Abstract
We investigated the effects of a hot-water extract of Artemisia iwayomogi, a plant belonging to family Compositae, on cardiac ventricular delayed rectifier K+ current (IK) using the patch clamp technique. The carbohydrate fraction AIP1 dose-dependently increased the heart rate with an apparent EC50 value of 56.1±5.5 µg/ml. Application of AIP1 reduced the action potential duration (APD) in concentration-dependent fashion by activating IK without significantly altering the resting membrane potential (IC50 value of APD50: 54.80±2.24, IC50 value of APD90: 57.45±3.47 µg/ml). Based on the results, all experiments were performed with 50 µg/ml of AIP1. Pre-treatment with the rapidly activating delayed rectifier K+ current (IKr) inhibitor, E-4031 prolonged APD. However, additional application of AIP1 did not reduce APD. The inhibition of slowly activating delayed rectifier K+ current (IKs) by chromanol 293B did not change the effect of AIP1. AIP1 did not significantly affect coronary arterial tone or ion channels, even at the highest concentration of AIP1. In summary, AIP1 reduces APD by activating IKr but not IKs. These results suggest that the natural product AIP1 may provide an adjunctive therapy of long QT syndrome.
Keywords: Artemisia iwayomogi, Delayed rectifier K+ current, Long QT syndrome, Ventricular myocyte
INTRODUCTION
Cardiac K+ currents are critical for regulation of action potential (AP) repolarization. The late phase of AP repolarization in cardiomyocytes is initiated in most species by activation of the delayed rectifier K+ current (IK). IK consists of two different components [1]: the rapidly activating IK (IKr) and a slowly activating IK (IKs), which can be distinguished based on electrophysiological kinetics, pharmacology and voltage-dependence [2-4]. Both currents play an important role in the repolarization of action potentials, and congenital or acquired prolongation of the QT interval in ECG is often brought about by a loss of function in this system [5]. Excessive AP duration (APD) prolongation causes long QT syndrome, which is associated with torsades de pointes, a ventricular tachyarrhythmia that can degenerate into ventricular fibrillation and cause sudden death [6,7]. Therefore, an activator of IKr or IKs channels may prove useful in the treatment of long QT syndrome resulting from an excessive pharmacological block of these channels or from mutations in the genes that encode the channel proteins [6].
Artemisia iwayomogi (A. messer-schmidtiana var. viridis Besser, Compositae), known as Haninjin or Dowijigi, is a perennial herb that is abundant in Korea and is used to treat various liver diseases, including hepatitis, in traditional medicine [8,9]. The AIP1 carbohydrate fraction has been purified from a crude water-soluble preparation from dried A. iwayomogi herb using size-exclusion chromatography [10]. AIP1 has various biological functions. For example, the AIP1 fraction increased antibody production and suppressed transplanted tumor cell growth [10]. The AIP1 fraction may also be involved in the survival of immune cells, either by suppressing apoptosis or by stimulating cell proliferation [11-13]. To date, however, the influence of AIP1 on cardiac function has not been elucidated. In the present study, we examined the effect of AIP1 on IK in ventricular cardiomyocytes. Our results demonstrated that AIP1 treatment decreased APD via activation of IKr, which may provide an adjunctive therapy of long QT syndrome.
METHODS
Single cell preparation of rabbit ventricular myocytes
Single ventricular myocytes were isolated from rabbit hearts as described previously [14]. Briefly, New Zealand White rabbits (1.5~2.0 kg) of either sex were anesthetized with sodium pentobarbitone (50 mg/kg) and were injected simultaneously with heparine (100 U/kg) into the ear vein. The procedure was carried out in accordance with the guidelines of the Committee for Animal Experiments of the Inje University College of Medicine. The hearts were quickly removed and cannulated by silicon tubing and retrogradely perfused via aorta on a Langendorff apparatus. Initially the heart was perfused with normal Tyrode solution for 4~5 min to clear the blood and then perfused with Ca2+-free normal Tyrode solution for 3 min. Then, the heart was perfused with enzyme solution containing 0.8 mg/ml collagenase for 20 min. Following the enzymatic treatment, the ventricle was dissected out and agitated mechanically with fire-polished Pasteur pipette in Kraft-Brühe (KB) solution. Single cells were stored in KB solution at 4℃ and used on the day of preparation.
Single cell preparation of arterial smooth muscle cells
Single smooth muscle cells were isolated from rabbit coronary arteries as described previously [15]. Briefly, the coronary arteries were isolated from hearts and cleaned of connective tissue in the normal Tyrode solution under a stereomicroscope. The arteries were transferred to normal Tyrode solution without CaCl2 (Ca2+-free solution) for 10 min and then incubated in the Ca2+-free solution containing papain (1 mg/ml) for 25 min. After the remaining enzyme was removed by washing in the Ca2+-free solution, the arteries were incubated in the Ca2+-free solution containing collagenase (2.8 mg/ml) for 20 min. Single smooth muscle cells were obtained by gentle agitation with a Pasteur pipette in Kraft-Brühe (KB) solution, stored at 4℃, and used on the day of preparation.
