Abstract
Evaluating the success of major funding programs from the National Institutes of Health (NIH) remains a vexing challenge. We propose a set of criteria to evaluate epidemiological studies that fit within the discovery, development, and delivery paradigm introduced by the NIH. We apply these criteria to the Nurses’ Health Study (NHS), a large epidemiological cohort study initiated in the 1970s to evaluate the associations between oral contraceptives and risk of breast cancer and between diet and other lifestyle factors and risk of cancer overall. Our evaluation suggests that the NHS has led to important changes in health practice, and it underscores the need to develop metrics that are suitable to the evaluation of large epidemiological cohort studies.
In its annual plan and budget proposal for 2004, the National Cancer Institute (NCI) identified this challenge: to “accelerate the engine of discovery; translate knowledge gained about the genetic, molecular, and cellular basis of cancer into the development of interventions to detect, diagnose, treat, and prevent cancer; and ensure that these interventions are delivered to all who need them” (1). But how is success in meeting this challenge to be measured? Research productivity has increasingly come to be measured by publication of scientific articles in peer-reviewed journals of varying impact factors and the extent to which other scientists cite these articles in their work. The National Institutes of Health (NIH), including the NCI, have used this metric in assessing new grant proposals and the success of grants it funded. However, other metrics—sensitive indicators of the development and delivery of improvements in health—are needed.
One innovative means of evaluating success in fostering scientific discovery was implemented by the NCI’s Division of Cancer Control and Population Sciences (DCCPS). It focuses on determining whether its initiatives soliciting grants on a thematic topic induce collaborative science in ways that the individual grants do not. This approach has been applied to recent DCCPS initiatives including the Transdisciplinary Tobacco Use Research Centers (TTURCs) (2). Four metrics have been used in the evaluation of TTURCs. These are: the pace at which new collaborative transdisciplinary projects are developed (intellectual integration); the speed of implementing the Centers; cumulative changes in the collaborative behaviors and values of participants in the Centers; and the extent to which there is a move to collaborative publications (2). This example serves as a model for applying metrics to evaluation of initiatives.
The extramural cancer epidemiology program in DCCPS and its extramural academic partners have been exploring how to assess NCI’s cancer epidemiology program according to the classical uses or purposes of epidemiology. Some of these uses or aims correspond to phases of the discovery, development, and delivery paradigm (3). For example, a purpose of epidemiology that corresponds to the discovery challenge is to explain the etiology of diseases and health conditions (4). Assessment of articles on this issue by defined criteria in the peer-reviewed literature may provide an excellent measurement tool for assessing discovery because scientific journals focus on direct scientific findings from research.
However, this approach cannot address the success of federal initiatives and investigator-initiated research in epidemiology in terms of development and delivery, and in this commentary we will propose additional criteria in these areas. We apply these criteria to the Nurses’ Health Study (NHS), a large prospective cohort study that was initiated in the 1976 and has followed 121 700 women who were 30–55 years of age at baseline to evaluate the associations between oral contraceptives and risk of breast cancer and between diet and other lifestyle factors and risk of cancer overall (5). Although the cohort is funded primarily by NCI, it receives focused support for the study of other endpoints from other pertinent NIH institutes. We discuss how our approach to evaluation of the NHS may be generally applicable to epidemiology.
Criteria for Evaluation of Epidemiological Studies and Their Application to the Nurses’ Health Study
The purpose of epidemiology that corresponds to discovery is to explain the etiology of diseases and health conditions by providing information about the distribution of cancer in populations, testing (or helping to formulate) hypotheses about disease etiology, and identifying new risk factors. We propose that success of the NHS in meeting this aim can be reasonably assessed based on the number of its publications, most of which address risk factors for cancer, and the impact factors of the journals in which they appear. Therefore, our assessment of discovery was based on a database of NHS publications that is maintained at Harvard University.
In the area of development, the aim of epidemiology is to provide the scientific basis for developing control measures and prevention strategies for groups and populations at risk and to develop needed public health measures and practices. The criteria we used in the area of development were: contributions of NHS publications to establishing factors that cause disease; development of risk models; development of clinical or prevention guidelines; and quantification of the preventable burden of disease (Table 1). We also sought to determine if NHS publications were used to justify the initiation of prevention and clinical trials and whether NHS findings were confirmed in these kinds of trials.
Table 1.
Metrics for applying public health evaluation to large cohort studies
| Purposes of epidemiology | Metrics for evaluation |
| Discovery—to explain the etiology of diseases and health conditions | A. Numbers of journal articles B. Impact factors of journals |
| Development—to provide a basis for developing control measures and prevention procedures for groups and populations at risk and for developing needed public health measures and practices | A. Contribution to determination of factors causing disease in human populations B. Scientific support for prevention and clinical guidelines C. Scientific support for prevention and clinical trials D. Quantification of preventable burden of disease E. Contributions to risk models |
| Delivery—implementation/use of findings pertaining to development and delivery by the public, clinicians, public health practitioners, policy makers, and others | A. Public awareness of the research B. Policy and regulatory applications C. Industry applications |
In terms of delivery, the goal of epidemiology can be considered to be implementation of the epidemiological findings by the public, clinicians, health practitioners, policy makers, industry, and others. Therefore, our delivery criteria were the use of NHS research findings by these end users in the areas of governmental policy, regulation, and industry applications (Table 1).
