Abstract
BACKGROUND
Contraceptive vaccines can provide valuable alternatives to current methods of contraception. We describe here the development of sperm-reactive human single chain variable fragment (scFv) antibodies of defined sperm specificity for immunocontraception.
METHODS
Peripheral blood leukocytes (PBL) from antisperm antibody-positive immunoinfertile and vasectomized men were activated with human sperm antigens in vitro, and the complementary DNA prepared and PCR-amplified using primers based on all the variable regions of heavy and light chains of immunoglobulins. The scFv repertoire was cloned into pCANTAB5E vector to create a human scFv antibody library.
RESULTS
Panning of the library against specific sperm antigens yielded several clones, and the four strongest reactive were selected for further analysis. These clones had novel sequences with unique complementarity-determining regions. ScFv antibodies were expressed, purified and analyzed for human sperm reactivity and effect on human sperm function. AFA-1 and FAB-7 scFv antibodies both reacted with fertilization antigen-1 antigen, but against different epitopes. YLP20 antibody reacted with the expected human sperm protein of 48 ± 5 kDa. The fourth antibody, AS16, reacted with an 18 kDa sperm protein and seems to be a human homologue of the mouse monoclonal recombinant antisperm antibody that causes sperm agglutination. All these antibodies inhibited human sperm function.
CONCLUSIONS
This is the first study to report the use of phage display technology to obtain antisperm scFv antibodies of defined antigen specificity. These antibodies will find clinical applications in the development of novel immunocontraceptives, and specific diagnostics for immunoinfertility.
Keywords: single chain variable fragment antibodies, sperm antigens, antisperm antibodies, infertility, immunocontraception
Introduction
The world population has exceeded 6.65 billion and is increasing by 1 billion every 12 years at the present rate (World POPClock Projection, 2008). Ninety-five percentage of this growth is in developing countries. Besides population explosion, unintended pregnancies are a major public health issue. It is estimated annually in the USA that half of all pregnancies are unintended, resulting in over 1 million elective abortions (Henshaw, 1998). In over half of these pregnancies, the women were using some type of contraception. Thus, it is imperative to develop better methods of contraception that are acceptable, both in the developing and developed countries.
Contraceptive vaccines (CVs) have been proposed as a valuable alternative. CVs may be more acceptable than the currently available methods due to high specificity, limited or no side effects, low cost and infrequent administration. Various targets are being explored in several laboratories. These include targeting gamete production (anti-GnRH), targeting gamete function [antisperm and anti-oocyte zona pellucida (ZP)], and targeting gamete outcome (anti-HCG) (Talwar, 1999; Naz et al., 2005). Of all these, CVs targeting sperm are especially interesting since the presence of antisperm antibodies (ASA) have been shown to be associated with involuntary infertility in humans, which provides a strong rationale and model for the antisperm CV development. Sperm have both auto- and isoantigenic potentials and can produce ASA in both men and women. Up to 70% of vasectomized men produce ASA, and 2–30% of cases of infertility may be associated with the presence of ASA in the male and/or female partner of an infertile couple (Clayton and Moore, 2001; Ohl and Naz, 1995; Pillai et al., 1996; Bohring and Krause, 2003). Several sperm antigens have been delineated and their complementary DNAs (cDNAs) have been cloned and sequenced. Active immunization with a few of them has been shown to cause a contraceptive effect.
Our laboratory has delineated two antigens, namely fertilization antigen-1 (FA-1) and YLP12, present on human sperm. FA-1 antigen is a glycoprotein of 50 ± 4 kDa that undergoes phosphorylation at tyrosine, serine and threonine residues during sperm capacitation and/or acrosome reaction (Naz and Zhu, 1997, 2002). The monoclonal and polyclonal antibodies to FA-1 antigen inhibit human sperm capacitation/acrosome reaction and human sperm–ZP binding. Vaccination of female mice with murine recombinant FA-1 antigen and FA-1 cDNA cause a long-term reversible contraceptive effect (Naz and Zhu, 1998). FA-1 antigen is involved in human involuntary immunoinfertility (Naz et al., 1993). The immunoinfertile men and women, and vasectomized men have antibodies reactive with the FA-1 antigen. YLP12 is a dodecamer peptide sequence, YLPVGGLRRIGG, present on human and murine sperm (Naz et al., 2000). This sequence is involved in binding to ZP protein, ZP3, of oocytes. Antibodies to synthetic YLP12 peptide inhibit sperm–oocyte binding in both species. The peptide and DNA vaccines based on YLP12 sequence cause immunocontraceptive effects in female mice (Naz and Chauhan, 2002). Immunoinfertile men have antibodies to YLP12 (Naz and Chauhan, 2001).
The progress in CV development has been hindered due to the variability of the immune response observed in individuals after vaccination (Talwar, 1999; Naz et al., 2005). It is envisaged that this concern may be obliterated by the passive immunization approach using the preformed antibodies. The antibody therapies have been tried and found to be successful against various infectious diseases, both in animals and humans. Some of the antibodies have become treatment modalities in the clinics (Riethmüller et al., 1993; Casadevall, 1999; Dunman and Nesin, 2003). Phage display technology (Smith, 1985) has been widely used to obtain a variety of engineered antibodies, including single chain variable fragment (scFv) antibodies against several antigens (Rader and Barbas, 1997; Ye et al., 2002; Park et al., 2007; Zhang et al., 2007; Zhou et al., 2007). ScFv is an antibody fragment that plays a major role in the antigen-binding activity, and is composed of variable heavy (VH) and variable light (VL) chains connected by a peptide linker. The most widely used peptide linker is a repeat of a 15-residue sequence of glycine and serine (Gly4Ser)3. This linker provides a flexibility to move ∼35°A to 40°A between the carboxyl terminal of VH and the amino terminus of VL chains for efficient antibody binding. Also, the linker length promotes more intra-domain than inter-domain disulfide bonding between VH and VL chains. The affinity and stability of scFv antibodies produced in bacteria are comparable with those of the native antibodies and are maintained by a strong disulfide bond. ScFv antibodies can be produced on a large scale using specially modified bacterial hosts and have an advantage over the whole immunoglobulin (Ig) molecule. ScFv antibodies lack the Fc portion that obliterates unwanted secondary effects associated with Fc, and due to its small size can be easily absorbed into tissues and gene manipulated (Yokota et al., 1992). The mouse monoclonal antibody can elicit strong anti-mouse antibody reactions, and the chimeric antibody can cause anti-chimeric response against murine antibody variable regions when injected into humans (Koren et al., 2002; Mirick et al., 2004; Sidhu and Fellouse, 2006). The xenogenic complementarity-determining regions (CDRs) of humanized antibodies can also evoke an anti-idiotypic response. In order to overcome these problems, antibodies have to be of human origin if to be used in humans. The potential poor immunogenicity and toxicity of an antigen, and ethical issues, limit immunizing humans to obtain human antibodies. However, the phage display technology can be employed to obtain these antibodies against target antigens if they exist involuntarily in humans, such as ASA in immunoinfertile men and women, and vasectomized men. This manuscript describes the application of phage display technology to isolate, produce and characterize fully functional human scFv antibodies against two well-characterized sperm antigens (FA-1 and YLP12) from immunoinfertile and vasectomized men, and examine their role in human sperm function. This is the first study to report the use the phage display technology to obtain antisperm scFv antibodies of defined antigen specificities from immunoinfertile/vasectomized men. The long-term objective of this study is to develop novel antisperm immunocontraceptives for humans.
Materials and Methods
Patient population and RNA isolation
Peripheral blood leukocytes (PBL) were isolated from heparinized blood of ASA—positive immunoinfertile and vasectomized men (n = 6, aged 27–37 years), and ASA-negative fertile (n = 4, aged 32–39 years) men by Ficoll-PaqueTM Plus gradient centrifugation (Amersham Biosciences AB, Uppsala, Sweden) following the manufacturer's protocol. The immunoinfertile and vasectomized men had ASA in their sera as revealed by the sperm immobilization technique (SIT), tray agglutination technique (TAT), and immunobead binding technique (IBT) (Isojima et al., 1987; Naz et al., 1993). All these sera demonstrated >20% sperm binding in IBT. The sera also demonstrated antibodies (IgG and IgA classes) to the purified cognate human sperm FA-1 antigen and synthetic YLP12 peptide in the enzyme linked immunosorbent assay (ELISA). The sera from fertile control men were negative for ASA when tested by SIT, TAT or IBT, and did not have antibodies to FA-1 antigen and YLP12 peptide. FA-1 antigen was purified from lithium diiodosalicylate (LIS)-solubilized human sperm extract (HSE) by using monoclonal immunoaffinity chromatography as described above. The purified FA-1 human sperm antigen showed a single band of 50 ± 4 kDa in the sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) after staining with silver nitrate. The YLP12 peptide was synthesized by solid-phase synthesis using Fmoc chemistry (Biosynthesis Inc., Lewisville, TX, USA) and had >90% purity. The study was approved by the Institutional Review Board (IRB) for human studies and the appropriate consent to participate in this study was obtained. PBL was washed (2×) in RPMI 1640 medium and 1 × 106 cells were then incubated (37°C, 5% CO2) with 8 µg each of FA-1 antigen, YLP12 peptide and HSE in a final volume of 200 µl RPMI 1640 medium containing 25 mmol/l Hepes buffer and l-glutamine, 10% fetal bovine serum, penicillin (100 U/ml) streptomycin (100 µg/ml) and kanamycin (100 µg/ml). After 5 days, the medium was changed to fresh medium of the same composition and incubated for an additional 2 days. The cells were then isolated, washed with RPMI 1640 medium and used for RNA isolation. RNA was isolated using guanidium thiocyanate-phenol RNA-STAT-60 (TEL-TEST Inc., Friendswood, TX, USA) following the manufacturer's protocol and was used for cDNA synthesis.
