Abstract
Background
A considerable number of Gram-negative bacteraemias occur outside intensive care units (ICUs). Inadequate antibiotic therapy in ICUs has been associated with adverse outcomes; however, there are no prospective studies in non-ICU patients.
Methods
A 6 month (1 August 2006–31 January 2007), prospective cohort study of non-ICU patients with Gram-negative bacteraemia in a tertiary-care hospital was performed. Inadequate empirical antibiotic therapy was defined as no antibiotic or starting a non-susceptible antibiotic within 24 h after the initial positive blood culture.
Results
Two hundred and fifty non-ICU patients had Gram-negative bacteraemia. The mean age was 56.4 (±16.1) years. The predominant bacteria in monomicrobial infections were Escherichia coli (24%), Klebsiella pneumoniae (18%) and Pseudomonas aeruginosa (8%). Sixty-one (24%) patients had polymicrobial bacteraemia. Seventy patients (28%) required ICU transfer and 35 (14%) died. Seventy-nine (31.6%) received inadequate empirical antibiotic therapy. These patients were more likely to have a hospital-acquired infection [odds ratio (OR) = 1.99, 95% confidence interval (CI) = 1.11–3.56, P = 0.02] and less likely to have E. coli monomicrobial bacteraemia [OR 0.40 (95% CI 0.19–0.86), P = 0.02]. There were no differences in occurrence of sepsis [72 (91.1%) patients with inadequate versus 159 (93.0%) with adequate therapy; P = 0.6], ICU transfer [20 (25.3%) versus 50 (29.2%); P = 0.5], post-bacteraemia length of stay (median = 6.8 versus 6.1 days; P = 0.09) or death [11 (13.9%) versus 24 (14.0%); P = 1.0].
Conclusions
Nearly one-third of the non-ICU patients with Gram-negative bacteraemia received inadequate empirical antibiotic therapy. There was no difference in adverse outcomes between patients receiving inadequate or adequate therapy in this study.
Keywords: bloodstream infection, Gram-negative bacteria, antibacterial agents, non-intensive care, mortality
Introduction
Approximately 250 000 episodes of bloodstream infections occur in the USA annually.1 Bloodstream infections have an overall mortality rate of 18%, making them one of the leading causes of death in the USA.2 Over the last two decades, Gram-negative bacteria have become a less frequent cause of bloodstream infections,3 since the increased use of indwelling vascular devices has resulted in a larger proportion of Gram-positive bacteraemias.1 However, there is evidence that Gram-negative bacteraemias are increasing once again.4 Antibiotic resistance among Gram-negative bacteria is also increasing.5 There has been limited development of new antibiotics with Gram-negative activity,6,7 which has made the treatment of Gram-negative bacteraemia more difficult.
Previous studies of bloodstream infections have focused primarily on intensive care unit (ICU)-acquired infections, because critically ill patients represent a well-defined and highly vulnerable population.8,9 However, bloodstream infections among hospitalized patients outside the ICU account for at least half of all nosocomial bloodstream infections.10 These infections in non-ICU patients have rarely been investigated separately.11,12 This is presumably because they were believed to be associated with less morbidity and mortality than in ICU patients, and also because the distribution of non-ICU patients in a hospital requires more workforce to conduct a prospective study. Little data are available on the demographic characteristics of non-ICU patients with Gram-negative bacteraemia and their clinical outcomes.
Several studies have demonstrated that inadequate empirical antibiotic treatment of bacteraemia is associated with poor outcome.13–16 These studies have mainly focused on ICU patients or have been carried out in diverse populations.17 Inadequate empirical treatment was reported in 23% to 30% of cases in previous studies. However, a 53% rate of inadequate treatment was reported in infections due to antibiotic-resistant organisms.18 If similar rates of inadequate treatment exist in non-ICU patients, empirical antibiotic prescribing practices would need to be re-examined.
In this study, we describe the epidemiology of Gram-negative bacteraemia in non-ICU patients at a tertiary-care hospital, investigate the frequency of inadequate antibiotic treatment, elicit predisposing factors for inadequate therapy and determine its impact on clinical outcomes.
Patients and methods
Setting
Barnes-Jewish Hospital (BJH), a 1250 bed teaching hospital, is the largest hospital in Missouri, USA, with a referral base that includes the Saint Louis metropolitan area, eastern Missouri and western Illinois.
Study design
We performed a prospective cohort study of patients with Gram-negative bacteraemia during a 6 month period from 1 August 2006 until 31 January 2007. An automated query of all non-ICU patients with a blood culture growing ≥1 species of Gram-negative bacilli was performed using electronic data from a BJC Healthcare clinical data repository and the results were sent daily to one of the investigators (J. M.).