In assessing the NHS in terms of development and delivery, we selected NHS publications for which we could identify bibliographic or other authoritative sources of evidence that the NHS addressed one or more of the criteria. We sought to identify examples that addressed each of the criteria, but we did not attempt to evaluate all NHS publications. Publications that referenced NHS articles and addressed a particular criterion for success were identified through Web of Science searches; through review of references in published guidelines from major cancer organizations, professional societies, and other organizations; and through review of references (including those to guidelines and clinical trials) that supported conclusions about health effects in International Agency for Research on Cancer (IARC) (101) reports and reports from the US Surgeon General. Additional selection standards were applied to determine if the references were evidence of NHS success. For example, if the reference was to a clinical guideline, publication of the guideline had to use results from the NHS as support. If the reference was to a trial, the NHS had to be cited in the introduction (ie, as part of the rationale for the trial). Selection of a reference that established causality required that the NHS had been used to provide positive support for the ultimate conclusion about causality.
Discovery
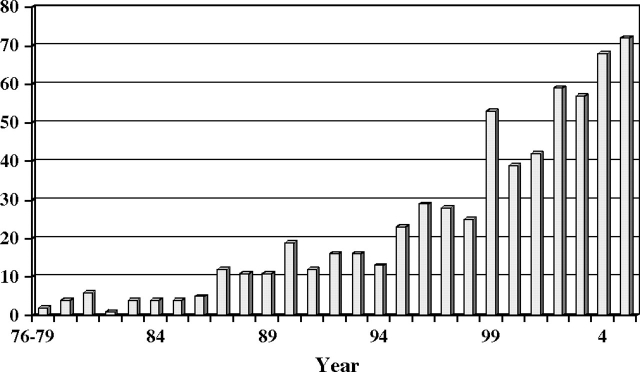
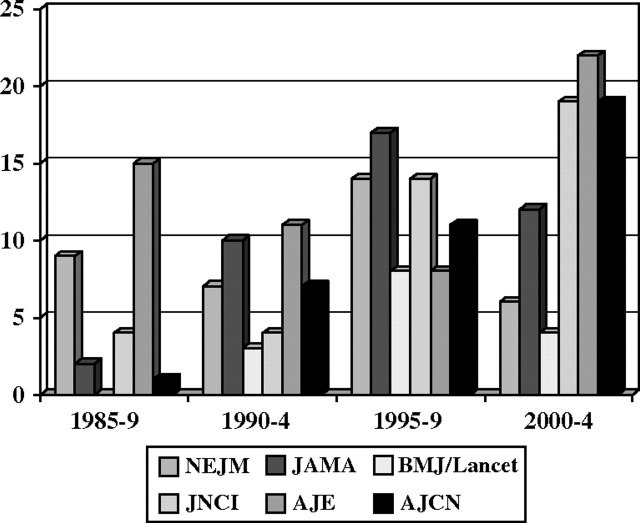
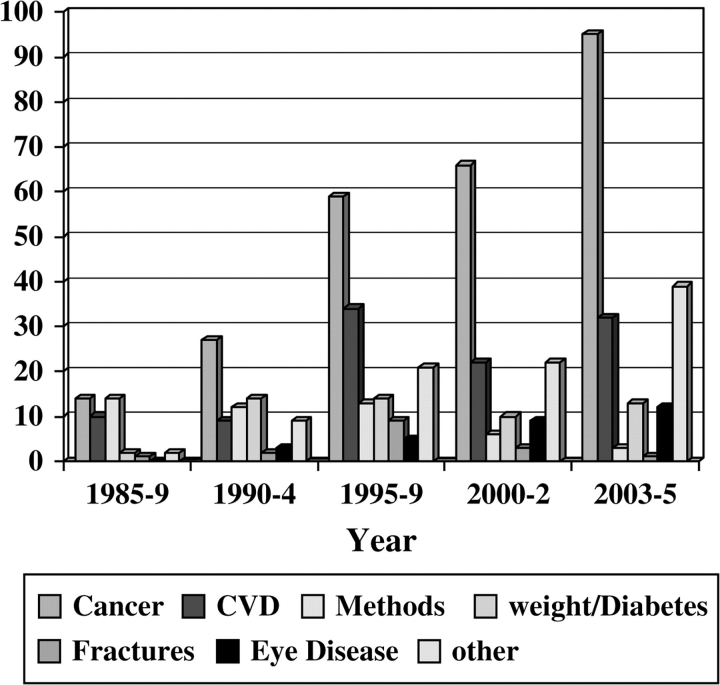
The early years of the NHS (extending to the first 10 years or so) led to relatively few publications (Figure 1), reflecting the young age of the women and the limited number of endpoints available for study. However, as of February 2005, after 28 years of follow-up, the findings from the NHS had led to numerous publications in a range of high-impact journals (Figure 2), including 36 publications in the New England Journal of Medicine, 41 in the Journal of the American Medical Association, and 41 in the Journal of the National Cancer Institute; 56 methodological pieces as well as papers reporting major findings appeared in the American Journal of Epidemiology. Reflecting the cohort’s status as a unique resource for the study of cancer in women and the fact that its primary funding was from the NCI, most of the publications addressed risk factors for cancer (Figure 3). Risk factors discovered using blood samples that were stored by the NHS for prospective evaluation of cancer risk included insulin-like growth factor (IGF) (for premenopausal breast cancer) (6); premenopausal estrogen, prolactin (7), and testosterone (8) (for breast cancer in general); and endogenous hormone levels (for receptor-positive postmenopausal breast cancer) (8). Nurses’ Health Study investigators also reported that folate, cysteine, (9) and vitamin D blood levels were associated with the risk of breast cancer (10,11), and the NHS also documented that radial scars on benign breast biopsies were associated with risk of subsequent breast cancer independent of other markers of proliferative disease in benign biopsies (12).
