Abstract
BACKGROUND
Evidence suggests that eutopic endometrium from women with endometriosis (US-E) has intrinsic functional anomalies compared with women without endometriosis (US-C). We hypothesized that differences in endometrial haptoglobin (eHp) mRNA and protein levels exist between eutopic endometrium from US-E and US-C and that inflammatory mediators may be involved.
METHODS
Endometrial stromal cells and tissue explants from US-E (n = 18) and US-C (n = 18) were cultured (24 h/48 h for cells/explants) with interleukin (IL)-1α, -1β, -6, -8 or tumor necrosis factor-alpha (TNF-α) at 0–100 ng/ml. eHp protein in media and mRNA levels were quantified by enzyme-linked immunosorbent assay and quantitative PCR.
RESULTS
In eutopic endometrial stromal cells from US-E, IL-1β, IL-6 and TNF-α (10 ng/ml) increased eHp mRNA levels (P = 0.002, P < 0.001 and P < 0.001, respectively) and eHp protein (P = 0.023, 0.031 and 0.006, respectively) versus control. In endometrial tissues from US-E, IL-1β, IL-6 and TNF-α increased eHp mRNA (P < 0.001, P = 0.017 and P < 0.001, respectively) and eHp protein (P < 0.001, P = 0.007 and 0.039, respectively) versus control. IL-1α and IL-8 had small or no effects on isolated endometrial cells or tissues. In US-C, IL-1β, IL-8 and TNF-α each reduced eHp mRNA in endometrial stromal cells (all P < 0.001) versus control; IL-1α and IL-6 had no effect. eHp mRNA increased in endometrial tissues from US-C in response to IL-1β (P = 0.008), IL-6 (P = 0.015) and TNF-α (P = 0.031) versus control; IL-1α or IL-8 had no effect.
CONCLUSIONS
Endometrium from US-E differentially responds to specific inflammatory cytokines by production of eHp. We propose that up-regulation of endometrial eHp by inflammatory mediators disrupts normal endometrial function and may facilitate the pathogenesis of endometriosis.
Keywords: cytokines, endometrium, haptoglobin, endometriosis
Introduction
Endometriosis
Endometriosis, characterized by the growth of endometrial glands and stroma outside the uterine cavity, is a gynecological disorder affecting millions of women of reproductive age worldwide (Farquhar, 2007; Meuleman et al., 2009). Women with endometriosis suffer from chronic, intractable pelvic pain and subfertility (Vigano et al., 2004). Despite the gravity of this disease, the mechanisms whereby retrogradely shed endometrial fragments (Sampson, 1927, 1940) avoid immune eradication, establish a vasculature at extra-uterine sites and form endometriotic lesions are poorly understood (Witz, 2002; Benagiano and Brosens, 2006).
While menstrual tissue and cells are present in peritoneal fluid from 50 to 75% of all women (D'Hooghe and Debrock, 2002; Sharpe-Timms, 2005), an enigma exists as to why some women develop endometriosis while others do not. Further, mechanisms underlying pain and reproductive failure in women with minimal or mild endometriosis are subtle and controversial and it is predicted that inflammatory factors are involved (D'Hooghe et al., 2003; Halis and Arici, 2004; Evans et al., 2007).
Eutopic endometrium in women with endometriosis
A growing body of evidence indicates that eutopic endometrium from women with endometriosis has intrinsic qualitative and quantitative differences when compared with endometrium of women without this disorder. Studies have shown that eutopic endometrium from women with endometriosis has abnormalities in structure, proliferation, immune components, adhesion molecules, proteolytic enzymes and inhibitors, steroid and cytokine production and responsiveness, and mRNA expression and protein production when compared with endometrium of women without this disorder (Vinatier et al., 2000; Sharpe-Timms, 2001; Kao et al., 2003; Ulukus et al., 2006; Hunter et al., 2008; Minici et al., 2008). Such anomalies have been considered contributory to the pathogenesis and pathophysiologies of endometriosis. Our research has shown that included among these differences is the fact that eutopic endometrium from women with endometriosis expresses significantly more endometrial haptoglobin (eHp) mRNA and protein than the trace amounts (or absence) of eHp found in endometrium from women without this disorder (Sharpe et al., 1993; Piva and Sharpe-Timms, 1999; Sharpe-Timms et al., 2000).
Inflammatory cytokines up-regulate haptoglobin production
Inflammatory cytokines stimulate hepatic haptoglobin (Hp, the most abundant form of Hp) production several fold (Oliviero et al., 1987; Baumann et al., 1990; Bowman, 1993). Local inflammatory cells (neutrophils, granulocytes and macrophages) secrete a number of cytokines into the bloodstream, most notable of which are interleukin-1 (IL-1), IL-6 and IL-8 and tumor necrosis factor-alpha (TNF-α). The liver responds to these stimuli by producing a large number of acute-phase reactants, including Hp.
There are many potential sources of inflammation in the uterine cavity (Oral et al., 1996; Rana et al., 1996; Koninckx et al., 1999; D'Hooghe et al., 2001; Harada et al., 2001). Yet, to date, the mechanisms by which inflammatory cytokines in the uterine environment affect the pathogenesis or pathophysiologies of endometriosis and whether eHp production plays a role in this mechanism have not been established. We hypothesize that endometriosis initiates with the cytokine-induced up-regulation of eHp production by eutopic endometrium. Hence, via the immunomodulatory and angiogenic properties of Hp (Langlois and Delanghe, 1996; Dobryszycka, 1997; Sharpe-Timms et al., 2002) these anomalies may play a role in the pathogenesis of endometriosis (Christodoulakos et al., 2007) and subsequently contribute to the sequela of pain and subfertility.
The objective of this study was to determine which specific endometriosis-associated inflammatory cytokines and chemokines (Oral et al., 1996; Rana et al., 1996; Koninckx et al., 1999) differentially up-regulate eHp production by eutopic endometrial tissues. This information may provide insights into modalities of therapeutic immunomodulation capable of restoring local homeostatic inflammatory mechanisms in the endometrium thereby preventing endometriosis from developing and/or treating endometriosis-related pain and subfertility.
Materials and Methods
Human subjects
Endometrial tissues were collected from fertile women undergoing laparoscopic tubal ligation or benign gynecological surgeries (e.g. benign ovarian cyst, leiomyoma) who had no evidence of endometriotic lesions (controls, n = 18) and from women undergoing diagnosis or treatment with laparoscopically documented endometriosis (n = 18). The American Society for Reproductive Medicine classification stage was only available for 2 of the 18 subjects and therefore could not be considered in the analyses. Future experiments will be required to determine a potential effect of classification stage. We acknowledge that not accounting for stage of endometriosis is a limitation of this study. The age (endometriosis 30 ± 7 years; control 31 ± 7 years) and race distribution (endometriosis and control: 89% Caucasian and 11% African American) were not different between groups. All subjects were menstruating and none was taking steroid hormones or steroid-modulating medications at the time of, or up to 6 months prior to, sample collection.