Inclusion and exclusion criteria
All adult patients admitted to non-ICU wards who presented with or developed Gram-negative bacteraemia (≥1 positive blood culture) were included. Polymicrobial infections were also included if at least one Gram-negative organism was present. Subsequent episodes of bacteraemia in study patients were excluded from the analysis. Patients who were bacteraemic as an outpatient (in clinics or in the emergency department) and who were discharged to home before the results of the culture were known were excluded. We also excluded patients who were initially identified as having a Gram-negative bacteraemia, but were determined to have Gram-positive organisms in the final laboratory identification (n = 4).
Data collection
Paper and electronic medical records of patients who met inclusion criteria were reviewed for demographics, medical history, home medication and possible sources of infection. Information on all positive clinical cultures other than blood cultures was also collected to determine any potential focus of infection. Charlson co-morbidity19 and McCabe severity of illness20 scores were computed for each patient. Patients’ vital signs, laboratory, pharmacy and radiological data were continuously reviewed during the admission. Medication information was entered sequentially as start and stop date and time for each antibiotic.
Key clinical outcomes measured included the development of hypotension, multiple organ dysfunction syndrome, acute respiratory distress syndrome, mechanical ventilation, any subsequent transfer to the ICU, length of hospital stay after detection of positive blood cultures and in-hospital mortality.
Definitions
Adequacy of antibiotic therapy was determined at various time periods: (i) within 24 h of the time the blood culture was drawn; (ii) within 24 h of notification of bacterial growth (which coincided with the notification of Gram's stain results); (iii) within 24 h of bacterial identification; and (iv) within 24, 48 and 72 h of notification of antibiotic susceptibility results. Inadequacy of antibiotic treatment was defined as no antibiotic or no susceptibility-matching antibiotic administered during each of these time periods in order to reflect the dynamics of inadequate treatment. Various time periods have been examined in the literature, including antibiotic treatment during a period of 24 h from the time of blood culture sampling,13,14,18,21,22 at the time when antibiotic susceptibility results are available15,23 or during 48 h from the time of notification of susceptibilities.17 We analysed inadequate treatment within 24 h of blood culture sampling, since this definition has been used in the largest number of studies. If antibiotic susceptibility testing was not performed, we decided on a case-by-case basis whether treatment could be considered adequate, based on the antibiogram for that particular organism at BJH. Multidrug resistance was defined using previously published criteria.24
Sepsis, sepsis-induced hypotension and multiple organ dysfunction syndrome were defined using established criteria.25 A bacteraemia was classified as community-acquired if the first positive blood culture occurred ≤48 h after hospital admission.26 Neutropenia was defined as white blood cell count <1.0 G/L. Medical immunosuppression was defined as receipt of prednisolone equivalent of ≥10 mg daily or any other immunosuppressant (e.g. cyclosporine, methotrexate etc.) during the 30 days prior to admission.
Microbiological methods
Work-up of all blood cultures was performed by the BJH Clinical Microbiology Laboratory. Blood cultures were incubated in the Bactec 9240 system (Becton–Dickinson Diagnostic Systems, Sparks, MD, USA). Standard microbiological methods for identification and antibiotic susceptibility testing were employed.27
In our institution, the microbiology laboratory notifies the clinician when a blood culture becomes positive. Following notification, the clinician is responsible for reviewing subsequent bacterial identification and antimicrobial susceptibility results in the hospital computer system.
Data analysis and statistical methods
Data entry was performed using Microsoft Access and Excel (Microsoft Corp., Redmond, WA, USA), and data analysis was performed using SPSS 14 (SPSS Inc., Chicago, IL, USA).
Univariate comparisons among categorical variables were performed using the χ2 test or Fisher’s exact test as appropriate. Comparisons among continuous independent variables were performed using Student’s t-test or Mann–Whitney U-test as appropriate. A two-sided P value of <0.05 was considered significant. Variables found to have a P < 0.1 on univariate testing were considered for entry into a forward stepwise multivariate logistic regression model. The study was approved by the Washington University Human Research Protection Office (No. 06-0638). Owing to the observational design of the study, informed consent was not required.
Results
The epidemiology of Gram-negative bacteraemia outside the ICU
Two hundred and ninety-four patients had a Gram-negative bacteraemia during the study period. Of these, 44 (15.0%) patients were ICU patients, leaving 250 patients for analysis (Table 1).
Table 1.