Figure 1.
Nurses’ Health Study: publications by year, 1976–2005.
Figure 2.
Nurses’ Health Study publications in high-impact journals, 1985–2004.
Figure 3.
Nurses’ Health Study: publications by topic.
Statistical methods have also been developed by the investigators of the NHS to address issues that have arisen in the analysis of the prospective data with repeated measures (13). These include approaches to measurement error correction (14), validation studies with variable number of observations (15), and polytomous regression with time-varying covariates (16).
Development
Establishing Causality.
Epidemiological findings are a critical component of established approaches to the assessment of disease causality (17). Several organizations, including the IARC and the Office of the US Surgeon General, impanel experts to evaluate the published scientific literature using a systematic series of criteria to reach conclusions about causality. Findings from the NHS on the associations of alcohol intake and exogenous hormone exposure with breast cancer risk (18) and the association of tubal ligation with ovarian cancer risk (19) have contributed substantially to conclusions about causality in reports from the IARC.
Scientific Support for Prevention and Public Health Guidelines.
For the study of many lifestyle factors in epidemiological investigations (eg, consumption of trans-fatty acids, weight gain, tubal ligation), randomized controlled trials are neither ethical nor practical. Making the case for the development of control and prevention efforts therefore involves assembling and assessing the evidence from epidemiological studies and other sources (20). Thus, preventive service and community practice guidelines often cite epidemiological findings as supportive evidence in lieu of or in addition to findings from randomized controlled trials (21,22). In Table 2, we list findings from the NHS that pertain to the association of behavioral and dietary risk factors with risk of cancer and their impact in terms of guidelines that draw on this evidence. For example, NHS data (23), along with findings from American Cancer Society (ACS) cohort studies and other epidemiological studies (73), led to recommendations by the ACS that people reduce alcohol intake and red meat consumption and increase physical activity (74).
Table 2.
Findings from the Nurses’ Health Study that had an impact on public health according to criteria proposed for evaluating the impact on development of large cohort studies*
| Outcome (reference) | Impact on public health (reference) | Development criterion |
| Related to smoking | ||
| Lung cancer, coronary heart disease, mortality (23,24) | 2001 Surgeon General’s report on women and smoking (25) | A |
| Coronary heart disease (26) | 1990 Surgeon General’s report on benefits of smoking cessation (27) | A |
| Stroke (29) | 2004 Surgeon General’s report on health consequences of smoking (28) | A |
| Related to physical activity | ||
| Colon cancer (30) | ACS guidelines on nutrition and physical activity (31), clinical trial on physical activity and colon cancer recurrence (32) | B |
| Colorectal adenoma (96) | Project PREVENT: a randomized trial to reduce multiple behavioral risk factors for colon cancer (34) | B |
| Postmenopausal breast cancer (35) | IARC report on potential for prevention of cancer through weight management and increased physical activity (36) | A, D |
| Diabetes (37,38) | American Diabetes Association prevention recommendation (39) | B |
| Coronary heart disease (40) | American Heart Association primary prevention recommendation (41) | B |
| Associated with obesity | ||
| Breast cancer (43) | Contributed to rationale for randomized controlled trial (97) | C |
| Breast cancer mortality (98) | Nutrition and physical activity during and after cancer treatment: guidance from ACS (44) | B |
| Total mortality (46) | Guidelines for screening for obesity from US Preventive Services Task Force (47) | B |
| Diabetes and hypertension (48,50) | Contributed to professional societies’ positions on weight and weight management (99,100) | B |
| Becoming obese (49) | Pediatric recommendation for limiting time spent watching television (51) | B |
| Related to oral contraceptives | ||
| Breast cancer (52) | Contributed to conclusion that oral contraceptives are causally related to breast cancer (101) | A |
| Decreased incidence of ovarian cancer (54) | Contributed to the conclusion that oral contraceptives protect against ovarian cancer (53) | A |
| Related to postmenopausal hormone therapy | ||
| Breast cancer (55) | Contributed to the conclusion that postmenopausal estrogens are carcinogenic to humans (58); estrogen plus progestin therapy risk confirmed in the Women’s Health Initiative (56) | A |
| Related to endogenous hormones | ||
| Breast cancer (57,58) | Randomized isoflavone intervention among premenopausal women (59); effects of the aromatase inhibitor letrozole on normal breast epithelial cell proliferation (60) | B, C |
| Breast cancer (6) | Trials initiated to evaluate action and pathways (61–63); growth hormone treatment and risk of solid tumors. A statement from the Drugs and Therapeutics Committee of the European Society for Paediatric Endocrinology (64) | B, C |
| Breast cancer (65) | Atypical hyperplasia added to risk prediction models (42) | C, E |
| Related to dietary factors | ||
| Coronary heart disease (66) | Trans fatty acids collaboration project phase 1: a study to assess the effect of the two different dietary sources of trans-fatty acids on cardiovascular risk factors in humans (67) | C |
| Coronary heart disease (66) | FDA rule making on trans-fatty acid labeling, nutrition content, and health claims (68) | B |
| Colon cancer (69) | Project PREVENT: a randomized trial to reduce multiple behavioral risk factors for colon cancer (34) | C |
| Physical activity and survival after breast cancer diagnosis (70) | Nutrition and physical activity during and after cancer treatment: an ACS guide (44) | B |
| Related to multiple factors | ||
| Coronary heart disease (72) | American Heart Association guide for improving cardiovascular health at the community level: a statement for public health practitioners, health-care providers, and health policy makers (33) | B, D |
Development criteria are as listed in Table 1. ACS = American Cancer Society; IARC = International Agency for Research on Cancer; FDA = Food and Drug Administration.