Endometrial biopsies were collected while patients were under general anesthesia using a Pipelle (Unimar, Wilton, CN, USA) endometrial suction curette as previously reported (Sharpe et al., 1993; Sharpe-Timms et al., 1998, 2000; Piva and Sharpe-Timms, 1999; Piva et al., 2001). Endometrial tissues were classified as menstrual (Days 1–5), proliferative (Days 6–14) or secretory (Days 16–28) cycle stages according to the date of the last menstrual period and by histological confirmation (Noyes et al., 1950). All samples were collected with a protocol approved by the University of Missouri Institutional Review Board-Health Sciences Section and with consent of the patient.
Endometrial stromal cell isolation, culture, cytokine treatment and eHp protein production
We have previously reported that the stromal component of ectopic endometriotic lesions, from women and from a surgically induced model of endometriosis in rats, synthesizes and secretes eHp (Sharpe and Vernon, 1993; Sharpe et al., 1993; Sharpe-Timms et al., 1998, 2000; Piva and Sharpe-Timms, 1999). Hence, we began our investigation to identify dose-related effects of IL-1α, IL-1β, IL-6, IL-8 and TNF-α on eHp production by endometrial stromal cells from women with (US-E) and without (US-C) endometriosis. Endometrial stromal cells were isolated as routinely performed in our laboratory (Sharpe and Vernon, 1993; Sharpe et al., 1993; Sharpe-Timms et al., 1998).
Briefly, endometrial tissue biopsies were minced and rinsed in phosphate-buffered saline (PBS) containing penicillin (100 U/ml) and streptomycin (100 µg/ml). The PBS was removed and replaced with 3 ml of fresh PBS with antibiotics. Tissues were then pre-incubated for 2 h in a shaking incubator at 37°C to remove serum proteins, followed by 1 h enzymatic digestion using 790 U/ml of collagenase Type I (Invitrogen, Carlsbad, CA, USA) and 0.02% DNase (Sigma, St.Louis, MO, USA) in phenol red-free Dulbecco's modified Eagle's medium (DMEM)/F12 (Media Tech Inc., Manassas, VA, USA) with 2% horse serum. Subsequently, a series of filtrations, sedimentations and selective attachment procedures were used to isolate the cells. Only primary cell cultures were used.
Endometrial stromal cells were plated at a density of 1 × 106 cells/well in six-well plates and cultured until near-confluence in phenol red-free DMEM/F12 with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, USA) and penicillin and streptomycin. Twenty-four hour prior to each experiment, media were replaced with 3 ml of DMEM/F12 with 1% ITS+ (insulin/transferring/selenium culture supplement; BD Biosciences, Bedford, MA, USA) and the same antibiotics. All cytokines were soluble in this media and the concentrations were created by using 10-fold serial dilutions; hence, the control medium (0 dose) was the medium without cytokines. Experiments were performed in this serum-free medium with 0, 0.1, 1.0, 10.0 and 100.0 ng/ml of IL-1α, IL-1β, IL-6, IL-8 or TNF-α (Millipore, Billerica, CA, USA) in a 5% CO2 incubator at 37°C. After 24 h, culture media were aspirated from the cell cultures, centrifuged to remove cellular debris, aliquoted and stored at −80°C to quantify eHp protein. The cells were then washed with sterile PBS while in their culture dish and lysed with lysis binding solution (Ambion, Austin, TX, USA) following the manufacturer's instructions, and stored in cryo vials at −80°C for eHp gene expression analysis.
The amount of eHp protein secreted into the media was quantified by an eHp enzyme-linked immunosorbent assay (ELISA) developed by our laboratory with assistance from Christopher C. Chadwick PhD at Life Diagnostics, Inc. (West Chester, PA, USA). This ELISA was designed to measure eHp in body fluid, culture media or tissue extracts. It is a sandwich ELISA utilizing Hp-coated ELISA plates (capture antibody) and a horse-radish peroxidase -conjugated Hp (detection antibody). We have previously documented that this Hp antibody recognizes de novo synthesized and secreted eHp (Sharpe-Timms et al., 1998). This new ELISA is more sensitive (1–100 ng/ml range) than a previous Hp ELISA developed and used in our laboratory (Piva et al., 2001).
For this ELISA, 96-well plates were coated overnight in 50 mM sodium carbonate (pH 9.6) with an Hp antibody (5 µg/ml, DAKO) using a volume of 200 µl/well. The next morning the plates were washed with water using a plate washer (BIOTEK, Winooski, VT, USA). Non-specific binding to the plates was blocked by addition of 250 µl of Coating Stabilizer & Block Buffer (BioDesign, Saco, ME, USA) for 30 min. The solution was then removed, and the plates were dried using a lyophilizer and stored with desiccant for up to 6 months.
To perform the ELISA, antigens (test sample or standard) were placed into the wells in 100 µl of a PBS buffer containing bovine serum albumin (0.07%) for 60 min at room temperature. The standard curve was made with dilutions of 0.625, 1.25, 2.50, 5.0, 10.0 and 20.0 ng/ml of Hp. After 60 min, the plates were washed with dH2O and then incubated with an Hp-HRP conjugate in 100 ul of Guardian Peroxidase Conjugate Stabilizer/Diluent (Thermo Fisher Scientific Pierce, Rockford, IL, USA) for 60 min at room temperature at a final dilution of 1: 2000. The plates were again washed as above prior to the addition of 100 µl of tetramethylbenzidine substrate (SureBlue, KLP, Inc., Gaithersburg, MD, USA) per well and incubate for 20 min at room temperature. The reaction was quenched by addition of 100 µl of 1 M HCl and the absorbance was measured at 450 nm. Total protein in the culture media was quantified using the DC protein assay (Biorad, Hercules, CA, USA). Results were expressed as eHp (pg/ml)/total protein (mg/ml).
Endometrial tissue explant culture, cytokine treatment and eHp protein production
Endometrial tissue biopsies were collected and cultured as explants as previously described (Sharpe et al., 1993; Sharpe and Vernon, 1993). Briefly, endometrial tissue biopsies were minced and rinsed in PBS with penicillin and streptomycin, the media replaced with 3 ml of fresh PBS with antibiotics and pre-incubated for 2 h in a shaking incubator at 37°C to remove serum proteins. Subsequently, the tissues were divided into pieces of 50–100 mg wet weight, placed into T-25 flasks containing 3 ml phenol red-free DMEM/F12 media supplemented with 1% ITS+ and antibiotics. IL-1α, IL-1β, IL-6, IL-8 and/or TNF-α were added to replicate cultures at 10.0 ng/ml; medium was added only for the 0 ng/ml experiments. On the basis of the dose–response studies in the isolated endometrial cells, the 10 ng/ml dose was chosen as this was an effective dose for cytokines to stimulate production of eHp by eutopic endometrial stromal cells.