Comparison of 250 non-ICU patients receiving inadequate versus adequate empirical antibiotic treatment for Gram-negative bacteraemia
| Total | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| n (%) (n = 250) | inadequate treatment (n = 79) | adequate treatment (n = 171) | P value | odds ratio (95% CI) | |
| Age, mean (±standard deviation), years | 56.4 (±16.1) | 55.3 years (±17.0) | 56.9 years (±15.8) | 0.5 | — |
| Male gender | 126 (50.4%) | 43 (54.4%) | 83 (48.5%) | 0.4 | — |
| Race | |||||
| White | 153 (61.2%) | ||||
| African-American | 94 (37.6%) | ||||
| other | 3 (1.2%) | ||||
| LTCF resident | 33 (13.2%) | 12 (15.2%) | 21 (12.3%) | 0.5 | — |
| Admitted within 3 months | 146 (58.4%) | 46 (58.2%) | 100 (58.5%) | 1.0 | — |
| BMI (median, range), kg/m2 | 26.4 (13.3–70.4) | 25.3 (17.0–70.4) | 27.3 (13.3–66.4) | 0.12 | — |
| Charlson co-morbidity score (median, range) | 4 (0–16) | 3 (0–16) | 4 (0–15) | 0.4 | — |
| McCabe severity of illness score (median, range) | 1 (1–3) | 1 (1–3) | 1 (1–3) | 0.2 | — |
| Congestive heart failure | 30 (12.0%) | 6 (7.6%) | 24 (14.0%) | 0.15 | — |
| Chronic pulmonary disease | 44 (17.6%) | 15 (19.0%) | 29 (17.0%) | 0.7 | — |
| Malignancy | 112 (44.8%) | 31 (39.2%) | 81 (47.4%) | 0.2 | — |
| leukaemia | 27 (10.8%) | 5 (6.3%) | 22 (12.9%) | 0.12 | — |
| metastatic solid tumour | 34 (13.6%) | 10 (12.7%) | 24 (14.0%) | 0.8 | — |
| neutropenia | 36 (14.4%) | 8 (10.1%) | 28 (16.4%) | 0.2 | — |
| chemotherapy ≤30 days prior to admission | 31 (12.4%) | ||||
| Received steroids ≤30 days prior to admission | 35 (14.0%) | ||||
| Other immunosuppressive therapy | 30 (12.0%) | ||||
| History of solid organ transplant | 10 (4.0%) | ||||
| Bone marrow transplant (this admission) | 10 (4.0%) | ||||
| Diabetes mellitus | 87 (34.8%) | 22 (27.8%) | 65 (38.0%) | 0.12 | — |
| Hyperglycaemia (>200 mg/dL) | 41 (16.4%) | 8 (10.1%) | 33 (19.3%) | 0.07 | — |
| Renal insufficiency (Cr >1.5 mg/dL) | 68 (27.2%) | 25 (31.6%) | 43 (25.1%) | 0.3 | — |
| Cerebrovascular disease | 28 (11.2%) | 7 (8.9%) | 21 (12.3%) | 0.4 | — |
| Hemiplegia | 15 (6.0%) | 8 (10.1%) | 7 (4.1%) | 0.06 | — |
| Liver disease | 26 (10.4%) | 12 (15.2%) | 14 (8.2%) | 0.09 | — |
| Mucositis at time of blood culture | 21 (8.4%) | 3 (3.8%) | 18 (10.5%) | 0.08 | 0.23 (0.06–0.84) |
| Source of bloodstream infection | |||||
| urinary tract | 67 (26.8%) | 14 (17.7%) | 53 (31.0%) | 0.03 | — |
| intravascular catheter | 40 (16.0%) | 18 (22.8%) | 22 (12.9%) | 0.047 | — |
| GI tract | 41 (16.4%) | ||||
| respiratory tract | 9 (3.6%) | ||||
| other source | 28 (11.2%) | ||||
| no source identified | 65 (26.0%) | ||||
| Hospital-acquired bacteraemia | 90 (36%) | 37 (46.8%) | 53 (31.0%) | 0.02 | 1.99 (1.11–3.56) |
| E. coli, monomicrobial infection | 59 (23.6%) | 10 (12.7%) | 49 (28.7%) | 0.006 | 0.40 (0.19–0.86) |
| K. pneumoniae, monomicrobial infection | 45 (18.0%) | 11 (13.9%) | 34 (19.9%) | 0.3 | — |
| P. aeruginosa, monomicrobial infection | 19 (7.6%) | 7 (8.9%) | 12 (7.0%) | 0.6 | — |
| Polymicrobial infection | 61 (24.4%) | 24 (30.4%) | 37 (21.6%) | 0.14 | — |
| Sepsis | 231 (92.4%) | 72 (91.1%) | 159 (93.0%) | 0.6 | — |
| Sepsis-induced hypotension | 105 (42.0%) | 32 (40.5%) | 73 (42.7%) | 0.7 | — |
| Outcomes | |||||
| multiple organ dysfunction syndrome | 11 (4.4%) | ||||
