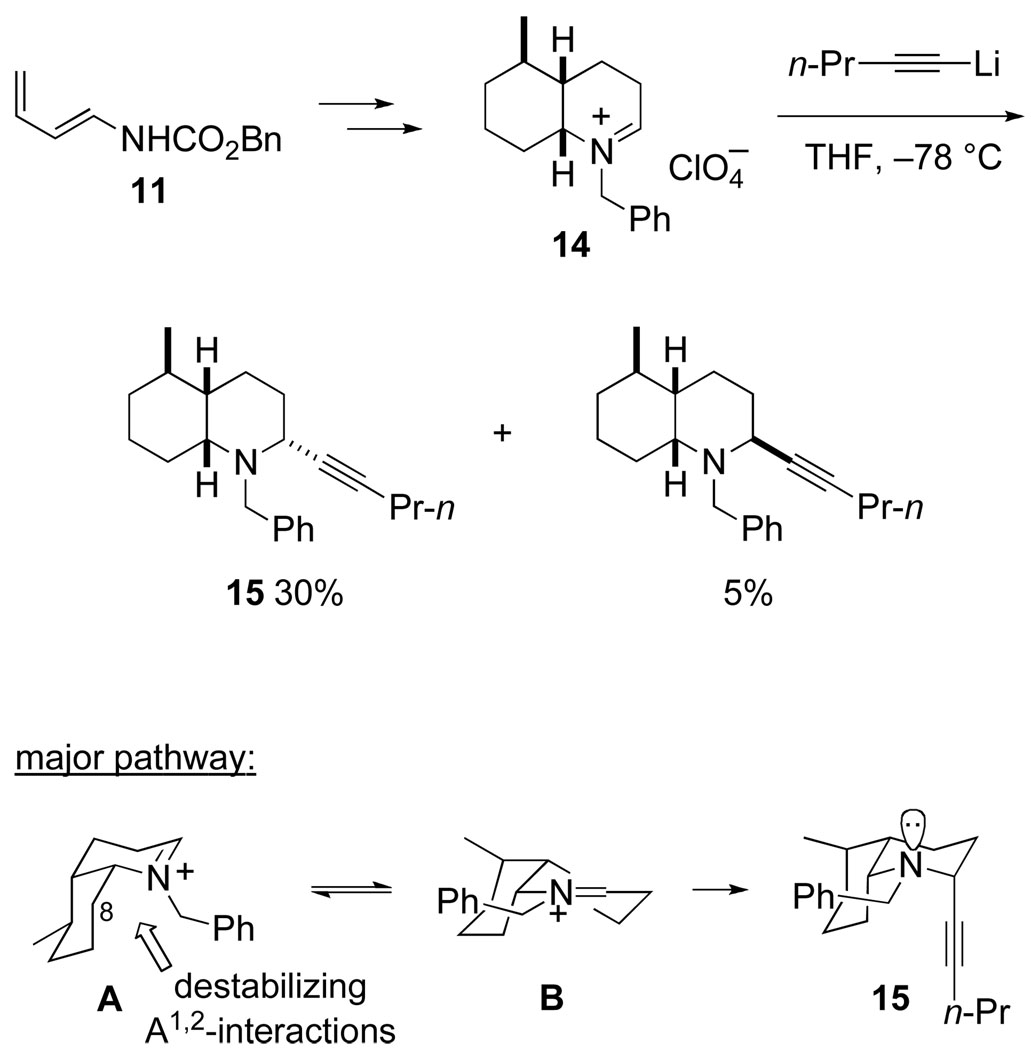
Our initial plan for preparing gephyrotoxin envisaged using a Diels–Alder reaction of diene carbamate 11 to construct the cis-octahydroquinoline moiety, to which we would append the pyrrolidine ring and its hydroxyethyl side chain from the sterically less-hindered β-face. We were quite surprised when Dr. Mitsunori Hashimoto’s early investigation of this plan in a model series showed that an alkyne nucleophile added preferentially to cis-octahydroquinolinium cation 14 from the concave α-face (Scheme 7). We had failed to appreciate that in conformer A destabilizing steric interactions between the N-benzyl substituent and C8 of the cis-octahydroquinolinium ring would result in nucleophilic addition taking place preferentially by way of the alternate cis-octahydroquinolinium ion conformer B, in which case a stereoelectronic preference for the nucleophile and developing non-bonded electron-pair on nitrogen to be aligned antiperiplanar42 would result in preferential formation of adduct 15.43
Scheme 7.
We surmised that one way to circumvent the stereoelectronic preference for adding nucleophiles from the undesired α-face of iminium ion intermediates such as B would be to employ a reaction in which the nitrogen of the iminium ion electrophile would not become pyramidalized during the formation of a σ-bond at the iminium ion carbon. A [3,3]-sigmatropic rearrangement of an unsaturated iminium ion, a reaction known since its early discovery by Geissman to be extremely facile,44 would fit this prescription (eq 4).45 To render such a transformation irreversible, we incorporated an oxygen substituent vicinal to the starting iminium nitrogen so the product of aza-Cope rearrangement would be trapped by an intramolecular Mannich cyclization (eq 5).
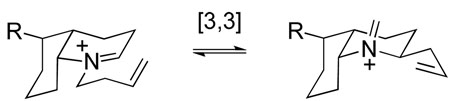 |
(4) |
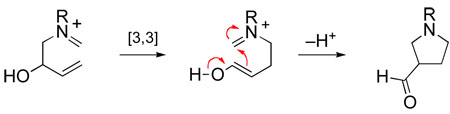 |
(5) |
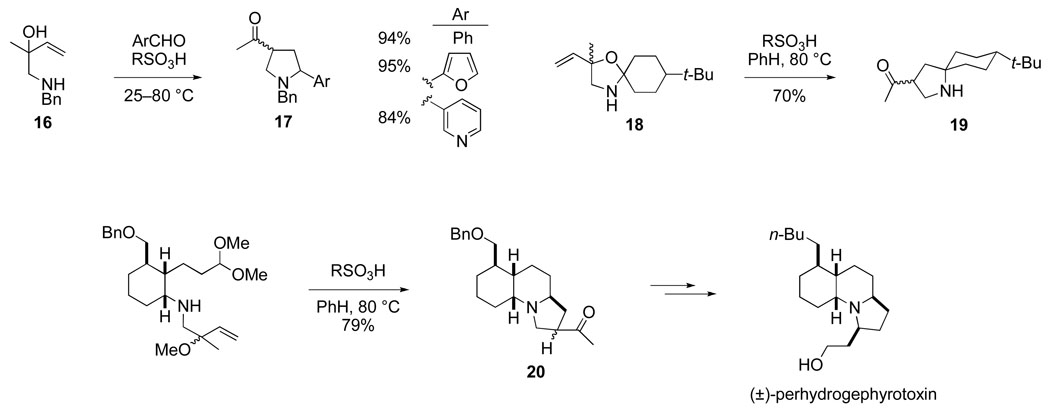
This cascade sequence, now commonly referred to as the aza-Cope–Mannich reaction, was first demonstrated for the synthesis of simple 3-acylpyrrolidines by Masa-aki Kakimoto in 1979 during a two-year period he spent in my laboratory while a Ph.D. student at Tokyo Institute of Technology (Scheme 8).46 As Kakimoto showed, simple 3-acylpyrrolidines are formed in good yields by direct acid-promoted condensation of homoallylic amines such as 16 with aldehydes, or preferably for ketones by rearrangement of 4-vinyloxazolidine intermediates (e.g., 18 → 19).47 Another co-worker at the time, Dr. Chikara Fukuya, quickly showed that the condensation could be carried out in an intramolecular fashion to form the gephyrotoxin ring system with high stereoselectivity. Dr. Fukuya subsequently elaborated aza-Cope–Mannich product 20 to complete in 1980 the first total synthesis of (±)-perhydrogephyrotoxin.48 Using an alternate strategy for introducing the pyrrolidine ring, Dr. Dominick Lesuisse completed a more concise construction of (±)-gephyrotoxin in 1983,43 shortly after Yoshito Kishi had reported the first total synthesis of this natural product.49
Scheme 8.
Because of the wide occurrence of pyrrolidine rings in both alkaloids and pharmaceutical agents, we recognized that the aza-Cope–Mannich reaction might have a much broader significance, being potentially useful for the synthesis of a variety of azacyclic ring systems. During the early and mid 1980s, my co-workers delineated many of the features of this cascade reaction (Scheme 9).50,51 The mild conditions of this transformation — neutral pH at room temperature or slightly above — are undoubtedly responsible for the success of the aza-Cope– Mannich reaction with aldehydes such as furfural and the reaction’s compatibility with acid-sensitive tertiary allylic alcohol functionality. The high preference for the iminium ion sigmatropic reorganization to take place by a chair topography, a feature critical to its use to assemble complex ring systems with high stereocontrol, was first defined by the stereoselective formation of pyrrolizidine 22 from E precursor 21, and the cognate transformation of the Z alkene analog to pyrrolizidine products epimeric at the methyl-bearing carbon.52 Another modification developed in these early studies is the use of a cyanomethyl group to generate an initial formaldiminium ion (e.g., 23 → 24 and 25 → 26), a strategy that is particularly useful when this group also serves to protect nitrogen in the construction of the allylic alcohol rearrangement precursor.53
Scheme 9.
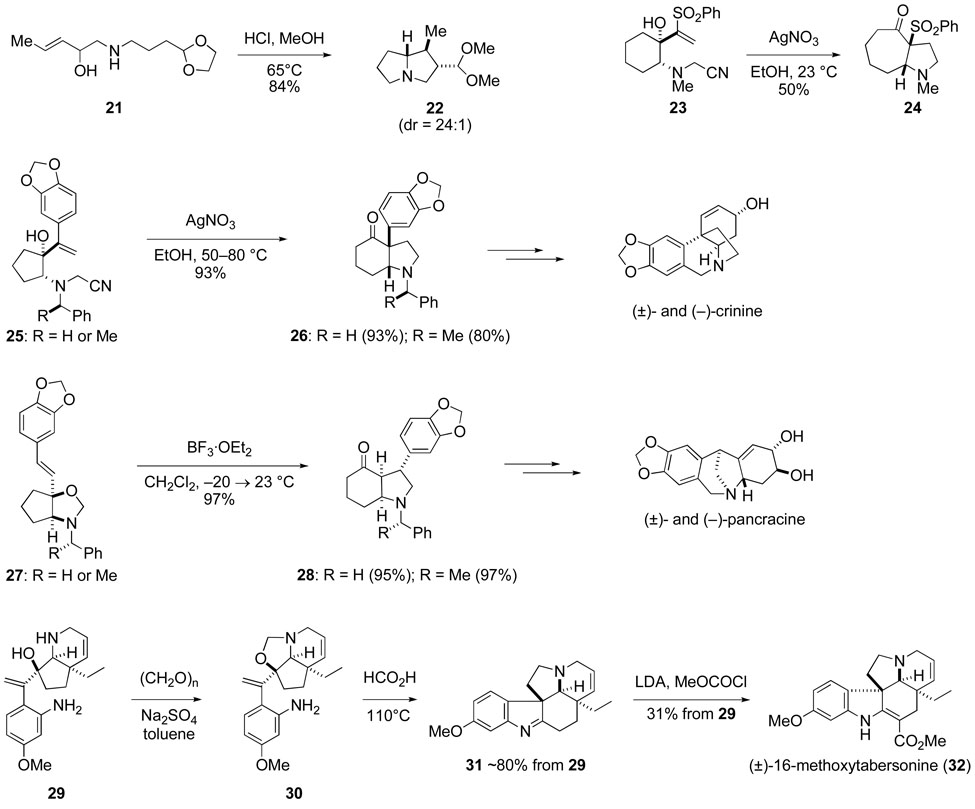
If the vicinal amine and alcohol groups are located on a ring, the aza-Cope–Mannich reaction couples pyrrolidine formation with one-carbon expansion of the starting ring, a feature illustrated in the conversion of 23 → 24, the transformation of cyclopentane derivatives 25 and 27 to cis-hydroindolones 26 and 28, and the construction of pentacyclic diamine 31 upon heating crude oxazolidine 30 in toluene in the presence of a trace of formic acid.54–56 Selectively combining an aldehyde or ketone with the vicinal amino alcohol by first generating an oxazolidine intermediate, as illustrated in this latter example, allows the aza-Cope–Mannich transformation to be carried out with substrates carrying a wide range of functionality, including primary amines and electron-rich styrene double bonds. The incorporation of the double bond in a suprafacial sense, as seen in the conversion of 27 → 28, is one of the distinctive features of this cascade transformation.