Purification of AIP1 fraction
AIP1 fraction was isolated from Artemisia iwayomogi as described by Koo et al. [10]. Briefly, a water-soluble crude extract was fractionated using Sephadex G-50 size exclusion chromatography with distilled water. The fractions containing carbohydrates or similar compounds were determined by the modified phenol/sulfuric acid method of total sugar determination and the fractions smaller than 1 kDa were pooled and used as the AIP1 fraction.
Electrophysiology
Whole cell patch clamp recordings were made using an Axon interface and Axopatch 1C amplifier (Axon Instruments, Union, CA). All experimental parameters, such as pulse generation and data acquisition, were controlled using PatchPro software, developed by our group. Action potentials were recorded by applying a square stimulus pulse of 1~2 nA and 3 ms duration. Patch pipettes were pulled from thin-walled borosilicate capillaries (Clark Electromedical Instruments, Pangbourne, UK) using a PP-83 vertical puller (Narishige, Tokyo, Japan). The voltage and current signals were filtered at 0.5~1.0 kHz and were sampled at a rate of 1~3 kHz. Electrode resistance before sealing was 3~4 MΩ, and after sealing it was <10 GΩ. The liquid-junction potentials between normal Tyrode and pipette solution, which were calculated based on ionic mobilities, were <5 mV. Since they were not large and liquid-junction potential between pipette solutions and intracellular solutions were not known, we did not make correction for junction potentials in presenting and analyzing data.
Measurement of arterial tone
The coronary arteries were isolated from the left anterior descending coronary artery and cleaned of connective tissue in the ice-cold Krebs-Henseleit (K-H) solution and continuously bubbled with the mixture of 95% O2 and 5% CO2, under a stereomicroscope. After isolation, the coronary artery was cut into rings of 5 mm length. Each ring was suspended between wires that were attached to the transducer for the measurement of isometric tension. Endothelial cells were disrupted. Successful removal of endothelium was assessed by showing that acetylcholine (10 µM) failed to relax arteries precontracted by 10 µM prostaglandin F2α. Arteries were allowed to equilibrate for at least 60 min before data collection. The rings were incubated in a chamber filled with K-H solution, continuously bubbled with the mixture of 95% O2 and 5% CO2, maintained at 37℃ during the stabilization and experiments.
Heart rate measurements
To measure the heart rate, the methodology of Langendorff's isolated perfused heart preparation was applied. The isolated hearts were mounted on a Langendorff perfusion system, then, perfused with K-H solution continuously bubbled with mixture of 95% O2 and 5% CO2. The heart remained at 37℃ throughout the entire experiment. Heart rate was determined by insertion of unipolar electrode in organ chamber solution to record the crude electrocardiogram (ECG) and analyzed with Powerlab (Chart 5) recording system.
Solutions and drugs
Normal Tyrode solution contained (in mM): NaCl, 140; KCl, 5.4; NaH2PO4, 0.33; CaCl2, 1.8; MgCl2, 0.5; HEPES, 5; glucose, 16.6; adjusted to pH 7.4 with NaOH. KB solution contained (in mM): KOH, 70; L-glutamate, 50; KH2PO4, 20; KCl, 55; taurine, 20; MgCl2, 3; glucose, 20; HEPES, 10; EGTA, 0.5; adjusted to pH 7.3 with KOH. K-H solution, containing (in mM) : NaCl, 120; KCl, 4.7; CaCl2, 1.8; MgSO4, 1.2; KH2PO4, 1.18; NaHCO3, 25; glucose, 16.5, continuously bubbled with the mixture of 95% O2 and 5% CO2; adjusted to pH 7.4 with NaOH after 1 hr of being bubbled with the gas mixture. The pipette-filled solution for recordings of IK in ventricular myocytes and K+ currents in vascular smooth muscle contained (in mM): K-aspartate, 115; KCl, 25; NaCl, 5; MgCl2, 1; Mg-ATP, 4; EGTA, 0.1; HEPES, 10; adjusted to pH 7.2 with KOH. The ventricular Ca2+ channel recording solution contained (in mM): NaCl, 140; CsCl, 10; NaH2PO4, 0.33; CaCl2, 1.8; MgCl2, 0.5; HEPES, 5; glucose, 16.6; adjusted to pH 7.4 with NaOH. The pipette-filled solution for recordings of Ca2+ current in ventricular myocytes contained (in mM): CsCl, 106; TEA-Cl, 20; NaCl, 5; Mg-ATP, 5; EGTA, 10; HEPES, 10; adjusted to pH 7.25 using CsOH. The vascular Ca2+ currents were recorded using perforated patch clamp technique. For the perforated-patch recordings of Ca2+ currents, the vascular smooth muscle cells bathed in solution contained (in mM): NaCl, 120; CaCl2, 2; CsCl, 5; TEA-Cl, 20; MgCl2, 0.5; HEPES, 10; glucose, 10; adjusted to pH 7.4 with NaOH. The pipette-filled solution contained (in mM): CsCl, 130; HEPES, 10; EGTA, 10; Mg-ATP, 5; adjusted to pH 7.2 with CsOH. Nystatin (200 µg/ml) was added to pipette solution. All pharmacological compounds were dissolved in water or dimethyl sulfoxide (DMSO) at >1,000 times to make stock solution. E-4031 (N-[4-[[1-[2-(6-methyl-2-pyridinyl)ethyl]-4-pi-peridinyl]carbonyl]phenyl]methanesulfonamide dihydrochroride) and Chromanol 293B were purchased from Sigma (St. Louis, MO, USA).