Scientific Support for Conducting Prevention or Clinical Trials.
Findings from observational studies provide essential rationale for conducting clinical trials. Findings from NHS that have been followed up in trials include those that showed the associations of endogenous estrogen levels (8,71), IGF (6), and combination hormone therapy (61) with increased risk of breast cancer. Development can also be measured by the extent to which epidemiological findings regarding protective or adverse effects are used to develop and test cancer control interventions at the level of individuals or communities or lead to the initiation of chemoprevention trials to reduce risk. The initiation of the Selenium and Vitamin E Cancer Prevention Trial (75), which built on epidemiological findings that found that lower selenium levels were associated with lower cancer risk, is an example of this use of epidemiological findings. Similar additional examples are the development of behavior change interventions in worksites (76) and for patients who have had a colon polyp removed (34).
Quantification of the Preventable Burden of Disease.
Determination of the preventable burden of disease is important to understanding disease inequalities in human populations and provides the basis for deciding which populations should be targeted for disease control and prevention. The preventable burden of disease depends on the prevalence of the risk factor for the disease in the population and the relative risk of disease among those exposed and not exposed to the risk factor. The preventable burden of disease has been estimated from NHS for several diseases, including heart disease (72) and diabetes (77), and the results suggest that modifiable risk factors are substantial contributors to the population burden of those diseases. Data from the NHS have contributed to estimates of the population-attributable risk (ie, the percentage of disease that could be prevented if the risk factor could be eliminated) for obesity and inactivity in relation to cancer, as summarized by the IARC (36).
Contributions to Risk Models.
The development of risk prediction models has drawn on the rich longitudinal data resources generated by cohort studies. For example, the Framingham Heart Study is the basis for the Framingham risk score for heart disease, which has been used extensively in clinical settings and validated in other populations (78). Risk prediction models, which permit the translation of data about risk factors into algorithms to identify high-risk subsets of the population, are a growing application of epidemiological data (79,80). Cancer-specific models have been developed from the prospective NHS data for breast cancer (81), ovarian cancer (82), and melanoma (83). Ongoing work will refine these models to include biomarkers and explore the usefulness of these models for predicting the development of disease. As Glasziou and Irwig (84) have pointed out, translating results from randomized controlled trials to therapy decisions for individual patients requires an assessment of underlying risk that can be obtained only from epidemiological studies.
Delivery
The extent to which delivery is successful can be assessed by use of the cohort study’s findings by end users. The public at large is an end user, and awareness of the results of the epidemiological research is an important prelude to behavior change. Much media attention has focused on the individual findings from the NHS cohort studies as they were published. This was often followed by more detailed coverage (in monthly magazines). To our knowledge, no database is available to place the coverage in context with that received by other NIH-funded research, and we did not apply this criterion to the NHS. Clinicians and public health practitioners are also end users. They tend to use guidelines developed from epidemiological studies rather than results of a single study as the evidence base for their practices. Therefore, individual NHS findings for these end users are not listed in Table 2. Finally, policy makers and industry are also end users. For example, findings on dietary trans-fat intake and increased risk of coronary heart disease (72) led the US Food and Drug Administration to change regulations such that as of January 2006 trans-fatty acids in foods had to be indicated on the package’s Nutrition Facts panels. The finding in the NHS of an association of higher vitamin A intake with increased risk of fractures (85,86) led manufacturers of multivitamins to reduce their vitamin A content.
Evaluation in Context
Although discovery is often the primary goal of scientific investigations, success is frequently narrowly defined in terms of numbers of journal articles and the perceived quality of the journal. Cohort studies in cancer epidemiology—which require large numbers of study participants and long time frames and lead to relatively few publications in the initial years, when there are few case subjects with the outcomes of interest—are criticized for their relatively high costs (87). The value of these studies has also been criticized on the basis that discovery is rare and because the time frame for investigation is such that these studies usually only confirm findings previously reported from weaker study designs.
Our analysis of the publication record of the NHS shows that the numbers of publications increased rapidly 10 years after the cohort’s establishment and that ultimately hundreds of publications reported findings from the cohort. This is consistent with the experience of other long-standing cohorts. For example, the Framingham Heart Study lists more than 1200 papers. Although the early focus of the Framingham Study was predominantly on the identification of risk factors for cardiovascular disease, studies of other chronic diseases (eg, osteoarthritis) were added over time. The Atherosclerosis Risk In Communities (ARIC) lists some 500 research publications over the life of the cohort to date. Almost all are cardiovascular in focus. The Rotterdam Study has focused on the incidence of major diseases in the elderly, and in the period from 1990 to 2004 it published some 500 papers addressing a broad range of endpoints and intermediate disease markers (88). In general, in long-established and well-conducted cohort studies the rate of production of publications tends to accelerate over time. The success of cohorts should be evaluated accordingly. Our analysis of the NHS reveals that this cohort has been successful in terms of discovery, development, and delivery. Moreover, we suggest that the approach to assessment that we outline can be used to evaluate other cohorts and other epidemiological studies.