Mechanistic studies are consistent with this pyrrolidine synthesis occurring by the sigmatropic rearrangement/Mannich cyclization pathway as suggested in eq 5, and not by an alternate aza-Prins cyclization/pinacol rearrangement sequence. The racemization that is observed in aza-Cope–Mannich reactions of some chiral, enantioenriched precursors, and the success of the aza-Cope–Mannich reaction with alkenes of widely varying nucleophilicity, including strongly electron-deficient ones (e.g., 23 → 24), are consistent with this mechanistic formulation.57
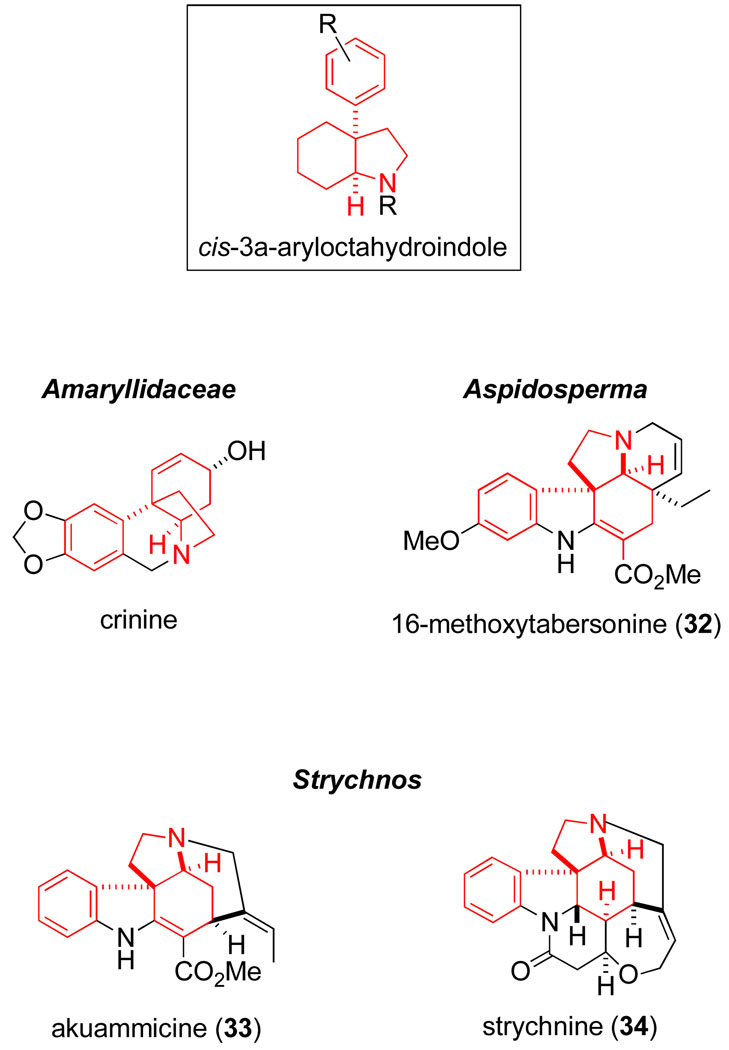
Of the several transformations that have been extensively studied in my research group over the years, the aza-Cope–Mannich reaction is the most robust. My appreciation of this fact developed as the scope of the aza-Cope–Mannich reaction was elaborated during various total synthesis ventures. Initially these studies focused on alkaloids of the Amaryllidaceaeliva,100,58–61 and Aspidosperma54,62,63 families (Scheme 9), and shortly thereafter Strychnos alkaloids.64–68 A common structural feature of these natural products is a cis-3a–aryloctahydroindole unit (Figure 1), a ring system that can be assembled with high stereoselectivity by the ring-enlarging variant of the aza-Cope–Mannich reaction. It was largely during these projects that my laboratory learned how to orchestrate multistep synthesis endeavors, and we began to appreciate the synergistic role that target-directed synthesis projects and exploratory reaction development studies can play in the evolution of a research program in synthetic organic chemistry.
Figure 1.
Representative alkaloids containing a cis-3a-aryloctahydroindole fragment prepared using aza-Cope–Mannich reactions.
It was only a few years after developing the aza-Cope–Mannich reaction that we began thinking about applying this transformation to prepare strychnine (34), which at the time had been synthesized only by Woodward in his landmark 1954 total synthesis.69 During our early Aspidosperma alkaloid total synthesis studies carried out by graduate students Robert Burk and Albert Robichaud and postdoctoral fellows Michael Sworin and Graeme Robertson,56,62,63 we learned how to assemble rather elaborate rearrangement precursors such as vinyloxazolidine 30 (see Scheme 9). Aza-Cope-Mannich rearrangement of this intermediate and concomitant intramolecular imine formation gives pentacyclic product 31, which in one additional step is converted to 16-methoxytabersonine (32).56
Early on, we envisaged strychnine evolving from the well known Wieland–Gumlich aldehyde 35, which in turn would arise from a pentacyclic precursor having the curane ring system such as 18-hydroxyakuammicine 36 (Scheme 10). Our early decision to target aldehyde 35 as the immediate precursor of strychnine rather than isostrychnine, the precursor in the Woodward synthesis,69 was based on the high yields that had been reported for the conversion of Wieland–Gumlich aldehyde to strychnine70 and the low efficiency of the isostrychnine to strychnine isomerization. The widely occurring Strychnos alkaloid akuammicine 33 was selected as our initial target. Two approaches for constructing potential akuammicine (and 18-hydroxyakuammicine) precursors using the aza-Cope–Mannich reaction are suggested in Scheme 10.
Scheme 10.
Steven Angle, the first of several Ph.D. students who pursued the total synthesis of strychnine, surveyed the more conservative strategy in which aza-Cope–Mannich reaction of amino cyclopentanol 40 would generate a cis-hydroindolone intermediate 38, bearing the side chain required for forming the bridging piperidine ring of the curane skeleton. He developed a route to aza-Cope–Mannich precursor 41 and showed that it is transformed in high yield to pentacyclic product 42 when heated in refluxing toluene in the presence of camphorsulfonic acid (eq 6).64 At the end of his Ph.D. studies, Steven Angle briefly examined, without success, closing the piperidine ring from intermediates derived from 42.
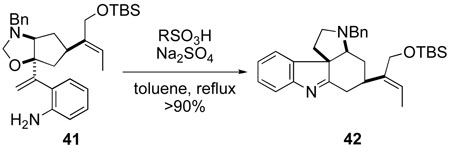 |
(6) |
Given more time, it is likely that the curane ring system could have been forged from tetracyclic intermediates such as 42. However, we were at the time gaining more confidence in the aza-Cope–Mannich transformation and chose to pursue the second approach adumbrated in Scheme 10 in which aza-Cope–Mannich reorganization would be carried out with a more elaborate precursor that already incorporates the piperidine ring. The challenge in this case is assembling the bridged-bicyclic intermediate 39, in particular how to incorporate the styrenyl functionality on the congested endo face of the azabicyclo[3.2.1]octane ring system. Postdoctoral fellow John Fevig eventually developed a concise way to prepare such aza-Cope–Mannich precursors by intramolecular aminolysis of epoxide intermediates in route to completing the first total synthesis of the Strychnos alkaloid (±)-dehydrotubifoline (Scheme 11).65 The pivotal aza-Cope–Mannich transformation (43 → 44) is realized in 88% yield when dienyl amino alcohol intermediate 43 is exposed to excess paraformaldehyde and 1 equiv of camphorsulfonic acid in refluxing acetonitrile. Shortly thereafter, graduate student Steven Knight used a related sequence to prepare (±)-akuammicine (33).66
Scheme 11.
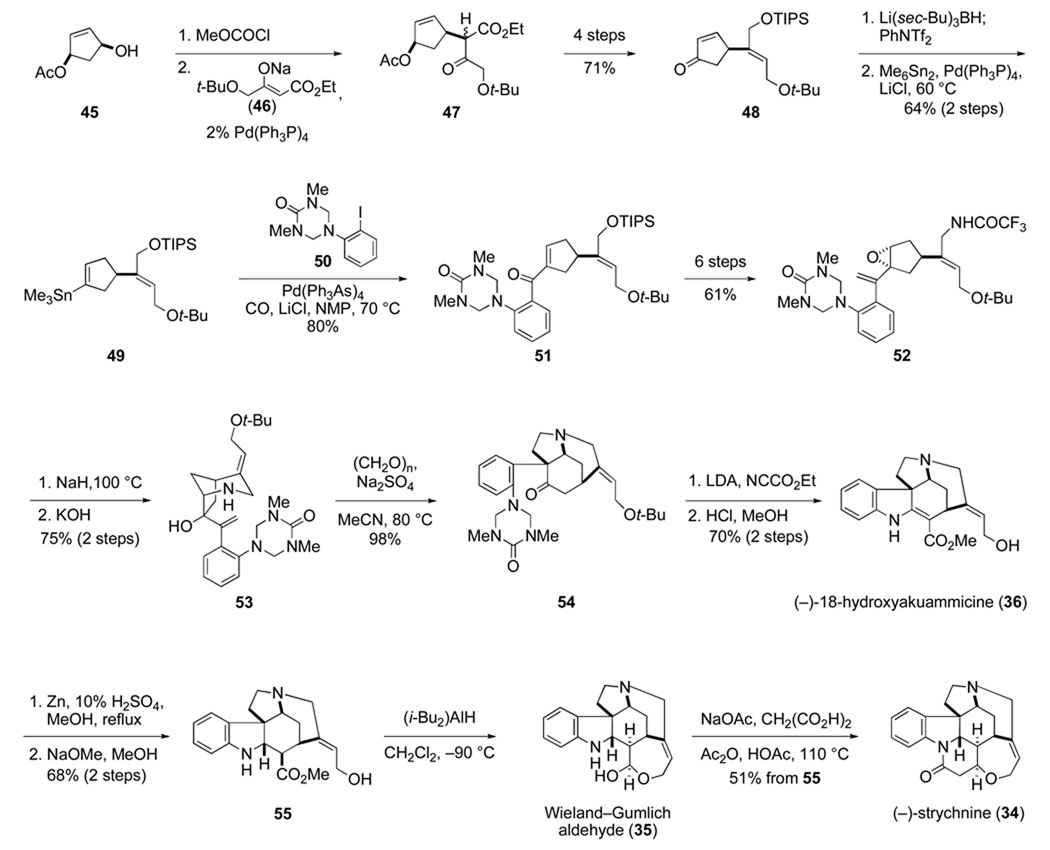
The stage was now set for addressing strychnine, a challenge taken up by Steven Knight and postdoctoral fellow Garry Pairaudeau. At this point, we had found that masking the aniline nitrogen as a triazone71 during assembly of the aza-Cope–Mannich precursor is ideal, and that the 4-aryltriazone unit is best incorporated by carbonylative Stille cross-coupling with a vinyl stannane intermediate generated from a cyclopentanone precursor. Thus, in the first stage of our strychnine synthesis, a cyclopentenone such as 48 (or an analogous cyclopentanone) would need to be assembled (Scheme 12). The differentially protected (Z)-3-hydroxy-1-hydroxymethyl-1-propenyl side chain of this intermediate would, at a late stage of the synthesis, be fashioned into the F and G rings of strychnine.
Scheme 12.
With surprising frequency an apparently simple synthetic objective can present a significant roadblock in a total synthesis endeavor. Such was the case in this undertaking, where developing an efficient stereocontrolled synthesis of cyclopentenone intermediate 48 was the key hurdle Steven Knight had to overcome. Numerous ways to couple a cyclopentene unit with a (Z)-2-butenyl fragment were investigated without success, with the mood in the laboratory becoming somewhat downbeat. Fortunately, Steven Angle, then a Professor at UC Riverside, was on sabbatical leave at Irvine at the time and suggested that we return to the strategy that he had developed during his Ph.D. studies in which a β-ketoester precursor is employed to fashion a less elaborate (Z)-butenyl side chain. Such an approach proved workable.67
Our total synthesis of (−)-strychnine thus begins with (1R,4S)-4-hydroxy-2-cyclopentenyl acetate (45), which is available on a large scale by desymmetrization of the meso-diacetate precursor with electric eel acetyl cholinesterase (Scheme 12).72 Palladium-catalyzed coupling of the allylic carbonate derivative of 45 with the β-ketoester enolate 46 gives cyclopentenyl acetate 47 in high yield. Anti-selective reduction of the β-ketoester side chain, followed by syn elimination of the β-hydroxyl ester products, are key steps in the four-step conversion of this intermediate to cyclopentenone 48.