Construction of human antibody phage display library
The first strand cDNA was synthesized by mixing 10 µg of total RNA from each PBL culture (1 × 106) with 1 µg of oligo (dT) 15 primer (500 µg/ml, Promega, Madison, WI, USA) in a 50 µl reaction volume. The contents were incubated at 70°C for 3 min and then chilled on ice. To this mixture, 10 µl of 5× first-strand buffer, 2.5 µl of 10 mM dNTP and 2.5 µl of moloney murine leukemia virus reverse transcriptase (200 units/μl, Invitrogen, Carlsbad, CA, USA) were added and mixed. The reaction was carried out at 37°C for 1 h and stopped by incubation at 70°C for 10 min. The mixture was stored at −20°C until further use.
The human antibody phage display library was constructed using the standard PCR procedure. The nucleotide sequences of all human antibody classes/subclasses (http://www.kabatdatabase.com) were collected and degenerate PCR primers were designed for amplification of VH and VL chains (Kabat et al., 1991). Briefly, PCR reactions were set to amplify 16 different VL and 8 different VH chains. PCR was performed using 3 µl of cDNA template, 1 U Taq polymerase, 10 mM dNTP, 25 mM MgCl2 and 50 pmol each of forward and reverse primers in a 50 µl reaction volume. After initial denaturation at 94°C for 2 min, PCR was performed for 35 cycles with denaturation at 94°C for 20 s, annealing at 55°C for 10 s and extension at 68°C for 30 s. The 330 bp PCR products from each reaction were mixed to form separate VH and VL pools, and the products were purified from the gel using QIATM quick gel extraction kit (QIAGEN Sciences, MD, USA). The scFv assembly of VH and VL chains, with a (G4S)3 linker between the chains, was performed using the splicing by overlapping extension PCR (SOE-PCR) procedure. The primers used in SOE-PCR contained overlapping linker sequences to allow the two genes to be spliced by an overlap extension. For SOE-PCR procedure, 20 ng of respective purified VH and VL products (mixed in equimolar ratio) were used as template for 25 cycles with denaturation at 94°C for 30 s, annealing at 55°C for 30 s and extension at 68°C for 50 s. A final PCR was performed to incorporate SfiI and NotI restriction sites to 5′ and 3′ ends of the assembled scFv, respectively. PCR was for 30 cycles at 94°C for 1 min, 55°C for 30 s and 72°C for 1 min. The final assembled PCR product (760 bp) was purified and then digested with restriction enzymes SfiI and NotI. One microgram of the purified digested PCR insert was ligated with 1.5 µg of SfiI—and NotI pre-digested and dephosphorylated pCANTAB5E (Amersham Biosciences AB), in a reaction volume of 50 µl containing 5 µl of l0× ligase buffer and 5 U of T4 DNA ligase (Fisher Scientific, Pittsburg, PA, USA). The ligation was performed overnight at 16°C, the residual ligase in the sample was inactivated (70°C, 10 min) and the reaction mixture was stored at −20°C. The ligated product was then transformed into electrocompetent TG1 cells (Stratagene, Cedar Creek, TX, USA) by electroporation programmed to give one pulse of 25 µF, 2.5 KV at 200 ohm. The transformed cells were suspended in 1 ml of 2× YT medium containing 2% glucose and incubated at 37°C for 1 h with shaking at 200 rpm, and then plated onto plates coated with SOB medium containing 100 µg/ml ampicillin and 2% glucose. Transformants were scraped using 2× YT medium containing 100 µg/ml ampicillin and 2% glucose (YT-AG), and stored as 1 ml aliquots in 30% glycerol at −80°C. For examining the transformation efficiency, 10 clones were randomly selected from the library and analyzed by PCR for the presence of scFv insert.
Screening of library and selection of specific clones
Phages displaying scFv antibodies were prepared from the primary library, and the phage rescue was performed using M13KO7 helper phage (Smith, 1985; Rader and Barbas, 1997). The phage titer was determined by infecting appropriate dilution of phages in exponentially growing Escherichia coli TG1 cells and plated in 2×YT-AG culture plates.
Phage panning was performed in 96-well microtiter plates following the standard protocol. Briefly, Nunc Maxisorb TM (Nunc Maxisorb, Roskilde, Denmark) plates were coated (10 µg /well) overnight at 4°C individually with each of purified cognate human sperm FA-1 antigen, synthetic YLP12 peptide or HSE diluted in 200 µl of 0.1 M carbonate buffer (pH 9.6). The wells were then washed (5×) with phosphate-buffered saline (PBS) containing 0.05% Tween-20 (PBS-T). To block the non-specific binding sites, the wells were incubated (37°C, 1 h) with PBS-T containing 1% bovine serum albumin (BSA) and then washed (5×) with PBS-T. The plates were incubated (37°C, 2 h) with 200 µl of the culture supernatant containing 1 × 1010 of the primary library phages. The wells were washed (3×) with PBS-T and then with PBS (3×). The bound scFv clones were eluted using three different procedures namely acidic, basic and antigen excess elutions. For acidic elution, glycine–HCl buffer (0.01 M, pH 2.2) was used, the basic elution was performed using Tris–HCl buffer (0.01 M, pH 9.0) and for the antigen excess elution 10× concentration of the respective antigen was used for elution. After three procedures, the eluted phages were immediately neutralized with 3 M Tris–HCl, (pH 8.0). The phage titer was determined by infecting E. coli TG1 cells as described before. For subsequent rounds of panning, the eluted phages were incubated with the coated wells as before, and the procedure was repeated twice, keeping all other conditions identical as before. The eluates from the third round of panning were pooled (all eluted using acidic, basic and antigen excess procedures) for each antigen (FA-1/YLP12/HSE) and used to infect E. coli TG1 cells to isolate the individual clones. The single clones picked from the third round of panning were phage rescued using M13KO7 helper phage as mentioned before, and resulting recombinant phages were screened using ELISA.
For ELISA, each well was coated overnight with the antigen (cognate FA-1 antigen/synthetic YLP12 peptide/HSE) (Naz and Chauhan, 2001). After blocking with PBS-T containing 1% BSA, the wells were incubated (37°C, 2 h) with the recombinant phages, washed and then incubated (37°C, 1.5 h) with horseradish-peroxidase (HRP)-conjugated anti-M13 monoclonal antibody (Amersham Biosciences AB) diluted 1:1000 in PBS-T containing 0.1% BSA. The wells were washed with PBS-T, and incubated (37°C, 20 min) with substrate solution (0.2 mg/ml, 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) diluted in citric acid buffer (0.05 M, pH 4.0) containing 30% H2O2. The reaction was stopped using 10% SDS, and the ELISA plate was read at 405 nm.
The clones showing stronger reactivity [>2 optical density (OD) of the control wells] to the respective antigen were selected and PCR amplified. The ∼850 bp PCR product was excised and the DNA was purified using QIA quick gel extraction kit. The eluted DNA was quantified and sequenced using ABI automated DNA sequencer (Biotech Core, Sunnyvale, CA, USA). The sequence analysis was done using IMGT®, the international ImMunoGeneTics information system (http://imgt.cines.fr) and gene tool software (www.biotools.com).
scFv antibody expression and purification
The individual colonies that showed the strongest reactivity with the sperm antigens (FA-1/YLP12/HSE) were selected, grown and the scFv antibodies were purified using RPASTM purification system with HiTrapTM anti-E-Tag Column (Amersham Biosciences AB) following the manufacturer's protocol. The protein concentration was determined using QuantiPro BCA kit (Sigma Chemical Co., Saint Louis, MO, USA), and the purity and authenticity of the antibodies were examined by using SDS–PAGE (Laemmli, 1970) and western blot procedures (Towbin et al., 1979). For SDS–PAGE, various concentrations (5–25 µg/lane) of the purified antibodies were run in 12% gel and the gel was stained with silver nitrate (Bio-Rad Labs, Hercules, CA, USA). For western blot procedure, the scFv antibodies were resolved in SDS–PAGE, transferred overnight to nitrocellulose membrane, the membranes were blocked with PBS containing 0.1% BSA, and then probed with anti-E-Tag mouse monoclonal antibody (1:2000 dilution), followed by incubation with alkaline phosphatase-conjugated goat anti-mouse IgG (H- and L-specific) (1:2000 dilution) (Southern Biotechnology Associates, Birmingham, AL, USA). The blot was washed and incubated with nitroblue tetrazolium–bromochloroindolyl phosphate (NBT–BICP) substrate solution to visualize the reactive protein bands. The blot treated similarly but not incubated with anti-E-Tag antibody, or the blot treated with a control scFv antibody (CAB-3) that did not react with any antigen (FA-1/YLP12/HSE) in the panning/ELISA procedure, served as controls.
The purified antibodies were concentrated by lyophilization and examined again for reactivity in ELISA against the respective antigen. Only the purified antibodies showing the expected band(s) and having high titers (>1:2056 ELISA titers) were used for further analysis.
Immunoreactivity of scFv antibodies with human sperm and respective sperm antigens
The immunoreactivity of scFv antibodies with human sperm and purified human FA-1 antigen was examined using western blot procedure, immunoprecipitation procedure and indirect immunofluorescence technique (IFT). For western blot procedure, LIS-solubilized HSE, methanol-chloroform (MC)-solubilized human sperm preparation or purified human FA-1 antigen were run by SDS–PAGE (10–20 µg/lane) on a 12% gel, and transferred overnight to nitrocelluose membrane. The membranes were blocked with PBS containing 0.1% BSA, probed with scFv antibody (5 µg/100 ml) and then with anti-E-Tag mouse monoclonal antibody (1:2000 dilution). This was followed by incubation with alkaline phosphatase-conjugated goat anti-mouse IgG (H- and L-specific) (1:2000 dilution). The blot was washed and incubated with substrate (NBT–BICP) solution to visualize the reactive protein bands. The blot treated with the control scFv antibody (CAB-3) served as control. The LIS-solubilized HSE (Naz and Zhu, 1997, 1998, 2002) and MC-solubilized human sperm preparation was prepared as described elsewhere (Svennerholm and Fredman, 1980). MC solubilized sperm preparation was used only for testing AS16 antibody, since the sperm agglutination antigen-1 (SAGA-1) antigen is present on the inner acrosomal membrane and MC may increase the solubilization of the inner acrosomal membrane proteins.
