Abstract
Carriage of a non–O ABO blood group and colonization by Helicobacter pylori are thought to be risk factors for pancreatic cancer. We examined these associations in a population-based case–control study of 373 case patients and 690 control subjects frequency matched on sex and age. Control subjects were selected by random-digit dialing. Seropositivity for H pylori and its virulence protein CagA was determined by enzyme-linked immunosorbent assay (ELISA). Increased risk of pancreatic cancer was associated with non–O blood group (adjusted odds ratio [OR] = 1.37, 95% confidence interval [CI] = 1.02 to 1.83, P = .034) and CagA-negative H pylori seropositivity (OR = 1.68, 95% CI = 1.07 to 2.66, P = .025), but no association was observed for CagA seropositivity (OR = 0.77, 95% CI = 0.52 to 1.16). An association between pancreatic cancer risk and CagA-negative H pylori seropositivity was found among individuals with non–O blood type but not among those with O blood type (OR = 2.78, 95% CI = 1.49 to 5.20, P = .0014; OR = 1.28, 95% CI = 0.62 to 2.64, P = .51, respectively). This study demonstrates an association between pancreatic cancer and H pylori colonization, particularly for individuals with non–O blood types.
CONTEXTS AND CAVEATS
Prior knowledge
Previous studies have shown an increased risk of pancreatic cancer for individuals with A, B, and AB blood type compared with those with blood type O. Gastric colonization with Helicobacter pylori is also associated with greater risk of pancreatic cancer.
Study design
The associations among blood type, H pylori seropositivity, and the H pylori virulence protein CagA were examined in a population-based case–control study.
Contribution
A statistically significant association between pancreatic cancer risk and H pylori seropositivity was found among individuals with non–O blood type (A, B, and AB) but not among those with O blood type. The association was greatest for non–O individuals colonized by CagA-negative H pylori, whereas CagA seropositivity was not associated with an increased risk.
Implications
The association among non–O blood type, H pylori colonization, and risk of pancreatic cancer is consistent with the hypothesis that the presence of A or B blood group antigens in gastrointestinal mucins influences the properties of H pylori binding. However, presence of the CagA virulence protein may temper the effect of H pylori on pancreatic cancer risk.
Limitations
Fewer than half of the potentially eligible individuals with pancreatic cancer could be interviewed because of serious illness and mortality. The results may thus be biased toward those individuals with better survival or less aggressive disease.
From the Editors
Human risk factors for pancreatic cancer include gastric colonization by Helicobacter pylori, dietary intake of N-nitrosamines or of nitrites, which form gastric N-nitrosamines, and cigarette smoking, which also delivers N-nitrosamines (1). We have hypothesized that pathophysiological actions of H pylori colonization enhance the pancreatic carcinogenic effect of N-nitrosamines conveyed by smoking or dietary sources (1). This effect is modulated by host inflammatory responses to the organism, by various virulence and other properties of the Helicobacter itself, and by host–organism interactions.
The association between ABO blood group and risk of pancreatic cancer has been known for more than 40 years but received little attention. A study in the United Kingdom (2) and a six-country study (3) observed an increased risk of pancreatic cancer for blood group A individuals (odds ratio [OR] = 1.18, 95% confidence interval [CI] = 1.01 to 1.39, P = .042; OR = 1.52, 95% CI = 0.87 to 2.67, P = .14, respectively). A study in Italy (4) found increased risk of pancreatic cancer among blood group B individuals (OR = 1.60, 95% CI = 1.25 to 2.04, P < .001), and a recent cohort study in the United States (5) found increased risk for individuals who self-reported blood types A, B, and AB compared with O (OR = 1.43, 95% CI = 1.14 to 1.81, P = .0024). Finally, a recent genome-wide association study “Panscan I” (6) identified single-nucleotide polymorphisms within the ABO locus as statistically significantly associated on a genome-wide basis (P = 10−7.3) with risk of pancreatic cancer.
