Abstract
Soluble Aβ oligomers are recognized as playing a key role in Alzheimer’s disease (AD) pathophysiology. Despite their significance, many investigators encounter difficulty generating reliable preparations for in vitro and in vivo experiments. Solutions of Aβ are often unstable and soluble conformer profiles inconsistent. In this study we describe detailed methods for preparing Aβ oligomers that are stable for several weeks and are enriched for low and high molecular weight oligomeric forms, including the 56-kDa form, a conformer implicated in AD-related cognitive impairment. We characterize their structural and functional properties using Western blot, dot blot, atomic force microscopy, Thioflavine T fluorescence, and primary neuronal culture toxicity assays. These synthetic preparations should prove valuable to many studying Aβ-mediated mechanisms underlying AD.
Keywords: amyloid-beta, atomic force microscopy, primary neurons
Introduction
As the leading cause of dementia in the United States, Alzheimer’s disease (AD) represents a critical public health concern. In the United States alone, 4.5 million people were inflicted with AD in the year 2000. This number is predicted to increase to 13.2 million by the year 2050 (Hebert et al., 2003). Patients experience a number of incapacitating symptoms including impairments in memory, movement, speaking and comprehension. However, it is the two classic neuropathological hallmarks of senile plaques comprised of fibrillar amyloid-beta (Aβ tangles consisting of hyperphosphorylated tau that are required for diagnosis. Additional neuropathological features include oxidative damage, neuronal and synaptic loss.
Due to its presence in extracellular senile plaques, Aβ has long been a suspected key culprit in the pathogenesis of AD. Additional genetic evidence from familial forms of AD also supports the role of Aβ in the disease. Mutations in the genes encoding presenilin 1, 2 and amyloid precursor protein (APP) all effectively lead to an increase in the generation of the pro-amyloidogenic Aβ1–42 peptide species and eventually an early-onset familial form of AD (Scheuner et al., 1996). The amyloid cascade hypothesis implicates Aβ peptide in the initiation of the other neuropathological features relating to tau dysfunction and cell loss (Gotz et al., 2001; Lewis et al., 2001; Oddo et al., 2004).
Aβ peptides arise from proteolytic cleavage of APP by two endoproteases, the β and γ-secretases, which liberate monomeric Aβ into the lumen of the endosome or into the extracellular space (Esler and Wolfe, 2001). Under physiological conditions, monomeric Aβ has the propensity to aggregate into soluble dimers, trimers, other low-n oligomers, spherical oligomers (Kayed et al., 2003), protofibrils (Nguyen and Hall, 2004) and insoluble amyloid fibrils. It was once believed that the aforementioned Aβ species followed a linear path towards Aβ fibril formation. However, recent evidence suggests that Aβ aggregation can proceed via different pathways and soluble oligomers are not an obligate intermediate of insoluble fibrils (Necula et al., 2007). Although a post-mortem signature, insoluble Aβ fibrils do not appear to be pathogenic, as clinical data indicates a poor correlation between the levels of Aβ fibrils and disease severity (Westerman et al., 2002). Instead, it is now more widely accepted that soluble oligomeric forms of Aβ impair cognitive function (Cleary et al., 2005) and it is the levels of these species, along with synapse loss, that correlate most accurately with the stage of neurological impairment (Lue et al., 1999; Scheff et al., 2005). Studies employing transgenic mouse models of AD and passive immunization strategies with anti-Aβ antibodies have demonstrated reversal of memory loss without an accompanying decrease in levels of amyloid plaque (Dodart et al., 2002; Kotilinek et al., 2002). Moreover, the amyloidogenic Tg2576 AD mouse model has demonstrated an age-related increase in a specific form of Aβ, dodecameric Aβ*56, concurrently with the onset of cognitive impairment (Lesne et al., 2006).
Soluble Aβ oligomers (AβOs) are present in the human AD brain and are biochemically analogous to synthetically generated AβOs (Gong et al., 2003). Incidentally, AβOs isolated from AD patients have demonstrated the capacity to bind at cultured neuronal synapses, similar to synthetically generated AβOs, and inhibit neural transmission (Lacor et al., 2004). Several investigators have reported methods to produce AβOs from synthesized Aβ peptide (Barghorn et al., 2005; Lambert et al., 1998; Lambert et al., 2001; Stine et al., 2003; Walsh et al., 1997). Preparations differ in their stability, enrichment of differently sized oligomeric forms, and most importantly, reproducibility. We have created a method for forming AβOs that are stable for several weeks at 4°C and these preparations are enriched for higher molecular weight oligomers, including the 56-kDa form. Herein, we describe the detailed methods to consistently generate the AβO preparation and characterize its structural and functional properties using SDS-PAGE with western analysis and silver stain, dot blot, ELISA, atomic force microscopy (AFM), Thioflavine T fluorescence, and cell viability assays.
Materials and Methods
Aβpreparation
The methods employed to generate monomeric and oligomeric Aβ preparations were modified from work previously described by the laboratories of Dr. William Klein and Dr. Heinz Hillen (Barghorn et al., 2005; Lambert et al., 2001). Human synthetic Aβ1–42 (American Peptide, Sunnyvale, CA) was suspended in 1,1,1,3,3,3 hexafluoro-2-propanol (or hexafluoroisopropanol HFIP; Sigma-Aldrich, St. Louis, MO) to 1 mM using a gastight syringe. Peptide samples were vortexed to obtain a homogenous solution, aliquoted into microfuge tubes and lyophilized via a Speed-Vac. The Aβ1–42 peptide films were stored desiccated at −20°C until further processed. Aβ monomer preparations were prepared immediately before use to minimize potential aggregation, where peptide films were resuspended to 5 mM in anhydrous dimethyl sulfoxide (DMSO), bath-sonicated for 10 min, then further diluted to 50 μg/ml (11 μM final concentration) and vortexed briefly. To form Aβ oligomers, peptide films were resuspended to 5 mM in DMSO, bath-sonicated for 10 min, diluted to 100 μM with cold PBS + 0.05% SDS, and vortexed for 30 sec. Aggregation was allowed to proceed for 24 h at 4°C before the peptide solution was further diluted with PBS to 50μg/ml and incubated for 2 weeks at 4°C to facilitate higher order aggregation. Following the 2-week incubation, oligomer preparations were ready, prior to use samples were centrifuged 13,000 rpm for 10 min at 4°C. Synthetic Aβ1–42 peptide was purchased from alternate vendors, Anaspec (Fremont, CA) and California Peptide Research (Napa, CA) for comparison to AβOs generated from Aβ1–42 purchased from American Peptide.