| transfer to ICU | 70 (28.0%) | 20 (25.3%) | 50 (29.2%) | 0.5 | — |
| mechanical ventilation after bacteraemia | 29 (11.6%) | ||||
| ARDS | 6 (2.4%) | ||||
| in-hospital mortality | 35 (14.0%) | 11 (13.9%) | 24 (14.0%) | 1.0 | — |
LTCF, long-term care facility; BMI, body mass index; GI tract, gastrointestinal tract; ICU, intensive care unit; ARDS, acute respiratory distress syndrome. Variables considered for entry in a forward stepwise multivariate logistic regression model included hospital-acquired infection; source, urinary tract; source, intravascular catheter; hemiplegia; E. coli, monomicrobial infection; hyperglycaemia; mucositis; and liver disease. The −2 log likelihood value for the final model was 293.796, and the Hosmer–Lemeshow goodness-of-fit χ2 test was 0.861 (P = 0.835).
There were 160 (64.0%) community-acquired and 90 (36.0%) hospital-acquired infections. The predominant organisms in monomicrobial bacteraemias were Escherichia coli (n = 59; 24%), Klebsiella pneumoniae (45; 18%) and Pseudomonas aeruginosa (19; 8%). Sixty-one bacteraemias were polymicrobial (24.4%) (Table 2). There were 12 (4.8%) multidrug-resistant organisms among the isolates.
Table 2.
Bacterial isolates in 250 non-ICU patients with Gram-negative bacteraemia
| Microorganism | n (%) (n = 274) |
|---|---|
| E. coli | 77 (28) |
| K. pneumoniae | 67 (24) |
| P. aeruginosa | 30 (11) |
| Enterobacter cloacae | 15 (5) |
| Proteus mirabilis | 13 (5) |
| Acinetobacter baumannii | 13 (5) |
| Klebsiella oxytoca | 8 (3) |
| Stenotrophomonas maltophilia | 6 (2) |
| Other Gram-negative microorganisms | 45 (16) |
Sixty-one (24.4%) of 250 Gram-negative bacteraemia episodes were polymicrobial infections. The most frequent among the 45 other Gram-negative organisms were Enterobacter aerogenes (n = 4), Achromobacter spp. (n = 3), Acinetobacter spp. (n = 3), Citrobacter freundii (n = 3), Citrobacter koseri (n = 3), Providencia spp. (n = 3), Pseudomonas spp. (n = 3), and Salmonella spp. (n = 3).
Two hundred and thirty-one (92.4%) patients were septic at the time of blood culture, 105 (42.0%) developed hypotension and 11 (4.4%) multiple organ dysfunction syndrome. Transfer to ICU was necessary in 70 (28.0%) patients. In-hospital mortality was 14.0% (n = 35).
The frequency of inadequate antibiotic treatment of Gram-negative bacteraemia
The antibiotics with Gram-negative activity that were most frequently prescribed during the 24 h period after the initial positive blood culture was drawn were cefepime (109; in 43.6% of episodes), ciprofloxacin (57; 22.8%), piperacillin/tazobactam (39; 15.6%), gentamicin (28; 11.2%), ceftriaxone (22; 8.8%), meropenem (9; 3.6%) and ampicillin/sulbactam (5; 2.0%). In 57 cases (22.8%), more than one antibiotic was given in this time period.
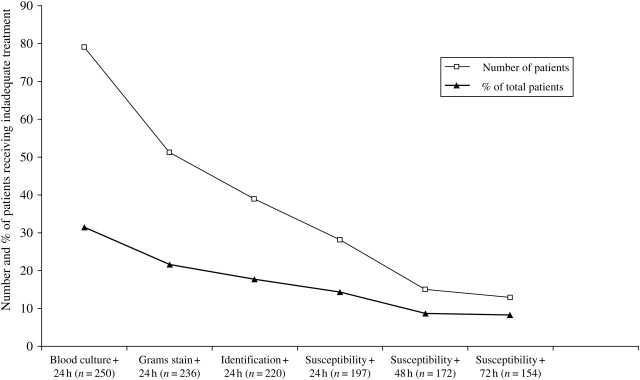
Seventy-nine (31.6%) patients received inadequate empirical antibiotic treatment. In 38 (48.1%) of these cases, inadequate treatment was due to failure to administer antibiotics with Gram-negative coverage within 24 h of the initial positive blood culture, and in 41 (51.9%) cases, it was due to a Gram-negative bacillus that was resistant to the prescribed antibiotic. Within 24 h after notification of antibiotic susceptibilities, 28 of 197 patients (14.2%) were still receiving inadequate antibiotic treatment (Figure 1).