For immunoprecipitation procedure, the scFv antibodies were conjugated to cyanogen bromide-activated Sepharose 4B and the conjugated beads were incubated with HSE overnight. To remove the unbound proteins, the beads were washed (5×) with RIPA buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, 0.1% SDS), and the beads were then boiled in Laemmli buffer, centrifuged and the supernatant was run in SDS–PAGE. The indirect IFT was used to localize the scFv antibody immunoreactive subcellular sites on human sperm. IFT was performed on both live and methanol-fixed human sperm. Semen was collected from fertile, healthy men by masturbation, following institutional IRB-approved protocol. Motile sperm cells, collected by use of the swim-up procedure, were washed (3×) with PBS (800g, 10 min), air-dried (2 × 104 sperm/well) on 10-well slide Teflon-coated slides (Polysciences, Inc., Warrington, PA, USA) at room temperature, fixed in methanol for 10 min and air-dried again. The slides were then rinsed with PBS, blocked with PBS containing 3% BSA for 45 min and then incubated with 15 µl of purified scFv antibodies. The slides were washed and incubated for 1.5 h with anti-E-Tag monoclonal antibody diluted (1:2000) in PBS. After thorough washing in PBS, the slides were incubated for 1.5 h with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (H- and L-chain specific) (1:40 dilution, Southern Biotechnology Associates). The slides were washed in PBS, mounted in PBS containing 90% glycerol and 1,4- diazabicyclo-[2,2,2]-octane (10 mg/ml), and examined using Zeiss confocal microscopy axiovert 100 M under FITC filter, and microphotographed using laser scanning microscope (LSM 510) (Version 3.2) at our microscope imaging facility.
For IFT using live sperm, the motile human sperm (1 × 106 sperm/ml) in Ham's F-10 medium containing 0.1% BSA were incubated (4°C, 1.5 h) with scFv antibodies (5–10 µg/ml), washed (3×) with PBS and air-dried on Teflon-coated slides. The rest of the procedure was same as described above for the methanol-fixed sperm.
Human sperm acrosome reaction
The effect of scFv antibodies on human sperm function (capacitation/acrosome reaction) was performed as described elsewhere (Naz et al., 2000). Briefly, the swim-up sperm were capacitated at 37°C for 6–8 h in 5% CO2 in air with 45 or 80 µg/ml of scFv antibody. The sperm were then washed to remove scFv antibody and incubated (37°C, 30 min) with calcium ionophore A23187 (Sigma) (5 µM final concentration) (Byrd et al., 1989). The sperm were washed (2×), methanol-fixed on the slides and the acrosome-reacted sperm were evaluated by using FITC-conjugated Pisum sativum agglutinin (ICN Biomedical, Inc., Aurora, OH, USA). Approximately 200–300 sperm were counted in each slide and each treatment was tested in 3–5 independent experiments on different days using sperm from three different men. Significance of differences was analyzed by using paired and unpaired Student's t-test. A P-value of <0.05 was considered significant.
Epitope analysis of scFv antibodies
Several peptides based upon immunodominant regions of published human (Naz and Zhu, 2002) and murine (Naz and Zhu, 1997) FA-1 amino acid sequences were synthesized by solid-phase synthesis using Fmoc chemistry (Biosynthesis Inc. or GenScript Corp., Piscataway, NJ, USA). The immunodominant regions were determined by using the online Invitrogen program (http://peptideselect.invitrogen.com/peptide/). The synthetic peptides were coated (10 µg/well) overnight in 96-well ELISA plate in 0.1 M carbonate buffer (pH 9.6). Non-specific binding sites were blocked using PBS-T containing 0.1% BSA. The wells were washed (3×) and incubated (37°C, 2 h) with the purified ScFv antibodies (5–20 µg/ml). The wells were washed (3×) again and incubated (37°C, 90 min) with HRP-conjugated anti-E-Tag antibody diluted 1:2000 in PBS-T containing 0.1% BSA. The wells were then incubated (37°C, 15 min) with substrate solution (0.2 mg/ml ABTS diluted in 0.05 M citric acid buffer, pH 4.0, containing 30% H2O2). The reaction was stopped using 10% SDS and read at 405 nm.
The absorbance reading of the control wells was subtracted from the absorbance reading of the peptide-coated wells, and the mean of the subtracted values was recorded. The absorbance readings were converted to standard deviation (SD) units by the formula: SD units = mean (test) − mean (control)/SD of control group. The test samples with >2 SD units were considered as positive.
Results
Preparation of scFv antibody library
The cDNA prepared from RNA of activated PBL was used as a template for PCR amplification. A collection of 66 human Vк chain sequences, 20 Vλ chain sequences, and 144 VH chain sequences from the Kabat database (Kabat et al., 1991) were used to design the consensus PCR primers. The human Vк are classified into four, Vλ into five and the VH into three subgroups. For amplification of VL chains, 11 different amplification reactions were performed using 11 forward primers and an equal mixture of two reverse primers. Similarly, five different PCRs were performed for amplification of VK chains using five different forward primers and an equal mixture of two reverse primers. The VH sequences were similarly amplified in eight different PCR reactions using eight forward primers and an equal mixture of two reverse primers. Each primer set produced a distinct band of predicted size of ∼330–350 bp. The Vк and Vλ sequence products obtained using all the primer pairs were pooled to form a VL pool. Similarly, products of all eight reactions performed to amplify VH were pooled to form a VH pool. Fifty nanogram DNA of each VL and VH pools were mixed to perform SOE-PCR. Ten nanogram of assembled scFv product were purified and used for construction of the library. The SfiI- and NotI-digested scFv repertoire was cloned into predigested SfiI- and NotI-digested and dephosphorylated vector, pCANTAB5E and transformed into E. coli TG1 cells to create the human scFv antibody library. The created library consisted of 3.8 × 105 primary transformants. PCR analysis of the individual clones (n = 10) randomly screened from the library showed 80% to be the expected recombinants, having a specific PCR product of 850 bp.
Panning of the library using sperm proteins
Rescue of phagemids encoding scFv fusion protein using helper phage (M13KO7) yielded phage particles displaying scFv. Selection of phages displaying scFv was carried out by performing three rounds of selection process comprising incubation of phages with sperm antigen (FA-1/YLP12/HSE)-coated wells, washing unbound non-specific phages and then elution of the bound specific phages. For the first round of panning, 1 × 1010 phages were added to each FA-1/YLP12/HSE-coated well. The titers of the eluted phages were 3.6 × 106 colony forming units (CFU)/ml for FA-1-coated wells, 2.4 × 106 CFU/ml for YLP12-coated wells and 1 × 107 CFU/ml for HSE-coated wells after the first round of panning. For the second round, the input phages from the first round of panning were used for incubation with the respective antigen-coated wells. The eluted phages had titers of 3 × 103, 3.6 × 106 and 9 × 106 CFU/ml for the FA-1, YLP12 and HSE, respectively. After the third round, using the eluate from the second round of panning, the titers were 1.3 × 102, 1.5 × 103 and 6 × 103 CFU/ml for FA-1, YLP12 and HSE, respectively. For each panning procedure, the specific phages were eluted using three strategies, namely low pH, high pH and antigen excess. The eluates after the third panning obtained from all the three elution procedures were pooled for each antigen, and the mixture was infected into E. coli TG1 cells to isolate individual colonies. Individual clones were grown and re-examined for their immunoreactivity with the respective sperm antigen. After these procedures, 9 clones were found to react with FA-1 antigen, 7 clones with YLP12 and 14 with HSE.
Five clones reacting the strongest with each antigen were selected, and their phagemids isolated and analyzed for nucleotide sequence. Analysis of these clones in immunogenetics database revealed them to be Ig sequence of human origin belonging to IgG class with к chain as their light chain.
Sequence analysis of selective recombinant scFv antibodies
Four of these clones, two reactive with FA-1 (AFA-1 and FAB-7), and one with YLP12 (YLP20) clones were selected because they reacted strongest with the FA-1 antigen and YLP12 peptide, respectively. The fourth clone, AS16, reactive with HSE was selected because it showed homology with a mouse antisperm monoclonal antibody (RASA) developed against a defined sperm antigen discussed below. AFA-1, FAB-7 and AS16 had IgG1, and the YLP12 scFv clone had IgG3 subclass heavy chain sequence. AFA-1, FAB-7 and YLP20 had light chain belonging to Igк3 subclass, and the AS16 clone had light chain belonging to Igк2 subclass. These scFv clones were further analyzed to determine homology with the consensus CDRs of human antibody sequences in the database. The scFv clone AFA-1 had 82% homology in CDR-1 region and 0% homology in CDR-2 and CDR-3 regions in VH region of human antibody sequence (accession no. M99637). It had 43%, 67% and 20% homology in CDR-1, CDR-2 and CDR-3 regions, respectively, in VL region of human antibody sequence (accession no. L19272) (Fig. 1). The scFv clone FAB-7 had 63%, 88% and 20% homology in CDR-1, CDR-2 and CDR-3 regions, respectively, in VH region of human antibody sequence (accession no. M99686). It had 100% homology in CDR-1 and CDR-2 regions and 90% homology in CDR-3 region in VL region of human antibody sequence (accession no. X12686) (Fig. 2). The scFv clone YLP20 had 75%, 13% and 23% homology in CDR-1, CDR-2 and CDR-3 regions, respectively, in VH region of human antibody sequence (accession no. M99679). It had 58%, 33% and 67% homology in CDR-1, CDR-2 and CDR-3 regions, respectively, in VL region of human antibody sequence (accession no. X12686) (Fig. 3). The scFv clone AS16 had 75%, 75% and 0% homology in CDR-1, CDR-2 and CDR-3 regions, respectively, in VH region of human antibody sequence (accession no. M62106). It had 82%, 100% and 45% homology in CDR-1, CDR-2 and CDR-3 regions, respectively, in VL region of human antibody sequence (accession no. X62106) (Fig. 4). The amino acid homology of clone AS16 was also compared with mouse antisperm scFv antibody RASA. The scFv clone AS16 had 88%, 75% and 0% homology in CDR-1, CDR-2 and CDR-3 regions, respectively, in VH region of this mouse antibody sequence (accession no. AF276797). It had 73%, 67% and 67% homology in CDR-1, CDR-2 and CDR-3 regions, respectively, in VL region of this mouse antibody sequence (accession no. AF276797).