SDS-PAGE with western analysis and silver stain
Aβ monomeric and oligomeric preparations were analyzed via SDS-PAGE using 12% Tris-Glycine gels. The Aβ samples were aged between 0 days to 8 weeks, added to sample buffer containing 2-mercaptoethanol, and boiled for 5 min. One microgram of each sample was loaded onto gels along with SeeBlue Plus2 (Invitrogen, Carlsbad, CA) pre-stained molecular weight markers, and electrophoretically separated at 100V. Gels were stained for total protein using BioRad Silver Stain Plus kit according to manufacturer’s protocol (BioRad, Hercules, CA) or were used for western blotting. Proteins were transferred from PAGs to polyvinylidene difluoride (PVDF) membranes at 4°C for either 1 h at 300 mAmps or overnight at 100 mAmps. PVDF membranes were subsequently blocked for 1 h at room temperature in Tris-buffered saline with 0.1% Tween-20 (TBST) and 5% non-fat dried milk. PVDF membranes were then incubated with the anti-APP/Aβ primary antibody 6E10 (Signet, Dedham, MA) at 1:1000 dilutions for 1 h at room temperature and washed 4 × 5 min with TBST. A horseradish peroxidase-conjugated rabbit anti-mouse secondary antibody was used at a 1:2000 dilution for 1 h at room temperature, washed as above, then washed 1 × 5 min in TBS. Immuno-positive Aβ species were visualized using chemiluminescence (Western Lightning Reagent, PerkinElmer, Waltham, MA). At least four independent AβO preparations were analyzed by Western blot with consistent results.
Dot blotting
Nitrocellulose membranes were wet with transfer buffer before 0.1μg of Aβ samples, which were aged between 0 days to 7 weeks, were spotted onto it in a grid-like pattern. Nitrocellulose membranes were blocked for 1 h at room temperature (~22°C) in TBST and 5% non-fat dried milk. Membranes were subsequently blotted using primary antibodies I11, an oligomer-specific antibody (kindly provided by Dr. Charles Glabe, UC Irvine), at 1:2000 or 6E10 at 1:5000 dilution overnight at 4°C and washed 4 × 5 min with TBST. A horseradish peroxidase-conjugated secondary antibody was used at a 1:2000 dilution for 1 h at room temperature, washed as above, then washed 1 × 5 min in TBS. Dot blots were visualized with chemiluminescence, and analyzed for total raw density (Labworks by UVP, Upland, CA) and total protein content with Amido Black assay (Dieckmann-Schuppert and Schnittler, 1997).
ELISA
Aβ samples, which were aged between 0 days to 8 weeks, were coated on plastic 96-well plates for 1 h at room temperature. Excess peptide was removed, wells washed once with TBS, then were blocked for 1 h at room temperature in 50% TBST and 50% casein blocker (Pierce, Rockford, IL). Wells were subsequently incubated with primary antibody NU-4, an oligomer-specific antibody (kindly provided by Dr. William Klein, Northwestern University), at 1:1000 for 1 h at room temperature and washed 5× with TBST. A horseradish peroxidase-conjugated secondary antibody was used at a 1:2000 dilution for 1 h at room temperature, washed as above, then washed 1× with TBS. HRP activity was detected using tetramethylbenzidine (TMB) substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD). Reactions were quenched after 15 min with 1M H3PO4 and plates read at 450nm.
Atomic Force Microscopy (AFM)
AβM and AβO preparations were aged 2 and 6 weeks. Aβ reverse peptides were prepared as AβOs, except their primary sequence is 42-1, and were aged for 2 weeks. Twenty microliters of Aβpeptide preparation was spotted onto each disc of freshly cleaved mica (Ted Pella, Redding, CA) and allowed to dry for 10 min before being washed twice with ddH20. AFM analysis of samples was carried out on a Nanoscope III (Digital Instruments/Veeco) instrument operated in tapping mode. PointProbe-Plus Silicon-SPM-Sensor 4.0 μm cantilevers (Nanosensors, Switzerland) with a spring constant of 40 N/m and resonant frequency of 330kHz were utilized. Micrographs were analyzed (Image SXM v1.89 analysis software) to determine the vertical distances (z-plane) of entities within an imaged field. Height analysis data were represented in a histogram to demonstrate the relative abundance of different heights. Mean heights from each micrograph were also calculated.
Thioflavine T fluorescence assay
A ThioT fluorescence assay was performed as described previously by Levine et al. with minor changes (LeVine, 1993). ThioT was purchased from Sigma. 150 μl of 50 μM ThioT (diluted in 50mM glycine-NaOH buffer, pH 8.5) was added to 50 μl of Aβ monomer or oligomer preparations of varying concentrations. Fluorescence was measured immediately on a SpectraMax Fluorometer (Molecular Devices, Sunnyvale, CA) with excitation and emission wavelengths of 450nm and 485nm, respectively, with automatic cutoff. The experiment was performed 3 times with each sample in duplicate.
Primary cortical cultures
Cortices from embryonic day 15 mice were micro-dissected, tissue washed in Hank’s Balance Salt Solution (HBSS), dissociated with 0.05% trypsin-EDTA for 5 min at 37°C and then trypsin deactivated using 3 washes of cold HBSS for 5 min each. Neurons were plated in polyethyleneimine-coated wells at a density of 2 × 105 cells/well in B-27-supplemented Neurobasal Media (with 0.5 mM L-glutamine plus L-glutamic acid). One-half of the B-27 supplemented neurobasal media (with 0.5mM L-glutamine) was changed every 4 days. Neurons were cultured at 37°C with 6% CO2.