The addition of several criteria to traditional measures of productivity based on the number of articles in journals of a given impact factor is appropriate for epidemiology. We extended our evaluation of scientific discovery to include evidence of uses of NHS and other cohort study data to explain disparities among populations with regard to the incidence of cancer and contributions to establishing causality. Our evaluation suggests that NHS findings have been important in explaining the lack of association between total fat intake and breast cancer risk, the direct association between physical activity and reduced risk of colon cancer, and the association between long-term intake of folate and methionine and reduced colon cancer risk. Our evaluation also suggests that NHS findings have helped to clarify the role of obesity and weight gain in increasing cancer risk among women and that they were critical in demonstrating that both alcohol consumption and adult weight gain are associated with increased risk of breast cancer.
Cohorts with a focus on multiple outcomes beyond cancer permit investigators to evaluate the risks and benefits of lifestyle and medications more comprehensively than cohorts with focus only on one disease. Evaluating the associations of multiple outcomes and lifestyle factors and medications with data on disease incidence avoids potential biases induced by behavior change after diagnosis of chronic diseases or the effects of comorbidities and different therapies administered after diagnosis. Also, NHS investigators have evaluated potential risk factors such as alcohol consumption (89), obesity (45), physical activity (90), and oral contraceptive use (91) in terms of total and cause-specific mortality, as well as incidence. Investigators have also evaluated a measure of diet quality in relation to incidence of chronic diseases (92).
Although we have identified some criteria that can be used to examine the productivity of a cohort such as the NHS across all domains and have found some data and bibliographic sources that would be useful in developing quantitative measures of discovery, developing adequate quantitative measures of the impact of scientific research in the development and delivery domains is difficult. Data sources for development and delivery applications, except for a few well-established searchable sources, such as the IARC monograph series and the Community Preventive Services Task Force, are not available. In addition, certain criteria typically can only be applied after the passage of sometimes substantial amounts of time. For example, one important criterion, the extent to which there is confirmation in clinical trials of epidemiological findings, can take years to assess because of the long time period required for sufficient scientific support to mount, execute, and analyze findings from trials.
Another possible criterion with which to evaluate the success of any large initiative is the extent of collaborative activities. These activities may involve pooling data on individual study participants from multiple cohort studies to improve sample size for specialized epidemiological investigations. Data from the NHS have been contributed to the Oxford University–led effort to combine data pertaining to the association of exogenous hormonal exposures (93) and endogenous hormones (94) with breast cancer risk, and NHS investigators are now participating in studies of gene–environment interactions that combine prospective data across several cohorts (95).
In summary, as financial resources for health research and their allocation change, and investigators must consider commitment to studies that can easily take an entire career, appropriate and comprehensive evaluation becomes increasingly important. We hope that the criteria suggested here and our application of them to NHS will initiate greater discussion of the issues relevant to evaluation, promote further improvements in the evaluation of large epidemiological studies beyond the confines of bibliographic analysis, and foster the development of improved data sources for evaluations.
Footnotes
This work was conducted while Dr Colditz was principal investigator on the NHS (CA89769) and under an interagency professional agreement with Epidemiology and Genetics Research Program, Division of Cancer Control and Population Science, National Cancer Institute.
References
- 1.National Cancer Institute . The Nation’s Investment in Cancer Research for Fiscal Year 2004. Bethesda, MD: National Cancer Institute, NIH; 2003. [Google Scholar]
- 2.Stokols D, Fuqua J, Gress J, et al. Evaluating transdisciplinary science. Nicotine Tob Res. 2003;5(suppl 1):S21–S39. doi: 10.1080/14622200310001625555. [DOI] [PubMed] [Google Scholar]
- 3.von Eschenbach AC. A vision for the National Cancer Program in the United States. Nat Rev Cancer. 2004;4(10):820–828. doi: 10.1038/nrc1458. [DOI] [PubMed] [Google Scholar]
- 4.Lilienfeld AM, Lilienfeld DE. Foundations of Epidemiology. 2nd ed. New York, NY: Oxford University Press; 1980. [Google Scholar]
- 5.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5(5):388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 6.Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 7.Hankinson SE, Willett WC, Michaud DS, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91(7):629–634. doi: 10.1093/jnci/91.7.629. [DOI] [PubMed] [Google Scholar]
- 8.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96(24):1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 9.Zhang SM, Willett WC, Selhub J, et al. Plasma folate, vitamin B6, vitamin B12, homocysteine, and risk of breast cancer. J Natl Cancer Inst. 2003;95(5):373–380. doi: 10.1093/jnci/95.5.373. [DOI] [PubMed] [Google Scholar]