Figure 1.
Inadequate antibiotic treatment among non-ICU patients with Gram-negative bacteraemia. Denominator changes due to patient discharge or death.
Factors associated with inadequate empirical antibiotic treatment of Gram-negative bacteraemia
Among patients receiving inadequate versus adequate empirical treatment within the first 24 h after the initial blood culture was drawn, there were no significant differences in mean age [55.3 years (±17.0) versus 56.9 years (±15.8), P = 0.5], male gender [43 (54.4%) versus 83 (48.5%), P = 0.4], body mass index (median 25.3 versus 27.3, P = 0.12), Charlson's score (median 3 versus 4, P = 0.4), McCabe score (median 1 versus 1, P = 0.2) (Table 1) or in type of service admitting the patient (data not shown). Patients with hospital-acquired bacteraemia were more often inadequately treated than those with community-acquired bacteraemia [37 (46.8%) versus 53 (31.0%) patients, P = 0.02].
E. coli was less likely to be the cause of inadequately treated bacteraemia [10 (12.7%) versus 49 (28.7%), P = 0.006]. Apart from resistance to ampicillin (58% of monomicrobial E. coli bacteraemias), E. coli were most often resistant to trimethoprim/sulfamethoxazole (21; 35.6%), ciprofloxacin (18; 30.5%), gentamicin (7; 11.9%) and piperacillin/tazobactam (2; 3.4%). Treatment was less often inadequate if the bloodstream infection had a urinary tract source [14 (20.9%) urinary versus 65 (35.5%) non-urinary source, P = 0.03].
In multivariate analysis, hospital-acquired bacteraemia [OR 1.99 (95% CI 1.11–3.56), P = 0.02] was associated with receiving inadequate empirical antibiotic treatment. Mucositis at the time of blood culture [OR 0.23 (95% CI 0.06–0.84), P = 0.03] and presence of E. coli monomicrobial bacteraemia [OR 0.40 (95% CI 0.19–0.86), P = 0.02] were more commonly associated with adequate antibiotic use (Table 1).
The outcome of inadequately empirically treated Gram-negative bacteraemia
Comparing the outcomes of inadequately versus adequately treated infections, there were no differences in transfer to the ICU [20 (25.3%) versus 50 (29.2%), P = 0.5], length of hospital stay after positive blood culture [median 6.8 days (range 1–89) versus 6.1 days (1–106), P = 0.09] or in-hospital mortality [11 (13.9%) versus 24 (14.0%), P = 1.0]. When adjusting the effect of inadequate treatment for the Charlson co-morbidity score, previous exposure to steroids and neutropenia (all of which had been found to be associated with mortality in univariate analysis), inadequate treatment did not remain in the final model (data not shown). There was no difference in mortality whether cefepime had been used for empirical treatment or not [17 (15.6%) patients exposed to cefepime versus 18 (12.8%) not exposed; P = 0.5].
Definitive treatment (defined as administration of an antibiotic that matched the bacteria’s susceptibility pattern within 24 h of notification of susceptibilities) was more often inadequate if empirical antibiotic treatment had been inadequate compared with if it had been adequate [20 (30.8%) with inadequate empirical therapy versus 8 (6.1%) with adequate empirical therapy, P < 0.001].
Discussion
Non-ICU patients account for approximately half of the bloodstream infections in the hospital.2,10 An even larger proportion of Gram-negative bacteraemias (62% to 95%) occurs in non-ICU patients.28–30 Nevertheless, bacteraemias have rarely been investigated outside the ICU,11,12,31 which may be due to the heterogeneity of non-ICU patients. To our knowledge, this is the first prospective study of Gram-negative bacteraemia in the non-ICU hospitalized population. During the study period, non-ICU patients accounted for 85% (250 of 294) of all Gram-negative bacteraemias in this hospital. The demographics, co-morbidities and microbiology of infections in this study are similar to retrospective studies of Gram-negative bacteraemias in hospitalized patients.28,29,32,33 Urinary tract infections were the predominant source of bacteraemia, and E. coli was the most frequently detected organism. This is in contrast to Gram-negative bacteraemias in ICU patients, which frequently originate from the respiratory34 or gastrointestinal tract35 and are more often caused by P. aeruginosa.31
Twenty-eight per cent of patients were transferred to the ICU after the bacteraemia had occurred. The in-hospital mortality was substantial (14%), but less than the 24% mortality rate in a Danish population-based study28 or in studies of ICU patients with Gram-negative bacteraemia (49% to 60%).34,35 This is likely due to differences in population characteristics including different levels of severity of underlying illnesses, but might also point to differences in the management of sepsis rather than antibiotic treatment.