Statistics
Origin 6.0 software (Microcal Software, Northampton, MA) was used for data analysis. Data are presented as mean±SEM. Statistical analyses were performed by using unpaired Student's t test. A value of p<0.05 was defined as statistically significant.
RESULTS
AIP1 increased the heart rate
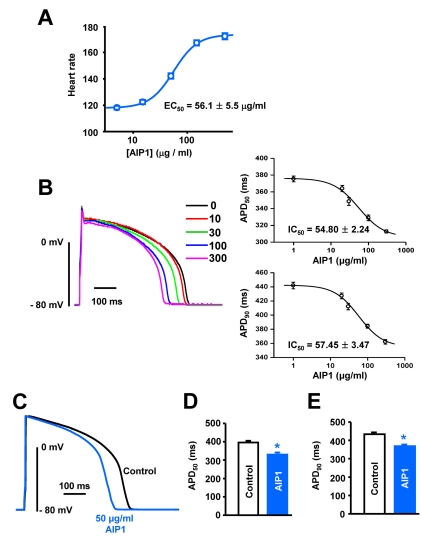
The application of AIP1 increased the heart rate dose-dependently (Fig. 1A). A non-linear square fit of the Hill equation yielded the EC50 of 56.1±5.5 µg/ml for the heart rate. This suggests that AIP1 activates IK in the sino-atrial (SA) node, which induces the reduction of APD in the SA node, resulting in the increased heart rate.
Fig. 1.
Effect of AIP1 on the heart rate and action potentials of ventricular myocytes. (A) Does-dependent effect of AIP1 on the heart rate (All n=4 hearts). (B) Dose-dependent effect of AIP1 on APD. Superimposed traces of APs obtained using the patch-clamp technique under control conditions and in various concentrations of AIP1 with an apparent IC50 value of APD50 and APD90 (n=4 cells). (C) Representative traces of APs under control conditions in the presence of 50 µg/ml AIP1. (D, E) Summary of the APD50 and APD90 as shown in (C). APD50 and APD90 mean the time required for repolarization to 50% and to 90% of basal membrane potential, respectively. *p<0.05 (n=6 cells).
AIP1 shortened APD in ventricular cardiomyocytes
We also tested the effects of AIP1 on APs using rabbit ventricular cardiomyocytes. APs were elicited every 5s using the current clamp mode for conventional whole-cell conditions. AIP1 shortened APD in dose-dependent manner without significantly affecting resting membrane potential (IC50 value of APD50: 54.80±2.24, IC50 value of APD90: 57.45±3.47 µg/ml, Fig. 1B). Considering the EC50 value of heart rate and IC50 values of APD50 and APD90, all recordings were performed with 50 µg/ml of AIP1. 50 µg/ml AIP1 decreased APD50 from 396.0±9.8 to 330.0±11.3 ms (Fig. 1C and D), and APD90 from 433.6±10.3 to 368.0±9.3 ms (Fig. 1C and E).