Cortical neuron viability assay
Day in-vitro (DIV) 8 cultures were treated with 1 μM Aβ oligomer preparations or Aβ42–1 reverse peptide control (prepared identically). At designated times after peptide treatment (36, 42, and 48 h), cultures were fixed with 4% paraformaldehyde and stained with Hoechst to visualize nuclei. Fluorescence and bright field photomicrographs were obtained by imaging 4 fields/well and 4 wells per condition. Neurons possessing condensed or irregularly shaped nuclei were counted as dead/dying.
SH-SY5Y cell culture and MTS viability assay
SH-SY5Y cells (ATCC, Manassas, VA) were plated at a density of 2×104 cells/well in 96-well plate formats (n=6 wells per experimental condition) and incubated overnight at 37°C in 5% CO2. Cells were maintained in DMEM/F12 + 10% FBS. AβOs or Aβ42–1 reverse peptide were aged for three weeks. Growth media was removed 24 hours after plating cells and replaced with experimental media composed of growth media containing AβOs or Aβ42–1 reverse peptide at 2 μM. Cells were incubated for 72 h and cell proliferation was assessed using the CellTiter 96 assay kit (Promega, Madison, WI) according to kit protocol. MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium), when added to phenazine methosulfate (PMS), produces a water-soluble formazan product. Absorbance at 490nm was used to quantify the relative numbers of live cells between treatment conditions. Cells were incubated with 20 μl MTS/PMS solution for 90 min at 37°C in 5% CO2. Absorbances were read at 490 nm on a plate reader (Bio-Tek Instruments, Winooski, VT).
Statistical analyses
Statistical analysis was performed using GraphPad Prism 5 software. Two-way analysis of variance with Bonferroni post-test was performed on the data sets indicated. Statistical differences with p-values of ≤ 0.05 were considered significant.
Results
Synthesis of amyloid-beta oligomer preparations
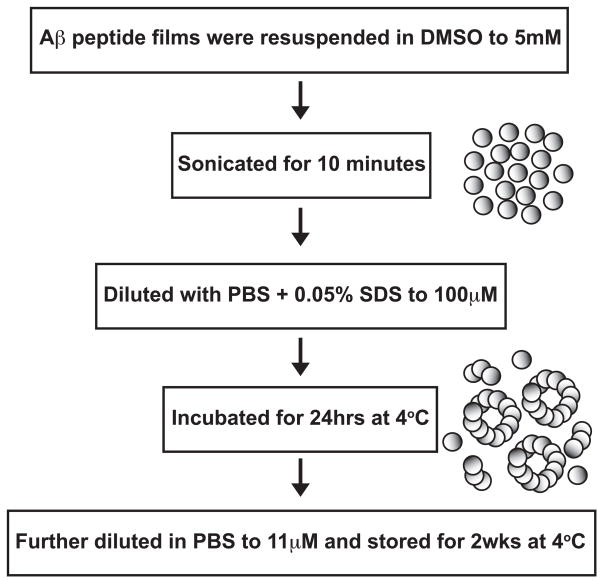
To achieve consistent AβO preparations, Aβ peptide was first treated with a fluorinated alcohol, HFIP, to break down any secondary structure, or “seeds”, inherent in the lyophilized peptide provided by the manufacturer (Zagorski and Barrow, 1992). The HFIP solvent was evaporated and peptide films were stored. A flow chart (Fig. 1) of the preparation methods outlines the steps used for generating the AβO preparations from the stored peptide films. A key step in their production was the inclusion 0.05% SDS, which is required for the induction of stable higher order oligomers.
Figure 1. Flow chart of Aβ oligomer preparation method.
Lyophilized Aβ1–42 peptide was treated with HFIP, which was subsequently evaporated to form clear peptide films. Aβ peptide films were resuspended to 5 mM in DMSO followed by bath sonication for 10 min. These were diluted to 100 μM with PBS + 0.05% SDS and incubated at 4° C for 24 h. This peptide solution was further diluted to 11 μM (50 μg/ml) and incubated at 4° C. After two weeks the Aβ preparation was enriched in high-order oligomeric species. These AβO preparations were then used in assays to identify their structural and functional characteristics.
Characterization of AβOs
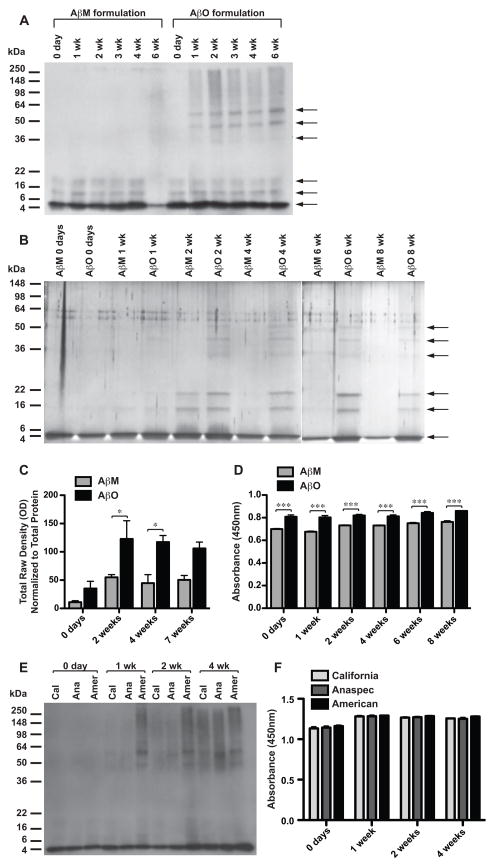
Aβ monomer (AβM) and AβO formulations (see Materials and Methods) were aged between 0 days and 8 weeks. It is important to note that AβMs were only aged as a structural comparison to the AβO formulation. Discrete bands of Aβ species were visualized using SDS-PAGE followed by Western blot analysis (Fig. 2A) and silver stain (Fig. 2B). Although western analysis is extremely sensitive, silver stain, a total protein stain, was employed because immunoreactivity of oligomers can be conformation dependent. In the AβM preparations, from 0 days to 4 weeks, monomers were abundant at the 4.5 kDa level; quantities of low-order oligomers were also detected. It has been shown that Aβ is present in solutions as a combination of both monomers and low-order oligomers, not as monomer alone (Bitan et al., 2003; Bitan et al., 2001; Walsh et al., 1997). After aging for 6 weeks, low levels of higher order conformers and insoluble species arose from AβM formulations; insoluble species resided in the stacker segment of the SDS-PAGE gel, not migrating into the resolving gel. The AβOs were consistent from preparation to preparation until 6 weeks. AβO preparations contained monomers and low-order oligomers, and high-order oligomers. The high molecular weight oligomers were comprised of species residing at approximately 36, 46, and 56 kDa levels that began to appear by 1 week. With western blot analysis at longer development times a 60-kDa band became visible (data not shown). These higher order oligomers continued to accumulate to maximum levels at approximately 2 weeks and remained at these levels until 6 weeks. The AβO preparations only began to form insoluble species after the 6-week time point.
