Abstract
Background:
Abdominal compartment syndrome is defined as the adverse physiologic effects of increased intra-abdominal pressure. Prolonged, unrelieved pressure may lead to respiratory compromise, renal impairment, cardiac failure, shock, and death. Abdominal compartment syndrome is diagnosed by measuring intra-cystic pressure as a reflection of intra-abdominal pressure. To examine the validity of the technique, we conducted a prospective study in surgical patients by directly measuring bladder and abdominal pressures simultaneously during laparoscopic cholecystectomy using a previously described technique.
Results:
In the present model, the bladder had higher baseline pressures than did the abdomen. Measurements across the bladder wall were not identical, but had high positive correlation coefficient when evaluated on an individual basis. Global analysis of the data for all patients showed a weak correlation coefficient.
Conclusion:
In the present study model, intra-cystic pressure did not reflect actual intra-abdominal pressure. In spite of some limitations in the study design, we feel that further research is warranted to identify other possible variables that may play a role in the relationship between the urinary bladder and the abdominal cavity pressures, providing better means for diagnosis of abdominal compartment syndrome.
Keywords: abdomen, bladder, compartment, pressure, syndrome
Introduction
Abdominal compartment syndrome (ACS) is defined as the adverse physiologic consequence of acutely increased intra-abdominal pressure (IAP). Prolonged, unrelieved increased IAP at greater than 20mmHg can produce pulmonary compromise, renal impairment, cardiac failure, shock, and death [1,2,3,4,5,6].
ACS is diagnosed by measuring intra-cystic pressure (ICP) as a reflection of IAP using a Foley catheter [4,7,8]. This technique was popularized by Kron et al [7] in 1984 after small animal studies. Human studies correlating ICP and IAP are lacking to date. To identify the relationship between the pressures across the urinary bladder wall, we simultaneously measured the pressures across the urinary bladder wall in 21 surgical patients in a prospective manner.
Materials and methods
After Institutional Review Board approval, and over the course of an 18-month period, we prospectively studied 21 patients who consented to participate the study as a part of their laparoscopic cholecystectomy. Pregnant women and children were excluded. No patient had a prior history of bladder dysfunction.
After induction of anesthesia, and while the patient was still in a supine position, the bladder was evacuated by a standard Foley catheter. The Foley catheter was then primed with 50 cm3 of sterile saline. The sterile tubing of the urinary drainage bag was cross-clamped just distal to the catheter aspiration port. The end of the sterile tubing was then connected to the Foley catheter. The clamp was released just enough to allow tubing proximal to the clamp to flow with fluid from the bladder, then reapplied. A 16-gauge needle was used to Y-connect a pressure transducer through the culture aspiration port of the tubing to the drainage bag. The symphysis pubis bone was used as the zero point, with the patient supine.
The laparoscopic equipment was calibrated before each operation. The operating room circulator was then asked to choose three progressive target abdominal pressures between 1 and 15 mmHg. Abdominal insufflation with carbon dioxide was started through a Veress needle. As the target pressure was reached, the bladder pressure was recorded after 5⌓10 s equilibration time at the end of the mechanical expiration. The procedure was repeated with every target abdominal pressure to ensure consistency. The compiled data was then analyzed using AB stat software [Version 1.93 (1996); Anderson-Bell, Arvada, CO 80005, USA] to identify the relationship between the ICP and IAP.
Results
Twenty females and one male were enroled in the study. Mean age was 32.5 years (range 19⌓61 years). Mean weight was 80.8 kg (range 53.1⌓131.7 kg). Mean height was 163 cm (range 155⌓180 cm). The target abdominal pressures and the corresponding bladder pressures are listed in Table 1. As shown, ICP and IAP were not identical. In every patient there was a positive baseline pressure inside the bladder when IAP was zero. Using regression analysis for individual subjects, pressures on either side of the bladder wall had high positive correlation (correlation coefficient 0.92⌓1.00). The lines generated by data points were parallel but not identical, with a wide range of interception (-3.2 to -30.5) and a variable regression coefficient (1.0⌓3.1).