Once in hand, cyclopentenone 48 was elaborated fairly quickly to (−)-strychnine. First (−)-18-hydroxyakuammicine (36) was prepared using chemistry developed during our earlier total synthesis of akuammicine. Key steps in the sequence are carbonylative Stille cross-coupling (49 + 50 → 51), base-promoted cyclization of cyclopentane epoxide 52 to form azabicyclooctane 53 after removal of the trifluoroacetyl group, and aza-Cope-Mannich rearrangement of the formaldiminium derived from 53 to give pentacyclic intermediate 54 in high yield. Guided by earlier studies in the Strychnos alkaloid area,73 we were able to saturate the double bond of 18-hydroxyakuammicine (36) and epimerize the α-ester product to yield intermediate 55. The final hurdle that had to be surmounted was defining conditions for selective reduction of the ester group of 55 to form Wieland-Gumlich aldehyde (35), an established precursor of strychnine.70 It turned out that diisobutylaluminum hydride at low temperature accomplishes this transformation quite cleanly.
The synthesis summarized in Scheme 12 is the first enantioselective total synthesis of (−)-strychnine, and at the time the most efficient construction of this benchmark natural product.67,68 Its significance, however, derives more from the large number of relatively new transformations utilized in the synthesis than from this milestone. Besides the aza-Cope– Mannich transformation, four catalytic reactions played pivotal roles: enzyme-catalyzed deacetylation to introduce the initial stereocenter, and three palladium-catalyzed coupling reactions –– Tsuji–Trost allylation, formation of a vinylstannane from an enol triflate, and carbonylative Stille cross-coupling.
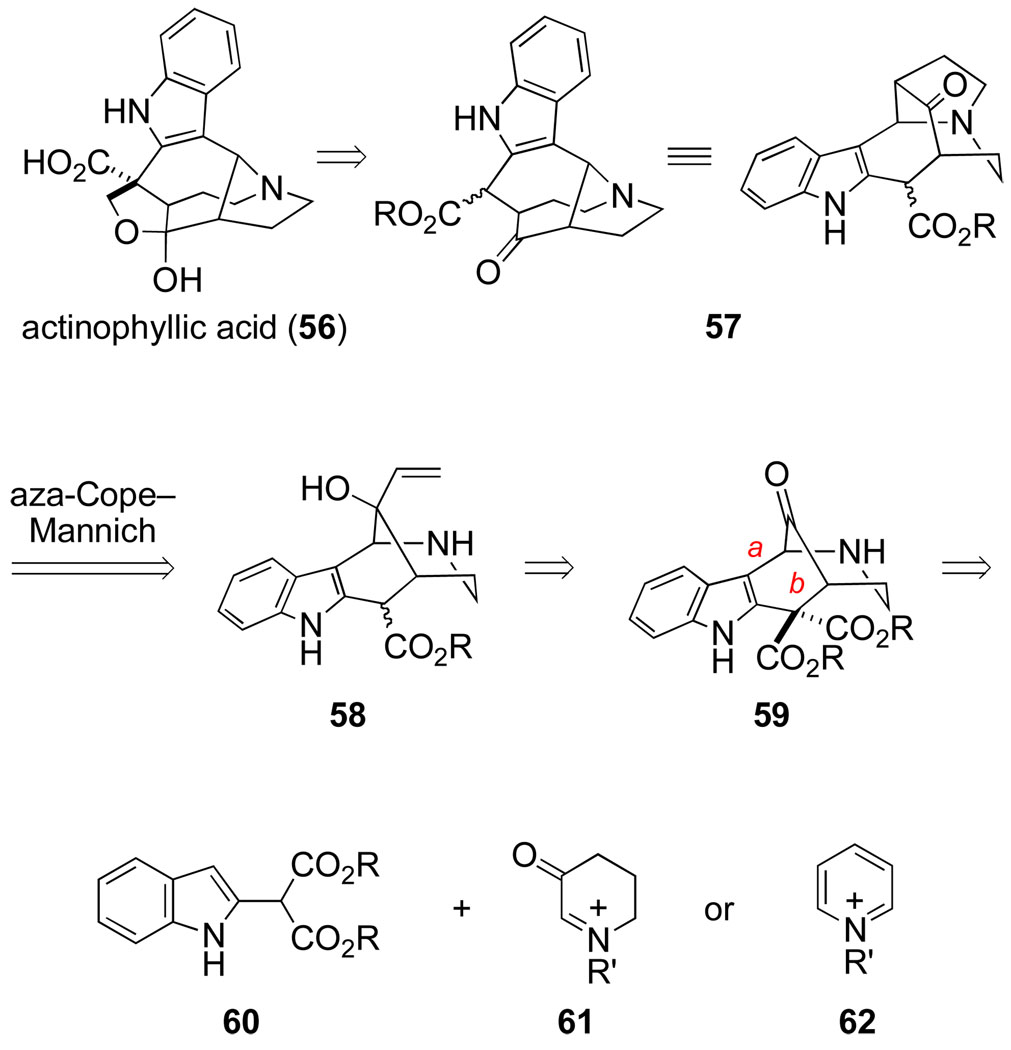
The ring-enlarging version of the aza-Cope–Mannich reaction is particularly useful when it allows a readily accessible precursor ring system to be transformed in one step to a more complex one. A good illustration is provided by our recent total synthesis of actinophyllic acid (56, Scheme 13),74 an indole alkaloid isolated by Quinn and co-workers from a screening campaign seeking natural inhibitors of carboxypeptidase U (TAFIa).75 The 1-azabicyclo[4.4.2]dodecane and 1-azabicyclo[4.2.1]nonane moieties that are signature features of its structure are found in no other indole alkaloid. This structurally unique alkaloid had just appeared in the literature when Jason Rohde joined my laboratory in mid 2005. I suggested actinophyllic acid to Jason as a potential postdoctoral project, with the notion that an aza-Cope– Mannich reaction might be employed to fashion the 1-azabicyclo[4.2.1]nonane fragment. He then developed an appealing plan in which an aza-Cope–Mannich reaction would be employed at a late stage to elaborate a tetracyclic intermediate having the ring system of uleine alkaloids to the pentacyclic core of actinophyllic acid (Scheme 13). Although several routes had been defined earlier for preparing the uleine alkaloid ring system,76 assembling tetracyclic intermediate 59 from an indole malonate precursor 60 looked particularly attractive.
Scheme 13.
At the outset, Jason Rohde examined assembling tetracyclic intermediate 59 from the conjugate base of malonate 60 and a pyridinium electrophile 62 (R’ = Tf, CO2CH2CCl3, etc.). However, this union did not take place at the malonate side chain, as we had anticipated, but rather at the β-carbon of the indole. It was apparent that our strategy needed to be modified so that intermediate 59 would be constructed by first forming bond a and then bond b, a thought process that eventually identified piperidone iminium ion 61 as the electrophilic fragment.
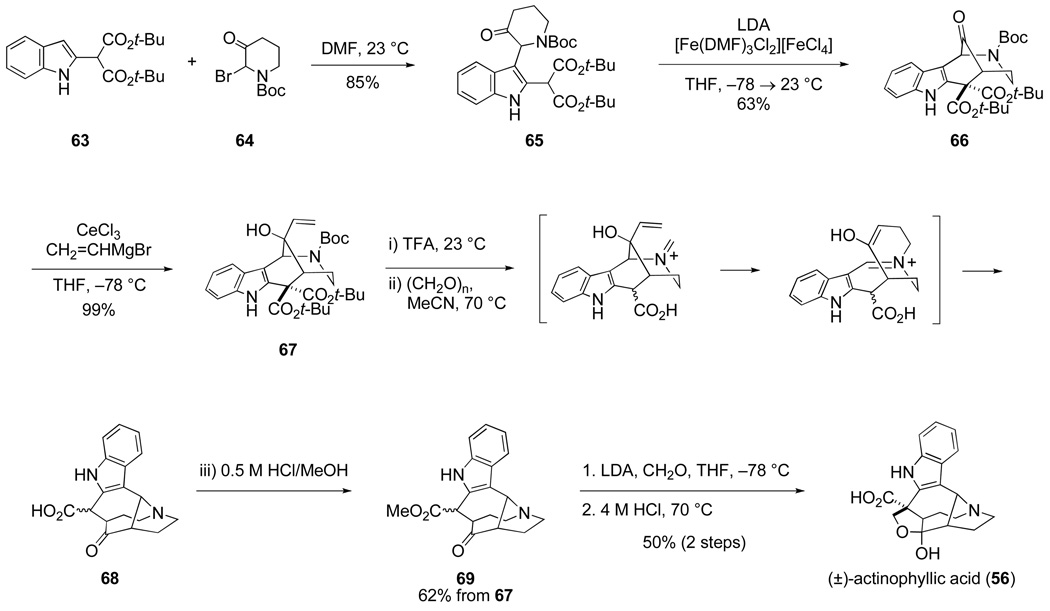
The total synthesis of (±)-actinophyllic acid that ultimately emerged is summarized in Scheme 14.74 The synthesis begins by condensing indole malonate 63 and 2-bromo-3-piperidone 64 in DMF to form indole derivative 65. Tetracyclic ketone 66 is then generated in one step by sequential reaction of intermediate 65 with 3.2 equiv of LDA and 3.5 equiv of the iron complex [Fe(DMF)3Cl2][FeCl4].77 Although the oxidative coupling of enolates and oxidative cyclization of two enolates was first described in 1977,78 this reaction has been underutilized in complex molecule construction.79 The cyclization of ketomalonate 65 to form tetracyclic ketone 66 is notable for both its high regioselectivity and compatibility with an electron-rich indole ring.
Scheme 14.
Second-year graduate student Connor Martin, who had recently joined the project, took over at this point. He subsequently developed several ways for advancing tetracyclic allylic alcohol 67 to actinophyllic acid. In the most concise and efficient sequence, this intermediate is exposed to trifluoroacetic acid at room temperature to cleave the tert-butoxycarbonyl group and the two tert-butyl esters, generating after decarboxylation an amino acid intermediate. After removing excess TFA in vacuo, the residue is dissolved in acetonitrile and paraformaldehyde is added to promote aza-Cope–Mannich elaboration to yield tetracyclic amino acid 68. Addition of acidic methanol to effect Fischer esterification gives amino ester 69 in 62% yield from this one-pot sequence. In two additional steps, this intermediate is converted to (±)-actinophyllic acid (56).
The synthesis of (±)-actinophyllic acid summarized in Scheme 14 proceeds by way of only seven isolated intermediates from o-nitrophenylacetic acid, the commercial precursor of indole malonate 63. It is rare in my experience that the first total synthesis of a structurally novel natural product is realized by a sequence this direct. The concise nature of this synthesis derives from the fact that all but one step form structural bonds (C–C or C–N) of actinophyllic acid. When the R enantiomer of tricyclic intermediate 65 is assembled, this sequence yields the natural levorotatory enantiomer of actinophyllic acid,80 confirming that the absolute configuration of (– )-actinophyllic acid is as depicted.81
I conclude our discussion of the aza-Cope–Mannich reaction by noting that this transformation has been utilized in original ways by other workers to assemble a variety of azacyclic structures. In the natural products arena, total syntheses of (–)-allokainic acid82 and (±)-epibatidine,83 a formal total synthesis of (–)-FR901483,84 and the construction of carbapenem precursors85 have been accomplished using this cascade transformation.