One of the major modifiable factors influencing the outcome of bacteraemia is the adequacy of antibiotic treatment.36 This was demonstrated in studies including ICU patients.13–17,23 However, no study has examined the effect of adequate antibiotic treatment on outcomes in non-ICU patients only. We demonstrated rates of inadequate empirical treatment during the first 24 h after the blood culture (31.6%) similar to the 30% to 37% reported from other prospective studies.15,17 In approximately half of the cases, inadequate treatment was due to failure to administer an antibiotic with Gram-negative activity.
Hospital-acquired bacteraemia was a risk factor for receiving inadequate empirical antibiotic treatment in our cohort. This has been noted previously13–15,21,22 and suggests that physicians are often unaware of the different microbiological patterns in the hospital versus the community. Increasing antibiotic resistance and lack of prescriber knowledge regarding appropriate antibiotics for likely in-hospital pathogens may lead to the institution of inadequate empirical antibiotic treatment. Decision support tools, based on local bacterial antimicrobial resistance patterns in association with clinical information and inclusion of Gram's stain results, may improve the choice of empirical therapy.37,38 Several other risk factors for inadequate treatment have been found, e.g. previous antibiotic treatment,13,14 hospital admission in the 90 days prior to the current admission,21 polymicrobial infections14 and Pseudomonas infections,22 which we did not find. Conversely, E. coli infection was associated with less risk of inadequate treatment, which has been reported before by others.13,22 E. coli is the most frequent cause of Gram-negative bacteraemia and is not as prone to multidrug resistance as other Gram-negative bacteria,33 which may explain why it is generally better covered by empirical antimicrobials. The finding that mucositis was protective against inadequate treatment might be related to mucositis being more often present in a subset of oncology patients and a tendency to start broad-spectrum antibiotics with Gram-negative activity earlier in this population.
In our cohort of patients, inadequate empirical treatment was not associated with deterioration of status (transfer to the ICU, length of hospital stay or increased in-hospital mortality). This is in contrast to many studies, in which inadequate treatment was associated with adverse outcomes.13–17,23 However, a few studies that included mixed ICU and non-ICU patient populations have not found this association.21,22 One possible explanation for our finding is that non-ICU patients in general have a lower severity of illness compared with ICU patients and, therefore, the role of the adequate antibiotic treatment may be less crucial.36 A study underlining this assumption showed that inadequate treatment was more frequently administered in less severely ill patients, with no discernable impact on outcomes.22 Interventions focused on optimizing treatment for non-ICU patients would likely have the greatest benefit in, for example, neutropenic patients, transplant patients and patients at risk for Pseudomonas bacteraemia.
In addition, we did not find that the use of cefepime for empirical treatment was associated with increased all-cause mortality as a recent meta-analysis has reported.39
There are some limitations to our study. First, this is a single, tertiary-care hospital and may reflect process issues unique to this facility. In our hospital, the clinician is only directly notified by the microbiology laboratory when a blood culture turns positive, but needs to look up subsequent bacterial identification and antimicrobial susceptibility results in the hospital computer system. This may cause delays in starting adequate antibiotic treatment. We also only collected crude mortality, not attributable mortality. The sample size is large for a single-centre prospective study but may still be too small to detect a difference in outcomes, as reported by Fraser et al.40 from a mixed ICU and non-ICU population.
One of the strengths of this prospective study is the detailed sequential analysis of the adequacy of antibiotic treatment at different time points. Previous studies of the adequacy of treatment have analysed one specific time frame and not taken into account the dynamic that is inherent in the processing of blood cultures and the notification of results to the treating physician. We also evaluated empirical and definitive therapy separately and controlled for baseline severity of illness.41 At our institution, antibiotic treatment is initiated by clinicians from various specialties and levels of professional experience, and is therefore diverse, which adds to the generalizability of our findings.