Table 1.
Pressure readings across the bladder wall
| Abdominal pressures (mmHg) | Bladder pressures (mmHg) | |||||||
| Patient | Baseline | 1st | 2nd | 3rd | Baseline | 1st | 2nd | 3rd |
| 1 | 0 | 5 | 9 | 12 | 11 | 11 | 16 | 19 |
| 2 | 0 | 5 | 10 | 15 | 16 | 17 | 20 | 24 |
| 3 | 0 | 7 | 9 | 13 | 12 | 17 | 18 | 18 |
| 4 | 0 | 4 | 9 | 13 | 12 | 14 | 16 | 18 |
| 5 | 0 | 5 | 10 | 15 | 18 | 19 | 23 | 26 |
| 6 | 0 | 8 | 14 | 15 | 10 | 12 | 17 | 19 |
| 7 | 0 | 5 | 11 | 14 | 7 | 11 | 13 | 13 |
| 8 | 0 | 5 | 10 | 15 | 16 | 18 | 20 | 25 |
| 9 | 0 | 8 | 11 | 14 | 15 | 19 | 20 | 22 |
| 10 | 0 | 5 | 10 | 15 | 4 | 7 | 10 | 13 |
| 11 | 0 | 6 | 10 | 14 | 9 | 16 | 19 | 23 |
| 12 | 0 | 8 | 11 | 14 | 12 | 18 | 22 | 24 |
| 13 | 0 | 5 | 10 | 15 | 13 | 17 | 18 | 21 |
| 14 | 0 | 7 | 10 | 14 | 9 | 10 | 12 | 13 |
| 15 | 0 | 7 | 10 | 13 | 12 | 17 | 21 | 21 |
| 16 | 0 | 5 | 10 | 15 | 4 | 7 | 12 | 18 |
| 17 | 0 | 8 | 11 | 14 | 7 | 10 | 12 | 14 |
| 18 | 0 | 8 | 11 | 13 | 9 | 14 | 17 | 20 |
| 19 | 0 | 5 | 10 | 15 | 8 | 8 | 12 | 16 |
| 20 | 0 | 6 | 9 | 13 | 10 | 15 | 19 | 21 |
| 21 | 0 | 5 | 7 | 15 | 15 | 21 | 22 | 25 |
Presented are three abdominal pressures plus baseline (abdominal pressure 0 mmHg) and the corresponding bladder pressures for each of 21 patients.
Global analysis of all data revealed a weak correlation between the pressures measured across the bladder wall (Fig. 1).
Figure 1.
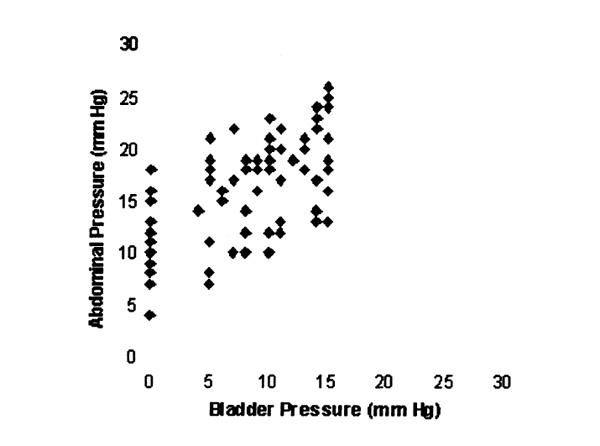
Scattergram of abdominal and bladder pressures.
Discussion
ACS is a poorly appreciated complication of increased IAP. Trauma, surgery, and certain medical conditions can be the culprit. Prolonged, unrelieved elevation of IAP can produce pulmonary compromise, renal impairment, cardiac failure, central nervous dysfunction, shock, and death [1,2,3,4,5,6].