- 10.Chen WY, Bertone-Johnson ER, Hunter DJ, Willett WC, Hankinson SE. Associations between polymorphisms in the vitamin D receptor and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2335–2339. doi: 10.1158/1055-9965.EPI-05-0283. [DOI] [PubMed] [Google Scholar]
- 11.Bertone-Johnson ER, Chen WY, Holick MF, et al. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1991–1997. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs T, Byrne C, Colditz G, Connolly J, Schnitt S. Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N Engl J Med. 1999;340(6):430–436. doi: 10.1056/NEJM199902113400604. [DOI] [PubMed] [Google Scholar]
- 13.Rosner B. Multivariate methods for binary longitudinal data with heterogeneous correlation over time. Stat Med. 1992;11(14–15):1915–1928. doi: 10.1002/sim.4780111412. [DOI] [PubMed] [Google Scholar]
- 14.Rosner B. Measurement error models for ordinal exposure variables measured with error. Stat Med. 1996;15(3):293–303. doi: 10.1002/(SICI)1097-0258(19960215)15:3<293::AID-SIM166>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 15.Perisic I, Rosner B. Comparisons of measures of interclass correlations: the general case of unequal group size. Stat Med. 1999;18(12):1451–1466. doi: 10.1002/(sici)1097-0258(19990630)18:12<1451::aid-sim142>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162(10):975–982. doi: 10.1093/aje/kwi309. [DOI] [PubMed] [Google Scholar]
- 17.Overall Evaluations of carcinogenicity: An update of IARC Monographs Volumes 1 to 42. Lyon, France: IARC; 1987. International Agency for Research on Cancer. [Google Scholar]
- 18.Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Hennekens CH, Speizer FE. Moderate alcohol consumption and the risk of breast cancer. N Engl J Med. 1987;316(19):1174–1180. doi: 10.1056/NEJM198705073161902. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson S, Hunter D, Colditz G, et al. Tubal ligation, hysterectomy and risk of ovarian cancer: a prospective study. JAMA. 1993;270:2813–2818. [PubMed] [Google Scholar]
- 20.Colditz GA, Sellers TA, Trapido E. Epidemiology—identifying the causes and preventability of cancer? Nat Rev Cancer. 2006;6(1):75–83. doi: 10.1038/nrc1784. [DOI] [PubMed] [Google Scholar]
- 21.Briss PA, Brownson RC, Fielding JE, Zaza S. Developing and using the Guide to Community Preventive Services: lessons learned about evidence-based public health. Annu Rev Public Health. 2004;25:281–302. doi: 10.1146/annurev.publhealth.25.050503.153933. [DOI] [PubMed] [Google Scholar]
- 22.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 suppl):21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 23.Kawachi I, Colditz G, Stampfer M, et al. Smoking cessation and time course of decreased risk of coronary heart disease in women. Arch Intern Med. 1994;154(2):169–175. [PubMed] [Google Scholar]
- 24.Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking cessation in relation to total mortality rates in women: a prospective cohort study. Ann Intern Med. 1993;119(10):992–1000. doi: 10.7326/0003-4819-119-10-199311150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Surgeon General’s Report—Women and Smoking. Washington, DC: Government Printing Office; 2001. U.S. Department of Health and Human Services. [Google Scholar]
- 26.Willett WC, Hennekens CH, Bain C, Rosner B, Speizer FE. Cigarette smoking and non-fatal myocardial infarction in women. Am J Epidemiol. 1981;113(5):575–582. doi: 10.1093/oxfordjournals.aje.a113134. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services. The Health Benefits of Smoking Cessation. A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; DHHS (CDC); 1990. pp. 90–8416. [Google Scholar]
- 28.U.S. Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 29.Colditz GA, Bonita R, Stampfer MJ, et al. Cigarette smoking and risk of stroke in middle-aged women. N Engl J Med. 1988;318(15):937–941. doi: 10.1056/NEJM198804143181501. [DOI] [PubMed] [Google Scholar]
- 30.Martinez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. Nurses’ Health Study Research Group. J Natl Cancer Inst. 1997;89(13):948–955. doi: 10.1093/jnci/89.13.948. [DOI] [PubMed] [Google Scholar]
- 31.Kushi LH, Byers T, Doyle C, et al. American Cancer Society guidelines on nutrition and physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56(5):254–281. doi: 10.3322/canjclin.56.5.254. quiz 313–314. [DOI] [PubMed] [Google Scholar]
- 32.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 33.Pearson TA, Bazzarre TL, Daniels SR, et al. American Heart Association guide for improving cardiovascular health at the community level: a statement for public health practitioners, healthcare providers, and health policy makers from the American Heart Association Expert Panel on Population and Prevention Science. Circulation. 2003;107(4):645–651. doi: 10.1161/01.cir.0000054482.38437.13. [DOI] [PubMed] [Google Scholar]
- 34.Emmons KM, McBride CM, Puleo E, et al. Project PREVENT: a randomized trial to reduce multiple behavioral risk factors for colon cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1453–1459. doi: 10.1158/1055-9965.EPI-04-0620. [DOI] [PubMed] [Google Scholar]
- 35.Rockhill B, Willett WC, Hunter DJ, Manson JE, Hankinson SE, Colditz GA. A prospective study of recreational physical activity and breast cancer risk. Arch Intern Med. 1999;159(19):2290–2296. doi: 10.1001/archinte.159.19.2290. [DOI] [PubMed] [Google Scholar]
- 36.Weight Control and Physical Activity. Lyon. France: International Agency for Research on Cancer; 2002. International Agency for Research on Cancer. [Google Scholar]