4. Pinacol-Terminated Cationic Cyclization Reactions
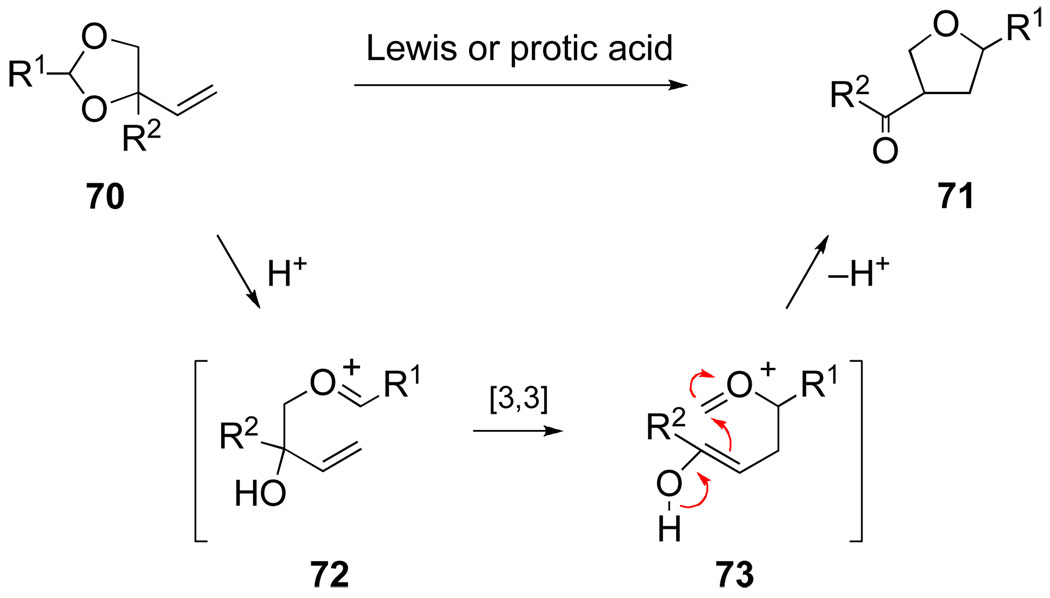
Soon after inventing the aza-Cope–Mannich reaction, we considered extending this reaction concept to the synthesis of five-membered ring oxygen heterocycles (Scheme 15). However, it was not until 1986 that graduate student Mark Hopkins first looked at this prospect. One reason for this delay was our concern that the transformation of an allylic acetal to a 3-acyltetrahydrofuran would require acidic reaction conditions, in contrast to the neutral conditions of the aza-Cope–Mannich reaction, heightening the prospect of competing ionization of the labile allylic C–O bond. Moreover, the [3,3]-sigmatropic rearrangement of unsaturated oxocarbenium ions (e.g., 72 → 73) had not been reported at that time, although it is now well established.86,87
Scheme 15.
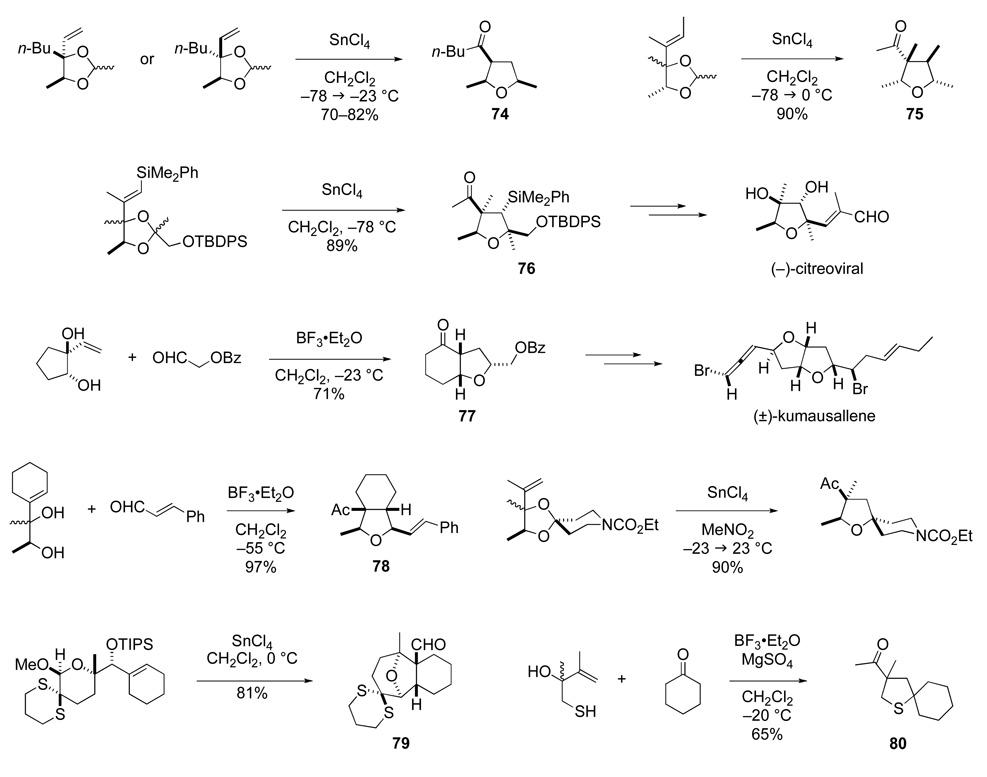
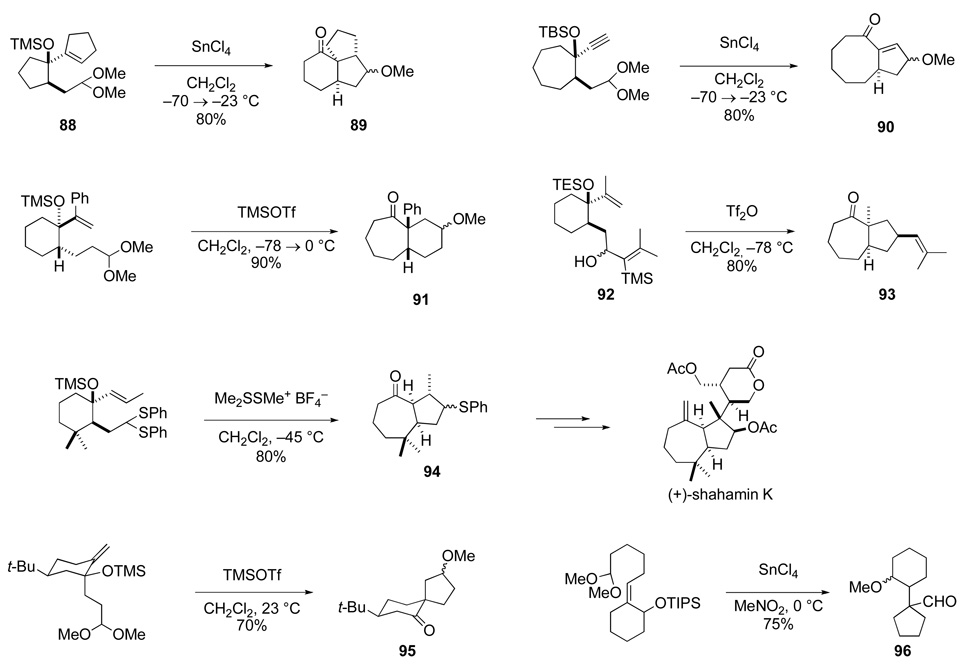
In his initial studies, Mark Hopkins was able to show that this construction of tetrahydrofurans is viable, and, under properly chosen conditions, proceeds with high stereoselectivity.88 Many aspects of the scope and limitations of this synthesis of tetrahydrofurans were defined over the next decade by the efforts of a number of my former co-workers.89–92 Representative examples of this transformation are illustrated in Scheme 16. Allylic acetals derived from both the syn- and anti-1,2-diol stereoisomers often give the 3-acyltetrahydrofuran product having a cis relationship of the acyl group and the side chains at C2 and C5 (e.g., 74 and 75). However, this result is not general, because the stereoelectronic effect of the allylic substituents that helps organize the transition structure is manifest to various degrees depending upon the nucleophilicity of the alkene and the reactivity of the oxocarbenium ion.93 Like the aza-Cope–Mannich reaction, a signal feature of this tetrahydrofuran synthesis is incorporation of the alkene in a suprafacial fashion (e.g., formation of 75 and 76). Often it is not necessary to preform an allylic acetal, as the direct condensation of allylic diols and aldehydes can deliver acyltetrahydrofuran products (e.g., 77 and 78). When the starting allylic diol is cyclic, a ring-enlarging furan annulation is realized, providing convenient access to a variety of cis-1-oxabicyclic ring systems (e.g., 77). If the initial oxocarbenium ion is incorporated in a ring, diverse oxacyclic structures such as 79 can be constructed.94 In addition, 3-acyltetrahydrothiophenes can be prepared in a similar manner (e.g., 80).95
Scheme 16.
The complete retention of configuration that is observed in the formation of tetrahydrofurans 81 and 82 (eq 7 and eq 8) suggests that this tetrahydrofuran synthesis does not proceed by the [3,3]-sigmatropic rearrangement/aldol pathway that we had initially envisaged (Scheme 15), but rather by a Prins cyclization/pinacol rearrangement pathway.89,96
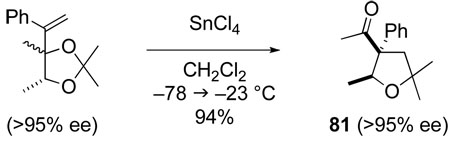 |
(7) |
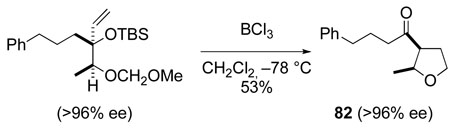 |
(8) |
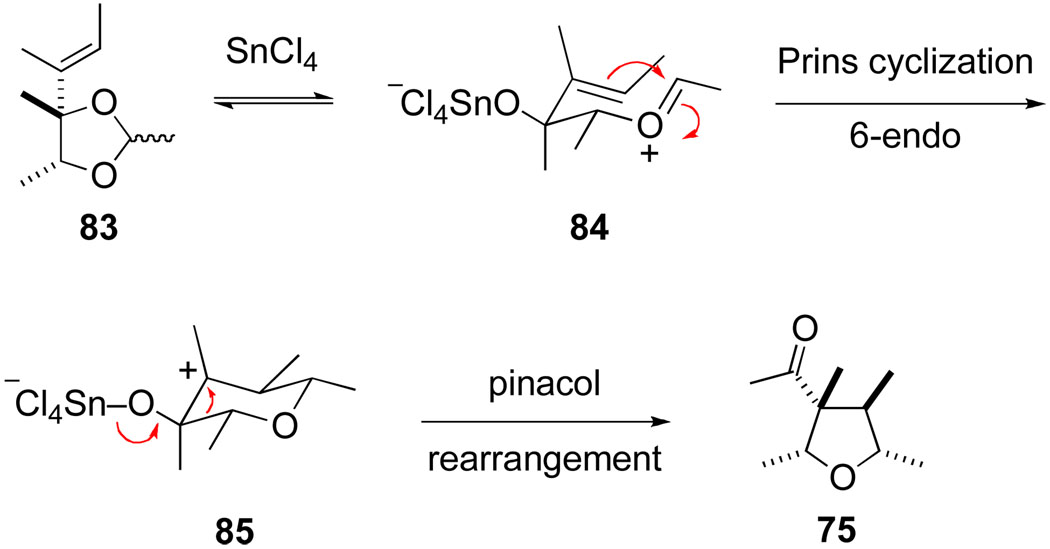
This Prins-pinacol sequence is depicted in Scheme 17 for the formation of tetrahydrofuran 75. Steric factors that contribute to the high stereoselectivity typically observed are also illustrated in this example: preferential Prins cyclization of an E oxocarbenium ion intermediate by a chair transition structure in which the 2-oxoallyl cation fragment is organized to minimize destabilizing allylic A1,3 interactions. The alternate oxocarbenium that could be generated by ring opening of acetal starting material 83 would be expected to undergo Prins cyclization only slowly, because the intramolecular Prins reaction in this case would involve a disfavored 5-endo-trig cyclization. As a result, there is no need to design the precursor so that intermediate 84 is generated selectively, an important feature contributing to the utility of this tetrahydrofuran synthesis.
Scheme 17.