Figure 2. Structural characteristics of AβO preparations.
Western blot analysis using anti-APP/Aβ antibody, 6E10 (A), and silver stain (B) of peptide samples separated by SDS-PAGE. Aβ1–42 samples prepared with PBS (no SDS) and aged at 4°C were termed “AβM” preparations. Aβ1–42 samples prepared with PBS and 0.05% SDS and aged at 4°C were termed “AβO” preparations. Conformers present in AβM and AβO preparations aged between 0 days and 8 weeks are shown (A, B). Arrows denote protein bands (B). C) Densitometry was performed on spots of differentially aged (0 days to 7 weeks) AβM and AβO preparations that were absorbed on nitrocellulose and blotted with an oligomer-specific antibody, I11. Densitometry data were normalized to total protein concentration. D) Differentially aged (0 days to 8 weeks) AβM and AβO preparations that were coated onto 96-well plates and an ELISA performed to measure oligomer immunoreactivity using oligomer-specific antibody, NU-4, and HRP-conjugated secondary. Absorbance was measured at 450 nm. Differentially aged (0 days to 4 weeks) AβO preparations generated from peptide purchased from different vendors (Cal–California Peptide Research, Ana–Anaspec, or Amer–American Peptide) were assessed using western analysis with anti-APP/Aβ antibody, 6E10 (E) and ELISA with oligomer-specific antibody, NU-4 (F). A two-way ANOVA with Bonferroni’s post-test was performed (“*” p<0.05; “***” p<0.001) (C, D, F).
Several proteins with unrelated primary sequences, form insoluble fibrous protein aggregates called amyloid, which is characterized by a cross-beta sheet quaternary structure. Amyloidogenic proteins include, but are not limited to Aβ, α-synuclein, huntingtin, prion, and islet amyloid polypeptide (IAPP). These amyloidogenic proteins have also been shown to form structurally similar soluble oligomers, which share an epitope recognized by oligomer-specific antibodies (Kayed et al., 2007; Kayed et al., 2003). In order to evaluate whether our AβO preparations share this common structure we performed dot-blot analysis using an oligomer specific antibody. Glabe and colleagues (2007) reported that rabbits immunized with IAPP oligomer mimics produced an immune response generating a serum termed I11, which recognizes prefibrillar oligomers derived from Aβ, α-synuclein and IAPP, but not monomers or fibrils (Kayed et al., 2007). Differentially aged AβM and AβO preps were spotted on nitrocellulose and probed with I11 to assess oligomeric content. Immunoreactivity was quantified with densitometry, data normalized for total protein content, and statistical significance determined (Fig. 2C). AβM and AβO preparations at 0 days were indistinguishable in oligomer-specific immunoreactivity, both measuring at basal levels. From day 0 to 2 weeks oligomeric content in AβO preparations increased 3.5-fold and this approximate level was sustained until 7 weeks. Oligomer-specific immunoreactivity in AβO preparations was significantly increased over AβM preparations at 2 and 4 weeks. Levels within AβM preparations increased slightly from day 0, but remained at a low level from 2–7 weeks. A two-way ANOVA demonstrated a significant effect of preparation type (p=0.0008) and time (p=0.0080).
To corroborate these findings a second oligomer-specific antibody was utilized, which was developed in the laboratory of Dr. William Klein. NU-4 recognizes Aβ oligomers, but not monomeric Aβ (Lambert et al., 2007). An enzyme-linked immunosorbent assay (ELISA) with NU-4 was employed to compare oligomeric content between differentially aged oligomeric and monomeric preparations (Fig. 2D). AβOs exhibited significantly higher NU-4 immunoreactivity than AβMs at 0 days through 8 weeks (type, p<0.0001). Increases in NU-4 immunoreactivity over the time points evaluated were subtle, but statistically significant (time, p=0.0001). To test whether observed differences were simply a consequence of differences in vehicle (presence or absence of SDS), Aβ reverse peptide aged for two weeks in phosphate buffered saline (PBS) with 0.05% SDS was also assessed, demonstrating only basal levels of NU-4 immunoreactivity (mean abs450 = 0.069).
To address whether AβO preparations generated from peptide purchased from alternate vendors are similar, western analysis and ELISA to assess oligomeric content were performed (Fig. 2E,F). Compared to AβOs generated from peptide purchased from American Peptide Company, those prepared from California Peptide Research and Anaspec peptides demonstrated a slightly delayed enrichment in higher-order oligomeric structures by western. However, when oligomeric content was assessed by ELISA, there was a typical increase over time (p<0.0001), but no difference was discerned between vendor sources (Two-way ANOVA, p=0.0762).