- 37.Manson JE, Rimm EB, Stampfer MJ, et al. A prospective study of physical activity and the incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 38.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282(15):1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 39.Diabetes prevention. American Diabetes Association. http://www.diabetes.org/diabetes-prevention.jsp. Accessed October 2007. [Google Scholar]
- 40.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341(9):650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 41.Krauss RM, Eckel RH, Howard B, et al. AHA dietary guidelines—revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Stroke. 2000;31(11):2751–2766. doi: 10.1161/01.str.31.11.2751. [DOI] [PubMed] [Google Scholar]
- 42.Fabian CJ, Kimler BF, Zalles CM, et al. Short-term breast cancer prediction by random periareolar fine-needle aspiration cytology and the Gail risk model. J Natl Cancer Inst. 2000;92(15):1217–1227. doi: 10.1093/jnci/92.15.1217. [DOI] [PubMed] [Google Scholar]
- 43.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. J Am Med Assoc. 1997;278(17):1407–1411. [PubMed] [Google Scholar]
- 44.Doyle C, Kushi LH, Byers T, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56(6):323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 45.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351(26):2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 46.Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med. 1995;333(11):677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 47.U.S. Preventive Services Task Force. Screening for Obesity in Adults. Rockville, MD: US Department of Health and Human Services, Agency for Healthcare Research and Quality; 2003. [Google Scholar]
- 48.Colditz GA, Willett WC, Stampfer MJ, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. 1990;132(3):501–513. doi: 10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- 49.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 50.Huang Z, Willett WC, Manson JE, et al. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128(2):81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 51.American Academy of Pediatrics. 2007. Television and the Family. http://www.aap.org/family/tv1.htm. [Google Scholar]
- 52.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- 53.International Agency for Research on Cancer. Hormonal Contraception and Post-menopausal Hormonal Therapy. Vol 72. Lyon, France: IARC, WHO; 1999. [Google Scholar]
- 54.Willett WC, Bain C, Hennekens CH, Rosner B, Speizer FE. Oral contraceptives and risk of ovarian cancer. Cancer. 1981;48(7):1684–1687. doi: 10.1002/1097-0142(19811001)48:7<1684::aid-cncr2820480735>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 55.Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332(24):1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 56.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 57.Hankinson SE, Willett WC, Manson JE, et al. Plasma sex steriod hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 58.Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 59.Maskarinec G, Williams AE, Inouye JS, Stanczyk FZ, Franke AA. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11(2):195–201. [PubMed] [Google Scholar]
- 60.Harper-Wynne C, Ross G, Sacks N, et al. Effects of the aromatase inhibitor letrozole on normal breast epithelial cell proliferation and metabolic indices in postmenopausal women: a pilot study for breast cancer prevention. Cancer Epidemiol Biomarkers Prev. 2002;11(7):614–621. [PubMed] [Google Scholar]
- 61.Guerrieri-Gonzaga A, Robertson C, Bonanni B, et al. Preliminary results on safety and activity of a randomized, double-blind, 2 x 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in premenopausal women. J Clin Oncol. 2006;24(1):129–135. doi: 10.1200/JCO.2005.02.9934. [DOI] [PubMed] [Google Scholar]
- 62.Kaaks R, Bellati C, Venturelli E, et al. Effects of dietary intervention on IGF-I and IGF-binding proteins, and related alterations in sex steroid metabolism: the Diet and Androgens (DIANA) Randomised Trial. Eur J Clin Nutr. 2003;57(9):1079–1088. doi: 10.1038/sj.ejcn.1601647. [DOI] [PubMed] [Google Scholar]
- 63.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003;12(8):721–727. [PubMed] [Google Scholar]
- 64.Juul A, Bernasconi S, Carel JC, Clayton PE, Kiess W, DeMuinck-Keizer Schrama S. Growth hormone treatment and risk of solid tumours. A statement from the Drugs and Therapeutics Committee of the European Society for Paediatric Endocrinology (ESPE) Horm Res. 2003;60(2):103–104. doi: 10.1159/000071879. [DOI] [PubMed] [Google Scholar]
- 65.London ST, Connolly JL, Schnitt SJ, Colditz GA. A prospective study of benign breast disease and the risk of breast cancer. J Am Med Assoc. 1992;267(7):941–944. [PubMed] [Google Scholar]
- 66.Willett WC, Stampfer MJ, Manson JE, et al. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet. 1993;341:581–585. doi: 10.1016/0140-6736(93)90350-p. [DOI] [PubMed] [Google Scholar]
- 67.Chardigny JM, Malpuech-Brugere C, Dionisi F, et al. Rationale and design of the TRANSFACT project phase I: a study to assess the effect of the two different dietary sources of trans fatty acids on cardiovascular risk factors in humans. Contemp Clin Trials. 2006;27(4):364–373. doi: 10.1016/j.cct.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Food and Drug Administration (Docket No. 95P-0197) Food labeling: health claims; oats and coronary heart disease. Fed Regist. 1997;62:3584–3601. [Google Scholar]
- 69.Giovannucci E, Stampfer M, Colditz G, et al. Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med. 1998;129:517–524. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 70.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 71.U.S. Preventive Services Task Force. Hormone therapy for the prevention of chronic conditions in postmenopausal women: recommendations from the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142(10):844–860. [PubMed] [Google Scholar]