After 48 h in an oscillating incubator with a gaseous atmosphere of 5% CO2, 55% N2, 45% O2, culture media were collected, centrifuged to remove cellular debris and frozen at −80°C. The concentration of eHp protein in the culture media was quantified by ELISA, as described above and normalized to the total protein in the culture media. After culture, tissue explants were snap-frozen in liquid nitrogen for gene analysis.
Real-time quantitative PCR for quantification of eHp mRNA levels in endometrial stromal cells and tissue explants
RNA from cultured endometrial stromal cells treated with vehicle or 10 ng/ml of each cytokine was extracted using the RNAqueous® -4PCR Kit (Ambion, Austin, TX, USA) and treated with DNAse using the TURBO DNA-free kit (Ambion) to remove possible genomic contamination. RNA from cultured tissue explants treated with vehicle or 10 ng/ml of each cytokine was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and treated with DNAse using the RNAse-free DNAse kit (Qiagen). All kits were used as per the manufacturer's instructions.
The yield and quantity of RNA were assessed by absorbance using a NanoDrop ND-1000 spectrophotometer (Wilmington, DE, USA) and by ethidium bromide-stained agarose gel electrophoresis. Complementary DNA (cDNA) was constructed from 500 ng mRNA using random primers and the First Strand cDNA Synthesis Kit for RT–PCR avian myeloblastosis virus (AMV) (Roche, Indianapolis, IN, USA) as per the manufacturer's instructions.
Expression levels of the eHp mRNA were assessed by performing a duplex quantitative PCR (Q-PCR) with an Applied Biosystems Incorporated (ABI) Prism 7500 sequence detection system and TaqMan methodologies. The duplex method allowed for the co-amplification of eHp gene and the endogenous 18S ribosomal gene in the same sample. The duplex Q-PCR assay was optimized following a standard curve method where liver cDNA was used as control template in 10-fold serial dilutions, and different concentrations of eHp primers (200 or 500 nM/reaction) and 18S primers (0.25, 0.5, 0.75, 1 µl/reaction) were assessed. Primers at 200 nM with 0.5 µl of 18 S yielded the most efficient duplex assay where the slopes of eHp and 18 S were, respectively, −3.42 and −3.39.
The eHp primers and probe were designed with assistance from ABI (Foster City, CA, USA). Like Hp, the eHp gene encodes a preproprotein which is processed to yield two different alpha chains (designated alpha 1 and alpha 2) and two identical beta chains which subsequently combine as a tetramer to produce haptoglobin (Piva and Sharpe-Timms, 1999). Our primer-probe set was designed in a region of the nucleotide sequence (NM_005143) to assure that our Q-PCR would detect all three eHp phenotypes (alpha 2-2, alpha 2-1 and alpha 1-1). The sequences were as follows:
| Sequence | Nucleotide position | |
|---|---|---|
| Forward primer | 5′-CTCTTCCAGAGGCAAGACCAA-3′ | 9–39 |
| Reverse primer | 5′-GAGCAGGAGGGCAATGACA-3′ | 62–80 |
| TaqMan MGB probe | 6FAM-AAGATGAGTGCCCTGGGAMGBNFQ | 42–59 |
Primers and the probe for 18S were obtained as a kit from ABI: the Eukaryotic 18S rRNA endogenous control with a VIC dye MGB Probe and limited primers (20x stock; sequence not provided by the manufacturer).
The following reagents were used for amplification in 20 µl of final reaction volume: TaqMan Universal Master Mix (Applied Biosystems), eHp primers (200 nM) and probe (250 nM), 0.5 µl of 18 S rRNA endogenous control, 2 µl cDNA (cells) or 4 µl of cDNA (explants) and nucleotide-free water. Thermocycling conditions were as follows: 2 min/50°C + 10 min/95°C + 40 cycles for 15 s/95°C + 1 min/60°C. Liver cDNA was used as positive control and water (no RNA) as blank negative control. The Q-PCR was performed in a 96-well clear optical reaction plate (ABI).
Statistical analyses
When necessary, data were log transformed to homogenize variances in eHp protein levels between subjects in the endometriosis group and account for a potential menstrual cycle stage effect. Log transformation was necessary since the eHp variance was proportional to the mean, which is quite common in dose–response studies covering a wide range of doses such as ours (Bland and Altman, 1996a, b, c) and has been previously used in our laboratory to analyze eHp (Endo-I) gene expression (Piva and Sharpe-Timms, 1999).
Linear mixed models (Verbeke and Molenberghs, 2000; McCulloch and Searle, 2001; Fitzmaurice et al., 2004) were used to study how the eHp protein response in uterine stromal cells varied across dose. In our statistical models, dose and cycle stage were considered fixed effects, while a heterogeneous variance compound symmetry covariance structure was used to account for correlated responses within a patient across doses, and unequal variances across dose. Model fit was assessed using the Pearson residuals, and models were fit using PROC GLIMMIX in SAS 9.1.3. F-tests (Type III) were used to determine the significance of fixed effects. If a significant dose effect was found, least-squares means were used to test for significant differences between all pairwise doses at a nominal error rate of 5%.
Log transformation was also performed on endometrial tissue explant eHp protein concentrations in culture media in response to 0 or 10 ng/ml of each cytokine tested, and differences were detected by a paired Student's t-test. The paired t-test examines the changes that occur before and after an experimental intervention on the same individuals to determine whether or not the treatment had a significant effect. Examining the changes, rather than the values observed before and after the intervention, removes the differences due to individual responses, producing a more sensitive or powerful test.
Relative levels of eHp mRNA in endometrial tissue explants and isolated stromal cells from women with endometriosis were calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001). The paired t-test was used to determine differences in relative eHp gene expression in response to 0 or 10 ng/ml of each cytokine tested.
Differences in eHp protein and mRNA levels in response to cytokines in women undergoing tubal sterilization (normal controls) were compared with those with benign gynecological problems using Student's t-test. No differences were found and hence the data in these two control groups were pooled. For all analyses, a P-value of 0.05 or less was considered significant.
Results
Dose-dependent endometrial stromal cell eHp protein production in response to cytokines
Production of eHp protein by US-E increased with cytokine dose when a response was found, but the effect of the menstrual cycle stage varied by cytokine. The results from the F-tests for dose effect and cycle effect showed the following. In women with endometriosis, there was a significant dose–response in US-E eHp protein production after treatments with IL-1β (P < 0.001), IL-6 (P = 0.004) and TNF-α (P < 0.001) compared with no cytokine treatment. IL-1β showed a cycle stage effect (increased in proliferative stage, P = 0.032) whereas TNF-α and IL-6 did not (P = 0.893 and 0.093, respectively). Subtle increases in US-E eHp protein by treatment with 100 ng/ml IL-1α (P = 0.083) or IL-8 (P = 0.183) were noted, but changes over the dose range were not significant (P = 0.134 and 0.637, respectively) and no cycle effect was found. Remarkably, none of these cytokines tested caused a significant increase in eHp protein production by US-C regardless of dose or cycle stage.