Figure 1:
The cDNA sequence and the corresponding amino acid (aa) sequence of single chain variable fragment (scFv) clone (AFA-1) reactive with FA-1 antigen
The aa sequence contains heavy chain (1–109 aa), linker (Gly4Ser)3 (110–124 aa) shown in shaded grey box, light chain (125–223 aa) and E-Tag (224–236 aa) sequence (shown after ↑ in italics). The aa sequence of immunoglobulin (Ig)G1 heavy chain includes framework region 1 (1–25 aa), framework region 2 (34–50 aa), framework region 3 (59–96 aa), and their corresponding CDR-1 (26–33 aa), CDR-2 (51–58 aa) and CDR-3 (97–109 aa) regions. Similarly, the aa sequence of Igк3 light chain includes framework region 1 (125–150 aa), framework region 2 (158–174 aa), framework region 3 (178–213 aa), and their corresponding CDR-1 (151–157 aa), CDR-2 (175–177 aa) and CDR-3 (214–223 aa) regions. All CDRs regions are enclosed in rectangular boxes.
Figure 2:
The cDNA sequence and the corresponding amino acid (aa) sequence of scFv clone (FAB-7) reactive with FA1 antigen
The aa sequence contains heavy chain (1–110 aa), linker (Gly4Ser)3 (111–125 aa) shown in shaded grey box, light chain (126–223 aa) and E-Tag (224–236 aa) sequence (shown after ↑ in italics). The aa sequence of IgG1 heavy chain includes framework region 1 (1–25 aa), framework region 2 (34–50 aa) and framework region 3 (58–95 aa), and their corresponding CDR-1 (26–33 aa), CDR-2 (51–57 aa) and CDR-3 (96–110 aa) regions. Similarly, the aa sequence of Igк3 light chain includes framework region 1 (126–151 aa), framework region 2 (159–175 aa) and framework region 3 (179–214 aa), and their corresponding CDR-1 (152–158 aa), CDR-2 (176–178 aa) and CDR-3 (215–223 aa) regions. All CDRs regions are enclosed in rectangular boxes.
Figure 3:
The cDNA sequence and the corresponding amino acid (aa) sequence of scFv clone (YLP20) reactive with YLP12 antigen
The aa sequence contains heavy chain (1–108 aa), linker (Gly4Ser)3 (109–123 aa) shown in shaded grey box, light chain (124–224 aa) and E-Tag (225–237 aa) sequence (shown after ↑ in italics). The aa sequence of IgG3 heavy chain includes framework region 1 (1–25 aa), framework region 2 (34–49 aa) and framework region 3 (58–95 aa), and their corresponding CDR-1 (26–33 aa), CDR-2 (50–57 aa) and CDR-3 (96–108 aa) regions. Similarly, the aa sequence of Igк3 light chain includes framework region 1 (123–149 aa), framework region 2 (157–173 aa) and framework region 3 (177–212 aa), and their corresponding CDR-1 (150–156 aa), CDR-2 (174–176 aa) and CDR-3 (213–224 aa) regions. All CDRs regions are enclosed in rectangular boxes.
Figure 4:
The cDNA sequence and the corresponding amino acid (aa) sequence of scFv clone (AS16) reactive with HSE
The aa sequence contains heavy chain (1–114 aa), linker (Gly4Ser)3 (115–129 aa) shown in shaded grey box, light chain (130–229 aa) and E-Tag (230–242 aa) sequence (shown after ↑ in italics). The aa sequence of IgG1 heavy chain includes framework region 1 (1–25 aa), framework region 2 (34–50 aa) and framework region 3 (59–96 aa), and their corresponding CDR-1 (26–33 aa), CDR-2 (51–58 aa) and CDR-3 (97–114 aa) regions. Similarly, the aa sequence of Igк2 light chain includes framework region 1 (130–153 aa), framework region 2 (165–181 aa) and framework region 3 (185–220 aa), and their corresponding CDR-1 (154–164 aa), CDR-2 (182–184 aa) and CDR-3 (221–229 aa) regions. All CDRs regions are enclosed in rectangular boxes.
Immunobiological characterization of scFv antibodies
E. coli containing recombinant phagemid of these four clones were grown, and the antibodies were expressed and purified using anti-E-Tag column. The purified scFv antibodies showed the expected single protein band of ∼28 kDa in SDS–PAGE after staining with silver nitrate (Fig. 5, lane a). The antibodies tend to aggregate into dimeric and sometimes into trimeric forms during freezing. Anti-E-Tag antibody specifically recognized all these forms in the western blot procedure (Fig. 5, lane b). The purified antibodies showing only the specific bands were used for further studies.
Figure 5:
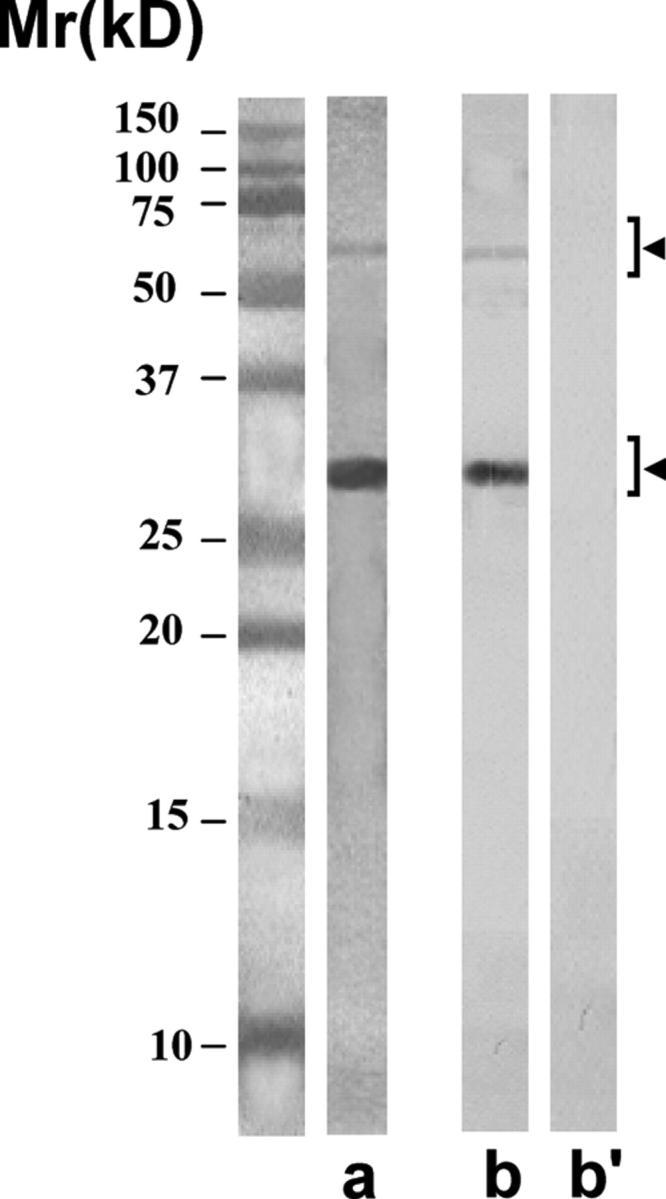
Purity of the scFv antibodies isolated by using anti-E-Tag antibody column
The purified scFv antibodies showed predominantly the expected single protein band of ∼28 kDa in SDS–PAGE after staining with silver nitrate (lane a). On freezing, the antibodies had a tendency to polymerize into dimeric form of ∼56 kDa, and sometimes into trimeric form of ∼84 kDa (not shown). All these forms were specifically recognized by the anti-E-Tag monoclonal antibody (lane b) and not by the myeloma control monoclonal antibody (lane b’) in the western blot procedure.
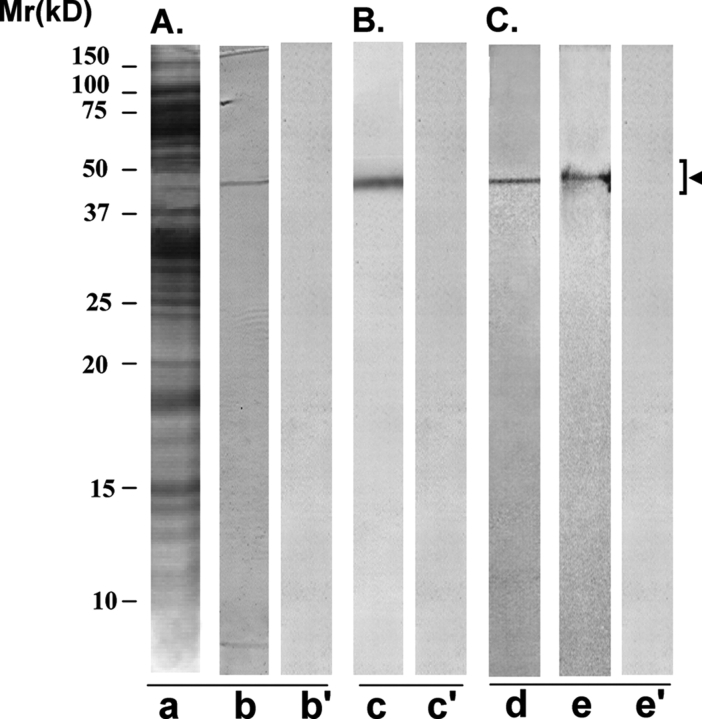
AFA-1 scFv antibody specifically recognized a protein band of 50 ± 4 kDa corresponding to FA-1 antigen in western blot procedure involving LIS-solubilized HSE (Fig. 6A, lane b). This band was not recognized by the control scFv antibody (CAB-3) (lane b’). AFA-1 antibody conjugated to Sepharose 4B immunobeads also specifically immunoprecipitated a protein band of 50 ± 4 kDa, corresponding to FA-1 antigen, from HSE (lane c), that was not immunoprecipitated by the control antibody immunobeads (lane c’). The cognate FA-1 antigen that was purified from LIS-solubilized HSE by immunoaffinity column involving a mouse monoclonal antibody (MA-24) conjugated to Sepharose 4B immunobeads, and showed a single band of 50 ± 4 kDa in SDS–PAGE (lane d). This band was specifically recognized by AFA-1 scFv antibody (lane e) and not by the control antibody (e’) in the western blot procedure.