In colonizing the human host, H pylori binds to gastric mucins, rather than directly to mucosal epithelium, to protect itself from luminal acidity and shedding (7). The most efficient binding occurs on mucin Lewis (b) antigens, with some secondary binding to H type 1 antigens (8). Both antigens contain the terminal Fucα1,2 residue on which binding occurs. Blood group A and B antigen determinants (GalNAcα1,3 and Galα1,3, respectively) are attached at the third position of the penultimate Galβ1,3 moiety, immediately adjacent to the Lewis (b) Fucα1,2 at the second position, and interact sterically with the Fucα1,2 residue (9). For example, some strains of H pylori that bind to the Lewis (b) antigen do not bind to A–Lewis (b) (9). Thus, we propose that the association between ABO blood group and risk of pancreatic cancer occurs through effects on colonization by H pylori. We therefore examined the association among carriage of ABO blood group, colonization with H pylori, and risk of pancreatic cancer in a case–control study in Connecticut in 2005–2009.
To identify case patients, study staff made frequent regular visits to the 30 general hospitals across the state of Connecticut between January 1, 2005, and August 31, 2009. Agreement for participation was obtained from physicians or physician practices for 83% of the 1092 potentially eligible individuals aged 35–83 years with newly diagnosed pancreatic cancer. Of 906 attempted in-person interviews, we completed 414 (46%). The remaining patients could not be located or contacted (n = 50), were too ill or had died before study contact (n = 333), or refused study participation (n = 109). Case patient eligibility was confirmed through examination of clinical and pathologic information. During the same period, we used pre-letter–assisted random-digit dialing methods to identify potential control subjects. An address was sought for each randomly selected landline telephone number through reverse-directory lookup in order to mail a study letter before telephone contact for eligibility. Control subjects, resident in Connecticut, were frequency matched to case patients by sex and age (35–51, 52–59, 60–64, 65–69, 70–74, 75–79, and 80–83 years). Most case patients had residential telephone numbers listed in the reverse directories, and thus, control subjects were additionally matched in overall distribution on the existence of a listed residential telephone number. We identified 1137 potentially eligible control subjects and interviewed 715 (63%) in person. Reasons for nonparticipation included inability to locate or contact (n = 140) and subject refusal (n = 282). At interview, subjects provided signed informed consent, after which the questionnaire interview was conducted, and venipuncture blood samples (93.5%) or saliva samples (5.9%) were obtained. The study was approved by the Human Investigation Committees of Yale University and the State of Connecticut Department of Public Health, as well as by the institutional review boards of the 30 Connecticut hospitals.
Following interview, blood and saliva samples were returned to our laboratory on freeze packs within 4 hours. Buffy coat, plasma, and erythrocytes were separated, aliquoted, and stored immediately at −80°C until analysis. Commercial immunoassay IgG enzyme-linked immunosorbent assay (ELISA) kits were used for the determination of seropositivity for H pylori (Diagnostic Automation, Inc, Calabasas, CA) and for CagA-positive H pylori strain (p120 kit of Viva Diagnostika; Inverness Medical Deutschland GmbH, Köln, Germany). Each 96-well plate included samples from a total of 43 mixed case patients and control subjects in duplicate, along with four calibration samples, two known positive samples, two known negative samples, and two blanks. All of the known samples yielded titer values well within their appropriate ranges, and the coefficients of variation for the calibration samples averaged 2.5% for the H pylori and 1.8% for the CagA ELISAs. Seropositivity was assigned for calculated titer levels exceeding the manufacturers’ recommended threshold values.
Over the lifetime in colonized normal adults, natural H pylori IgG seroreversion occurs to a substantially greater degree than CagA seroreversion (10,11). We therefore considered CagA seropositivity to indicate history of colonization by CagA-positive strains of H pylori, whether or not H pylori seropositivity was present. Finally, ABO blood group was determined by standard erythrocyte antiserum agglutination methods on the fresh blood specimens using a commercial kit (Eldon Biologicals A/S, Gentofte, Denmark).
For the present analysis, we included all 373 case patients and 690 control subjects who provided blood samples. Control subjects were adequately matched to case patients on age at interview (mean 66.9 vs 68.3 years, respectively) and sex (percent male 56.5 vs 57.6, respectively). Unconditional logistic regression methods were used to estimate odds ratios and 95% confidence intervals. All analyses were adjusted for age at interview (continuous), sex, years of cigarette smoking (continuous), and ELISA plate number. All P values are two-sided.