Our study is the first to prospectively describe the epidemiology of Gram-negative bacteraemias in non-ICU patients. The frequency of inadequate empirical antibiotic treatment is similar to data from ICUs. The administration of inadequate treatment did not confer worse patient outcomes. Therefore, although adequate antibiotic therapy is an important factor, our findings suggest that there are other factors that may be more important in determining the prognosis in the non-ICU population.
Funding
J. M. received a research grant from the Swiss National Science Foundation (PBBSB-113014). D. K. W. (K23 AI050585-02) and V. J. F. (IK24 AI 06779401) are funded through NIH grants. D. K. W. and V. J. F. received a CDC Prevention Epicenter Programme grant (CDC 1U1CI000033301). The study was performed without industry support.
Transparency declarations
D. K. W. is a Consultant for 3M Healthcare, Novabay Pharmaceuticals and Enturia, Inc., and receives research funding from Sage Products, Inc. and 3M Healthcare. V. J. F. is a Consultant for Steris and Verimetrix, and Member of the Speakers Bureau for Pfizer, Merck and Cubist Pharmaceuticals. All other authors have no conflict of interest to declare.
Acknowledgements
We thank Cherie Hill, Stacy Leimbach and Dorothy Sinclair for the invaluable help in data management.
References
- 1.Pittet D, Wenzel RP. Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch Intern Med. 1995;155:1177–84. doi: 10.1001/archinte.155.11.1177. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein MP, Towns ML, Quartey SM, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 3.Gaynes R, Edwards JR and the National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by Gram-negative bacilli. Clin Infect Dis. 2005;41:848–54. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 4.Albrecht SJ, Fishman NO, Kitchen J, et al. Reemergence of Gram-negative health care-associated bloodstream infections. Arch Intern Med. 2006;166:1289–94. doi: 10.1001/archinte.166.12.1289. [DOI] [PubMed] [Google Scholar]
- 5.D’Agata EM. Rapidly rising prevalence of nosocomial multidrug-resistant, Gram-negative bacilli: a 9-year surveillance study. Infect Control Hosp Epidemiol. 2004;25:842–6. doi: 10.1086/502306. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 7.Rice LB. Challenges in identifying new antimicrobial agents effective for treating infections with Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis. 2006;43(Suppl 2):S100–5. doi: 10.1086/504487. [DOI] [PubMed] [Google Scholar]
- 8.Warren DK, Zack JE, Elward AM, et al. Nosocomial primary bloodstream infections in intensive care unit patients in a nonteaching community medical center: a 21-month prospective study. Clin Infect Dis. 2001;33:1329–35. doi: 10.1086/322483. [DOI] [PubMed] [Google Scholar]
- 9.Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271:1598–601. doi: 10.1001/jama.271.20.1598. [DOI] [PubMed] [Google Scholar]
- 10.Edmond MB, Wallace SE, McClish DK, et al. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–44. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 11.Suljagic V, Cobeljic M, Jankovic S, et al. Nosocomial bloodstream infections in ICU and non-ICU patients. Am J Infect Control. 2005;33:333–40. doi: 10.1016/j.ajic.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Garrouste-Orgeas M, Chevret S, Mainardi JL, et al. A one-year prospective study of nosocomial bacteraemia in ICU and non-ICU patients and its impact on patient outcome. J Hosp Infect. 2000;44:206–13. doi: 10.1053/jhin.1999.0681. [DOI] [PubMed] [Google Scholar]
- 13.Bouza E, Sousa D, Munoz P, et al. Bloodstream infections: a trial of the impact of different methods of reporting positive blood culture results. Clin Infect Dis. 2004;39:1161–9. doi: 10.1086/424520. [DOI] [PubMed] [Google Scholar]
- 14.Harbarth S, Garbino J, Pugin J, et al. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003;115:529–35. doi: 10.1016/j.amjmed.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim EH, Sherman G, Ward S, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–55. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 16.Kollef MH, Sherman G, Ward S, et al. Inadequate antimicrobial treatment of infections. Chest. 1999;115:462–74. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- 17.Leibovici L, Shraga I, Drucker M, et al. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med. 1998;244:379–86. doi: 10.1046/j.1365-2796.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 18.Kang CI, Kim SH, Park WB, et al. Bloodstream infections caused by antibiotic-resistant Gram-negative bacilli; risk factors for mortality and impact of inappropriate antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49:760–6. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.McCabe WR, Jackson GG. Gram-negative bacteremia. Arch Intern Med. 1962;110:847–55. [Google Scholar]