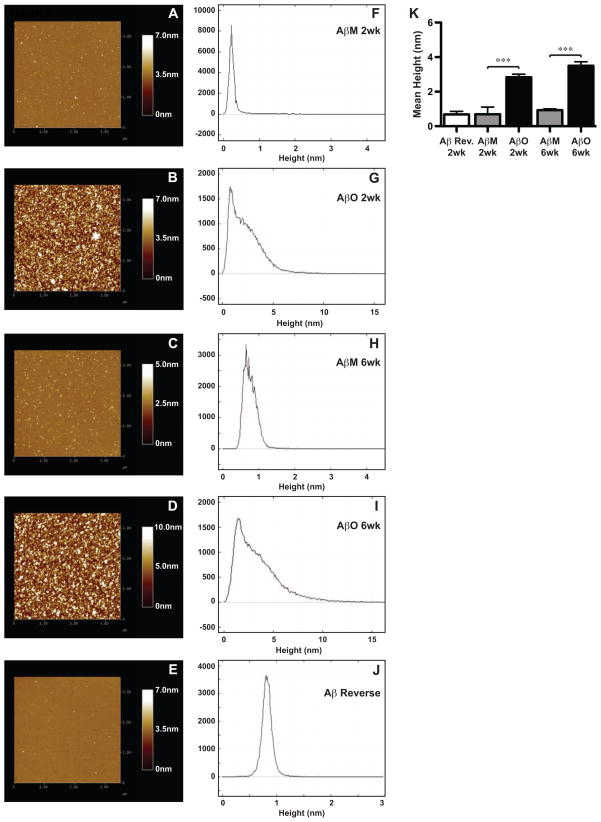
Since the oligomer-specific antibodies likely recognize multiple molecular species of soluble prefibrillar oligomers, our goal was to determine the precise composition of the AβO preparations. SDS-PAGE alone is not always sufficient to determine the properties of synthetic Aβ as it is possible that some assemblies dissociate during the procedure (Stine et al., 2003). Therefore, atomic force microscopy (AFM) was utilized to determine the structural properties of these AβO preparations. AβM, AβO and Aβ reverse peptide preparations were spotted on freshly cleaved mica and analyzed with AFM (Fig. 3). Representative atomic force micrographs from 2 and 6 week old preparations are depicted; height of species was color coded with higher values represented with lighter shades (Fig. 3A–E). Prior publications have reported a range of z-height values for individual monomers to range between 0.7 to 2.0 nm and oligomers between 2.0 to 6.0 nm (Mastrangelo et al., 2006; Stine et al., 2003). Atomic force micrographs of the AβO preparations were collected and section analysis was performed to determine the relative height diversity, which is displayed in frequency distributions (Fig. 3F–J). The mean height of each preparation was also calculated (Fig. 3K). Aβ reverse peptide had a mean height of 0.683 nm, which was similar to AβM preparations at 2 weeks (0.693 nm) and 6 weeks (0.933 nm). Entities from AβO preparations exhibited a significantly higher mean height of 2.83 nm at 2 weeks and 3.50nm at 6 weeks, which were not significantly different from each other. The frequency distribution data demonstrate that AβMs and Aβ reverse preps are predominately composed of a single peak. AβOs contain a peak similar to the monomer preps, but also harbor lower quantities of numerous species within the height range of both low and high-order Aβ oligomers (Fig. 3G,I) (Mastrangelo et al., 2006; Stine et al., 2003). The AFM data from the AβO preparations substantiate our SDS-PAGE results, which demonstrated abundance of monomers and less intense bands of low- and high-order oligomers. The structures present on the atomic force micrographs were sphere shaped, not elongated, as fibrils would characteristically appear. These data demonstrate the AβO preparations included monomers, low-order and high-order oligomers, but no fibrils.
Figure 3. AβO preparations analyzed by atomic force microscopy.
Aβ reverse peptide, AβO, and AβM preparations were aged for 2 and 6 weeks and subsequently analyzed by atomic force microscopy (AFM). (A–E) Representative micrographs were acquired over a 3.5-μm2 area. Colors depicting height or total z-range are shown on individual scales. (F-J) Section analysis was performed to determine the relative abundance of heights of different Aβ entities present in a micrograph and represented as a frequency distribution. (K) The mean height was collected across multiple atomic force micrographs for each condition and graphed. A one-way ANOVA (p<0.0001) with Bonferroni’s post-test was performed (“***” p<0.001).
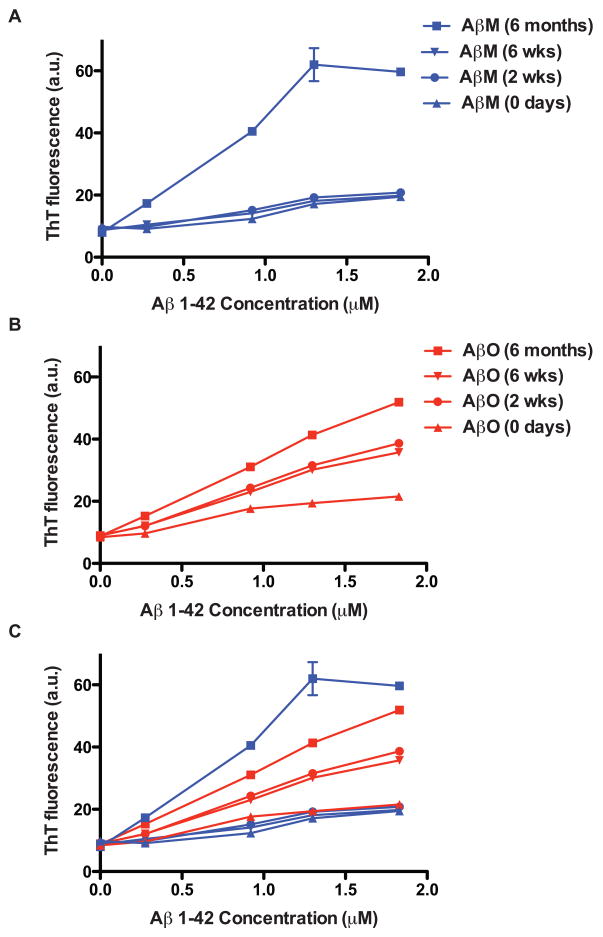
Thioflavine T (ThioT) and its derivatives have long been known to associate with the β-sheet structure of fibrilar amyloid. Upon interaction with aggregated Aβ peptides, the excitation spectrum of ThioT transforms from that of the free dye with a new excitation peak at 450nm (LeVine, 1993). Fluorescence emission can be measured at 482–485 nm. Fluorescence intensity has also been shown to be dependent upon the concentration of aggregated Aβ peptides (LeVine, 1993). ThioT fluorescence assays have classically been used to detect the presence and quantity of fibrils, however it has been shown that ThioT also detects AβOs. Maezawa and colleagues determined the binding capacity and affinity of several small molecule Aβ-specific ligands for AβOs using surface plasmon resonance. Binding affinity for ThioT specified by the dissociation constant (KD) was determined to be in the nanamolar range (498.0 nmol/L) (Maezawa et al., 2008). Using a classic ThioT fluorescence assay they found oligomers and fibrils generated from equal molar concentrations of Aβ1–42 had an equivalent binding capacity (Maezawa et al., 2008). These data, as well as a study where ThioT was shown to bind to protofibrils (Walsh et al., 1999), demonstrate the ability of ThioT to interact with oligomers as well as fibrils.