Effect of AIP1 on IK in ventricular cardiomyocytes
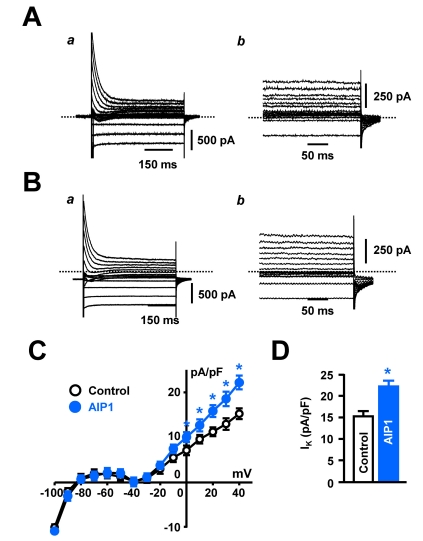
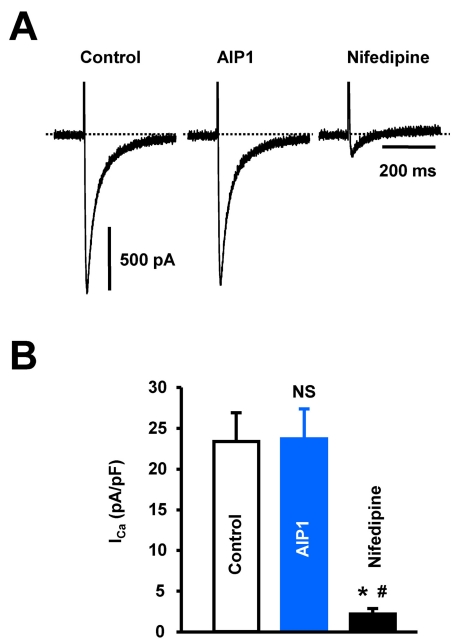
Shortening of APD is closely related to the activation of IK or inhibition of Ca2+ current (ICa). Thus, we recorded whole-cell IK current using the patch-clamp technique. ICa was inhibited by pretreatment with the L-type Ca2+ channel inhibitor, nifedipine (10 µM). IK was elicited by 500-ms depolarizing pulses between -120 and +40 mV from a holding potential of -80 mV. As shown in Fig. 2A (control) and 2B (after application of AIP1), AIP1 (50 µg/ml) increased the IK over the range that the current was activated (I-V relationship, Fig. 2C), from 15.3±1.2 to 22.2±1.5 pA/pF at +40 mV (Fig. 2D). We also confirmed the involvement of ICa in shortening of APD. ICa was elicited by one-step 400-ms depolarizing pulses from a holding potential of -50 mV (to inhibit the Na+ and T-type Ca2+ currents) to 0 mV. The application of AIP1 did not affect ICa (Fig. 3). From these results, we suggest that AIP1 increased IK, and did not inhibit ICa, which was followed by shortening of APD.
Fig. 2.
AIP1 increased IK in ventricular cardiomyocyte. (A, B) Whole-cell current of ventricular cardiomyocytes under control conditions (A) and in the presence of 50 µg/ml AIP1 (B). Panel (a) was original recording traces and panel (b) was magnified traces of the panel (a). (C) Summary of whole-cell I-V relationship under control conditions (○ and in the presence of 50 µg/ml AIP1 (•). (D) Statistical summary of IK at +40 mV. *p<0.05 (n=5 cells).
Fig. 3.
The effect of AIP1 on ICa in ventricular cardiomyocyte. (A) ICa in ventricular cardiomyocytes under control conditions and in the presence of 50 µg/ml AIP1. ICa was confirmed using the ICa inhibitor, nifedipine (10 µM). (B) Statistical summary of ICa at 0 mV. NS, not significant (Control vs AIP1, n=4 cells). *p<0.05 (Control vs nifedipine, n=4 cells). #p<0.05 (AIP1 vs nifedipine, n=4 cells).