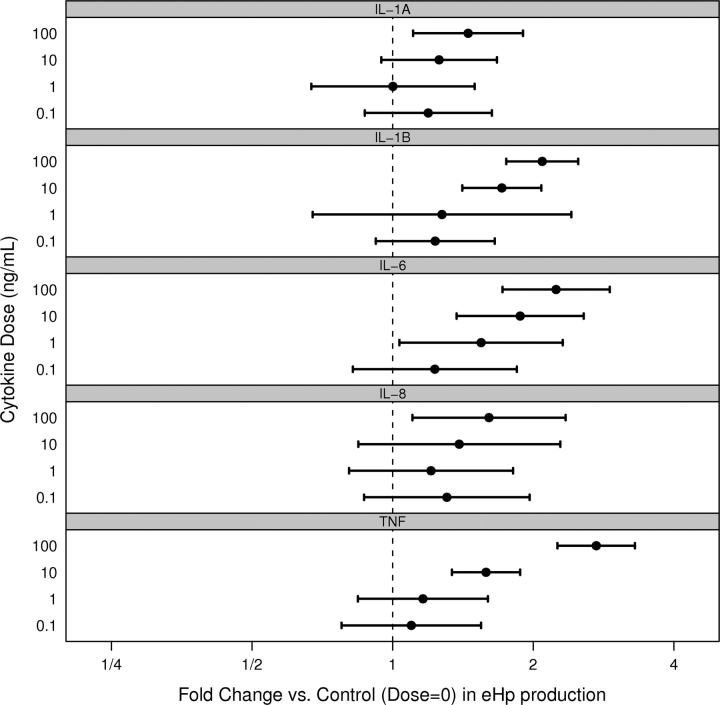
The dose-related fold change in eHp protein production by the stromal cells for each cytokine tested, using the least-square means of pairwise differences, is shown in Fig. 1. Both IL-1β and TNF-α significantly increased eHp at doses ≥10 ng/ml, whereas IL-6 significantly increased eHp at doses ≥1 ng/ml and thus the 10 ng/ml dose was selected for further experiments.
Figure 1.
Fold change in eHp protein production by endometrial stromal cells from women with endometriosis (US-E) in response to IL-1α, IL-1β, IL-6, IL-8 or TNF-α. Each horizontal interval bar depicts the estimated 95% confidence interval (CI) for the mean fold change for a given dose of cytokine (0.1, 1, 10 or 100) versus no dose. The black circle in each interval is the estimated mean fold change. A broken vertical line is shown at fold change = 1, as this aids in the interpretation of the CIs. Intervals which overlap the broken line imply that the associated dose for that cytokine is not significantly different from the control (α = 0.05). The fold change was tested using the least-square means of pairwise differences.
Cytokine-specific effects on endometrial stromal cell eHp protein and mRNA production
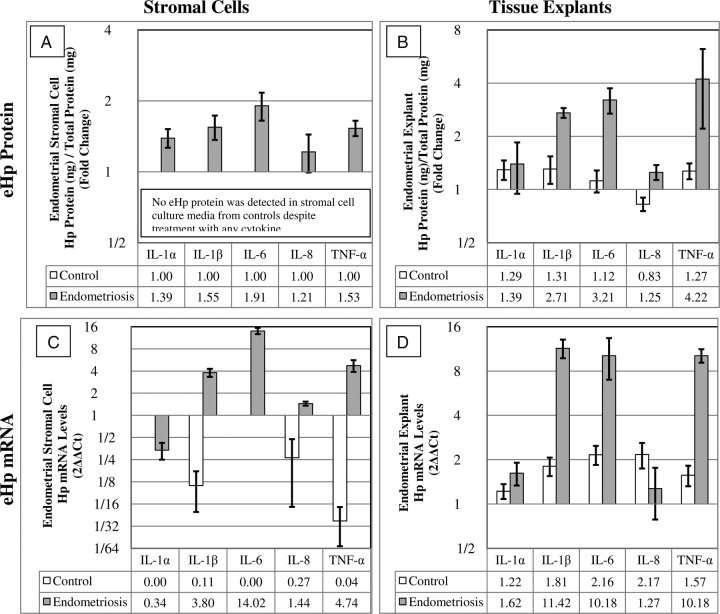
The effects of 10 ng/ml of the five cytokines on the fold change in eHp protein production and mRNA levels compared with no cytokine treatment in endometrial stromal cells and endometrial tissue explants from women with and without endometriosis are shown in Fig. 2. A summary for the raw data is shown in Table I.
Figure 2.
Relative fold change in eHp protein production (A, B) and mRNA levels (C, D) from endometrial stromal cells (A, C) and endometrial tissue explants (B, D) from women with (gray bars) and without (white bars) endometriosis in response to 0 or 10 ng/ml cytokine treatment. The mRNA levels were quantified by real-time PCR and calculated using the 2−ΔΔCt method, relative to no treatment and therefore by nature are log base two. Hence for comparison purposes, the eHp protein data [eHp (pg/ml)/total protein (mg/ml)] were transformed to log base two. (Fold = eHp at 10 ng/ml cytokine/eHp at 0 ng/ml cytokine). Both isolated US-E (A) and endometrial tissue explants (B) from women with endometriosis (gray bars) produced more significant eHp protein (A, B) and had higher levels of eHp mRNA (B, D) in response to 10 ng/ml IL-1β, IL-6 and TNF-α, but not IL-1α or IL-8 compared with culture media without cytokines and compared with women without endometriosis (A, B, C, D; white bars). Large (>4-fold; P < 0.001) decreases in eHp mRNA levels were noted when US-C (C, white bars) were treated IL-1β (P < 0.001), IL-8 (P < 0.001) or TNF-α (P < 0.001). Each bar represents the mean and SE analyses from samples of 4 to 6 women per cytokine (Raw data shown in Table 1).
Table I.
Effects of cytokine on eHp protein production by endometrial tissue explants and endometrial stromal cells from women with (UE) and without endometriosis (UC).
| IL-1α |
IL-1β |
IL-6 |
IL-8 |
TNF-α |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | ||
| Tissue explants | UC | 13 844 (2031) | 17 775 (2495) | 13 106 (1225) | 16 490 (2449) | 13 675 (1993) | 17 081 (71) | 14 432 (1422) | 11 957 (1397) | 13 572 (1462) | 16 766 (417) |
| UE | 24 183 (4377) | 33 772 (8722) | 63 308 (1431) | 171 504 (2855) | 60 583 (4092) | 192 338 (11 478) | 50 560 (13 399) | 59 336 (14 836) | 47 201 (18 344) | 156 538 (36 414) | |
| Stromal cells | UC | 0 (NA) | 0 (NA) | 0 (NA) | 0 (NA) | 0 (NA) | 0 (NA) | 0 (NA) | 0 (NA) | 0 (NA) | 0 (NA) |
| UE | 695 (105) | 955 (159) | 728 (130) | 1136 (284) | 582 (156) | 1434 (422) | 582 (156) | 931 (319) | 746 (149) | 1177 (307) | |
IL, interleukin; TNF-α, tumor necrosis factor-alpha.