Associations between non–O ABO blood group, seropositivity for H pylori and for CagA-positive H pylori strain, and pancreatic cancer risk are shown in Table 1. CagA seropositivity was not associated with increased risk of pancreatic cancer, whereas CagA-negative H pylori seropositivity and non–O blood group were. The association was greatest for seropositive non–O individuals (OR = 1.88, 95% CI = 1.14 to 3.08, P = .013), particularly those seropositive for CagA-negative H pylori (OR = 2.78, 95% CI = 1.49 to 5.20, P = .0014). The two-sided P value for whether risk from CagA-negative H pylori seropositivity differed according to O or non–O blood group (OR = 1.28 vs 2.78) was .083.
Table 1.
Association between Helicobacter pylori colonization and non–O blood group with risk of pancreatic cancer*
| Model | Risk factors | No. of case patients (n = 373) | No. of control subjects (n = 690) | OR (95% CI) | P |
| 1 | Non–O blood group | 224 | 375 | 1.37 (1.02 to 1.83) | .034 |
| 2 | H pylori+† | 80 | 120 | 1.34 (0.94 to 1.92) | .11 |
| 3 | Non–O blood group | 224 | 375 | 1.37 (1.03 to 1.84) | .033 |
| H pylori+ | 80 | 120 | 1.35 (0.94 to 1.93) | .10 | |
| 4 | H pylori−, non–O blood group | 178 | 314 | 1.36 (0.98 to 1.88) | .068 |
| H pylori+, O blood group | 34 | 59 | 1.30 (0.76 to 2.24) | .34 | |
| H pylori+, non–O blood group | 46 | 61 | 1.88 (1.14 to 3.08) | .013 | |
| 5 | H pylori+, CagA−‡ | 47 | 58 | 1.68 (1.07 to 2.66) | .025 |
| 6 | H pylori+, CagA− | 47 | 58 | 1.63 (1.02 to 2.59) | .039 |
| CagA+ | 55 | 108 | 0.83 (0.55 to 1.24) | .36 | |
| 7 | Non–O blood group | 224 | 375 | 1.37 (1.02 to 1.83) | .036 |
| H pylori+, CagA− | 47 | 58 | 1.63 (1.02 to 2.59) | .040 | |
| CagA+ | 55 | 108 | 0.83 (0.55 to 1.25) | .36 | |
| 8 | H pylori−, CagA−, non–O group | 164 | 287 | 1.43 (1.02 to 2.01) | .040 |
| H pylori+, CagA−, O blood group | 17 | 30 | 1.28 (0.62 to 2.64) | .51 | |
| H pylori+, CagA−, non–O group | 30 | 28 | 2.78 (1.49 to 5.20) | .0014 | |
| CagA+ | 55 | 108 | 1.01 (0.64 to 1.59) | .96 |
Case patients were aged 35–83 y with newly diagnosed pancreatic cancer and frequency matched on sex and age to 690 control subjects. Unconditional logistic regression models were used to obtain the odds ratios (ORs) and 95% confidence intervals (CIs). All models were adjusted for age at interview (continuous), sex, and years of cigarette smoking (continuous), and with indicator terms for enzyme-linked immunosorbent assay plate number. Each row in the table is a separate adjusted model that includes all of the risk factors in the row; the referents in each model are individuals not exposed to any of the risk factors shown in that model. P values, calculated by the Wald ratio, are two-sided. CagA is seropositivity for an H pylori virulence protein.
H pylori titer considered positive at the manufacturer’s threshold of greater than or equal to 1.1 units.
CagA titer considered positive at the manufacturer's threshold of greater than or equal to 7.5 units.
To our knowledge, this study is the largest analysis of the association between pancreatic cancer risk and H pylori colonization to date. One limitation, though, is that even with very rapid access to patient information, we were able to interview fewer than half of the potentially eligible individuals with pancreatic cancer. More than 30% of potential case patients were too ill or deceased by the time we could approach them for interview, only a few weeks after diagnosis. Our results may therefore be biased toward those of individuals with better survival or less aggressive disease. However, we are not aware of major effects of ABO blood group or H pylori seropositivity on survival with pancreatic cancer. In the recent genome-wide association study (6), the risk effect for having non–O blood type was appreciably stronger in the included cohort studies than in the case–control studies, suggesting that the association does apply to patients with short survival. The magnitude of the effect for non–O blood group in this study (OR = 1.37) is very close to the recently published cohort study value (OR = 1.43) (5) and also suggests little bias among our study subjects.