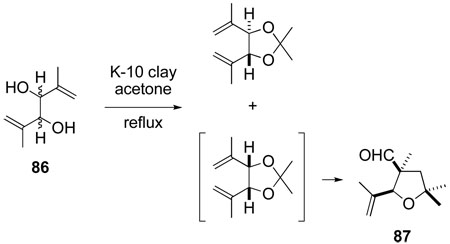
It was not until 1992, as a result of a substructure search of Chemical Abstracts, which had become common by that time, that we became aware that this tetrahydrofuran synthesis had been discovered earlier during attempted preparation of acetals from allylic diols.97 In the initial report, Mousset described the reaction of a mixture of syn and anti diene diols 86 with acetone in the presence of an acidic clay catalyst to generate the acetonide from the syn diol stereoisomer and a 3-formyltetrahydrofuran, subsequently characterized as 87, from the stereoisomeric acetonide intermediate (eq 9).98 In a series of largely overlooked pioneering papers published during the 1970s, Mousset and co-workers outlined several of the key features of this synthesis of 3-acyltetrahydrofurans, for which both a Prins-pinacol mechanism and a concerted mechanism in which a tetrahydropyranyl cation intermediate is generated directly from an allylic acetal precursor were proposed.99
 |
(9) |
Our early demonstration that the tetrahydrofuran synthesis we have been considering takes place by a Prins-pinacol mechanism was quite important to the development of this line of research in my laboratory. Foremost, this conclusion suggested that exocyclic as well endocyclic Prins cyclizations could initiate such cascade sequences. Thus, we began in the late 1980s to more broadly develop cationic cyclization reactions terminated by pinacol rearrangements. Representative examples of carbocyclic ring systems that can be prepared in this fashion, typically with high stereoselectivity, are shown in Scheme 18. One useful expression of this chemistry couples carbocyclic ring formation with expansion of a starting ring by one-carbon (e.g., formation of 89–91, 93 and 94).100–104 Although we have generally employed an acetal as the precursor to an oxocarbenium ion intermediate, the initial cationic cyclization is brought about more selectively with a thiocarbenium ion (e.g., formation of 94).104,105 As expected, other electrophiles such as an allyl cation in the conversion of 92 → 93 can be employed also.106 In addition, Prins-pinacol reactions can be used to form other carbocyclic motifs, such as spiro (e.g., 95)107 or attached (e.g., 96)108 ring systems. Stereoselection in the processes depicted in Scheme 18 is dictated by the topography of the Prins cyclization step, with pinacol rearrangement of the derived cationic intermediate occurring more rapidly than loss of its relative configuration.107 As in the related reactions that form tetrahydrofurans, the topography of the Prins cyclization step is organized by the interplay of steric and stereoelectronic factors.
Scheme 18.
Within a few years of initiating our investigations into the synthesis of cyclic ethers, we began exploring the scope and limitations of Prins-pinacol reactions in the context of natural products total synthesis.109 Early on, graduate student Mark Brown employed the benzyl congener of hydrobenzofuranone 77 (see Scheme 16) to complete the first total synthesis of the halogenated marine lipid (±)-trans-kumausyne.110 Shortly thereafter, postdoctoral fellow Timothy Griese followed up the initial investigations of graduate student Kira Hutchinson to accomplish the first total synthesis of (±)-kumausallene from hydrobenzofuranone 77, establishing in the process the relative configuration of the bromoallene side chain of this marine lipid.111 Several years later, a Prins-pinacol reaction to form the highly substituted 3-acetyltetrahydrofuran 76 was developed as the central step of an enantioselective total synthesis of (–)-citreoviral.112
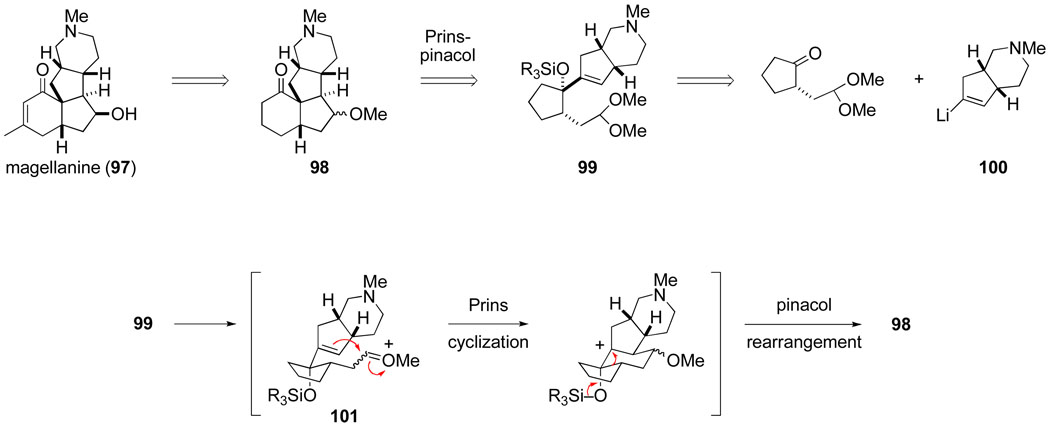
Almost immediately after postdoctoral fellow Paul Howard initiated his studies in 1987 to extend pinacol-terminated Prins cyclizations to the synthesis of carbocycles,100 we envisaged applying such reactions to the synthesis of the members of the structurally distinctive magellanane group of Lycopodium alkaloids.113 The transformation of cyclopentene acetal 88 to the angularly fused tricyclic product 89 (see Scheme 18) provided some precedent for the convergent approach to magellanine (97) outlined in retrosynthetic fashion in Scheme 19. Assuming Prins cyclization of oxocarbenium ion intermediate 101 took place from the less-hindered convex face of the cis-hydropyrindine moiety, tetracyclic product 98 would evolve with the proper orientation of the critical quaternary and adjacent secondary carbon stereocenters.
Scheme 19.
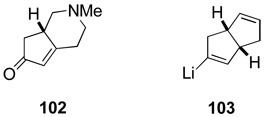
Postdoctoral fellow Gavin Hirst examined several approaches for assembling vinyl halide precursors of the cis-hydropyrindine nucleophile 100. Although azabicyclic enone 102 could be prepared from 1-methyl-4-piperidone, its stereoselective conversion to a halide precursor of lithium reagent 100 was never accomplished in satisfactory fashion. Making a sacrifice in convergency, the synthesis plan was modified to replace cis-hydropyrindine nucleophile 100 with the cis-hexahydropentalene analog 103.
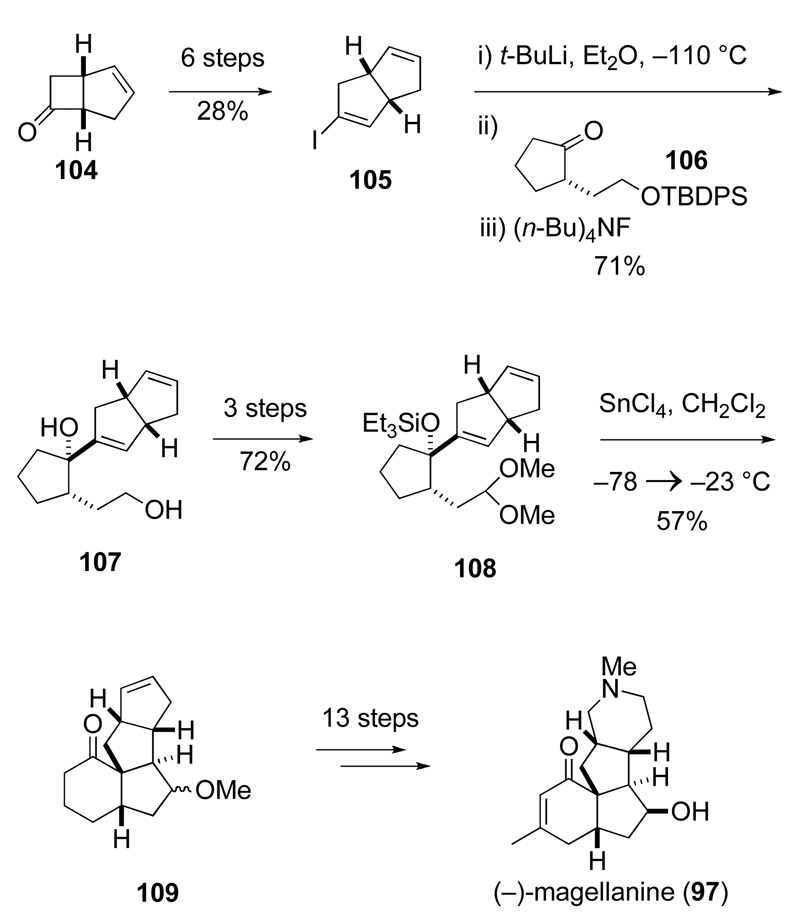
Graduate student Theodore Johnson, Jr., who succeeded Hirst on the project, successfully completed in late 1992 the inaugural total syntheses of (–)-magellanine (97) and the related ketone, (–)-magellaninone.114 Key stages of this synthesis are summarized in Scheme 20. (1R,5S)-Bicyclo[3.2.0]heptenone (104),115 an intermediate produced at the time on large scale by Glaxo, served as the starting material for the preparation of both the enantioenriched iodide 105 and cyclopentanone 106. Addition of lithium reagent 103, generated from its iodide precursor 105, occurred cleanly from the face opposite the side chain of cyclopentanone 106 to form diol 107 after removal of the silyl protecting group. Prins-pinacol reaction of siloxy dienyl acetal 108, available in three additional steps from 107, with 1.1 equiv of SnCl4 provides carbotetracycle 109 in 57% yield, as a 2:1 mixture of β and α methoxy epimers. In a multistep sequence of conventional reactions, the β-methoxy epimer of product 109 is elaborated to (–)-magellanine (97) and the α-epimer to the congeneric ketone, (–)-magellaninone, thereby completing the first total syntheses of Lycopodium alkaloids of the magellanane group. The stiff price paid for not being able to bring a preformed piperidine ring into the central Prins-pinacol transformation is the many steps required to elaborate intermediate 109 to (–)-magellanine (97).
Scheme 20.
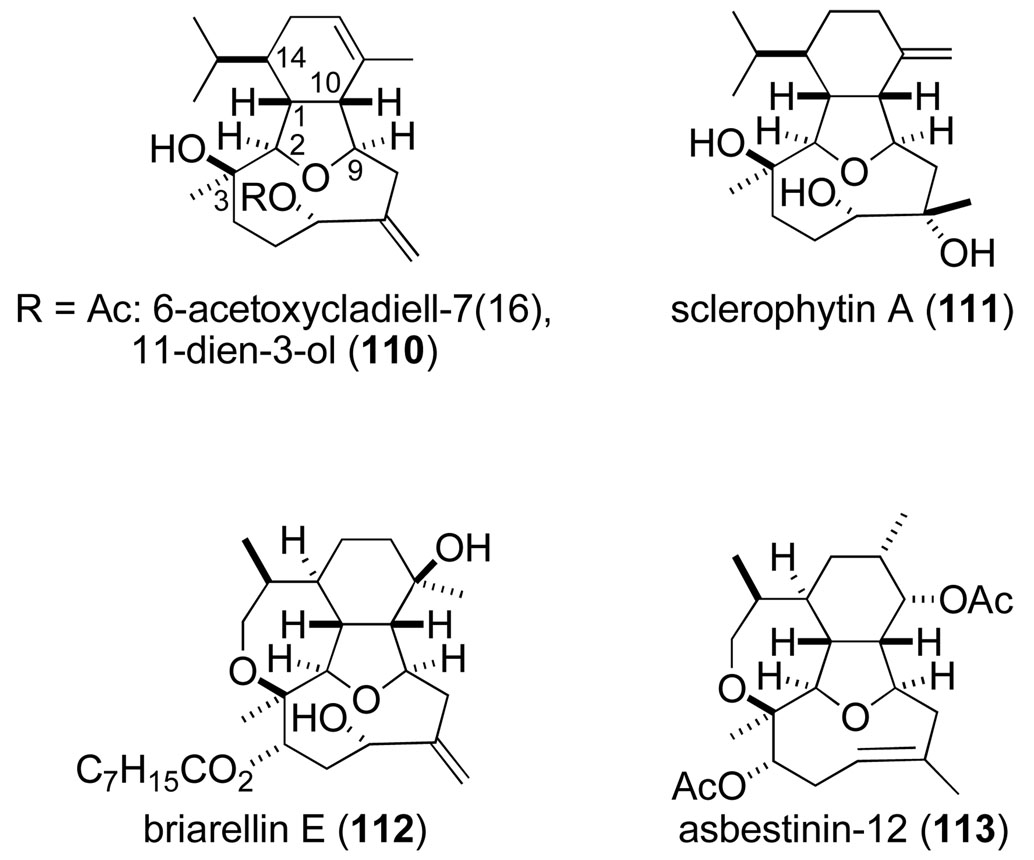
Also in the early 1990s, we initiated studies to examine the potential utility of the Prins-pinacol synthesis of tetrahydrofurans for preparing members of the C2,C11-cyclized cembranoid family of oxacyclic marine diterpenes.116 The cladiellins (also known as the eunicellins) are the structurally simplest members of this group (e.g., 110 and 111). The cladiellins and the more elaborate briarellins (e.g., 112) and asbestinins (e.g., 113) have in common an oxatricyclic ring system composed of hexahydroisobenzofuran and oxacyclononane units, as well as six stereogenic carbons (C1–3, C9, C10, and C14; Figure 2).