Results are expressed as mean (eHp pg/ml/total protein (mg/ml) with the SE at both the 0 and 10 ng/ml dose after 24 h in culture. Stromal cell protein measurements in the UC group were below the detection level and are reported as 0. The subsequent statistical analyses for this raw data took into account the paired nature of the data (i.e. cells measured before and after treatment), but this pairing cannot be summarized in a table such as this.
Only US-E (Fig. 2A, gray bars) produced more eHp protein in response to IL-1α (P = 0.032), IL-1β (P = 0.023), IL-6 (P = 0.031) and TNF-α (P = 0.006) compared with no treatment; yet IL-8 had no significant effect on US-E eHp protein production (P = 0.816). When US-C were treated with any of the five cytokines tested, no eHp protein was found in the culture media (Fig. 2A, absence of white bars). Comparisons in eHp protein response to any cytokine tested between US-E and US-C could not be made statistically as no detectable eHp protein was produced detected in US-C.
Significant elevations in eHp mRNA levels were observed in US-E (Fig. 2C, gray bars) in response to IL-1β (P = 0.002), IL-6 (P < 0.001) and TNF-α (P < 0.001). A much smaller but still significant increase was seen in US-E mRNA after IL-8 treatment (P = 0.012). Levels of eHp mRNA in US-E in response to IL-1α did not change significantly (P = 0.161). Large (>4-fold) significant (P < 0.001) decreases in eHp mRNA levels were noted when US-C (Fig. 2C, white bars) were treated with IL-1β (P < 0.001), IL-8 (P < 0.001) or TNF-α (P < 0.001). No eHp mRNA was detected when US-C were treated with IL-1α or IL-6.
Comparing eHp mRNA levels in US-E versus US-C (Fig 2C, gray versus white bars) in response to IL-1β, IL-8 and TNF-α, eHp mRNA levels were increased in US-E but decreased in US-C (P < 0.001). IL-6 treatment significantly increased eHp mRNA levels compared with no treatment in US-E, but as no eHp mRNA was detected in US-C, a P-value could not be calculated. No significant difference between US-E and US-C eHp mRNA levels was detected in response to IL-1α.
Cytokine-specific effects on endometrial tissue explant eHp protein and mRNA production
In endometrial tissue explants from women with endometriosis, significant elevations in eHp protein production were found in response to IL-1β (P < 0.001), IL-6 (P < 0.007) and TNF-α (P = 0.039) compared with no treatment (Fig. 2B, gray bars). IL-8 elicited a smaller increase in eHp protein production (P = 0.033) and the response to IL-1α was not significant (P = 0.186).
When endometrial tissue explants from women without endometriosis were treated with the cytokines (Fig. 2B, white bars), small non-significant increases in eHp protein were noted after IL-1α, IL-1β, IL-6 and TNF-α treatment. A small and highly variable decrease in eHp production was noted after IL-8 treatment (P = 0.158), but this was not significant.
Comparing eHp protein production by endometrial tissue explants from women with endometriosis to those without endometriosis (Fig. 2B, gray versus white bars), IL-1β (P = 0.017), IL-6 (P = 0.005) and TNF-α (P = 0.039) significantly increased eHp protein production in the endometriosis group. No difference in eHp protein was found between the two study groups after IL-1α treatment. As IL-8 elicited a small increase in eHp protein production within endometrium from women with endometriosis (P = 0.033) and a small, non-significant decrease in eHp in women without endometriosis (P = 0.158), the dichotomy of the responses caused a statistical difference between the two subject groups (P = 0.020).
In endometrial explants, women with endometriosis elevated levels of eHp mRNA (>10-fold) were found, relative to no treatment (Fig. 2D, gray bars), in response to IL-1β (P < 0.001), IL-6 (P = 0.017) and TNF-α (P < 0.001) treatment, but not IL-1α (P = 0.102) or IL-8 (P = 0.628). Subtle but significant increases in eHp mRNA levels (1.5–2.6-fold) were also found in endometrial explants from women without endometriosis relative to no treatment in response to IL-1β (P = 0.008), IL-6 (P = 0.015) and TNF-α (P = 0.031) treatment, but not IL-1α (P = 0.265) or IL-8 (P = 0.54) (Fig. 2D, white bars).
Comparing eHp mRNA concentrations from endometrial tissue explants from women with endometriosis to those without endometriosis (Fig. 2D, gray versus white bars), IL-1β (P < 0.001), IL-6 (P = 0.021) and TNF-α (P < 0.001) significantly increased eHp mRNA in the endometriosis group. No differences in eHp mRNA concentrations were found between the two study groups after IL-1α or IL-8 treatment.
Discussion
These studies are the first to our knowledge to show that exposure to the inflammatory cytokines IL-1β, IL-6 and TNF-α significantly up-regulates eHp mRNA expression, protein synthesis and secretion, by isolated endometrial stromal cells from women with endometriosis (US-E) in a dose-dependent fashion. Only supra-physiological concentrations of IL-1α or IL-8 (100 ng/ml) elicited eHp protein production by these cells. Exposure to 10 ng/ml of IL-1β, IL-6 and TNF-α, but not IL-1α or IL-8, consistently and significantly up-regulated eHp mRNA expression and protein production not only by isolated endometrial stromal cells, but also by endometrial tissue explants from women with endometriosis. Comparing eHp mRNA and protein levels by isolated stromal cells versus endometrial tissue explants, the patterns of response to the cytokines tested were similar. However, the magnitude of the fold change in eHp mRNA and protein was significantly more robust in the endometrial tissue explants compared with the endometrial isolated stromal cells. These differences may be attributable to paracrine interactions between the endometrial cell types (epithelial and stromal cells), endothelial cells and/or resident immune cells in the tissue explants which were absent in the isolated stromal cell cultures.