We measured ThioT fluorescence from differentially aged AβO and AβM preparations (Fig. 4). Peptides aged for 6 months were included to demonstrate ThioT fluorescence in the presence of fibrils. AβMs aged for 0 days, 2 and 6 weeks demonstrated only a basal level of fluorescence (Fig. 4A). We observed the same basal fluorescence from AβOs at 0 days. AβO preparations aged for 2 and 6 weeks exhibited a similar level of fluorescence emission suggesting similar levels of oligomers were present (Fig. 4B). AβOs and AβM aged for 6 months had higher levels of fluorescence than the AβOs aged for 2 and 6 weeks indicating further aggregation had occurred.
Figure 4. Thioflavine T fluorescence assay of AβO preparations.
AβO (A) and AβM (B) preparations aged for 0 days, 2 weeks, 6 weeks, and 6 months were added at different concentrations to free ThioT dye in a black 96-well microplate. Wells were excited at 450 nm and emission was read at 485 nm on a fluorometer. Relative fluorescence of each preparation is shown in arbitrary units (a.u.). (C) AβO and AβM data were overlaid for comparison.
In aggregate, these data demonstrate the structural consistency and reproducibility of preparations of Aβ oligomers aged from 2 to 6 weeks. We next sought to determine if these synthetic peptide preparations possessed toxic properties similar to oligomers isolated from the AD brain.
Neurotoxic action of AβO preparations
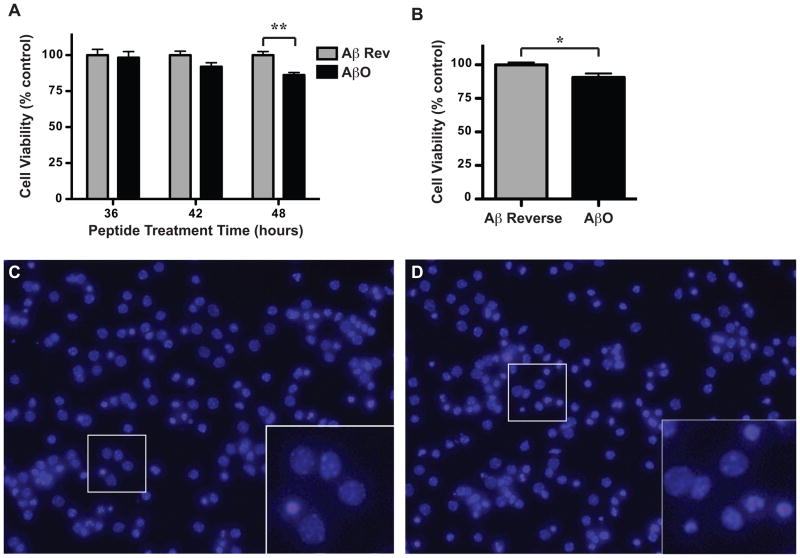
To determine whether the AβO preparations possessed a biologically relevant function we tested their effect on the viability of primary neuronal cultures. Cultures were derived from the cortices of embryonic day 15 mice and allowed to mature to DIV8 before being treated with 1 μM AβOs (aged 2 weeks) or Aβ42–1 reverse peptide control (prepared identically, aged 2 weeks). At designated times after peptide treatment (36, 42, and 48 h), cells were fixed, stained with Hoechst to visualize nuclei and enumerated as “live” or “dead”. An effect of the AβO treatment was not observed 36 h after treatment, but there was a trending decrease in viability at 42 h, which reached significance by 48 h (Fig. 5A). Two-way ANOVA showed a significant effect of treatment and time-point. Representative images of Aβ42–1 reverse peptide-treated (Fig. 5C) and AβO-treated (Fig. 5D) cortical cultures are shown with insets magnified to demonstrate the visible difference between healthy and dying/dead cells. Live, healthy cells harbor large round nuclei with one or more nucleoli present, whereas dead, or dying cells have irregularly shaped nuclei and condensed chromatin (insets Fig. 5C and D). To corroborate these findings we performed an alternate viability assay using the SH-SY5Y neuronal cell line treated with AβOs or Aβ42–1 reverse peptide control. Cell viability was assessed using a modified MTT reduction assay (CellTiter 96; Promega). This assay utilizes a tetrazolium compound (MTS), which is bioreduced in live cells into a soluble formazan product causing a quantifiable color change to occur. The AβO treatment decreased cell viability, which was observed 72 h after treatment (Fig. 5B). A t-test demonstrated a significant effect of the AβO treatment.
Figure 5. AβO preparations are neurotoxic.