Figure 2.
Structures of representative C2,C11-cyclized cembranoid oxacyclic natural products.
When David MacMillan began his graduate training in my laboratory in 1991, he examined whether cis-oxabicyclic ring systems, including cis-hexahydroisobenzofurans, could be prepared by Prins-pinacol reactions. An early verification of this tactic was the formation of oxabicyclic product 78 (Scheme 16).
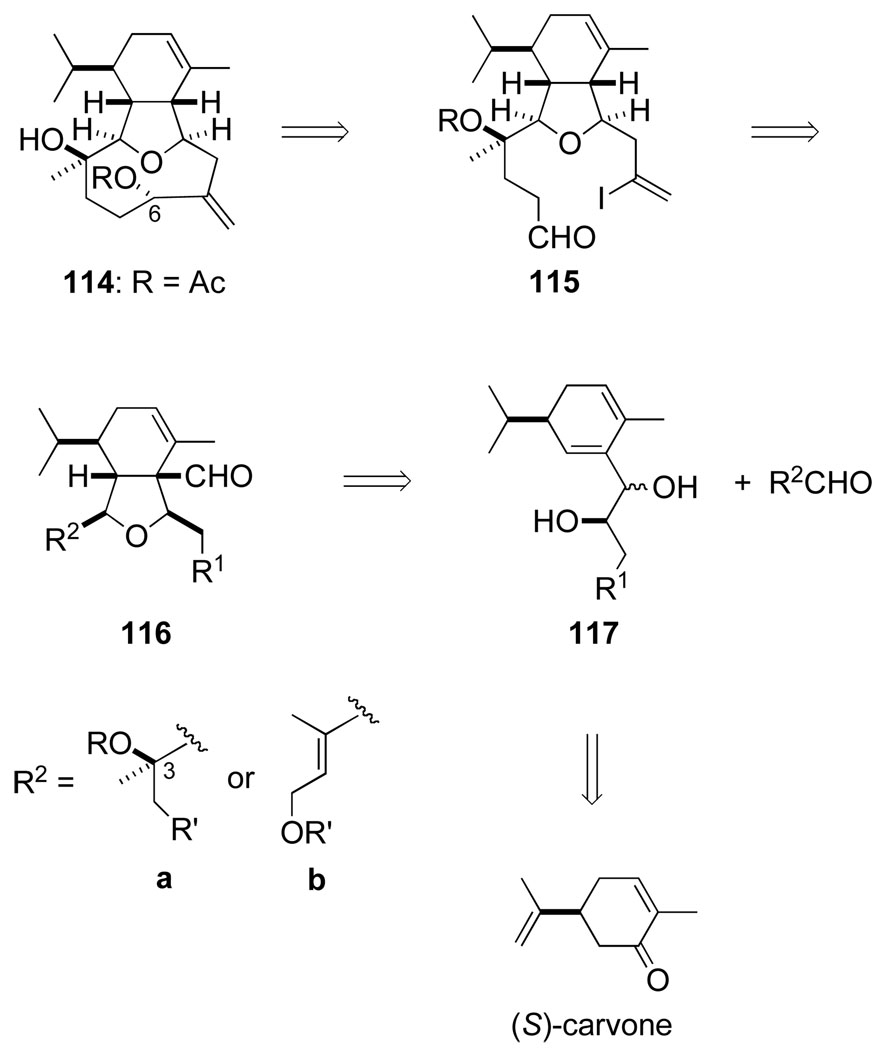
By early 1992 we had devised a strategy whereby the cladiellin diterpenes would be assembled from (S)-carvone (Scheme 21). We envisaged constructing the hydrobenzofuran moiety and its five stereocenters by Prins-pinacol reaction of dienyl diol 117 and an aldehyde. At the outset, we hoped that this aldehyde could incorporate the common C3 stereocenter of these marine diterpenes. This plan would defer two pivotal conversions — removing the extraneous carbonyl group from Prins-pinacol product 116 and closing the nine-membered oxacyclic ring — to late stages of the synthesis. Although postponing such critical transformations involved an element of risk, others had carried out both transformations previously with fairly elaborate structures. Photodeformylation of β,γ-unsaturated aldehydes had been extensively studied by Jeger and Schaffner; critical to our needs, this reaction had been shown under many conditions to take place with retention of configuration.117 Although what stereoselectivity would be realized at C6 in forming the oxacyclononane ring from intermediate 115 was unclear at the outset, Kishi’s pioneering use of chromium-promoted alkenyl halide/aldehyde coupling reactions to form medium rings gave us confidence that at least the efficiency of this demanding ring closure might be satisfactory.118
Scheme 21.
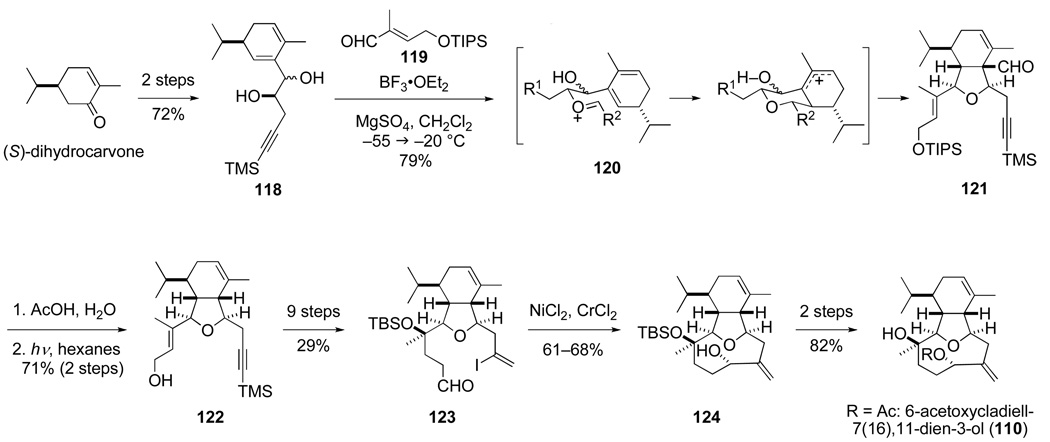
The total synthesis of 6-acetoxycladiell-7(16),11-dien-3-ol (110) completed by David MacMillan in 1995, which constituted the first total synthesis success in this area of marine diterpenes, is summarized in Scheme 22.119,120 Although Prins-pinacol condensation of alkynyl diene diol 118 with aldehydes having oxygen substituents at the α-carbon proved to be problematic, this condensation with (E)-α,β-unsaturated aldehyde 119 proceeds smoothly to deliver cis-hexahydroisobenzofuran 121 as a single stereoisomer in 79% yield. Stereoselection in this conversion derives from preferential Prins cyclization of an E oxocarbenium ion intermediate by chair conformer 120 from the face of the diene opposite the isopropyl substituent. After removing the TIPS protecting group, the extraneous carbonyl fragment is removed by photochemical deformylation, which proceeds with retention of configuration to form cis-hexahydroisobenzofuran 122 in good yield. After advancing this product to aldehyde vinyl iodide 123, Nozaki–Hiyama–Kishi cyclization118,121 successfully closes the nine-membered ring to give tricyclic allylic alcohol 124 in 61–68% yield. Stereoselection in this ring closure is exceptional, with a >20:1 preference for forming the allylic alcohol stereocenter with the desired relative configuration being realized; an after the fact rationalization of this stereoselectivity has been advanced.120 In two additional steps, intermediate 124 is converted in high yield to 6-acetoxycladiell-7(16),11-dien-3-ol (110).
Scheme 22.
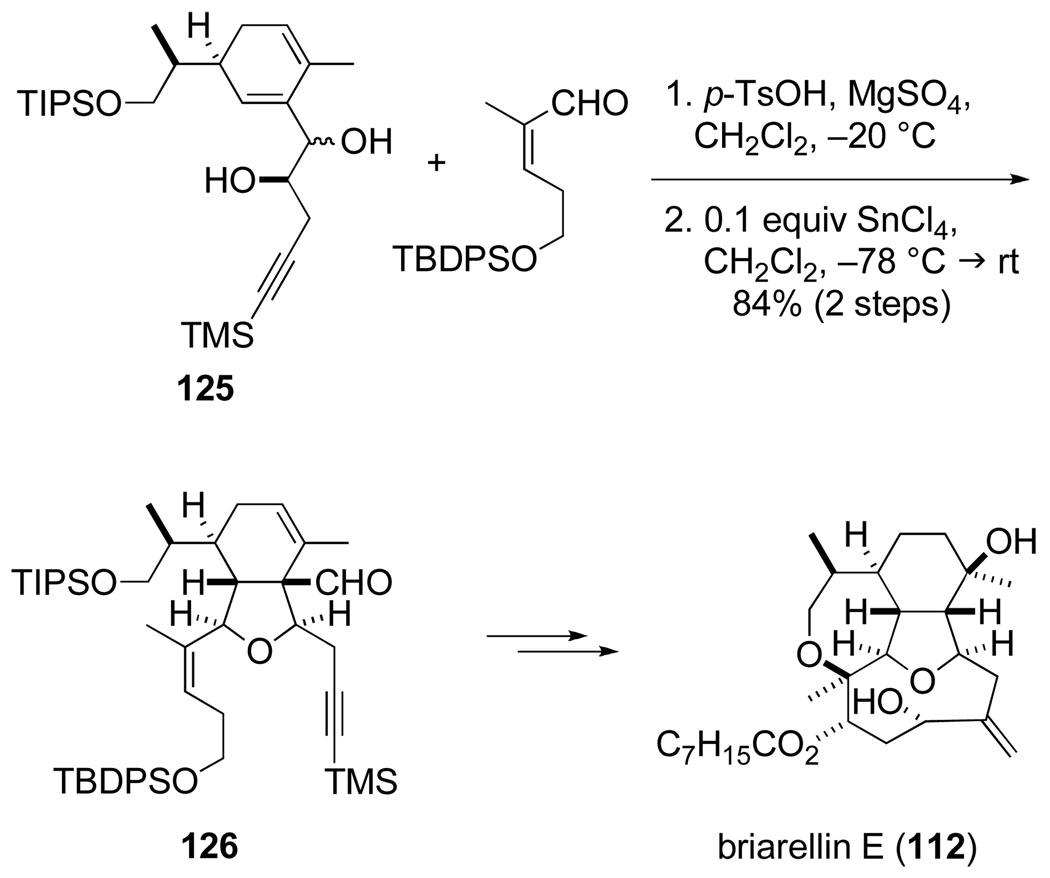
Our synthesis of 6-acetoxycladiell-7(16),11-dien-3-ol (110) was subsequently streamlined by graduate student Lewis Pennington, who showed that a (Z)-enal can participate without loss of double bond configuration in the pivotal Prins-pinacol step.120,122 This discovery paved the way for our use of this tactic with the more highly functionalized alkynyl diene diol 125 to provide hexahydroisobenzofuran 126, a central intermediate in the first total synthesis of the briarellin diterpenes achieved in 2002 by postdoctoral researcher Olivier Corminboeuf (Scheme 23).123,124
Scheme 23.
5. Concluding Remarks
In target-directed organic synthesis, molecular rearrangements have more often been undesired events to fear, rather than central elements of synthesis strategy. The research program that is the subject of this account provides a demonstration of the value of designed molecular rearrangements in target-directed synthesis. Before a new transformation can reliably serve as a foundation for a total synthesis program, its mechanism and scope and limitations must be understood. The effort that we expended in defining these features of the aza-Cope–Mannich reaction and pinacol-terminated cyclization reactions has been key to their successful use in demanding total synthesis contexts. I am confident that the interface of fundamental investigations of chemical reactivity and target-directed synthesis will continue to be a fertile arena for future progress in organic synthesis.