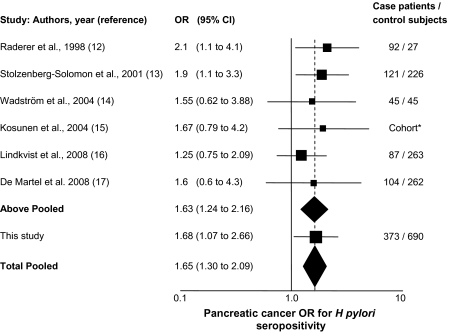
Overall, this study provides evidence in support of the association between H pylori seropositivity and increased risk of pancreatic cancer. Six previous studies have examined this relationship, and an inverse variance-weighted pooled analysis of the seven studies demonstrates a statistically significant combined association (P < .001) (Figure 1). This study also confirms the association of pancreatic cancer with non–O blood group and suggests that the effect of H pylori seropositivity is particularly manifested in non–O individuals. This finding is consistent with our hypothesis that the presence of terminal A or B blood group antigens in gastrointestinal mucins influences the properties of the adjacent H pylori binding.
Figure 1.
Forest plot of the odds ratio (OR) of pancreatic cancer according to Helicobacter pylori seropositivity. The solid squares are centered on the point estimate from each study, and the horizontal line through each square indicates the 95% confidence interval (CI) for the study estimate. The area of each square represents the weight of the study in the meta-analysis. The center of each diamond indicates the inverse variance-weighted summary estimate of the effect size; the horizontal tips represent the 95% confidence interval; the area represents the combined areas of the solid squares included in the summary. The vertical dashed line is at the center of the total pooled odds ratio (OR = 1.65). The seven studies show little heterogeneity (Q statistic P value = .93). All of the studies except Kosunen et al. (15) ascertained seropositivity for H pylori. Kosunen et al. (15) examined incidence of pancreatic cancer in a cohort of individuals colonized by H pylori. In this cohort, 4137 of the total 18 012 carriers had successful H pylori eradication, and in the figure, the odds ratio and confidence interval for this study represent noneradication of H pylori. Fixed-effects inverse-weighting meta-analysis summary calculations on the log odds ratios (18) for the diamonds in the figure were performed in Excel.
The present work provides little evidence for an association between CagA seropositivity and risk of pancreatic cancer. CagA-positive Helicobacter strains appear more likely to cause chronic atrophic gastritis and gastric cancer than CagA-negative ones (19–21). This fact is consistent with our etiologic theory (1), in which the hypo- or achlorhydria associated with CagA-positive H pylori corpus-predominant gastritis would not lead to enhanced secretin stimulation of the pancreatic ductal epithelium and thus not increase the risk of pancreatic cancer.
Funding
US National Cancer Institute-National Institutes of Health (5R01CA098870 to H.A.R.).
Footnotes
The authors assume full responsibility for analyses and interpretation of these data. No funders of the study had any involvement in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The cooperation of 30 Connecticut hospitals, including Stamford Hospital, in allowing patient access, is gratefully acknowledged. This study was approved by the State of Connecticut Department of Public Health Human Investigation Committee. Certain data used in this study were obtained from the Connecticut Tumor Registry in the Connecticut Department of PublicHealth.