Primary cortical cultures derived from embryonic day 15 mice were allowed to mature to DIV8 before being treated with 1 μM AβOs or Aβ42-1 reverse peptide control (prepared identically) both aged to 2 weeks. A) At 36, 42, or 48 h after treatment, cells were stained with Hoechst to visualize nuclei, enumerated as “live” or “dead”, and data plotted as cell viability as a percent of control. Two-way ANOVA with Bonferroni post-test was performed (“**” indicate p<0.01). Representative images of Aβ42-1 reverse peptide-treated (C) and AβO-treated (D) cortical cultures are depicted (20×) with insets magnified (40×). B) SH-SY5Y cells were treated with media containing 2 μM AβOs or Aβ42-1 reverse peptide control aged for 3 weeks. At 72 h following peptide treatment cell viability was assessed using an MTS reduction assay (Promega, Madison, WI) in which the conversion of MTS into formazan occurs only in metabolically active cells. Absorbance was measured at 490nm and viability expressed as a percentage of control. A t-test was performed (“*” indicate p=0.0212).
Discussion
Here we describe a protocol designed to consistently generate stable neurotoxic AβO preparations. We have employed a combination of immunological, biochemical, biophysical and cell biological methods to characterize the synthetic AβO preparations. The ease of generation and reproducibility of these peptide conformers lend their use in a variety of in vitro and in vivo applications.
There is a wealth of scientific evidence supporting the significance of soluble Aβ oligomers in the pathophysiology of AD (Cleary et al., 2005; Gong et al., 2003; Klyubin et al., 2005; Lesne et al., 2006; Shankar et al., 2008). Oligomers obtained from the AD brain have been shown to be biochemically analogous to synthetically generated AβOs (Gong et al., 2003). Additionally, AβOs from AD patients have demonstrated the ability to bind at neuronal synapses, similar to synthetic AβOs, and inhibit neural transmission (Lacor et al., 2004). A dodecameric form of Aβ, isolated from an AD mouse model, was implicated as the specific form causing cognitive impairment (Lesne et al., 2006). This evidence supports the continued study of oligomeric forms of Aβ and approaches designed to block their neurotoxic effects. Therefore, a straightforward method to produce reliable preparations is valuable to the field.
Our AβOs are unique from other synthetic Aβ preparations reported in the literature in that they contain discrete bands of both low- and high-order oligomers, which are reproducible and stable from 2 to 6 weeks at 4°C. This is unlike synthetic Aβ-derived diffusible ligands (ADDLs) (Lambert et al., 1998; Lambert et al., 2001), which have been reported to vary in composition based on incubation times (Shankar and Walsh, 2009). The AβO preparation technique is similar to the method by which the Aβ1–42 globulomers (Barghorn et al., 2005) are generated due to the use of SDS to induce aggregation. However, upon SDS-PAGE analysis Aβ1–42 globulomers migrate as two bands corresponding to 38 and 48 kDa. Upon cross-linking with glutaraldehyde it was shown that the dual globulomer band is actually one species with a molecular weight of 60 kDa when analyzed via size exclusion chromatography. The high-order oligomeric species in our AβO preparations are comprised of bands that run at approximate molecular weights of 36, 46, 56 and 60 kDa on SDS-PAGE. The differences between the globulomer protocol and the one described here, which include variations in Aβ concentration and aggregation conditions, account for the disparate outcomes. Additionally, the species in our AβO preparations may have distinct conformations compared to globulomer species because ThioT dye binds significantly to our preps (Fig. 4), whereas it was reported globulomers do not bind ThioT (Gellermann et al., 2008).
It is clear in this study that SDS plays a key role as an inducer of Aβ aggregation, and perhaps even stabilization. The sole difference between the AβM and AβO preparations is the low concentration of SDS in the latter. The molecular composition of SDS (C12H25SO4Na) gives it amphiphilic properties; a sulfate group as a head followed by a 12-unit hydrocarbon tail. Thus, SDS is commonly employed as a detergent for multiple applications. Aggregation properties of Aβ were examined in the presence of certain detergents (including SDS) and membrane-mimicking environments (Yamamoto et al., 2004). Their data suggest that at low concentrations of SDS, the area between water and the surface of the detergent micelles is a favored environment for the induction of Aβ aggregation (Yamamoto et al., 2004). The micellar structure and negative charge of SDS is likely to alter the hydrophobic microenvironment, creating preferential conditions to initiate the formation of oligomeric Aβ.
The question could be posed that since SDS is a detergent not found under physiologic conditions how are these oligomers physiologically relevant? SDS is used as an initiator or seed for the building of Aβ oligomers, there are endogenous components of cells that could also participate in the induction of AβO formation. GM1 ganglioside is an integral component of the plasma membrane, particularly enriched in lipid rafts. GM1 ganglioside has been shown to bind Aβ and has been suggested to act as a seed to initiate aggregation (Yanagisawa et al., 1995). In vitro aggregation studies with Aβ and different concentration of GM1 ganglioside verified it as an inducer of aggregation (Yamamoto et al., 2004). This is just one example of an endogenous molecule that could act comparably to the in vitro role of SDS.
AβOs demonstrated temporally dissimilar patterns of recognition by I11 and NU-4 oligomer-specific antibodies. These differences could be accounted for if I11 and NU-4 bind distinct oligomeric epitopes. This is likely since I11 is a polyclonal antibody, thus may recognize multiple oligomeric epitopes. NU-4 has been shown to recognize low molecular weight forms of synthetic ADDLs with high specificity, among others (Lambert et al., 2007). By western analysis (Fig. 2A), we demonstrated that levels of low-order oligomers appear early and are relatively stable throughout the time points tested in both AβM and AβO preparations. These Aβ species, could therefore, account for the nearly stable immunoreactivity of NU-4 observed from 0 days to 8 weeks. The increase in NU-4 immunoreactivity detected in AβOs over AβMs could represent a difference in conformation induced by the presence of SDS.