No eHp protein was detected in control endometrial stromal cell (US-C) culture media, and IL-1β, IL-6 and TNF-α actually decreased eHp mRNA in these cells. In the control endometrial tissue explant cultures, some changes in eHp protein were noted in response to the cytokines (10 ng/ml) compared with no treatment, but they were not significantly different. Interestingly, like endometrial explant tissues from women with endometriosis, IL-1β, IL-6 and TNF-α, but not IL-1α or IL-8, elicited a significant increase in eHp mRNA levels in control endometrial tissue compared with no treatment. The fact that no significant amounts of eHp protein were found while there was a significant increase in eHp mRNA in control tissue explants suggests that eHp is not being fully transcribed and/or secreted into the culture media. Alternatively, the eHp protein produced by control samples (US-C) may somehow have been degraded compared with the endometriosis samples (US-E). Alternatively, control endometrial epithelial cells and/or resident immune cells may be suppressing eHp production by tissue explants in women without endometriosis. Additional studies using a co-culture model to test the paracrine effects between endometrial and other cell types in the endometrium may provide further insights into the cytokine regulation of eHp production.
The specific cytokines and chemokines evaluated here (IL-1α, IL-1β, IL-6, IL-8 or TNF-α) were selected as the focus of our study because of their well-known associations with the pathogenesis and/or pathophysiologies of endometriosis (Oral et al., 1996; Koninckx et al., 1998; Gazvani and Templeton, 2002). Yet, there is significant debate in the literature as to which cytokines are truly involved in endometriosis and at what concentrations. Many studies are based on levels of cytokines measured in peritoneal fluid or sera, often reported at pg/ml levels (Koninckx et al., 1998, 1999; Harada et al., 2001; Braun et al., 2002; Bedaiwy and Falcone, 2003; Kalu et al., 2007); less is known about concentrations of these cytokines within the local endometrial environment (Vinatier et al., 1996). It is possible that cytokines are more concentrated in the microenvironment of the uterine cavity compared with the significantly greater volume of the peritoneal cavity.
The dose–response range of 0, 0.1, 1.0, 10.0 and 100.0 ng/ml was selected based on prior reports from our laboratory and from others, which were focusing on the effects of these cytokines on various secretory products produced by eutopic endometrial stromal cells from women with endometriosis (US-E). It may be argued that the cytokine concentrations at ng/ml, which are significantly greater than those found in peritoneal fluid, may not have biological relevance, despite the statistical significance in our experiments. Yet, precedence has been set that cytokine concentrations at ng/ml levels cause biologically relevant effects in endometrial stromal cell and tissue culture models. For example, at concentrations ranging between 1 and 100 ng/ml IL-6 increased endometrial stromal cell eHp production in a dose-dependent fashion (Piva et al., 2001). Others have also reported that endometrial stromal cells from women with endometriosis (US-E), but not stromal cells from controls (US-C), which were treated with IL-1β or TNF-α at ng/ml concentrations increased production of matrix metalloproteinase enzymes (Braundmeier and Nowak, 2006), glutathione (Lee et al., 2009) and RANTES (Fang et al., 2009). These cytokine-induced responses are believed to facilitate peritoneal remodeling for invasion of ectopic endometrium, maintenance of endometrial stromal cell viability and survival, and enhanced chemotactic activity, respectively, which are associated with the establishment, progression and inflammatory sequelae of endometriosis.
Ultimately, caution must be taken when interpreting the concentrations and the effects of various cytokines on different tissues, cells and environments. And certainly, other cytokines and chemokines at different concentrations, and the synergistic or antagonistic interactions of these inflammatory mediators (Sakamoto et al., 2003; Yamauchi et al., 2004) may also be involved in regulation of endometrial eHp production. Further studies are warranted to expand dosing studies as well as decipher the complex interactions between cytokines and chemokines and eHp production as well as to determine the source of the cytokines eliciting these responses.
IL-1β was the only cytokine tested that showed both a dose-related increase in eHp production and a menstrual cycle stage effect. We observed that endometrial cells collected during the proliferative stage showed a greater change in eHp production in response to IL-1β treatment than those collected in the menstrual or secretory stages. Menstrual cycle stage variations occur in the presence or production of cytokines in the endometrium, and we recognize such differences and their complexity on endometrial function. However, this study focused on cytokine-induced eHp mRNA and protein production by the endometrium of women with and without endometriosis. Studies of the effects of endogenous cytokines on eHp production in vivo in animal models remain to be investigated.
Our current results show that isolated, purified endometrial stromal cells from women with endometriosis (US-E), but not those without (US-C), do in fact express elevated levels of both the eHp mRNA and protein in response to IL-6 treatment. These results were ascertained by using Q-PCR and a new, more sensitive, second-generation Hp ELISA developed in our laboratory. These new findings supersede our previous report that IL-6 treatment in vitro did not up-regulate eHp mRNA and protein production by eutopic endometrial cells of women with endometriosis, as detected by semi-quantitative RT–PCR with Southern blot analysis and a first-generation eHp ELISA developed in our laboratory (Piva et al., 2001). A likely explanation for this discrepancy lies within our development of the more sensitive RNA and protein assays used in the current study.
Others have reported that Hp protein is present in rabbit and human oviduct, endometrium and decidua. In the peri-implantation period in rabbits, expression of Hp mRNA and protein was found in the endometrial epithelial surface and mucosal folds (Hoffman et al., 1996; Olson et al., 1997) and high amounts of Hp protein were reported in the microenvironment surrounding the oocyte/embryo (Herrler et al., 2003, 2004). In humans, Hp protein was immunolocalized in normal endometrium during the period of uterine receptivity and was elevated in the decidua (Berkova et al., 2001). The peri-implantation period is associated with an inflammatory challenge to the uterine environment which may also regulate endometrial Hp production. Concurrent inflammation and the presence of Hp protein in the endometrium in the peri-implantation period in rabbits and in the endometrium of women with endometriosis further supports a role for Hp in immunomodulation to protect the embryonic allograft and to avoid rejection in the peritoneal cavity, respectively.
In summary, we and others have previously demonstrated that established peritoneal endometriotic lesions (Sharpe et al., 1993; Piva and Sharpe-Timms, 1999; Sharpe-Timms et al., 2000; Piva et al., 2002; Eyster et al., 2007) and retrogradely shed endometrial fragments found in peritoneal fluid of women with endometriosis (Sharpe-Timms, 2005) synthesize and secrete significant amounts of a uniquely glycosylated eHp. And further, IL-6 differentially up-regulates eHp production by endometriotic cells in vitro (Piva et al., 2001). Our current results show that (i) basal levels of eutopic endometrial eHp mRNA and protein in women with endometriosis are significantly greater than those in endometrium from women without this disorder and (ii) specific inflammatory mediators, in a dose-dependent manner, play a significant role in regulating expression of eHp in eutopic endometrium in a subset of women who have endometriosis.
We hypothesize that the differential production of uniquely glycosylated eHp by eutopic endometrium from women with endometriosis in response to inflammatory cytokines, with its immunomodulatory properties (Langlois and Delanghe, 1996; Dobryszycka, 1997; Sharpe-Timms et al., 2002), may be involved in the pathogenesis and pathophysiologies of endometriosis. Further knowledge of such mechanisms may provide insights into modalities of therapeutic immunomodulation, capable of restoring homeostatic local inflammatory mechanisms in the endometrium, thereby leading to development of novel medical approaches to prevent the establishment of endometriosis and more effectively manage this disease.