Figure 6:
Immunoreactivity pattern of AFA-1 scFv antibody with HSE and FA-1 antigen
The purified scFv antibody was examined for its immunoreactivity with LIS-solubilized HSE (A and B) and purified cognate human sperm FA-1 antigen (C), using western blot (A and C) and immunoprecipitation (B) procedures. (A) SDS–PAGE of HSE revealed several protein bands of various molecular identities after silver staining (lane a). AFA-1 scFv antibody specifically recognized a protein band of 50 ± 4 kDa, corresponding to FA-1 antigen on western blot of HSE (lane b). Control scFv antibody did not react with any specific band on the western blot (lane b’). (B) AFA-1 scFv antibody Sepharose 4B immunobeads reacted with a specific protein in HSE that on elution with glycine–HCl (0.1 M, pH 2.8) showed a single band of 50 ± 4 kDa, corresponding to FA-1 antigen, in SDS–PAGE (lane c). Control scFv antibody Sepharose 4B immunobeads did not react with any protein in HSE (lane c’). (C) The cognate FA-1 antigen purified from HSE using immunoaffinity column involving mouse monoclonal antibody MA-24 showed a single band of 50 ± 4 kDa in SDS–PAGE (lane d) that was specifically recognized by AFA-1 scFv antibody (lane e) and not by the control scFv antibody (lane e’) in the western blot procedure.
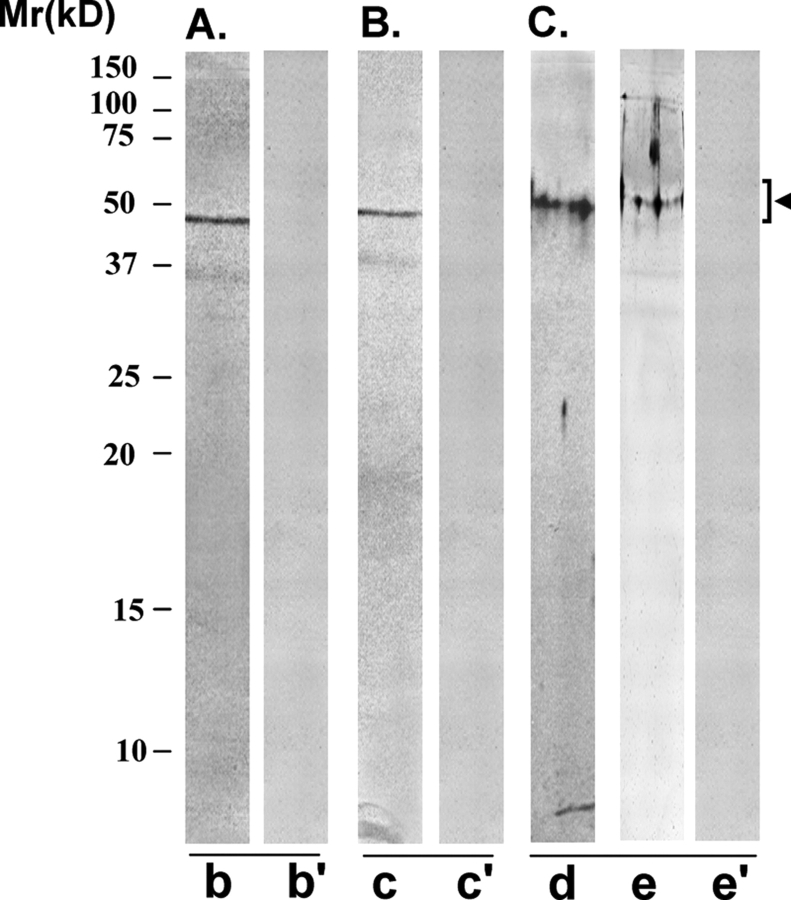
FAB-7 scFv antibody, which was also identified using the FA-1 antigen in the panning procedure, showed a similar immunoreactive pattern with HSE and FA-1 antigen (Fig. 7) as observed with the AFA-1 antibody, described above (Fig. 6). FAB-7 scFv antibody specifically recognized a protein band of 50 ± 4 kDa, corresponding to FA-1 antigen, in the western blot of HSE (Fig. 6A, lane b). Control antibody (CAB-3) did not react with any specific band in the western blot procedure (lane b’). FAB-7 scFv antibody-conjugated to Sepharose 4B immunobeads immunoprecipitated a specific protein of 50 ± 4 kDa, corresponding to FA-1 antigen, from HSE (lane c). Control antibody conjugated immunobeads did not immunoprecipitate any specific protein from HSE (lane c’). The purified cognate FA-1 antigen was specifically recognized by FAB-7 antibody (lane e) and not by the control antibody (lane e’) in the western blot procedure.
Figure 7:
Immunoreactivity pattern of FAB-7 ScFv antibody with HSE and FA-1 antigen
The purified scFv was examined for its reactivity with LIS-HSE (A and B) and purified cognate human sperm FA-1 antigen (C), using western blot (A and C) and immunoprecipitation (B) procedures. (A) FAB-7 scFv antibody specifically recognized a protein band of 50 ± 4 kDa, corresponding to FA-1 antigen, on western blot of HSE (lane b). Control scFv antibody did not react with any specific band on the western blot (lane b’). (B) FAB-7 scFv antibody Sepharose 4B immunobeads reacted with a specific protein in HSE that on elution with glycine–HCl (0.1 M, pH 2.8) showed a single band of 50 ± 4 kDa, corresponding to FA-1 antigen, in SDS–PAGE (lane c). Control scFv antibody Sepharose 4B immunobeads did not react with any protein in HSE (lane c’). (C) The cognate FA-1 antigen purified from HSE using immunoaffinity column involving mouse monoclonal antibody MA-24 showed a single band of 50 ± 4 kDa in SDS–PAGE (lane d), that was specifically recognized by FAB-7 ScFv antibody (lane e) and not by the control scFv antibody (lane e’) in the western blot procedure.
YLP20 antibody specifically reacted with a protein band of 48 ± 5 kDa in the western blot of HSE (Fig. 8A, lane b), which was not recognized by the control antibody (lane b’). YLP20 antibody-conjugated to Sepharose 4B immunobeads (lane c) and not the control antibody immunobeads (lane c’), immunoprecipitated a protein of 48 ± 5 kDa from HSE.
Figure 8:
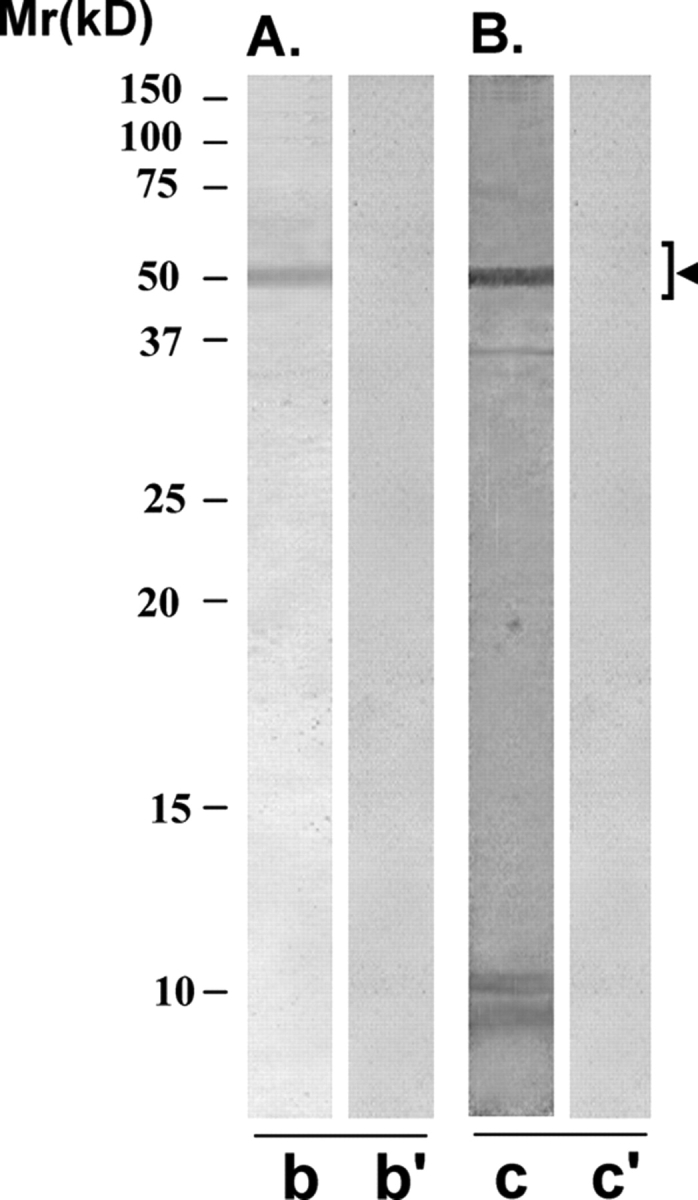
Immunoreactivity pattern of YLP20 scFv antibody with HSE
The purified scFv was examined for its reactivity with LIS-solubilized HSE using western blot (A) and immunoprecipitation (B) procedures. (A) YLP20 scFv antibody specifically recognized a protein band of 48 ± 5 kDa, corresponding to YLP12 antigen, on western blot of HSE (lane b). Control scFv antibody did not react with any specific band on the western blot (lane b’). (B) YLP20 scFv antibody Sepharose 4B immunobeads reacted with a specific protein in HSE that on elution with glycine–HCl (0.1 M, pH 2.8) showed a single band of 48 ± 5 kDa in SDS–PAGE (lane c). Control scFv antibody Sepharose 4B immunobeads did not react with any protein in HSE (lane c’).