- 21.McDonald JR, Friedman ND, Stout JE, et al. Risk factors for ineffective therapy in patients with bloodstream infection. Arch Intern Med. 2005;165:308–13. doi: 10.1001/archinte.165.3.308. [DOI] [PubMed] [Google Scholar]
- 22.Scarsi KK, Feinglass JM, Scheetz MH, et al. Impact of inactive empiric antimicrobial therapy on inpatient mortality and length of stay. Antimicrob Agents Chemother. 2006;50:3355–60. doi: 10.1128/AAC.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Micek ST, Lloyd AE, Ritchie DJ, et al. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother. 2005;49:1306–11. doi: 10.1128/AAC.49.4.1306-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lockhart SR, Abramson MA, Beekmann SE, et al. Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol. 2007;45:3352–9. doi: 10.1128/JCM.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 26.Diekema DJ, Beekmann SE, Chapin KC, et al. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol. 2003;41:3655–60. doi: 10.1128/JCM.41.8.3655-3660.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Sixteenth Informational Supplement M100-S16. Wayne, PA, USA: CLSI; 2006. [Google Scholar]
- 28.Pedersen G, Schonheyder HC, Sorensen HT. Antibiotic therapy and outcome of monomicrobial Gram-negative bacteraemia: a 3 year population based study. Scand J Infect Dis. 1997;29:601–6. doi: 10.3109/00365549709035903. [DOI] [PubMed] [Google Scholar]
- 29.Uzun O, Akalin HE, Hayran M, et al. Factors influencing prognosis in bacteremia due to Gram-negative organisms: evaluation of 448 episodes in a Turkish university hospital. Clin Infect Dis. 1992;15:866–73. doi: 10.1093/clind/15.5.866. [DOI] [PubMed] [Google Scholar]
- 30.Pittet D, Li N, Woolson RF, et al. Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin Infect Dis. 1997;24:1068–78. doi: 10.1086/513640. [DOI] [PubMed] [Google Scholar]
- 31.Arvanitidou M, Katikaridou E, Douboyas J, et al. Epidemiological characteristics of nosocomial bacteraemias among ICU and non-ICU patients in a tertiary-care hospital in Greece. J Hosp Infect. 2005;59:70–2. doi: 10.1016/j.jhin.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 32.Harbarth S, Rohner P, Auckenthaler R, et al. Impact and pattern of Gram-negative bacteraemia during 6 y at a large university hospital. Scand J Infect Dis. 1999;31:163–8. doi: 10.1080/003655499750006218. [DOI] [PubMed] [Google Scholar]
- 33.Diekema DJ, Pfaller MA, Jones RN, et al. Survey of bloodstream infections due to Gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin Infect Dis. 1999;29:595–607. doi: 10.1086/598640. [DOI] [PubMed] [Google Scholar]
- 34.Sligl W, Taylor G, Brindley PG. Five years of nosocomial Gram-negative bacteremia in a general intensive care unit: epidemiology, antimicrobial susceptibility patterns, and outcomes. Int J Infect Dis. 2006;10:320–5. doi: 10.1016/j.ijid.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Gardiner DF, Scholand SJ, Babinchak T. Mortality and Gram-negative rod bacteraemia in the intensive care unit. J Hosp Infect. 2006;62:453–7. doi: 10.1016/j.jhin.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Harbarth S, Nobre V, Pittet D. Does antibiotic selection impact patient outcome? Clin Infect Dis. 2007;44:87–93. doi: 10.1086/510075. [DOI] [PubMed] [Google Scholar]
- 37.Mullett CJ, Thomas JG, Smith CL, et al. Computerized antimicrobial decision support: an offline evaluation of a database-driven empiric antimicrobial guidance program in hospitalized patients with a bloodstream infection. Int J Med Inf. 2004;73:455–60. doi: 10.1016/j.ijmedinf.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Hautala T, Syrjälä H, Lehtinen V, et al. Blood culture Gram stain and clinical categorization based empirical antimicrobial therapy of bloodstream infection. Int J Antimicrob Agents. 2005;25:329–33. doi: 10.1016/j.ijantimicag.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Yahav D, Paul M, Fraser A, et al. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis. 2007;7:338–48. doi: 10.1016/S1473-3099(07)70109-3. [DOI] [PubMed] [Google Scholar]
- 40.Fraser A, Paul M, Almanasreh N, et al. Benefit of appropriate empirical antibiotic treatment: thirty-day mortality and duration of hospital stay. Am J Med. 2006;119:970–6. doi: 10.1016/j.amjmed.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 41.McGregor JC, Rich SE, Harris AD, et al. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin Infect Dis. 2007;45:329–37. doi: 10.1086/519283. [DOI] [PubMed] [Google Scholar]