AIP1 increased activity of IKr, rather than IKs in ventricular cardiomyocytes
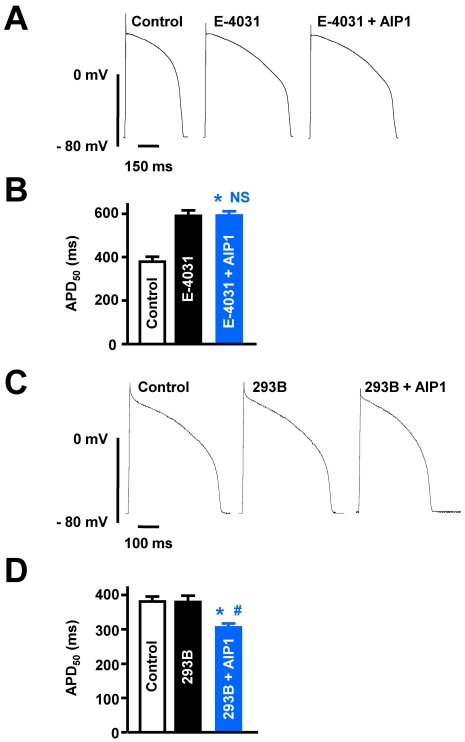
As described above, IK consists of two different components, IKr and IKs. To date, many specific inhibitors have been developed to distinguish between IKr and IKs. For example, E-4031 and chromanol 293B are known to be specific inhibitors of IKr and IKs, respectively. Therefore, to examine the relative selectivity of AIP1 for IKr and IKs, we pretreated cells with 10 µM E-4031 to block IKr or 20 µM chromanol 293B to block IKs before applying AIP1. Application of E-4031 itself increased the APD50 from 379.3±23.0 to 591.7±25.3 ms (Fig. 4A and 4B). Under these conditions, additional application of AIP1 did not reduce the APD (593.3±19.0 ms, Fig. 4A and 4B). Previous studies have suggested that chromanol 293B-induced inhibition of IKs has only minimal effects on an in vitro system, when measuring APD in various species, including rabbit, without β-adrenergic stimulation [16-18]. Consistent with these findings, our recordings also showed that chromanol 293B alone had no effect on APD (Control: 381.6±15.1 ms, chromanol 293B: 380.5±18.3 ms) (Fig. 4C and 4D). However, additional application of AIP1 reduced APD50 to a similar level as shown in Fig. 1 (322.1±11.3 ms, Fig. 4C and 4D). These findings suggest that AIP1-induced shortening of APD is attributable to activation of IKr, rather than IKs.
Fig. 4.
Effects of AIP1 on IKr and IKs. (A) Representative traces of action potentials obtained under control conditions, in the presence of 10 µM E-4031, and with additional application of 50 µg/ml AIP1. (B) Summary of APD50 as shown in (A). *p<0.05 (Control vs E-4031 +AIP1, n=6 cells). NS, not significant (E-4031 vs E-4031+AIP1, n=6 cells). (C) The traces of action potentials obtained under control conditions, in the presence of 20 µM chromanol 293B, and with additional application of 50 µg/ml AIP1. (D) Summary of APD50 as shown in (C). *p<0.05 (Control vs chromanol 293B+AIP1, n=4 cells). #p<0.05 (chromanol 293B vs chromanol 293B+AIP1, n=4 cells).
Effect of AIP1 on coronary arteries
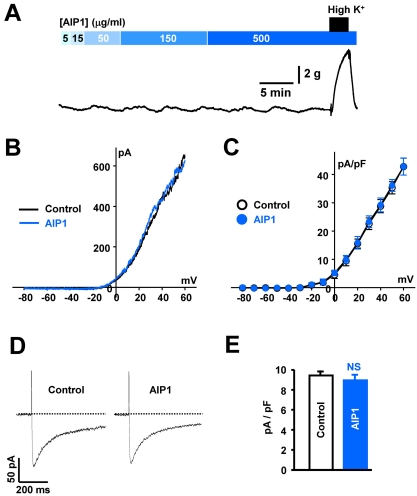
We also investigated the effect of AIP1 on vascular smooth muscle by measuring the changes in arterial tone and whole-cell current. AIP1 did not significantly affect arterial tone, even at the highest concentration of 500 µg/ml (Fig. 5A). Likewise, AIP1 had no effect on the activity of voltage-dependent K+ channels in arterial smooth muscle cells, as determined by measuring the whole-cell current using a ramp (Fig. 5B) or step protocol (Fig. 5C). Furthermore, vascular ICa, which was elicited by one-step 600-ms depolarizing pulse from a holding potential of -60 mV to 0 mV, was not affected by the application of AIP1 (Fig. 5D and E). These results suggested that AIP1 was not involved in the relaxation of coronary arterial smooth muscle by activation of K+ channels.
Fig. 5.
AIP1 did not affect coronary vascular tone or ion channels. (A) Representative traces showing the effect of various concentrations of AIP1 on vascular tone in coronary arteries. At the end of each experiment, 60 mM high K+ solution was applied to test the effectiveness of arteries (n=4 tissues). (B) Voltage-dependent K+ currents recorded using the ramp-pulse protocol (from -80 to +60 mV for 280 ms) under control conditions (black line, n=5 cells) and in the presence of 50 µg/ml AIP1 (blue line, n=5 cells). (C) I-V relationship of voltage-dependent K+ currents obtained by step voltages (from -80 and +60 mV in steps of 10 mV) under control conditions (○, n=5 cells) and in the presence of 50 µg/ml AIP1 (•, n=5 cells). (D) vascular Ca2+ currents obtained by one step voltage (-60 mV to 0 mV) under control condition and in the presence of 50 µg/ml AIP1. (E) Statistical summary of ICa at 0 mV. NS, not significant (Control vs AIP1, n=3 cells).