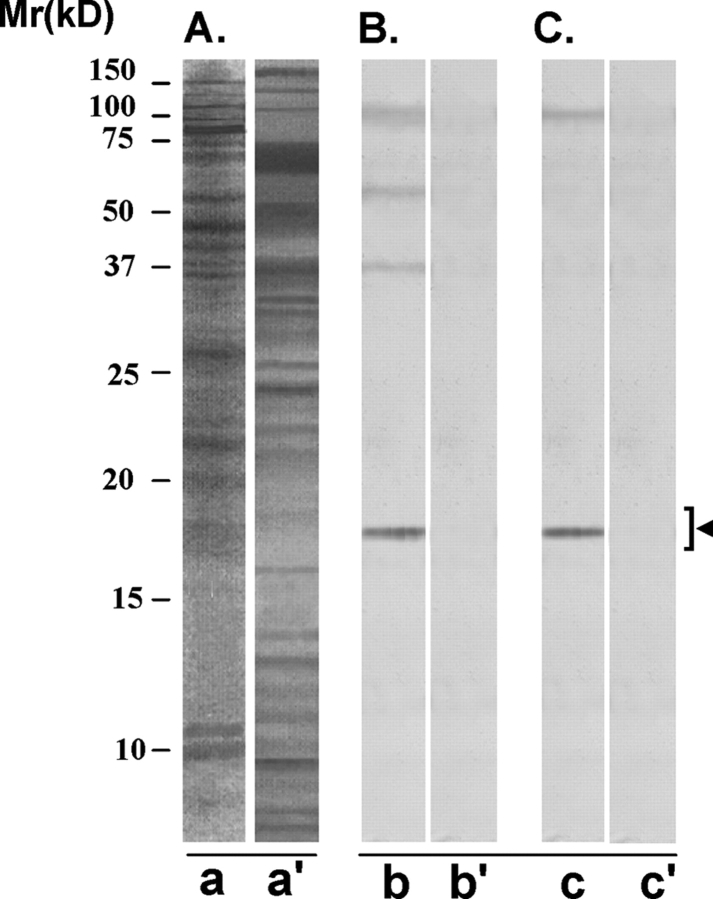
AS16 antibody recognized four protein bands, one major (18 kDa) and three minor (37, 55 and 110 kDa) on the western blot of LIS-solubilized HSE (Fig. 9B, lane b’). Using the western blot of MC-solubilized human sperm preparation, AS16 antibody reacted with a major (18 kDa) and a minor (100 kDa) protein band (lane c). These protein bands were not recognized by the control antibody (lanes b' and c’).
Figure 9:
Immunoreactivity pattern of AS-16 scFv antibody with HSE
The purified scFv antibody was examined for its reactivity with LIS-solubilized HSE (B) and MC-solubilized human sperm preparation (C), in the western blot procedure. (A) SDS–PAGE of LIS-solubilized human sperm preparation (lane a) and MC-solubilized human sperm preparation (lane a’) revealed several protein bands of various molecular identities after silver staining. (B) AS16 scFv antibody specifically recognized four protein bands of 18 (major band), 37, 55 and 100 kDa, respectively, on the western blot of LIS-solubilized human sperm preparation (lane b). Control scFv antibody did not react with any band on the western blot (lane b’). (C) AS-16 ScFv antibody recognized two protein bands of 18 (major band) and 100 kDa (minor band), in the western blot of MC-solubilized human sperm preparation (lane c). Control scFv antibody did not react with any specific band on the western blot (lane c’). The 18 kDa protein was the major band specifically recognized in both the LIS-solubilized human sperm preparation (lane a) and MC-solubilized human sperm preparation (lane a’) and it corresponds to SAGA-1 sperm protein.
In the indirect IFT, all the three antibodies that were examined, namely AFA-1, FAB-7 and YLP20, reacted with both the methanol-fixed and unfixed live human sperm (Fig. 10). AFA-1 (a and a’) and FAB-7 (b and b’) antibodies predominantly reacted with post-acrosomal, midpiece and tail regions of methanol-fixed (a and b) and unfixed live human sperm (a' and b’). YLP20 antibody reacted with acrosomal, midpiece and tail regions of methanol-fixed (c) and unfixed live (c’) human sperm.
Figure 10:
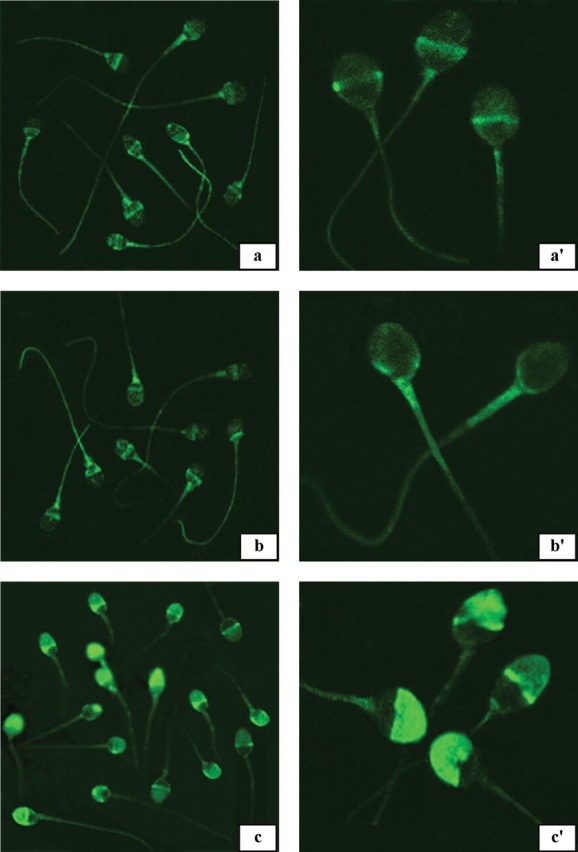
Epifluorescent photomicrographs indicating the indirect immunofluorescent reaction pattern of AFA-1, FAB-7 and YLP20 scFv antibodies with methanol-fixed (a, b and c) and unfixed live human sperm (a', b', and c’)
AFA-1 (a and a’) and FAB-7 (b and b’) antibodies predominantly reacted with post-acrosomal, midpiece and tail regions of methanol-fixed (a and b) and unfixed live human sperm (a' and b’). YLP20 antibody reacted with acrosomal, midpiece and tail regions of methanol-fixed (c) and unfixed live (c’) human sperm. Magnifications: a, b and c ×698; a', b' and c' ×1924.
Effect of antibodies on human sperm capacitation/acrosome reaction
To examine the effect of antibodies on human sperm function, the antibodies (AFA-1/FAB-7/YLP20) were incubated with sperm during capacitation, and the percentage of sperm undergoing acrosome reaction was determined (Table I). Following treatment with ionophore, an average of 74% sperm underwent acrosome-reaction when they were capacitated in the presence of 85 µg/ml of control antibody (CAB-3). Capacitation of sperm with the AFA-1/FAB-7/YLP20 antibody caused a concentration-dependent inhibition in percentage of acrosome-reacted sperm compared with control antibody (Table I). The highest inhibition in acrosome reaction was induced by AFA-1 antibody, especially at 80 µg/ml concentration. There was no effect of any of these antibodies on sperm motility or viability, and they did not cause agglutination of sperm. AS16 caused agglutination of sperm, thus was not tested for its effects on sperm capacitation/acrosome reaction.
Table I.
Effect of various single chain variable fragment (ScFv) antibodies on human sperm acrosome reaction.
| Antibody | Concentration (μg/ml) | Acrosome-reacted sperm (%) |
|---|---|---|
| 1. AFA-1 | 45 | 50 ± 6a |
| 80 | 36 ± 7b | |
| 2. FAB-7 | 45 | 58 ± 8c |
| 80 | 44 ± 6b | |
| 3. YLP20 | 45 | 52 ± 7c |
| 80 | 42 ± 3b,d | |
| 4. Bovine Serum Albumin/ Control antibody | 85 | 74 ± 5e |
a,b,c,d,eValues with different superscripts are significantly different, P < 0.01 to P < 0.001.
Epitope analysis of FA-1 reactive antibodies
Both AFA-1 and FAB-7 antibodies demonstrated similar reaction patterns with HSE, FA-1 antigen and human sperm, but revealed different nucleotide and amino acid sequences in the CDRs. It was hypothesized that these antibodies may be directed against different epitopes of FA-1 antigen. To investigate, several peptides based on the immunodominant regions of mouse and human FA-1 sequences were synthesized and examined for their immunoreactivity with AFA-1 and FAB-7 antibodies. AFA-1 antibody showed a positive reaction (>2 SD units) only with peptides based on human FA-1200-219aa and mouse FA-1117-136aa sequences. This epitope was designated as FA-1a (Table II). FAB-7 antibody showed a positive reaction only with peptides based on human FA-182-97aa and mouse FA-12-19aa sequences. This epitope was designated as FA-1b. These antibodies did not react with other human and mouse peptide sequences (data not shown). YLP20 antibody did not react with these peptides, and AFA-1 and FAB-7 antibodies did not react with YLP12 peptide (Table II).
Table II.
Immunoreactivity of ScFv antibodies with various synthetic peptides.
| Antibody | FA-1a | FA-1b | YLP12 | FA-1 antigen | Control |
|---|---|---|---|---|---|
| AFA-1 | 23.6–37.1a | 1.4 | 0.1 | 91.0a | 0.1 |
| FAB-7 | 1.8 | 48.7–60.2a | 1.1 | 89.5a | 0.3 |
| YLP20 | 0.1 | 0.2 | 27.2a | 1.3 | 0.5 |
| Control antibody | 0.1 | 0.7 | 0.3 | 0.9 | 0.1 |
aImmunoreactivity is expressed as SD units. Values with superscript a are positive (>2 SD units), and the others are negative (<2 SD units). FA-1a epitope refers to peptides based on human FA-1200–219 aa and mouse FA-1117–136 aa sequences, and FA-1b epitope refers to peptides based on human FA-182–97 aa and mouse FA-12–19 aa sequences. Control peptide is based on human FA-1220–240 aa sequence.