The adverse physiologic effects of ACS can be divided into systemic and local effects [1,2,3]. The systemic hemodynamic effects are very complex in nature. Usually, the patient manifests low cardiac output and high systemic vascular resistance in the context of high central venous and pulmonary capillary wedge pressure. Increased IAP reduces venous return and increases intra-thoracic pressure, with impairment of ventricular compliance. In effect, there will be decreased stroke volume and low cardiac output, with compensatory increase in peripheral vascular resistance. Also, there is a reduction in both static and dynamic compliance of the lungs and perfusion of the kidneys, as well as of other retroperitoneal and intraperitoneal organs, except for the adrenals. High intra-thoracic pressure interferes with cerebral venous return, with decreased cerebral perfusion leading to brain dysfunction. On the other hand, local effects of ACS are probably best reflected by wound dehiscence secondary to direct pressure on the wound, as well as interference with local blood supply.
The incidence of ACS varies between 15 and 38% of all surgical patients admitted to intensive care units [2,9]. A high index of suspicion is imperative for optimal outcome. If not recognized and treated in timely manner, ACS can result in multiorgan system failure and death. Animal studies have shown that IAP higher than 20 mmHg results in abdominal compartment syndrome [4,5,8]. The adverse effects are reversible with the relief of pressure, if done at the proper time. Several clinical reports [1,2,3,6,7,9,10,11,12,13,14,15,16] have emphasized the importance of early recognition of this syndrome.
Traditionally, IAP has been measured indirectly through the urinary bladder using a Foley catheter. This technique was adopted to avoid direct invasive techniques, and was subsequently popularized by Kron et al [7] in 1984. Those authors measured IAP in 17 postoperative patients using the technique described above under Materials and methods. Ten patients had IAP of 10⌓15 mmHg. None of them developed renal insufficiency. In contrast, seven patients had IAP of more than 25 mmHg and had a drop in urine output. Three patients were not explored and died of acute renal failure. The other four patients were explored and had a prompt diuresis. However, two of the four succumbed later to sepsis. Kron et al concluded that, in the early postoperative period, IAP higher than 25 mmHg requires re-exploration and decompression of the abdomen.
Iberti et al [8] in 1987 conducted another study in a canine model. IAP was established by infusing warm saline in 500 cc increments, to total of 5 l. IAPs were measured using a separate transducer through the abdominal wall. Simultaneously, the bladder pressures were measured through a transuretheral catheter after a 5-min equilibration period. As opposed to the technique described by Kron et al [7], Iberti et al did not prime the Foley catheter with sterile saline. Pressures obtained through the bladder were not significantly different from the abdominal pressures, and therefore those authors concluded that the bladder could be used to measure the actual IAP accurately [8].
These results could not be reproduced at the range of IAPs generated in our study. We were limited by IAP of 15 mmHg, because it would not be ethical to subject our patients to ACS for the sake of the study. Accepting this limitation, and realizing that abdomen⌓bladder relationship might or might not differ at higher pressures, we identified the following interesting findings: (1) The urinary bladder had a higher baseline pressure than the abdomen in every patient compared. This may be related to the intrinsic detrusor muscle activity that might have been stimulated by the infused volume, rate of infusion, or even the temperature of the saline used to prime the Foley catheter. (2) On an individual basis, pressures measured across the bladder wall were not similar, but had high positive correlation coefficients. Although the lines generated by the data points were parallel, they were not identical. Also, there was a wide range of interception (-3.2 to -30.5) and a variable regression coefficient (1.0⌓3.1). This variability might be explained by the first finding, above. (3) Global analysis of the data from all patients showed a weak correlation between pressures measured across the bladder wall. This might be related to the biologic variation between our study subjects with regard to age, sex, weight, height, body mass index, or even history of pregnancy in females.
These findings eliminated our ability to predict IAP using ICP, and raise the question of other as yet unknown variables that may play an important role in determining the relationship between the abdominal cavity and the urinary bladder.
Conclusion
In the present study model, ICP did not reflect actual IAP. In spite of some limitations in the study design, we feel that further research is warranted to identify other possible variables that might play a role in the relationship between the urinary bladder and the abdominal cavity, providing better means for diagnosis of ACS. We are currently embarking on a prospective study on severely injured trauma patients in an attempt to answer some of these questions.