DISCUSSION
Our results indicate that AIP1 shortened APD by increasing the activity of IKr in rabbit ventricular myocytes, whereas IKs was largely unaffected. Furthermore, AIP1 was not involved in the relaxation of coronary arterial smooth muscle by activation of K+ channels.
Previous studies have examined the biological function of the polysaccharide fraction AIP1 isolated from A. iwayomogi. For example, both AIP1 and the ethanol-soluble fraction of the hot-water extract of A. iwayomogi lowered serum total-cholesterol levels and inhibited fibrosis and lipid oxidation in fibrotic rats [9]. Treatment of mouse thymocytes with AIP1 resulted in the suppression of cell death and improved cell survival, by suppression of the Fas/FasL-dependent apoptotic pathway [12,13,19]. Furthermore, treatment of mouse spleen cells with the AIP1 fraction suppressed apoptosis via the modulation of apoptosis-related genes [11]. AIP1 has been shown to inhibit mast cell-derived immediate-type allergic reactions by lowering intracellular Ca2+, inhibiting histamine release, and attenuating the activation of p38, a mitogen-activated protein kinase (MAPK) [20]. AIP1 also reduced allergic asthma via the down-regulation of tumor necrosis factor-alpha (TNF-α) expression [21]. AIP1 may also act as a potent anti-oxidant. Active compounds from A. iwayomogi are potent scavengers of ONOO-, and inhibitors of inducible nitric oxide synthase [22,23]. Until now, the focus of the pharmacological effects of AIP1 has been on its anti-tumor effects, immunomodulating activity, and liver protective mechanisms.
In this study, for the first time, we demonstrated the effect of AIP1 on cardiac ion channels. Our major findings were that AIP1 reduces APD by activating IKr in rabbit ventricular cardiomyocytes. IK consists of two components in cardiomyocytes, IKr and IKs, which have been well characterized using the patch-clamp technique, on the basis of their different electrophysiological kinetics, pharmacology and rectification properties [1-4]. Candidate genes that may encode IKr and IKs channel proteins have been identified. The human ether-a-go-go-related gene (hERG) encodes the pore-forming α-subunit of cardiac IKr channel, possibly in connection with the β-subunit KCNE2, whereas KCNQ1 and minK (KCNE1) co-assemble to form the IKs channel [24-30]. Class III antiarrhythmic drugs, notably E-4031, sotalol, and dofetilide reduce IKr activity to help prevent arrhythmia. However, excessive blocking of IKr or mutation of hERG causes long QT syndrome [31], which is linked to an increased incidence of sudden cardiac death. Therefore, if IKr blockers are used as antiarrhythmic drugs, channel activators would be helpful to reverse the effects of overdose. Furthermore, channel activators could also be used for the solution of inherited long QT syndrome. Our study clearly showed that AIP1 reduced APD by activating IKr, but not IKs. This result would provide an adjunctive therapy for treating long QT syndrome with the natural product, AIP1. Moreover, co-treatment with AIP1 may reduce the cardiac toxicity caused by hERG channel inhibition by several drugs.
The lack of effect of AIP1 on vascular smooth muscle may be the result of the different expression of Kv subtypes. IKr, which consists of hERG (Kv11.1) and auxiliary KCNE2 (MiRP1), has been identified mainly in the brain and heart, and not vascular smooth muscle. Instead, vascular smooth muscle cells express the Kv1.1, Kv1.2, Kv1.4, Kv1.5, Kv1.6, Kv2.1, Kv4.3, and Kv9.3 subunits, as well as the auxiliary Kvβ1, Kvβ2, and Kvβ3 [32-34]. Therefore, the main target for increasing the activity of IKr by AIP1 might be restricted to hERG (Kv11.1) and/or auxiliary KCNE2 (MiRP1). Future experiments are planned to investigate the specific effects of AIP1 on hERG channels using cell lines over-expressing the hERG channel. Additionally, we did not address the detailed mechanism underlying the AIP1-induced increase in IKr activity; therefore further studies into drug-channel interactions or signaling mechanisms are required in the near future.
ACKNOWLEDGEMENTS
This research was supported by a Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (313-2008-2-E00046, R0A-2007-000-20085-0 and R13-2007-023-00000-0).