AβO-specific immunoreactivity by I11 increases from 0 days to 2 weeks and then remains unaltered between 2 to 7 weeks. High-order oligomers (36–60 kDa) appear during this time. Therefore, it is probable that these species account for the temporal increase in I11 immunoreactivity. As I11 is only reliable on a dot blot, it will be difficult to determine what other types of conformers underlie the observed increase. It is important to reiterate that AβM preparations are not consistent or stable during in vitro aging. This variability makes comparisons of the aged AβM preparations from one experiment to another challenging. However, they remain a useful comparator for the AβO preparations, as they do not appear to form high-order oligomers. Instead, the monomer and low-order oligomers tend to be converted to insoluble species that do not enter the resolving gel during SDS-PAGE.
The ease of preparation, reproducibility and stability of the AβO preparations make them applicable in a variety of in vitro and in vivo procedures in the study of Alzheimer’s disease. The AβOs contain a variety of oligomeric forms, both low and high-order, that could act as the antigen in vitro for the isolation of aptamers or oligomer-specific antibodies from phage-display libraries. AβOs paired with an adjuvant to induce an active Aβ-specific immune response to generate antibodies in vivo could yield novel immuno-therapeutics. Due to their neurotoxic properties, the AβOs could also be employed in screens of small molecule inhibitors to test their efficacy at subverting neuronal death. These applications involve both isolating and testing potential Alzheimer’s disease treatments. This synthetic preparation should prove to be a valuable tool to many studying Alzheimer’s disease.
Acknowledgments
The authors would like to thank Dr. Seymon Papernov (University of Rochester Laboratory for Laser Energetics) for instruction on how to perform atomic force microscopy, Dr. Charles Glabe (University of California-Irvine) for providing the I11 antibody, Dr. William Klein (Northwestern University) for providing the NU-4 antibody, and Ms. Rita Giuliano (University of Rochester Medical Center) for guidance on culturing mouse primary neurons. This work was supported by NIH F31-NS059283 to DAR, and NIH R01-AG023593 and NIH R21-AG031878 to WJB
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer’s disease. J Neurochem. 2005;95:834–47. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid beta -protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc Natl Acad Sci U S A. 2003;100:330–5. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan G, Lomakin A, Teplow DB. Amyloid beta-protein oligomerization: prenucleation interactions revealed by photo-induced cross-linking of unmodified proteins. J Biol Chem. 2001;276:35176–84. doi: 10.1074/jbc.M102223200. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Dieckmann-Schuppert A, Schnittler HJ. A simple assay for quantification of protein in tissue sections, cell cultures, and cell homogenates, and of protein immobilized on solid surfaces. Cell Tissue Res. 1997;288:119–26. doi: 10.1007/s004410050799. [DOI] [PubMed] [Google Scholar]
- Dodart J-C, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reversesmemory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nat Neurosci. 2002;5:452–7. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Esler WP, Wolfe MS. A portrait of Alzheimer secretases--new features and familiar faces. Science. 2001;293:1449–54. doi: 10.1126/science.1064638. [DOI] [PubMed] [Google Scholar]
- Gellermann GP, Byrnes H, Striebinger A, Ullrich K, Mueller R, Hillen H, Barghorn S. Abeta-globulomers are formed independently of the fibril pathway. Neurobiol Dis. 2008;30:212–20. doi: 10.1016/j.nbd.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer’s disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci U S A. 2003;100:10417–22. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, Rasool S, Gurlo T, Butler P, Glabe CG. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–9. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, Rowan MJ. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–61. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci. 2002;22:6331–5. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci USA. 1998;95:6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Velasco PT, Chang L, Viola KL, Fernandez S, Lacor PN, Khuon D, Gong Y, Bigio EH, Shaw P, De Felice FG, Krafft GA, Klein WL. Monoclonal antibodies that target pathological assemblies of Abeta. J Neurochem. 2007;100:23–35. doi: 10.1111/j.1471-4159.2006.04157.x. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Viola KL, Chromy BA, Chang L, Morgan TE, Yu J, Venton DL, Krafft GA, Finch CE, Klein WL. Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J Neurochem. 2001;79:595–605. doi: 10.1046/j.1471-4159.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- LeVine H., 3rd Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–10. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–62. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Hong HS, Liu R, Wu CY, Cheng RH, Kung MP, Kung HF, Lam KS, Oddo S, Laferla FM, Jin LW. Congo red and thioflavin-T analogs detect Abeta oligomers. J Neurochem. 2008;104:457–68. doi: 10.1111/j.1471-4159.2007.04972.x. [DOI] [PubMed] [Google Scholar]
- Mastrangelo IA, Ahmed M, Sato T, Liu W, Wang C, Hough P, Smith SO. High-resolution atomic force microscopy of soluble Abeta42 oligomers. J Mol Biol. 2006;358:106–19. doi: 10.1016/j.jmb.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282:10311–24. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- Nguyen HD, Hall CK. Molecular dynamics simulations of spontaneous fibril formation by random-coil peptides. Proc Natl Acad Sci U S A. 2004;101:16180–5. doi: 10.1073/pnas.0407273101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–70. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Walsh DM. Alzheimer’s disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine WB, Jr, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–22. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274:25945–52. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem. 1997;272:22364–72. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22:1858–67. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Hasegawa K, Matsuzaki K, Naiki H, Yanagisawa K. Environment- and mutation-dependent aggregation behavior of Alzheimer amyloid beta-protein. J Neurochem. 2004;90:62–9. doi: 10.1111/j.1471-4159.2004.02459.x. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Odaka A, Suzuki N, Ihara Y. GM1 ganglioside-bound amyloid beta-protein (A beta): a possible form of preamyloid in Alzheimer’s disease. Nat Med. 1995;1:1062–6. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- Zagorski MG, Barrow CJ. NMR studies of amyloid beta-peptides: proton assignments, secondary structure, and mechanism of an alpha-helix----beta-sheet conversion for a homologous, 28-residue, N-terminal fragment. Biochemistry. 1992;31:5621–31. doi: 10.1021/bi00139a028. [DOI] [PubMed] [Google Scholar]