Discussion
Using phage display technology involving cDNA cloned from lymphocytes of immunoinfertile and vasectomized men, we have isolated, characterized and produced fully functional human scFv antibody fragments against two well-characterized sperm specific antigens, namely FA-1 and YLP12. The role of FA-1 antigen and YLP12 sequence in human sperm function and human sperm–oocyte ZP binding is well documented (Naz and Zhu, 1997, 1998, 2002; Naz et al., 2000; Naz and Chauhan, 2002). The immunoinfertile men and women, and vasectomized men have circulating and testis antibodies to these antigens (Naz et al., 1993; Naz and Chauhan, 2001). Vaccination with these antigens causes a contraceptive effect and the combination vaccination results in an enhanced reduction in fertility (Naz and Zhu, 1998; Naz and Chauhan, 2002).
For construction of the antibody library, immunoinfertile/vasectomized men that had high titers of ASA, especially against FA-1 antigen and YLP12 peptide, were selected. PBL were activated with sperm antigens (FA-1/YLP12/HSE) to enrich them with specific RNAs. PCR primers were constructed to amplify at least 90% of the human antibody sequences available in the Kabat database (Kabat et al., 1991). The phages displaying recombinant scFv antibodies were made from the primary library and used for panning against the sperm antigens (FA-1/YLP12/HSE). The positive clones, after three rounds of panning, were selected, sequenced and analyzed using the immunogenetics database.
Of the 30 clones reacting positively with these antigens, four strongest reacting were selected for further analysis. Of these four, two were reactive with FA-1 antigen, one with YLP12 and one with HSE. The nucleotide and amino acid sequences of these clones did not show a complete homology with any of the existing sequences in the human database, indicating them to be novel. The antigen binding site is comprised of six CDRs, three each contributed by heavy and light chain, and confer specificity for binding to a specific epitope. All these antibody clones had a similarity in the framework region with other human antibody sequences in the database, but their CDR regions were novel, indicating that these were directed against different antigenic determinants.
Three antibodies (AFA-1/FAB-7/AS16) had heavy chain belonging to IgG3 and one (YLP20) had heavy chain belonging to IgG1 subclass. On the basis of the nucleic acid homology, the human Ig heavy chains consist of 50 functional gene segments belonging to seven gene families (VH1–VH7). The majority (86%) of the contribution is provided by three families (VH1, VH3 and VH4) (Cook and Tomlinson, 1995) and our four clones fall in these families. Antibodies reactive with the FA-1/YLP12/HSE in the sera of immunoinfertile and vasectomized men primarily belong to the IgG class, and we have examined these antibodies for subclass specificity. The light chain of all the four clones belonged to κ-chain family. The relative roles of heavy and light chains, and their classes and subclasses in antigen recognition and binding, as pertaining to sperm immunity, have not been determined.
These four clones were expressed to produce and purify larger quantity of antibodies for various immunobiological assays. Antibodies had an E-Tag sequence at the end that made it easy to purify them using an anti-E-Tag monoclonal antibody column. The purified antibodies showed the expected single band of 28 kDa in the silver-stained gel that was specifically recognized by the anti-E-Tag antibody. The antibodies had a tendency to polymerize (dimers and occasionally trimers) during freezing. This has been reported to occur with several other scFv antibodies (Arndt et al., 1998; Deng et al., 2003). The yield of antibodies using our vector system was low. Various methods such as high-level expression vectors, increase of linker length and reversing the domain orientation from VH–VL to VL–VH have been used to increase the expression level for several scFv antibodies (Nieba et al., 1997; Hamilton et al., 2001).
The authenticity of clones (AFA-1 and FAB-7) obtained by panning against cognate FA-1 antigen was confirmed by: (i) immunoreactivity of AFA-1/FAB-7 antibodies with the specific expected band of ∼50 ± 4 kDa, corresponding to FA-1 antigen, in the western blot and immunoprecipitation procedures; (ii) binding of these antibodies with the post-acrosomal, midpiece and tail regions of unfixed live and methanol-fixed human sperm, the subcellular sites of human sperm previously shown to have FA-1 antigen expression; and (iii) binding of these antibodies with the purified cognate human sperm FA-1 antigen. Both these antibodies, AFA-1 and FAB-7, showed a similar immunoreactivity pattern in all these assays.
The authenticity of YLP20 clone obtained by panning against synthetic dodecamer YLP12 peptide was confirmed by: (i) immunoreactivity of YLP20 antibody with a specific protein band of ∼48 ± 5 kDa, corresponding to YLP12 antigen, in the western blot and immunoprecipitation procedures; and (ii) binding of the antibodies to the acrosomal, midpiece and tail regions of live and methanol-fixed human sperm, the subcellular sites of human sperm previously shown to have YLP12 expression. YLP12 dodecamer amino acid sequence is a part of a 72 ± 5 kDa protein that is synthesized during spermatogenesis in the testis and later gets modified/cleaved to form a 48 ± 5 kDa protein in the mature ejaculated sperm cell (Naz et al., 2000; Naz and Chauhan, 2001, 2002).
There were several clones reactive with HSE. The clone AS16 was selected for further analysis. This clone demonstrated 67–88% homology in CDRs of both heavy and light chains with a RASA. RASA is a mouse scFv monoclonal antibody directed against a tissue-specific epitope of human SAGA-1 that is a sperm glycoform of CD52 antigen (Diekman et al., 1997; Norton et al., 2001). Antibodies against the SAGA-1 cause agglutination of human sperm. As with FA-1 and YLP12, SAGA-1 has been proposed as a candidate for immunocontraception. Besides having homology in CDRs with RASA, AS16 scFv antibodies reacted with the specific protein of ∼18 kDa in HSE, corresponding to SAGA-1 and caused agglutination of human sperm. AS16 seems to be a human homolog of the mouse monoclonal scFv, RASA.
All the four antibodies (AFA-1/FAB-7/YLP20/AS16) inhibited human sperm function. AS16 caused an agglutination of sperm and the other three antibodies (AFA-1/FAB-7/YLP20) inhibited human sperm capacitation/acrosome reaction. The polymerized antibodies demonstrated more agglutination than the freshly isolated antibodies. In general, scFv fragments should not agglutinate sperm, as they are single chain Igs. It may be a tendency of the antibodies to aggregate into dimeric and trimeric forms that cause sperm agglutinating activity. Polyclonal antibodies raised in rabbits/mice against FA-1 antigen and YLP12 also inhibit human sperm capacitation/acrosome reaction. These three antibodies did not agglutinate or immobilize sperm when they were in monomeric form. Both AFA-1 and FAB-7 antibodies had different sequences in their CDRs. The data indicate that these two antibodies are directed against two different epitopes of FA-1 antigen. AFA-1 antibody is directed against an epitope, designated as FA-1a, which is present on human FA-1200-219aa and mouse FA-1117-136aa sequences. FAB-7 antibody is directed against an epitope, designated as FA-1b, which is present on human FA-182-97aa and mouse FA-12-19aa sequences. In these two epitope regions, the human and mouse FA-1 sequences have 60–61% homology. These data also indicate that these antibodies are directed against the polypeptide sequence and not against the carbohydrate moiety of the FA-1 glycoprotein. The findings may also indirectly suggest that these epitopes may be more immunogenic and produce antibodies in human immunoinfertility and after vasectomy. Indeed, in a recent study, we found that 24.6–41.8% of the sera from immunoinfertile women have antibodies reactive with synthetic peptides based on sequences of these two epitopes (Williams et al. in press).
In conclusion, using phage display technology involving cDNA isolated from lymphocytes of immunoinfertile and vasectomized men, we have isolated four novel fully functional scFv human antibodies reactive with well-characterized human sperm antigens (FA-1/YLP12/SAGA-1). These antibodies inhibit human sperm function, thus may find applications in immunocontraceptive development and specific diagnosis of immunoinfertility. There are a few reports on the development of human hybridoma secreting ASA (Isojima et al., 1987) and antibody Fab fragments by combinatorial phage display reactive with human spermatozoa (Clayton et al., 1998). This is the first report on the development of human scFv ASA of defined specificity related to immunoinfertility/vasectomy. Their in vivo efficacy as passively administered immunocontraceptives is presently being explored. Various methods such as PEGylation (Chapman, 2002), fusion with serum albumin (Smith et al., 2001) and multimerization (Hudson and Kortt, 1999) are being examined to increase the efficacy and half-life of these genetically engineered recombinant antibodies.
Funding
National Institutes of Health (HD24425).
Acknowledgements
We sincerely thank Professor Dana Ohl, MD, for providing blood from several vasectomized and fertile men from his Male Fertility Clinic at the University of Michigan, Ann Arbor, MI, USA. We also thank members of Andrology Laboratory at the University of Michigan and Don Zeh at Andrology Laboratory at West Virginia University for providing semen specimens and technical assistance in antisperm antibody analysis and sperm functional tests. The technical assistance of Dr C. Rajesh is gratefully acknowledged. We also thank Renée Zell and Hilary Steele for providing typing assistance.