ABBREVIATIONS
- AP
action potential
- APD
action potential duration
- IKr
rapidly activating delayed rectifier K+ current
- IKs
slowly activating delayed rectifier K+ current
References
- 1.Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng J, Laurita KR, Rosenbaum DS, Rudy Y. Two components of the delayed rectifier K+ current in ventricular myocytes of the guinea pig type. Theoretical formation and their role in repolarization. Circ Res. 1995;77:140–152. doi: 10.1161/01.res.77.1.140. [DOI] [PubMed] [Google Scholar]
- 3.Heath BM, Terrar DA. Separation of the components of the delayed rectifier potassium current using selective blockers of IKr and IKs in guinea-pig isolated ventricular myocytes. Exp Physiol. 1996a;81:587–603. doi: 10.1113/expphysiol.1996.sp003961. [DOI] [PubMed] [Google Scholar]
- 4.Heath BM, Terrar DA. The deactivation kinetics of the delayed rectifier components IKr and IKs in guinea-pig isolated ventricular myocytes. Exp Physiol. 1996b;81:605–621. doi: 10.1113/expphysiol.1996.sp003962. [DOI] [PubMed] [Google Scholar]
- 5.Chiang CE, Roden DM. The long QT syndromes: genetic basis and clinical implications. J Am Coll Cardiol. 2000;36:1–12. doi: 10.1016/s0735-1097(00)00716-6. [DOI] [PubMed] [Google Scholar]
- 6.Salata JJ, Jurkiewicz NK, Wang J, Evans BE, Orme HT, Sanguinetti MC. A novel benzodiazepine that activates cardiac slow delayed rectifier K+ currents. Mol Pharmacol. 1998;53:220–230. doi: 10.1124/mol.54.1.220. [DOI] [PubMed] [Google Scholar]
- 7.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 8.Lee KR, Zee OP, Kwak JH, Kim YS, Park HK, Koo KA, Youn HJ. The polysaccharide fractions of Artemisia species. Korean J Pharmacogn. 1993;24:289–295. [Google Scholar]
- 9.Park EJ, Nan JX, Kim JY, Kang HC, Choi JH, Lee SJ, Lee BH, Lee JH, Kim YC, Sohn DW. The ethanol-soluble part of a hot-water extract from Artemisia iwayomogi inhibits liver fibrosis induced by carbon tetrachloride in rats. J Pharm Pharmacol. 2000;52:875–881. doi: 10.1211/0022357001774561. [DOI] [PubMed] [Google Scholar]
- 10.Koo KA, Kwak JW, Lee KR, Zee OP, Woo ER, Park HK, Youn HJ. Antitumor and immuno-modulating activities of the polysaccharide fractions from Artemisia selengenesis and Artemisia iwayomogi. Arch Pharm Res. 1994;17:371–374. [Google Scholar]
- 11.Hwang JS, Chung HK, Bae EK, Lee AY, Ji HJ, Park DW, Jung HJ, Cho CW, Choi HJ, Lee DS, Lee KR, Youn HJ. The polysaccharide fraction AIP1 from Artemisia iwayomogi suppresses apoptotic death of the mouse spleen cells in culture. Arch Pharm Res. 2003;26:294–300. doi: 10.1007/BF02976958. [DOI] [PubMed] [Google Scholar]
- 12.Hwang JS, Ji HJ, Koo KA, Lee NH, Yeo HK, Cheong SW, Park JH, Oh GS, Yoon CS, Youn HJ. AIP1, a water-soluble fraction from Artemisia iwayomogi, suppresses thymocyte apoptosis in Vitro and down-regulates the expression of Fas gene. Biol Pharm Bull. 2005;28:921–924. doi: 10.1248/bpb.28.921. [DOI] [PubMed] [Google Scholar]
- 13.Ji HJ, Yeo HK, Lee NH, Hwang JS, Koo KA, Cheong SW, Park JH, Oh GS, Yoon CS, Youn HJ. A carbohydrate graction, AIP1, from Artemisia iwayomogi down-regulated Fas gene expression and suppresses apoptotic death of the thymocytes induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biotechnol Lett. 2005;27:253–257. doi: 10.1007/s10529-004-8294-2. [DOI] [PubMed] [Google Scholar]
- 14.Kim NR, Youm JB, Joo H, Kim EY, Han J. Protein kinase C activates ATP-sensitive potassium channels in rabbit ventricular myocytes. Korean J Physiol Pharmacol. 2005;9:187–193. [Google Scholar]
- 15.Park WS, Ko JH, Kim NR, Son YK, Kang SH, Warda M, Jung ID, Park YM, Han J. Increased inhibition of inward rectifier K+ channels by angiotensin II in small-diameter coronary artery of isoproterenol-induced hypertrophied model. Arterioscler Thromb Vasc Biol. 2007;27:1768–1775. doi: 10.1161/ATVBAHA.107.143339. [DOI] [PubMed] [Google Scholar]