References
- Burch JM, Moore EE, Moore FA, Franciose R. The abdominal compartment syndrome. Surg Clin North Am. 1996;76:833–842. doi: 10.1016/s0039-6109(05)70483-7. [DOI] [PubMed] [Google Scholar]
- Nathens AB, Brenneman FD, Boulanger BR. The abdominal compartment syndrome. Can J Surg. 1997;40:254–262. [PMC free article] [PubMed] [Google Scholar]
- Watson RA, Howdieshell TR. Abdominal compartment syndrome. Southern Med J. 1998;91:326–332. doi: 10.1097/00007611-199804000-00002. [DOI] [PubMed] [Google Scholar]
- Harman PK, Kron IL, McLachlan HD, Freedlender AE, Nolan SP. Elevated intra-abdominal pressure and renal function. Ann Surg. 1982;196:594–597. doi: 10.1097/00000658-198211000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashtan J, Green J, Parsons EQ, Holcroft JW. Hemodynamic effects of increased abdominal pressure. J Surg Res. 1981;30:249–255. doi: 10.1016/0022-4804(81)90156-6. [DOI] [PubMed] [Google Scholar]
- Schein M, Wittmann DH, Aprahamian CC, Condon RE. The abdominal compartment syndrome: the physiological and clinical consequences of elevated intra-abdominal pressure. J Am Coll Surg. 1995;180:745–753. [PubMed] [Google Scholar]
- Kron IL, Harman PK, Nolan SP. The measurement of intra-abdominal pressure as a criterion for abdominal re-exploration. Ann Surg. 1984;199:28–30. doi: 10.1097/00000658-198401000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iberti TJ, Kelly KM, Gentili DR, Hirsch S, Benjamin E. A simple technique to accurately determine intra-abdominal pressure. Crit Care Med. 1987;15:1140–1142. doi: 10.1097/00003246-198712000-00014. [DOI] [PubMed] [Google Scholar]
- Meldrum DR, Moore FA, Moore EE, et al. Prospective characterization and selective management of the abdominal compartment syndrome. Am J Surg. 1997;174:667–673. doi: 10.1016/s0002-9610(97)00201-8. [DOI] [PubMed] [Google Scholar]
- Reeves ST, Pinosky ML, Byrne TK, Norcross ED. Abdominal compartment syndrome. Can J Anaesth. 1997;44:308–312. doi: 10.1007/BF03015370. [DOI] [PubMed] [Google Scholar]
- Bendaham J, Coetzee CJ, Papagianopoulos C, Muller R. Abdominal compartment syndrome. J Trauma Injury Infect Crit Care. 1995;38:152–153. doi: 10.1097/00005373-199501000-00034. [DOI] [PubMed] [Google Scholar]
- Eddy VA, Key SP, Morris JA. Abdominal compartment syndrome: etiology, detection, and management. J Tennessee Med Assoc. 1994;February:55–57. [PubMed] [Google Scholar]
- Williams M, Simms HH. Abdominal compartment syndrome: case reports and implications for management in critically ill patients. Am Surg. 1997;63:555–558. [PubMed] [Google Scholar]
- Morris JA, Eddy VA, Blinman TA, Rutherford EJ, Sharp KW. The staged celiotomy for trauma. Issues in unpacking and reconstruction. Ann Surg. 1993;217:576–586. doi: 10.1097/00000658-199305010-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivatury RR, Porter JM, Simon RJ, et al. Intra-abdominal hypertension after life-threatening penetrating abdominal trauma: prophylaxis, incidence, and clinical relevance to gastric mucosal pH and abdominal compartment syndrome. J Trauma Injury Infect Crit Care. 1998;44:1016–1023. doi: 10.1097/00005373-199806000-00014. [DOI] [PubMed] [Google Scholar]
- Mayberry JC, Mullins RJ, Crass RA, Trunkey DD. Prevention of abdominal compartment syndrome by absorbable mesh prosthesis closure. . Arch Surg. 1997;132:957–962. doi: 10.1001/archsurg.1997.01430330023003. [DOI] [PubMed] [Google Scholar]