I conclude by noting that soon after we became involved in natural products total synthesis, one of our objectives became to use such endeavors as platforms for evaluating how reactions that we were studying, or other recently developed or neglected transformations, performed with complex polyfunctional substrates. In my view, there is no better way to gain an understanding of the reliability and robustness of new chemistry. As a result, we were often content to incorporate steps that at the outset would be considered high risk in order to gain the opportunity to learn more about their scope. I hope that this approach to natural products total synthesis is apparent in the total syntheses considered in this essay.
Acknowledgments
The research described in this account was made possible by the dedication and creativity of the remarkable group of co-workers that I have had the privilege to work with at UC Irvine over the past 38 years. I have been able to acknowledge some of their contributions in this account. To these, and to my other former coworkers whose contributions could not be discussed in this short account, I extend my profound thanks.
The research program that I have described would not have been possible without long-term support from the National Science Foundation and the National Institutes of Health (National Institute of General Medical Sciences and National Institute of Neurological Disorders and Stroke). I also wish to acknowledge the University of California, Irvine, particularly my Chemistry Department colleagues, who have provided a highly supportive environment for my research program over the years.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dedication. I dedicate this article to my companion for a majority of my life, Joanne Overman. What I have accomplished in my professional career would not have been possible without her support.
References
- 1.This account is a personal reflection on how a research program developed in my laboratory. As a result, I have cited literature that influenced the evolution of our studies. Citations to the work of others who worked in these areas are poorly annotated; more scholarly documentation of these contributions is found in the original accounts of the work highlighted in this essay and in our review publications.
- 2.Whitlock HW, Jr, Overman LE. J. Am. Chem. Soc. 1971;93:2247–2253. [Google Scholar]
- 3.Whitlock HW, Jr, Overman LE. J. Org. Chem. 1969;34:1962–1963. [Google Scholar]
- 4.Breslow R, Overman LE. J. Am. Chem. Soc. 1970;91:1075–1077. doi: 10.1021/ja00707a062. [DOI] [PubMed] [Google Scholar]
- 5.Breslow R, Baldwin S, Flechtner T, Kalicky P, Liu S, Washburn W. J. Am. Chem. Soc. 1973;95:3251–3262. doi: 10.1021/ja00791a031. [DOI] [PubMed] [Google Scholar]
- 6.Overman LE. J. Chem. Soc., Chem. Commun. 1972:1196–1198. [Google Scholar]
- 7.Overman LE, Campbell CB. J. Org. Chem. 1974;39:1474–1481. [Google Scholar]
- 8.Cramer F, Pawelzik K, Baldauf HJ. Chem. Ber. 1958;91:1049–1054. [Google Scholar]
- 9.Overman LE. J. Am. Chem. Soc. 1974;96:597–599. [Google Scholar]
- 10.Overman LE. J. Am. Chem. Soc. 1976;98:2901–2910. [Google Scholar]
- 11.Clizbe LA, Overman LE. Org. Synth. 1978;58:4–11. Coll. Vol. 6 pp 507–511. [Google Scholar]
- 12.Carpenter NC, Overman LE. The Allylic Trihaloacetimidate Rearrangement. In: Overman LE, editor. Organic Reactions. Vol. 66. New York: John Wiley and Sons; 2005. pp. 1–107. [Google Scholar]
- 13.Henry PM. Acc. Chem. Res. 1973;6:16–24. [Google Scholar]
- 14.Overman LE, Campbell CB, Knoll FM. J. Am. Chem. Soc. 1978;100:4822–4834. [Google Scholar]
- 15.Overman LE. Angew. Chem., Int. Ed. Eng. 1984;23:579–586. [Google Scholar]
- 16.Overman LE. Acc. Chem. Res. 1980;13:218–224. [Google Scholar]
- 17.Metz P, Mues C, Schoop A. Tetrahedron. 1992;48:1071–1080. [Google Scholar]
- 18.(a) Anderson CE, Overman LE. J. Am. Chem. Soc. 2003;125:12412–12413. doi: 10.1021/ja037086r. [DOI] [PubMed] [Google Scholar]; (b) Kirsch SF, Overman LE, Watson MP. J. Org. Chem. 2004;69:8101–8104. doi: 10.1021/jo0487092. [DOI] [PubMed] [Google Scholar]; (c) Watson MP, Overman LE, Bergman RG. J. Am. Chem. Soc. 2007;129:5031–5044. doi: 10.1021/ja0676962. [DOI] [PubMed] [Google Scholar]
- 19.(a) Overman LE, Knoll FM. J. Am. Chem. Soc. 1980;102:1454–1456. [Google Scholar]; (b) Overman LE, Jacobsen EJ. J. Am. Chem. Soc. 1982;104:7225–7231. [Google Scholar]
- 20.(a) Mizoroki T, Mori K, Ozaki A. Bull. Chem. Soc. Jpn. 1971;44:581. [Google Scholar]; (b) Heck RF, Nolley JP., Jr J. Org. Chem. 1972;37:2320–2322. [Google Scholar]; (c) Heck RF. Palladium-Catalyzed Vinylation of Organic Halides. In: Dauben WG, editor. Organic Reactions. Vol. 27. New York: John Wiley and Sons; 1982. pp. 345–390. [Google Scholar]
- 21.For the first report of sequential (cascade) Heck cyclizations, see: Abelman MM, Overman LE. J. Am. Chem. Soc. 1988;110:2328–2329..
- 22.For the first reports, see: Sato Y, Sodeoka M, Shibasaki M. J. Org. Chem. 1989;54:4738–4739. Carpenter NE, Kucera DJ, Overman LE. J. Org. Chem. 1989;54:5846–5848..
- 23.Link JT. Org. React. 2002;60:157–534. [Google Scholar]
- 24.Dounay AB, Overman LE. “The Asymmetric Intramolecular Mizoroki-Heck Reaction in Natural Product Synthesis” Chapter 16. In: Oestreich M, editor. The Mizoroki–Heck Reaction. West Sussex, UK: Wiley–VCH; 2009. pp. 533–556. [Google Scholar]
- 25.Overman LE, Clizbe LA. J. Am. Chem. Soc. 1976;98:2352–2355. [Google Scholar]
- 26.Overman LE, Kakimoto M. J. Org. Chem. 1978;43:4564–4567. [Google Scholar]
- 27.Tsuboi S, Stromquist P, Overman LE. Tetrahedron Lett. 1976;17:1145–1148. [Google Scholar]
- 28.Overman LE, Tsuboi S. J. Am. Chem. Soc. 1977;99:2813–2815. [Google Scholar]
- 29.Overman LE, Tsuboi S, Roos JP, Taylor GF. J. Am. Chem. Soc. 1980;102:747–754. [Google Scholar]
- 30.Overman LE, Marlowe CM, Clizbe LA. Tetrahedron Lett. 1979;20:599–603. [Google Scholar]
- 31.Overman LE, Clizbe LA, Freerks RL, Marlowe CK. J. Am. Chem. Soc. 1981;103:2807–2816. [Google Scholar]
- 32.Danishefsky SJ, Kitahara T. J. Am. Chem. Soc. 1974;96:7807–7808. [Google Scholar]
- 33.(a) Oppolzer W, Fröstl W. Helv. Chim. Acta. 1975;58:587–589. doi: 10.1002/hlca.19750580232. [DOI] [PubMed] [Google Scholar]; (b) Oppolzer W, Fröstl W. Helv. Chim. Acta. 1975;58:590–593. doi: 10.1002/hlca.19750580232. [DOI] [PubMed] [Google Scholar]; (c) Oppolzer W, Fröstl W. Helv. Chim. Acta. 1975;58:593–595. doi: 10.1002/hlca.19750580232. [DOI] [PubMed] [Google Scholar]
- 34.Overman LE, Taylor GF, Jessup P. J. Tetrahedron Lett. 1976;17:3089–3092. [Google Scholar]
- 35.Overman LE, Taylor GF, Houk KN, Domelsmith LS. J. Am. Chem. Soc. 1978;100:3182–3189. [Google Scholar]
- 36.Overman LE, Freerks RL, Petty CB, Clizbe LA, Ono RK, Taylor GF, Jessup PJ. J. Am. Chem. Soc. 1981;103:2816–2822. [Google Scholar]
- 37.Jessup PJ, Petty CB, Roos J, Overman LE. Org. Synth. 1979;59:1–9. Coll. Vol. 6, pp 95–101. [Google Scholar]
- 38.Overman LE, Jessup PJ. Tetrahedron Lett. 1977;18:1253–1256. [Google Scholar]
- 39.Overman LE, Jessup PJ. J. Am. Chem. Soc. 1978;100:5179–5185. [Google Scholar]
- 40.Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. New York: Wiley; 1989. [Google Scholar]
- 41.Daly JW. J. Nat. Prod. 1998;61:162–172. doi: 10.1021/np970460e. [DOI] [PubMed] [Google Scholar]
- 42.See, inter alia: Stevens RV, Lee AWM. J. Am. Chem. Soc. 1979;115:7032–7035. Ziegler FE, Spitzner EB. J. Am. Chem. Soc. 1973;95:7147–7149. doi: 10.1021/ja00802a040..
- 43.Overman LE, Lesuisse D, Hashimoto M. J. Am. Chem. Soc. 1983;105:5373–5379. [Google Scholar]
- 44.Horowitz RM, Geissman TA. J. Am. Chem. Soc. 1950;72:1518–1522. [Google Scholar]
- 45.For reviews, see: Heimgartner H, Hansen HJ, Schmid H. In: Iminium Salts in Organic Chemistry. Part 2. Böhme H, Viehe HG, editors. New York: Wiley; 1979. pp. 655–732. Przheval'skii NM, Grandberg II. Russ. Chem. Rev. 1987;5:477. Blechert S. Synthesis. 1989:71–82..