- 72.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343(1):16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 73.Longnecker MP. Alcoholic beverage consumption in relation to risk of breast cancer: meta-analysis and review. Cancer Causes Control. 1994;5(1):73–82. doi: 10.1007/BF01830729. [DOI] [PubMed] [Google Scholar]
- 74.Byers T, Nestle M, McTiernan A, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2002;52(2):92–119. doi: 10.3322/canjclin.52.2.92. [DOI] [PubMed] [Google Scholar]
- 75.Klein EA. Clinical models for testing chemopreventative agents in prostate cancer and overview of SELECT: the Selenium and Vitamin E Cancer Prevention Trial. Recent Results Cancer Res. 2003;163:212–225. doi: 10.1007/978-3-642-55647-0_19. discussion 264–266. [DOI] [PubMed] [Google Scholar]
- 76.Sorensen G, Stoddard AM, LaMontagne AD, et al. A comprehensive worksite cancer prevention intervention: behavior change results from a randomized controlled trial (United States) J Public Health Policy. 2003;24(1):5–25. [PubMed] [Google Scholar]
- 77.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifetsyle, and risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 78.D’Agostino RB Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 79.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 80.Freedman AN, Seminara D, Gail MH, et al. Cancer risk prediction models: a workshop on development, evaluation, and application. J Natl Cancer Inst. 2005;97(10):715–723. doi: 10.1093/jnci/dji128. [DOI] [PubMed] [Google Scholar]
- 81.Colditz G, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am J Epidemiol. 2000;152(10):950–964. doi: 10.1093/aje/152.10.950. [DOI] [PubMed] [Google Scholar]
- 82.Rosner B, Colditz G, Webb P, Hankinson S. Mathematical models of ovarian cancer incidence in the Nurses’ Health Study. Epidemiology. 2004;16(4):508–515. doi: 10.1097/01.ede.0000164557.81694.63. [DOI] [PubMed] [Google Scholar]
- 83.Cho E, Rosner BA, Feskanich D, Colditz GA. Risk factors and individual probabilities of melanoma for whites. J Clin Oncol. 2005;23(12):2669–2675. doi: 10.1200/JCO.2005.11.108. [DOI] [PubMed] [Google Scholar]
- 84.Glasziou PP, Irwig LM. An evidence based approach to individualising treatment. BMJ. 1995;311:1356–1359. doi: 10.1136/bmj.311.7016.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feskanich D, Singh V, Willett WC, Colditz GA. Vitamin A intake and hip fractures among postmenopausal women. JAMA. 2002;287(1):47–54. doi: 10.1001/jama.287.1.47. [DOI] [PubMed] [Google Scholar]
- 86.Feskanich D, Willett WC, Colditz GA, Calcium vitamin D, milk consumption hip fractures: a prospective study among postmenopausal women. Am J Clin Nutr. 2003;77(2):504–511. doi: 10.1093/ajcn/77.2.504. [DOI] [PubMed] [Google Scholar]
- 87.Hennekens CH, Burling JE. Epidemiology in Medicine. Boston, MA: Little, Brown and Co.; 1987. [Google Scholar]
- 88.Hofman A. The Incidence of Major Diseases in the Elderly, The Rotterdam Study 1990-2004. Rotterdam, The Netherlands: Erasmus University; 2004. [Google Scholar]
- 89.Fuchs CS, Stampfer MJ, Colditz GA, et al. Alcohol consumption and mortality among women. N Engl J Med. 1995;332(19):1245–1250. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- 90.Rockhill B, Willett WC, Manson JE, et al. Physical activity and mortality: a prospective study among women. Am J Public Health. 2001;91(4):578–583. doi: 10.2105/ajph.91.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colditz GA. Oral contraceptive use and mortality during twelve years of follow-up: the Nurse’ Health Study. Ann Intern Med. 1994;120(10):821–826. doi: 10.7326/0003-4819-120-10-199405150-00002. [DOI] [PubMed] [Google Scholar]
- 92.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 93.Collaborative group on hormonal factors in breast cancer. Breast cancer and hormone replacement therapy. Combined reanalysis of data from 51 epidemiological studies involving 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350(9084):1047–1059. [PubMed] [Google Scholar]
- 94.The Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 95.Hunter DJ, Riboli E, Haiman CA, et al. A candidate gene approach to searching for low-penetrance breast and prostate cancer genes. Nat Rev Cancer. 2005;5(12):977–985. doi: 10.1038/nrc1754. [DOI] [PubMed] [Google Scholar]
- 96.Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes Control. 1996;7(2):253–263. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- 97.Irwin ML, Yasui Y, Ulrich CM. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA. 2003;289(3):323–330. doi: 10.1001/jama.289.3.323. [DOI] [PubMed] [Google Scholar]
- 98.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(7):1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 99.Klein S, Sheard NF, Pi-Sunyer X. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. A statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Am J Clin Nutr. 2004;80(2):257–263. doi: 10.1093/ajcn/80.2.257. [DOI] [PubMed] [Google Scholar]
- 100.Klein S, Burke LE, Bray GA. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110(18):2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 101.IARC Monographs on the Evaluation of Carcinogenic Risks to Human. Vol. 99. Lyon, France: IARC; 2007. International Agency for Research on Cancer. Combined estrogen-progestogen contraceptives and combined estrogen-progestogen menopausal therapy. http://monographs.iarc.fr/ENG/Monographs/vol91/index.phpAccessed June 16, 2008. [PMC free article] [PubMed] [Google Scholar]