References
- 1.Risch HA. Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J Natl Cancer Inst. 2003;95(13):948–960. doi: 10.1093/jnci/95.13.948. [DOI] [PubMed] [Google Scholar]
- 2.Macafee AL. ABO blood groups and carcinoma of pancreas. Ulster Med J. 1964;33(2):129–131. [PMC free article] [PubMed] [Google Scholar]
- 3.Vioque J, Walker AM. Cancer de pancreas y tipologia sanguinea ABO: un estudio de casos y controles. Med Clin (Barc) 1991;96(20):761–764. [PubMed] [Google Scholar]
- 4.Annese V, Minervini M, Gabbrielli A, Gambassi G, Manna R. ABO blood groups and cancer of the pancreas. Int J Pancreatol. 1990;6(2):81–88. doi: 10.1007/BF02933042. [DOI] [PubMed] [Google Scholar]
- 5.Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101(6):424–431. doi: 10.1093/jnci/djp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies ABO blood group susceptibility variants for pancreatic cancer. Nat Genet. 2009;41(9):986–990. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azevedo M, Eriksson S, Mendes N, et al. Infection by Helicobacter pylori expressing the BabA adhesin is influenced by the secretor phenotype. J Pathol. 2008;215(3):308–316. doi: 10.1002/path.2363. [DOI] [PubMed] [Google Scholar]
- 8.Alkout AM, Blackwell CC, Weir DM, et al. Isolation of a cell surface component of Helicobacter pylori that binds H type 2, Lewis a, and Lewis b antigens. Gastroenterology. 1997;112(4):1179–1187. doi: 10.1016/s0016-5085(97)70129-x. [DOI] [PubMed] [Google Scholar]
- 9.Aspholm-Hurtig M, Dailide G, Lahmann M, et al. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;35(5683):519–522. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- 10.Kist M, Strobel S, Kirchner T, Dammann HG. Impact of ELISA and immunoblot as diagnostic tools one year after eradication of Helicobacter pylori in a multicentre treatment study. FEMS Immunol Med Microbiol. 1999;24(2):239–242. doi: 10.1111/j.1574-695X.1999.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosenstock S, Jørgensen T, Andersen L, Bonnevie O. Seroconversion and seroreversion in IgG antibodies to Helicobacter pylori: a serology based prospective cohort study. J Epidemiol Community Health. 2000;54(6):444–450. doi: 10.1136/jech.54.6.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raderer M, Wrba F, Kornek G, et al. Association between Helicobacter pylori infection and pancreatic cancer. Oncology. 1998;55(1):16–19. doi: 10.1159/000011830. [DOI] [PubMed] [Google Scholar]
- 13.Stolzenberg-Solomon RZ, Blaser MJ, Limburg PJ, et al. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J Natl Cancer Inst. 2001;93(12):937–941. doi: 10.1093/jnci/93.12.937. [DOI] [PubMed] [Google Scholar]
- 14.Wadström T, Fryzek JP, Demirjian S, et al. Antibodies to Helicobacter bilis in patients with pancreatic carcinoma. Helicobacter. 2004;9(5):538–539. [Google Scholar]
- 15.Kosunen TU, Pukkala E, Seppälä K, et al. The effect of eradication therapy for Helicobacter infection on the incidence of gastric and other cancers. Helicobacter. 2004;9(5):534. [Google Scholar]
- 16.Lindkvist B, Johansen D, Borgström A, Manjer J. A prospective study of Helicobacter pylori in relation to the risk for pancreatic cancer. BMC Cancer. 2008;8:321. doi: 10.1186/1471-2407-8-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Martel C, Llosa AE, Friedman GD, et al. Helicobacter pylori infection and development of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1188–1194. doi: 10.1158/1055-9965.EPI-08-0185. [DOI] [PubMed] [Google Scholar]
- 18.Fleiss JL. Statistical Methods for Rates and Proportions. 2nd ed. New York, NY: John Wiley & Sons; 1982. [Google Scholar]
- 19.Palli D, Masala G, Del Giudice G, et al. CagA+ Helicobacter pylori infection and gastric cancer risk in the EPIC-EURGAST study. Int J Cancer. 2007;120(4):859–867. doi: 10.1002/ijc.22435. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki G, Cullings H, Fujiwara S, et al. Low-positive antibody titer against Helicobacter pylori cytotoxin-associated gene A (CagA) may predict future gastric cancer better than simple seropositivity against H. pylori CagA or against H. pylori. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1224–1228. doi: 10.1158/1055-9965.EPI-06-1048. [DOI] [PubMed] [Google Scholar]
- 21.Suriani R, Colozza M, Cardesi E, et al. CagA and VacA Helicobacter pylori antibodies in gastric cancer. Can J Gastroenterol. 2008;22(3):255–258. doi: 10.1155/2008/521724. [DOI] [PMC free article] [PubMed] [Google Scholar]