- 46.Kakimoto M, Overman LE. J. Am. Chem. Soc. 1979;101:1310–1312. [Google Scholar]
- 47.Overman LE, Kakimoto M, Okawara M. Tetrahedron Lett. 1979;42:4041–4044. [Google Scholar]
- 48.Fukaya C, Overman LE. J. Am. Chem. Soc. 1980;102:1454–1456. [Google Scholar]
- 49.Fujimoto R, Kishi Y, Blount JF. J. Am. Chem. Soc. 1980;102:7154–7156. [Google Scholar]
- 50.Overman LE, Kakimoto M, Okazaki M, Meier GP. J. Am. Chem. Soc. 1983;105:6622–6629. [Google Scholar]
- 51.Overman LE. Acc. Chem. Res. 1992;25:352–359. [Google Scholar]
- 52.Doedens RJ, Meier GP, Overman LE. J. Org. Chem. 1988;53:685–690. [Google Scholar]
- 53.Overman LE, Jacobsen EJ. Tetrahedron Lett. 1982;23:2741–2744. [Google Scholar]
- 54.(a) Overman LE, Mendelson LT. J. Am. Chem. Soc. 1981;103:5579–5581. [Google Scholar]; (b) Overman LE, Mendelson LT, Flippin LA. Tetrahedron Lett. 1982;23:2733–2736. [Google Scholar]; (c) Overman LE, Jacobsen EJ. Tetrahedron Lett. 1982;23:2737–2740. [Google Scholar]; (d) Overman LE, Jacobsen EJ, Doedens RJ. J. Org. Chem. 1983;48:3393–3400. [Google Scholar]; (e) Overman LE, Okazaki M, Jacobsen EJ. J. Org. Chem. 1985;50:2403–2405. [Google Scholar]
- 55.Overman LE, Shim J. J. Org. Chem. 1991;56:5005–5007. [Google Scholar]
- 56.Overman LE, Sworin M, Burk RM. J. Org. Chem. 1983;48:2685–2690. [Google Scholar]
- 57.Jacobsen EJ, Levin J, Overman LE. J. Am. Chem. Soc. 1988;110:4329–4336. [Google Scholar]
- 58.Overman LE, Mendelson LT, Jacobsen EJ. J. Am. Chem. Soc. 1983;105:6629–6637. [Google Scholar]
- 59.Overman LE, Sugai S. Helv. Chim. Acta. 1985;68:745–749. [Google Scholar]
- 60.Overman LE, Shim J. J. Org. Chem. 1993;58:4662–4672. [Google Scholar]
- 61.Overman LE, Wild H. Tetrahedron Lett. 1989;30:647–650. [Google Scholar]
- 62.Overman LE, Sworin M. Tetrahedron. 1981;37:4041–4045. [Google Scholar]
- 63.Overman LE, Robertson GM, Robichaud AJ. J. Am. Chem. Soc. 1991;113:2598–2610. [Google Scholar]
- 64.Overman LE, Angle SR. J. Org. Chem. 1985;50:4021–4028. [Google Scholar]
- 65.Fevig JM, Marquis RM, Jr, Overman LE. J. Am. Chem. Soc. 1991;113:5085–5086. [Google Scholar]
- 66.Angle SR, Fevig JM, Knight SD, Marquis RM, Jr, Overman LE. J. Am. Chem. Soc. 1993;115:3966–3976. [Google Scholar]
- 67.Knight SD, Overman LE, Pairaudeau G. J. Am. Chem. Soc. 1993;115:9293–9294. [Google Scholar]
- 68.Knight SD, Overman LE, Pairaudeau G. J. Am. Chem. Soc. 1995;117:5776–5788. [Google Scholar]
- 69.(a) Woodward RB, Cava MP, Ollis WD, Hunger A, Daeniker HU, Schenker K. J. Am. Chem. Soc. 1954;76:4749–4751. [Google Scholar]; (b) Woodward RB, Cava MP, Ollis WD, Hunger A, Daeniker HU, Schenker K. Tetrahedron. 1963;19:247–288. [Google Scholar]
- 70.Anet FAL, Robinson R. Chem. Ind. 1953:245. [Google Scholar]
- 71.Knapp S, Hale JJ, Bastos M, Molina A, Chen KY. J. Org. Chem. 1992;57:6239–6256. [Google Scholar]
- 72.Deardorff DR, Windham CQ, Craney CL. Org. Synth. 1995;73:25–32. Col. Vol. 9, 1998, 487–493. [Google Scholar]
- 73.(a) Edwards PN, Smith GF. J. Chem. Soc. 1961:152–156. [Google Scholar]; (b) Wenkert E, Sklar R. J.Org. Chem. 1966;31:2689–2691. doi: 10.1021/jo01346a513. [DOI] [PubMed] [Google Scholar]; (c) Hymon JR, Schmid H. Helv. Chim. Acta. 1966;49:2067–2071. doi: 10.1002/hlca.19660490534. [DOI] [PubMed] [Google Scholar]
- 74.Martin CL, Overman LE, Rohde JM. J. Am. Chem. Soc. 2008;130:7568–7569. doi: 10.1021/ja803158y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carrol AR, Hyde E, Smith J, Quinn RJ, Guymer G, Foster PI. J. Org. Chem. 2005;70:1096–1099. doi: 10.1021/jo048439n. [DOI] [PubMed] [Google Scholar]
- 76.For recent examples and leading references, see: Bennasar M-L, Roca T, García-Díaz D. J. Org. Chem. 2008;73:9033–9039. doi: 10.1021/jo801998h. Amat M, Perez M, Llor N, Escolano C, Luque FJ, Molins E, Bosch J. J. Org. Chem. 2004;69:8681–8693. doi: 10.1021/jo0487101..
- 77.Tobinaga S, Kotani E. J. Am. Chem. Soc. 1972;94:309–310. [Google Scholar]
- 78.Ito Y, Konoike T, Harada T, Saegusa T. J. Am. Chem. Soc. 1977;99:1487–1493. [Google Scholar]
- 79.For notable examples, see: Cohen T, McNamara K, Kuzemko MA, Ramig K, Landi JJ, Jr, Dong Y. Tetrahedron. 1993;49:7931–7942. Paquette LA, Bzowej EI, Branan BM, Stanton KJ. J. Org. Chem. 1995;60:7277–7283. Baran PS, Hafensteiner BD, Ambhaikar NB, Guerrero CA, Gallagher JD. J. Am. Chem. Soc. 2006;128:8678–8693. doi: 10.1021/ja061660s..
- 80.Martin CL. unpublished studies. UC Irvine: 2009. [Google Scholar]
- 81.Taniguchi T, Martin CL, Monde K, Nakanishi K, Berova N, Overman LE. J. Nat. Prod. 2009;72:430–432. doi: 10.1021/np800665s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agami C, Cases M, Couty F. J. Org. Chem. 1994;59:7937–7940. [Google Scholar]
- 83.Armstrong A, Bhonoah Y, Shanahan SE. J. Org. Chem. 2007;72:8019–8024. doi: 10.1021/jo701536a. [DOI] [PubMed] [Google Scholar]
- 84.Brummond KM, Hong S. J. Org. Chem. 2005;70:907–916. doi: 10.1021/jo0483567. [DOI] [PubMed] [Google Scholar]
- 85.Sakurai O, Horikawa H, Iwasaki T. J. Chem. Soc., Chem. Commun. 1995:2527–2529. [Google Scholar]
- 86.First recognized as a competing process in Prins cyclizations, see: Lolkema LDM, Semeyn C, Ashek L, Hiemstra H, Speckamp WN. Tetrahedron. 1994;50:7129–7140..
- 87.For a recent study and leading references, see: Jasti R, Rychnovsky SD. J. Am. Chem. Soc. 2006;128:13640–13648. doi: 10.1021/ja064783l..
- 88.Hopkins MH, Overman LE. J. Am. Chem. Soc. 1987;109:4748–4749. [Google Scholar]
- 89.Hopkins MH, Overman LE, Rishton GM. J. Am. Chem. Soc. 1991;113:5354–5365. [Google Scholar]
- 90.Overman LE, Rishton GM. Org. Synth., Coll. Vol. 9. New York: Wiley; 1998. pp. 4–9. [Google Scholar]
- 91.Herrinton PM, Hopkins MH, Mishra P, Brown MJ, Overman LE. J. Org. Chem. 1987;52:3711–3712. [Google Scholar]
- 92.Brown MJ, Harrison T, Herrinton PM, Hopkins MH, Hutchinson KD, Mishra P, Overman LE. J. Am. Chem. Soc. 1991;113:5365–5378. [Google Scholar]
- 93.Cohen F, MacMillan DWC, Overman LE, Romero A. Org. Lett. 2001;3:1225–1228. doi: 10.1021/ol0157003. [DOI] [PubMed] [Google Scholar]
- 94.Overman LE, Velthuisen EJ. Org. Lett. 2004;6:3853–3856. doi: 10.1021/ol0482745. [DOI] [PubMed] [Google Scholar]
- 95.Ponce AM, Overman LE. J. Am. Chem. Soc. 2000;122:8672–8676. [Google Scholar]
- 96.Gasparski CM, Herrinton PM, Overman LE, Wolfe JP. Tetrahedron Lett. 2000;41:9431–9435. [Google Scholar]
- 97.Hopkins MH, Overman LE, Rishton GM. J. Am. Chem. Soc. 1992;114:10093. [Google Scholar]
- 98.(a) Martinet P, Mousset G, Michel M. C. R. Acad. Sci. Paris, Ser. C. 1969;268:1303–1306. [Google Scholar]; (b) Martinet P, Mousset G. Bull. Soc. Chim. Fr. 1970:1071–1076. [Google Scholar]
- 99.(a) Martinet P, Mousset G. Bull. Soc. Chim. Fr. 1971:4093–4096. [Google Scholar]; (b) Mousset G. Bull.Soc. Chim. Fr. 1971:4097–4106. [Google Scholar]; (c) Chambenois D, Mousset G. C. R. Acad. Sci. Paris, Ser. C. 1972;274:715–718. [Google Scholar]; (d) Chambenois D, Mousset G. C. R. Acad. Sci. Paris, Ser. C. 1972;274:2088–2091. [Google Scholar]; (e) Chambenois D, Mousset G. Bull. Soc. Chim. Fr. 1974:2969–2974. [Google Scholar]; (f) Malardeau C, Mousset G. Bull. Soc. Chim. Fr. 1977:988–992. [Google Scholar]
- 100.Hirst GC, Howard PN, Overman LE. J. Am. Chem. Soc. 1989;111:1514–1515. [Google Scholar]
- 101.Johnson T, Overman LE. Tetrahedron Lett. 1991;32:7361–7364. [Google Scholar]
- 102.Ando S, Minor KP, Overman LE. J. Org. Chem. 1997;62:6379–6387. [Google Scholar]
- 103.Gahman TC, Overman LE. Tetrahedron. 2002;58:6473–6483. [Google Scholar]
- 104.Lebsack AD, Overman LE, Valentegovich RJ. J. Am. Chem. Soc. 2001;123:4851–4852. doi: 10.1021/ja015802o. [DOI] [PubMed] [Google Scholar]
- 105.Burke BJ, Lebsack AD, Overman LE. Synlett. 2004:1387–1393. [Google Scholar]
- 106.Overman LE, Wolfe JP. J. Org. Chem. 2002;67:6421–6429. doi: 10.1021/jo025927r. [DOI] [PubMed] [Google Scholar]
- 107.Minor KP, Overman LE. Tetrahedron. 1997;53:8927–8940. [Google Scholar]
- 108.(a) Overman LE, Pennington LD. Can. J. Chem. 2000;78:732–738. [Google Scholar]; (b) Overman LE, Velthuisen EJ. J. Org. Chem. 2006;71:1581–1587. doi: 10.1021/jo0522862. [DOI] [PubMed] [Google Scholar]
- 109.For a recent review, see: Overman LE, Pennington LD. J. Org. Chem. 2003;68:7143–7157. doi: 10.1021/jo034982c..
- 110.Brown MJ, Harrison T, Overman LE. J. Am. Chem. Soc. 1991;113:5378–5384. [Google Scholar]
- 111.Grese TA, Hutchinson KD, Overman LE. J. Org. Chem. 1993;58:2468–2477. [Google Scholar]
- 112.Hanaki N, Link JT, MacMillan DWC, Overman LE, Trankle WG, Wurster JA. Org. Lett. 2000;2:223–226. doi: 10.1021/ol991315q. [DOI] [PubMed] [Google Scholar]
- 113.MacLean DB. Alkaloids. Vol. 115. Academic Press; 1993. pp. 2992–2993. [Google Scholar]
- 114.Hirst GC, Johnson TO, Overman LE. J. Am. Chem. Soc. 1993;115:2992–2993. [Google Scholar]
- 115.Collington EW, Wallis CJ, Waterhouse I. Tetrahedron Lett. 1983;24:3125–3128. [Google Scholar]
- 116.For reviews, see: Sung P-J, Chen M-C. Heterocycles. 2002;57:1705–1715. Bernardelli P, Paquette LA. Heterocycles. 1998;49:531–556. Rodríguez AD. Tetrahedron. 1995;51:4571–4618. doi: 10.1016/0040-4020(95)00216-U..
- 117.See, inter alia: Hill J, Iriarte J, Schaffner K, Jeger O. Helv. Chim. Acta. 1966;49:292–311. Baggiolini E, Hamlow HP, Schaffner K. J. Am. Chem. Soc. 1970;92:4906–4921..
- 118.(a) Rowley M, Kishi Y. Tetrahedron Lett. 1988;29:4909–4912. [Google Scholar]; (b) Rowley M, Tsukamoto M, Kishi Y. J. Am. Chem. Soc. 1989;111:2735–2737. [Google Scholar]
- 119.MacMillan DWC, Overman LE. J. Am. Chem. Soc. 1995;117:10391–10392. [Google Scholar]
- 120.MacMillan DWC, Overman LE, Pennington LD. J. Am. Chem. Soc. 2001;123:9033–9044. doi: 10.1021/ja016351a. [DOI] [PubMed] [Google Scholar]
- 121.Takai K, Kimura K, Kuroda T, Hiyama T, Nozaki H. Tetrahedron Lett. 1983;24:5281–5284. [Google Scholar]
- 122.Corminboeuf O, Overman LE, Pennington LD. Org. Lett. 2003;5:1543–1546. doi: 10.1021/ol034384k. [DOI] [PubMed] [Google Scholar]
- 123.Corminboeuf O, Overman LE, Pennington LD. J. Am. Chem. Soc. 2003;125:6650–6652. doi: 10.1021/ja035445c. [DOI] [PubMed] [Google Scholar]
- 124.The first total synthesis of an asbestinin diterpene was completed in 2005, see: Crimmins MT, Ellis JM. J. Am. Chem. Soc. 2005;127:17200–17201. doi: 10.1021/ja056921x..