- 16.Lengyel C, Iost N, Virag L, Varro A, Lathrop DA, Papp JG. Pharmacological block of the slow component of the outward delayed rectifier current (IKs) fails to lengthen rabbit ventricular muscle QTc and action potential duration. Br J Pharmacol. 2001;132:101–110. doi: 10.1038/sj.bjp.0703777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volders PG, Stengl M, van Opstal JM, Gerlach U, Spätjens RL, Beekman JD, Sipido KR, Vos MA. Probing the contribution of IKs to canine ventricular repolarization: Key role for beta-adrenergic receptor stimulation. Circulation. 2003;107:2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- 18.Guerard NC, Traebert M, Suter W, Dumotier BM. Selective block of IKs plays a significant role in MAP triangulation induced by IKr block in isolated rabbit heart. J Pharmacol Toxicol Methods. 2008;58:32–40. doi: 10.1016/j.vascn.2008.05.129. [DOI] [PubMed] [Google Scholar]
- 19.Chung HK, Bae EK, Ji HJ, Hwang JS, Park DW, Kim EJ, Jung HJ, Choi HJ, Lee DS, Youn HJ. An oligosaccharide fraction from Korean mugwort herb suppresses death of the mouse thymocytes in culture by down-regulating the Fas death receptor gene. Biotechnol Lett. 2003;25:1549–1553. doi: 10.1023/a:1025482516404. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Choi CH, Kim SY, Eun JS, Shin TY. Anti-allergic effects of Artemisia iwayomogi on mast cell-mediated allegy model. Exp Biol Med. 2005;230:82–88. doi: 10.1177/153537020523000111. [DOI] [PubMed] [Google Scholar]
- 21.Lee JA, Sung HN, Jeon CH, Gill BC, Oh GS, Youn HJ, Park JH. A carbohydrate fraction, AIP1 from Artemisia iwayomogi suppresses pulmonary eosinophilia and Th2-type cytokine production in an ovalbumin-induced allergic asthma. Down-regulation of TNF-α expression in the lung. Int Immunopharmacol. 2008;8:117–125. doi: 10.1016/j.intimp.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Ahn H, Kim JY, Lee HJ, Kim YK, Ryu JH. Inhibitors of inducible nitric oxide synthase expression from Artemisia iwayomogi. Arch Pharm Res. 2003;26:301–305. doi: 10.1007/BF02976959. [DOI] [PubMed] [Google Scholar]
- 23.Kim AR, Zou YN, Park TH, Shim KH, Kim MS, Kim ND, Kim JD, Bae SJ, Choi JS, Chung HY. Active components from Artemisia iwayomogi displaying ONOO-scavenging activity. Phytother Res. 2004;18:1–7. doi: 10.1002/ptr.1358. [DOI] [PubMed] [Google Scholar]
- 24.Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induced a slow voltage-gated potassium current. Science. 1988;242:1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- 25.Folaander K, Smith JS, Antanavage J, Bennett C, Stein RB, Swanson R. Cloning and expression of the delayed rectifier IKs channel from neonatal rat heart and diethylstilbestrol-primed rat uterus. Proc Natl Acad Sci USA. 1990;87:2975–2979. doi: 10.1073/pnas.87.8.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman LS, Kass RS. Expression of a minimal K+ channel protein in mammalian cells and immunolocalization in guinea pig heart. Circ Res. 1993;73:968–973. doi: 10.1161/01.res.73.5.968. [DOI] [PubMed] [Google Scholar]
- 27.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between and inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 28.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KvLQT1 and mink (IKs) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Feng Z, Kondo CS, Sheldon RS, Duff HJ. Developmental changes in the delayed rectifier K+ channels in mouse heart. Circ Res. 1996;79:79–85. doi: 10.1161/01.res.79.1.79. [DOI] [PubMed] [Google Scholar]
- 30.Hansen RS, Olesen SP, Ronn LC, Grunnet M. In vivo effects of the IKr agonist NS 3623 on cardiac electrophysiology of the guinea pig. J Cardiovasc Pharmacol. 2008;52:35–41. doi: 10.1097/FJC.0b013e31817dd013. [DOI] [PubMed] [Google Scholar]
- 31.Heath BM, Terrar DA. Protein kinase C enhances the rapidly activating delayed rectifier potassium current, IKr, through a reduction in C-type inactivation in guinea-pig ventricular myocytes. J Physiol. 2000;522:391–402. doi: 10.1111/j.1469-7793.2000.t01-2-00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan XJ, Wang J, Juhaszova M, Golovina VA, Rubin LJ. Molecular basis and function of voltage-gated K+ channels in pulmonary arterial smooth muscle cells. Am J Physiol. 1998;274:L621–L635. doi: 10.1152/ajplung.1998.274.4.L621. [DOI] [PubMed] [Google Scholar]
- 33.Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX. Chronic hypoxia decrease K(V) channel expression and function in pulmonary artery myocytes. Am J Physiol. 2001;280:L801–L812. doi: 10.1152/ajplung.2001.280.4.L801. [DOI] [PubMed] [Google Scholar]
- 34.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]