Funding
This research was supported by the National Institutes of Child Health and Human Development (HD040849 to KST).
References
- Baumann H, Jahreis GP, Morella KK. Interaction of cytokine- and glucocorticoid-response elements of acute- phase plasma protein genes. J Biol Chem. 1990;265:22275–22281. [PubMed] [Google Scholar]
- Bedaiwy MA, Falcone T. Peritoneal fluid environment in endometriosis: clinicopathological implications. Minerva Ginecol. 2003;55:333–345. Review. [PubMed] [Google Scholar]
- Benagiano G, Brosens I. History of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:449–463. doi: 10.1016/j.bpobgyn.2006.01.007. Review. [DOI] [PubMed] [Google Scholar]
- Berkova N, Lemay A, Dresser DW, Fontaine JY, Kerizit J, Goupil S. Haptoglobin is present in human endometrium and shows elevated levels in the decidua during pregnancy. Mol Hum Reprod. 2001;7:747–754. doi: 10.1093/molehr/7.8.747. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Logarithms. BMJ. 1996a;312:700. doi: 10.1136/bmj.312.7032.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Transforming data. BMJ. 1996b;312:770. doi: 10.1136/bmj.312.7033.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ. 1996c;12:1079. doi: 10.1136/bmj.312.7038.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman BH. Haptoglobin. In: Bowman BH, editor. Hepatic Plasma Proteins. San Diego: Academic Press; 1993. pp. 159–167. [Google Scholar]
- Braun DP, Ding J, Dmowski WP. Peritoneal fluid-mediated enhancement of eutopic and ectopic endometrial cell proliferation is dependent on tumor necrosis factor-alpha in women with endometriosis. Fertil Steril. 2002;78:727–732. doi: 10.1016/s0015-0282(02)03318-6. [DOI] [PubMed] [Google Scholar]
- Braundmeier AG, Nowak RA. Cytokines regulate matrix metalloproteinases in human uterine endometrial fibroblast cells through a mechanism that does not involve increases in extracellular matrix metalloproteinase inducer. Am J Reprod Immunol. 2006;56:201–214. doi: 10.1111/j.1600-0897.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- Christodoulakos G, Augoulea A, Lambrinoudaki I, Sioulas V, Creatsas G. Pathogenesis of endometriosis: the role of defective ‘immunosurveillance. Eur J Contracept Reprod Health Care. 2007;12:194–202. doi: 10.1080/13625180701387266. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Debrock S. Endometriosis, retrograde menstruation and peritoneal inflammation. Hum Reprod Update. 2002;8:84–88. doi: 10.1093/humupd/8.1.84. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Bambra CS, Xiao L, Hill JA. The effect of menstruation and intrapelvic injection of endometrium on peritoneal fluid parameters in the baboon. Am J Obstet Gynecol. 2001;184:917–925. doi: 10.1067/mob.2001.111715. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Debrock S, Hill JA, Meuleman C. Endometriosis and subfertility: is the relationship resolved? Sem Reprod Med. 2003;21:243–254. doi: 10.1055/s-2003-41330. Review. [DOI] [PubMed] [Google Scholar]
- Dobryszycka W. Biological functions of haptoglobin–new pieces to an old puzzle. Eur J Clin Chem Clin Biochem. 1997;35:647–654. [PubMed] [Google Scholar]
- Evans S, Moalem-Taylor G, Tracey DJ. Pain and endometriosis. Pain. 2007;132(Suppl. 1):S22–S25. doi: 10.1016/j.pain.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Eyster KM, Klinkova O, Kennedy V, Hansen KA. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil Steril. 2007;88:1505–1533. doi: 10.1016/j.fertnstert.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Fang CL, Han SP, Fu SL, Wang W, Kong N, Wang XL. Ectopic, autologous eutopic and normal endometrial stromal cells have altered expression and chemotactic activity of RANTES. Eur J Obstet Gynecol Reprod Biol. 2009;143:55–60. doi: 10.1016/j.ejogrb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Farquhar C. Endometriosis. BMJ. 2007;334:249–253. doi: 10.1136/bmj.39073.736829.BE. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. New York: Wiley; 2004. [Google Scholar]
- Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002;123:217–226. doi: 10.1530/rep.0.1230217. Review. [DOI] [PubMed] [Google Scholar]
- Halis G, Arici A. Endometriosis and inflammation in infertility. Ann NY Acad Sci. 2004;1034:300–315. doi: 10.1196/annals.1335.032. Review. [DOI] [PubMed] [Google Scholar]
- Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001;76:1–10. doi: 10.1016/s0015-0282(01)01816-7. Review. [DOI] [PubMed] [Google Scholar]
- Herrler A, von Rango U, Beier HM. Embryo-maternal signaling: how the embryo starts talking to its mother to accomplish implantation. Reprod Biomed Online. 2003;6:244–256. doi: 10.1016/s1472-6483(10)61717-8. [DOI] [PubMed] [Google Scholar]
- Herrler A, Krusche CA, Müller-Schöttle F, Beier HM. Haptoglobin expression and release by rabbit oviduct and endometrium, its localization in blastocyst extra-embryonic matrix and fluid during preimplantation time. Hum Reprod. 2004;19:2730–2737. doi: 10.1093/humrep/deh517. [DOI] [PubMed] [Google Scholar]
- Hoffman LH, Winfrey VP, Blaeuer GL, Olson GE. A haptoglobin-like glycoprotein is produced by implantation-stage rabbit endometrium. Biol Reprod. 1996;55:176–184. doi: 10.1095/biolreprod55.1.176. [DOI] [PubMed] [Google Scholar]
- Hunter RHF, Cicinelli E, Einer-Jensen N. Peritoneal fluid as an unrecognized vector between female reproductive tissues. Acta Obstet Gynecol Scand. 2008;86(3):260–265. doi: 10.1080/00016340601155098. [DOI] [PubMed] [Google Scholar]
- Kalu E, Sumar N, Giannopoulos T, Patel P, Croucher C, Sherriff E, Bansal A. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. Am J Obstet Gynaecol Res. 2007;33:490–495. doi: 10.1111/j.1447-0756.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Kennedy SH, Barlow DH. Endometriotic disease: the role of peritoneal fluid. Hum Reprod Update. 1998;4:741–751. doi: 10.1093/humupd/4.5.741. Review. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Kennedy SH, Barlow DH. Pathogenesis of endometriosis: the role of peritoneal fluid. Gynecol Obstet Invest. 1999;47(Suppl. 1):23–33. doi: 10.1159/000052856. Review. [DOI] [PubMed] [Google Scholar]
- Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42:1589–1600. [PubMed] [Google Scholar]
- Lee SR, Kim SH, Lee HW, Kim YH, Chae HD, Kim CH, Kang BM. Increased expression of glutathione by estradiol, tumor necrosis factor-alpha, and interleukin 1-beta in endometrial stromal cells. Am J Reprod Immunol. 2009;62:352–356. doi: 10.1111/j.1600-0897.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McCulloch CE, Searle SR. Generalized, Linear, and Mixed Models. New York: John Wiley and Sons; 2001. [Google Scholar]
- Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D'Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 2009;92:68–74. doi: 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- Minici F, Tiberi F, Tropea A, Orlando M, Gangale MF, Romani F, Campo S, Bompiani A, Lanzone A, Apa R. Endometriosis and human infertility: a new investigation into the role of eutopic endometrium. Hum Reprod. 2008;3:530–537. doi: 10.1093/humrep/dem399. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AF, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- Oliviero S, Morrone G, Cortese R. The human haptoglobin gene: transcriptional regulation during development and acute phase induction. EMBO J. 1987;6:1905–1912. doi: 10.1002/j.1460-2075.1987.tb02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson GE, Winfrey VP, Matrisian PE, Melner MH, Hoffman LH. Specific expression of haptoglobin mRNA in implantation-stage rabbit uterine epithelium. J Endocrinol. 1997;152:69–80. doi: 10.1677/joe.0.1520069. [DOI] [PubMed] [Google Scholar]
- Oral E, Olive DL, Arici A. The peritoneal environment in endometriosis. Review Hum Reprod Update. 1996;2:385–398. doi: 10.1093/humupd/2.5.385. [DOI] [PubMed] [Google Scholar]
- Piva M, Sharpe-Timms KL. Peritoneal endometriotic lesions differentially express a haptoglobin-like gene. Mol Hum Reprod. 1999;5:71–78. doi: 10.1093/molehr/5.1.71. [DOI] [PubMed] [Google Scholar]
- Piva M, Horowitz GM, Sharpe-Timms KL. Interleukin-6 differentially stimulates haptoglobin production by peritoneal and endometriotic cells in vitro: a model for endometrium–peritoneum interaction in endometriosis. J Clin Endocrinl Metab. 2001;86:2553–2561. doi: 10.1210/jcem.86.6.7613. [DOI] [PubMed] [Google Scholar]
- Piva M, Moreno JI, Sharpe-Timms KL. Glycosylation and over-expression of endometriosis-associated peritoneal haptoglobin. Glycoconj J. 2002;19:33–41. doi: 10.1023/a:1022580813870. [DOI] [PubMed] [Google Scholar]
- Rana N, Braun DP, House R, Gebel H, Rotman C, Dmowski WP. Basal and stimulated secretion of cytokines by peritoneal macrophages in women with endometriosis. Fertil Steril. 1996;65:925–930. [PubMed] [Google Scholar]
- Sakamoto Y, Harada T, Horie S, Iba Y, Taniguchi F, Yoshida S, Iwabe T, Terakawa N. Tumor necrosis factor-alpha-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-kappa B activation: gonadotropin-releasing hormone agonist treatment reduced IL-8 expression. J Clin Endocrinol Metab. 2003;88:730–735. doi: 10.1210/jc.2002-020666. [DOI] [PubMed] [Google Scholar]
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422. [Google Scholar]
- Sampson JA. The development of the implantation theory for the origin of peritoneal endometriosis. Am J Obstet Gynecol. 1940;40:549–557. [Google Scholar]
- Sharpe-Timms KL. Endometrial anomalies in women with endometriosis. Annals NY Acad Sci. 2001;934:131–147. doi: 10.1111/j.1749-6632.2001.tb03797.x. [DOI] [PubMed] [Google Scholar]
- Sharpe-Timms KL. Haptoglobin expression by shed endometrial tissue fragments found in peritoneal fluid. Fertil Steril. 2005;84:22–30. doi: 10.1016/j.fertnstert.2005.02.014. :35–7. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Vernon MW. Polypeptides synthesized and released by rat ectopic uterine tissue differ from those of the uterus in culture. Biol Reprod. 1993;48:1334–1340. doi: 10.1095/biolreprod48.6.1334. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Zimmer RL, Griffin WT, Penney LL. Polypeptides synthesized and released by human endometriosis tissue differ from those of the uterine endometrium in culture. Fertil Steril. 1993;60:839–851. doi: 10.1016/s0015-0282(16)56285-2. [DOI] [PubMed] [Google Scholar]
- Sharpe-Timms KL, Piva M, Ricke EA, Surewicz K, Zhang YL, Zimmer RL. Endometriotic lesions synthesize and secrete a haptoglobin-like protein. Biol Reprod. 1998;58:988–994. doi: 10.1095/biolreprod58.4.988. [DOI] [PubMed] [Google Scholar]
- Sharpe-Timms KL, Ricke EA, Piva M, Horowitz GM. Differential in vivo expression and localization of endometriosis protein-I (ENDO-I), a haptoglobin homologue, in endometrium and endometriotic lesions. Hum Reprod. 2000;15:101–105. doi: 10.1093/humrep/15.10.2180. [DOI] [PubMed] [Google Scholar]
- Sharpe-Timms KL, Zimmer RL, Ricke EA, Piva MA, Horowitz GM. Endometriotic haptoglobin binds peritoneal macrophages and alters their function in endometriosis. Fertil Steril. 2002;78:810–819. doi: 10.1016/s0015-0282(02)03317-4. [DOI] [PubMed] [Google Scholar]
- Ulukus M, Cakmak H, Arici A. The role of endometrium in endometriosis. J Soc Gynecol Invest. 2006;13:467–476. doi: 10.1016/j.jsgi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer; 2000. [Google Scholar]
- Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18:177–200. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Vinatier D, Dufour P, Oosterlynck D. Immunological aspects of endometriosis. Hum Reprod Update. 1996;2:371–384. doi: 10.1093/humupd/2.5.371. Review. [DOI] [PubMed] [Google Scholar]
- Vinatier D, Cosson M, Dufour P. Is endometriosis an endometrial disease? Eur J Obstet Gynecol Reprod Biol. 2000;91:113–125. doi: 10.1016/s0301-2115(99)00263-8. [DOI] [PubMed] [Google Scholar]
- Witz CA. Pathogenesis of endometriosis. Gynecol Obstet Invest. 2002;53(Suppl. 1):52–62. doi: 10.1159/000049425. Review. [DOI] [PubMed] [Google Scholar]
- Yamauchi N, Harada T, Taniguchi F, Yoshida S, Iwabe T, Terakawa N. Tumor necrosis factor-alpha induced the release of interleukin-6 from endometriotic stromal cells by the nuclear factor-kappa B and mitogen-activated protein kinase pathways. Fertil Steril. 2004;82(Suppl. 3):1023–1028. doi: 10.1016/j.fertnstert.2004.02.134. [DOI] [PubMed] [Google Scholar]