References
- Arndt KM, Muller KM, Pluckthun A. Factors influencing the dimer to monomer transition of an antibody single-chain Fv fragment. Biochemistry. 1998;37:12918–12926. doi: 10.1021/bi9810407. [DOI] [PubMed] [Google Scholar]
- Bohring C, Krause W. Immune infertility: towards a better understanding of sperm (auto)-immunity. The value of proteomic analysis. Hum Reprod. 2003;18:915–924. doi: 10.1093/humrep/deg207. [DOI] [PubMed] [Google Scholar]
- Byrd W, Tsu J, Wolf D. Kinetics of spontaneous and induced acrosomal loss in human sperm incubated under capacitating and noncapacitating conditions. Gamete Res. 1989;22:109–122. doi: 10.1002/mrd.1120220111. [DOI] [PubMed] [Google Scholar]
- Casadevall A. Passive antibody therapies: progress and continuing challenges. Clin Immunol. 1999;93:5–15. doi: 10.1006/clim.1999.4768. [DOI] [PubMed] [Google Scholar]
- Chapman AP. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv Drug Deliv Rev. 2002;54:531–545. doi: 10.1016/s0169-409x(02)00026-1. [DOI] [PubMed] [Google Scholar]
- Clayton R, Moore H. Experimental models to investigate the pathology of antisperm antibodies: approaches and problems. Hum Reprod. 2001;7:457–459. doi: 10.1093/humupd/7.5.457. [DOI] [PubMed] [Google Scholar]
- Clayton R, Cooke ID, Partridge LJ, Moore HD. A combinatorial phage display for the generation of specific Fab fragments recognizing human spermatozoa and inhibiting fertilizing capacity in vitro. Biol Reprod. 1998;59:1180–1186. doi: 10.1095/biolreprod59.5.1180. [DOI] [PubMed] [Google Scholar]
- Cook GP, Tomlinson IM. The human immunoglobulin VH repertoire. Immunol Today. 1995;16:237–242. doi: 10.1016/0167-5699(95)80166-9. [DOI] [PubMed] [Google Scholar]
- Deng XK, Nesbit LA, Morrow KJ. Recombinant single-chain variable fragment antibodies directed against Clostridium difficile toxin B produced by use of an optimized phage display system. Clin Diagn Lab Immunol. 2003;10:587–595. doi: 10.1128/CDLI.10.4.587-595.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekman AB, Westbrook-Case VA, Naaby-Hansen S, Klotz KL, Flickinger CJ, Herr JC. Biochemical characterization of sperm agglutination antigen-1, a human sperm specific antigen implicated in gamete interactions. Biol Reprod. 1997;57:1136–1144. doi: 10.1095/biolreprod57.5.1136. [DOI] [PubMed] [Google Scholar]
- Dunman PM, Nesin M. Passive immunization as prophylaxis: When and where will this work? Curr Opin Pharmacol. 2003;3:486–496. doi: 10.1016/j.coph.2003.05.005. [DOI] [PubMed] [Google Scholar]
- Hamilton S, Odili J, Gundogdu O, Wilson GD, Kupsch JM. Improved production by domain inversion of single-chain Fv antibody fragment against high molecular weight proteoglycan for the radioimmunotargeting of melanoma. Hybrid Hybridomics. 2001;20:351–360. doi: 10.1089/15368590152740752. [DOI] [PubMed] [Google Scholar]
- Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30:24–29. [PubMed] [Google Scholar]
- Hudson PJ, Kortt AA. High avidity scFv multimers; diabodies and triabodies. J Immunol Methods. 1999;231:177–189. doi: 10.1016/s0022-1759(99)00157-x. [DOI] [PubMed] [Google Scholar]
- Isojima S, Kameda K, Tsuji Y, Shigeta M, Ikeda Y, Koyama K. Establishment and characterization of a human hybridoma secreting monoclonal antibody with high titers of sperm immobilizing and agglutinating activities against human seminal plasma. J Reprod Immunol. 1987;10:67–78. doi: 10.1016/0165-0378(87)90051-9. [DOI] [PubMed] [Google Scholar]
- Kabat EA, Wu TT, Perry HM, Gottesmann KS, Foeller C. 5th edn. Bethesda, Maryland: National Center for Biotechnology Information, National Library of Medicine; 1991. Sequences of Proteins of Immunological Interest, [Google Scholar]
- Koren E, Zuckerman LA, Mire-Sluis AR. Immune responses to therapeutic proteins in humans–clinical significance, assessment and prediction. Curr Pharm Biotechnol. 2002;3:349–360. doi: 10.2174/1389201023378175. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mirick GR, Bradt BM, Denardo SJ, Denardo GL. A review of human anti-globulin antibody (HAGA, HAMA, HACA, HAHA) responses to monoclonal antibodies. Not four letter words. Q J Nucl Med Mol Imaging. 2004;48:251–257. [PubMed] [Google Scholar]
- Naz RK, Chauhan SC. Presence of antibodies to sperm YLP12 synthetic peptide in sera and seminal plasma of immunoinfertile men. Mol Hum Reprod. 2001;7:21–26. doi: 10.1093/molehr/7.1.21. [DOI] [PubMed] [Google Scholar]
- Naz RK, Chauhan SC. Human sperm-specific peptide vaccine that causes long-term reversible contraception. Biol Reprod. 2002;67:674–680. doi: 10.1095/biolreprod67.2.674. [DOI] [PubMed] [Google Scholar]
- Naz RK, Zhu X. Fertilization antigen-1: cDNA cloning, testis-specific expression, and immunocontraceptive effects. Proc Natl Acad Sci USA. 1997;94:4704–4709. doi: 10.1073/pnas.94.9.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz RK, Zhu X. Recombinant fertilization antigen-1 causes a contraceptive effect in actively immunized mice. Biol Reprod. 1998;59:1095–1100. doi: 10.1095/biolreprod59.5.1095. [DOI] [PubMed] [Google Scholar]
- Naz RK, Zhu X. Molecular cloning and sequencing of cDNA encoding for human FA-1 antigen. Mol Reprod Dev. 2002;63:256–268. doi: 10.1002/mrd.90010. [DOI] [PubMed] [Google Scholar]
- Naz RK, Ahmad K, Menge AC. Antiidiotypic antibodies to sperm in sera of fertile women that neutralize antisperm antibodies. J Clin Invest. 1993;92:2331–2338. doi: 10.1172/JCI116837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz RK, Gupta SK, Gupta JC, Vyas HK, Talwar GP. Recent advances in contraceptive vaccine development: a mini-review. Hum Reprod Update. 2005;20:3271–3283. doi: 10.1093/humrep/dei256. [DOI] [PubMed] [Google Scholar]
- Naz RK, Zhu X, Kadam AL. Identification of human sperm peptide sequence involved in egg binding for immunocontraception. Biol Reprod. 2000;62:318–324. doi: 10.1095/biolreprod62.2.318. [DOI] [PubMed] [Google Scholar]
- Nieba L, Honegger A, Krebber C, Plückthun A. Disrupting the hydrophobic patches at the antibody variable/constant domain interface: improved in vivo folding and physical characterization of an engineered scFv fragment. Protein Eng. 1997;10:435–444. doi: 10.1093/protein/10.4.435. [DOI] [PubMed] [Google Scholar]
- Norton EJ, Diekman AB, Westbrook VA, Flickinger CJ, Herr JC. RASA, a recombinant single-chain variable fragment (scFv) antibody directed against the human sperm surface: implications for novel contraceptives. Hum Reprod. 2001;16:1854–1860. doi: 10.1093/humrep/16.9.1854. [DOI] [PubMed] [Google Scholar]
- Ohl DA, Naz RK. Infertility due to antisperm antibodies. Urology. 1995;46:591–602. doi: 10.1016/S0090-4295(99)80282-9. [DOI] [PubMed] [Google Scholar]
- Park KJ, Lee SH, Kim TI, Lee HW, Lee CH, Kim EH, Jang JY, Choi KS, Kwon MH, Kim YS. A human ScFv antibody against TRAIL receptor 2 induces autophagic cell death in both TRAIL-sensitive and TRAIL-resistant cancer cells. Cancer Res. 2007;67:7327–7334. doi: 10.1158/0008-5472.CAN-06-4766. [DOI] [PubMed] [Google Scholar]
- Pillai S, Wright D, Gupta A, Zhou G, Hull G, Jiang H, Zhang H. Molecular weights and isoelectric points of sperm antigens relevant to autoimmune infertility in men. J Urol. 1996;155:1928–1933. [PubMed] [Google Scholar]
- Rader C, Barbas CF., III Phage display of combinatorial antibody libraries. Curr Opin Biotechnol. 1997;8:503–508. doi: 10.1016/s0958-1669(97)80075-4. [DOI] [PubMed] [Google Scholar]
- Riethmüller G, Schneider-Gädicke E, Johnson JP. Monoclonal antibodies in cancer therapy. Curr Opin Immunol. 1993;5:732–739. doi: 10.1016/0952-7915(93)90129-g. [DOI] [PubMed] [Google Scholar]
- Sidhu SS, Fellouse FA. Synthetic therapeutic antibodies. Nat Chem Biol. 2006;2:682–688. doi: 10.1038/nchembio843. [DOI] [PubMed] [Google Scholar]
- Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;288:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Popplewell A, Athwal D, Chapman AP, Heywood S, West SM, Carrington B, Nesbitt A, Lawson AD, Antoniw P, et al. Prolonged in vivo residence times of antibody fragments associated with albumin. Bioconjug Chem. 2001;12:750–756. doi: 10.1021/bc010003g. [DOI] [PubMed] [Google Scholar]
- Svennerholm L, Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980;617:97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- Talwar GP. Vaccines and passive immunological approaches for the control of fertility and hormone-dependent cancers. Immunol Rev. 1999;171:173–192. doi: 10.1111/j.1600-065x.1999.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Samuel A, Naz RK. Presence of antisperm antibodies reactive with peptide epitopes of FA-1 and YLP12 in sera of immunoinfertile women. Am J Reprod Immunol. doi: 10.1111/j.1600-0897.2008.00581.x. in press. [DOI] [PubMed] [Google Scholar]
- World POPClock Projection. US Census Bureau [online]. http://www.census.gov/ipc/www/popclockworld.html. (13 February 2008, date last accessed) [Google Scholar]
- Ye Z, Hellstrom I, Hayden-Ledbetter M, Dahlin A, Ledbetter JA, Hellstrom KE. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat Med. 2002;8:343–348. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]
- Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52:3402–3408. [PubMed] [Google Scholar]
- Zhang W, Matsumoto-Takasaki A, Kusada Y, Sakaue H, Sakai K, Nakata M, Fujita-Yamaguchi Y. Isolation and characterization of phage-displayed single chain antibodies recognizing nonreducing terminal mannose residues. 2. Expression, purification, and characterization of recombinant single chain antibodies. Biochemistry. 2007;46:263–270. doi: 10.1021/bi0618767. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Drummond DC, Zou H, Hayes ME, Adams GP, Kirpotin DB, Marks JD. Impact of single-chain Fv antibody fragment affinity on nanoparticle targeting of epidermal growth factor receptor expressing tumor cells. J Mol Biol. 2007;371:934–947. doi: 10.1016/j.jmb.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]