Abstract
Objectives
The susceptibility of clinical isolates of Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA), to host-derived cationic antimicrobial peptides was investigated.
Methods
We examined the susceptibility of 190 clinical strains of methicillin-susceptible S. aureus (MSSA) and 304 strains of MRSA to two different classes of cationic antimicrobial peptides: LL-37 and human β-defensin-3 (hBD3). Out of the total 494 clinical strains, a random selection of 54 S. aureus strains was examined to establish the relationship between the net charge, or zeta potential, of each strain and its susceptibility to hBD3 or LL-37. To further confirm bacterial susceptibility to either hBD3 or LL-37, we concurrently measured: (i) percentage survival after in vitro bacterial exposure and (ii) MBCs for both MRSA and MSSA strains.
Results
Of the 54 randomly selected S. aureus strains, those MRSA strains resistant to LL-37 showed significantly higher zeta potentials than those susceptible to LL-37 (P < 0.05). In contrast, there was no difference in bacterial zeta potentials for MRSA strains that showed either resistance or susceptibility to hBD3. In addition, resistance to LL-37, but not to hBD3, as determined by either percentage survival or MBC, was significantly elevated in highly methicillin-resistant strains of S. aureus when compared with MSSA strains (P < 0.01).
Conclusions
Clinical strains of MRSA, but not MSSA, that demonstrated an increased net charge also showed elevated resistance to LL-37, but not to hBD3.
Keywords: cathelicidin family peptide, human β-defensin, clinical isolates, antibiotic susceptibility, innate immunity
Introduction
In humans, antimicrobial peptide production, which occurs within the innate immune system at the epidermal and mucosal epithelial surfaces, also involves phagocytes, such as neutrophils.1 Human epithelia are known to produce four distinct β-defensins (hBD1–4) and one cathelicidin family peptide (LL-37), which have all been identified as cationic antimicrobial peptides.2 As bacteria first penetrate the host's immune defence system via epidermal and mucosal epithelia, these antimicrobial peptides play necessary and significant roles in the host's innate immune defence against the initial colonization of bacteria at these sites. Staphylococcus aureus is a major pathogen causing infectious disease in humans. Therefore, it is important to understand whether S. aureus, including methicillin-resistant S. aureus (MRSA), possesses resistance not only to antibiotics, but also to host-derived antimicrobial peptides. We previously demonstrated that bacterial net charge, including that of S. aureus, affects the resistance of a given bacterium to cationic antimicrobial peptides.3,4 Therefore, the present study has extended these results by examining the susceptibility of 494 strains of S. aureus clinical isolates to two cationic antimicrobial peptides, hBD3 and LL-37, in relation to the variance of bacterial net charges.
Materials and methods
Bacterial strains and subject populations
Between 1999 and 2001, 494 S. aureus strains were isolated from various clinical sources at the Hiroshima Prefecture Hospital in Hiroshima City, Japan. Informed consent agreements were provided to all subjects prior to bacterial sampling. Each strain was independently isolated from a particular lesion. Not more than two lesions were chosen for bacterial sampling from each patient. S. aureus was grown aerobically in Trypticase soy (TS) broth (BBL) at 37°C, unless otherwise stated.
Zeta potential determination
Of 494 clinical isolates of S. aureus, a random selection of 54 S. aureus strains [15 strains of methicillin-susceptible S. aureus (MSSA) and 39 strains of MRSA] was specifically examined to first establish susceptibility to both LL-37 and hBD3. Then, the degree of such susceptibility was correlated with bacterial net charge and the level of methicillin resistance of the bacterial cells, as measured by the MIC of methicillin. To evaluate the net charge of bacterial cells, a Zeecom™ zeta potential analyser (Microtec, Nition; Funabashi, Japan) was used, as previously described.4
Assay to determine the antibacterial activity of antimicrobial peptides
Percentage survival
The antibacterial assay was performed following the protocol described previously.5 Briefly, overnight cultures of bacterial strains were harvested, washed with PBS and suspended in sodium phosphate. The bacterial suspension (105–106 cells) was inoculated into 200 µL of sodium phosphate with or without synthetic hBD3 (2 mg/L) or LL-37 (1 mg/L) and incubated aerobically for 2 h at 37°C. Synthetic peptides of hBD3 and LL-37 were generated, as described previously.5 Dilutions of the reaction mixture (100 µL) were applied onto agar plates and incubated at 37°C overnight. The number of cfu was determined as the total number of colonies identified on each plate. The antibacterial effect was calculated as the ratio of surviving cells (percentage survival) to the total number of bacteria incubated in control sodium phosphate solution after exposure to antimicrobial peptides.
Minimal bactericidal concentration
We also measured the MBC (>99.9% killing rate) using a modified method as described by Harder et al.6 The bacterial suspension (104 cells) was applied to 100 µL of sodium phosphate with or without hBD3, at 1, 2, 4, 8 and 16 mg/L or LL-37, at 0.5, 1, 2, 4 and 8 mg/L and incubated for 2 h at 37°C. Immediately following incubation, the bacterial mixture was plated onto TS agar (TSA) plates. To measure the total bacterial cells, the reaction mixture without peptides was similarly diluted and poured onto TSA plates. After counting the colonies grown on the TSA plates, MBC was defined as the concentration that killed >99.9% of total bacteria.
MIC of methicillin for S. aureus
The MIC of methicillin (Sigma-Aldrich) was determined using the microdilution method, as described previously.7
Results
Zeta potential of clinical S. aureus strains
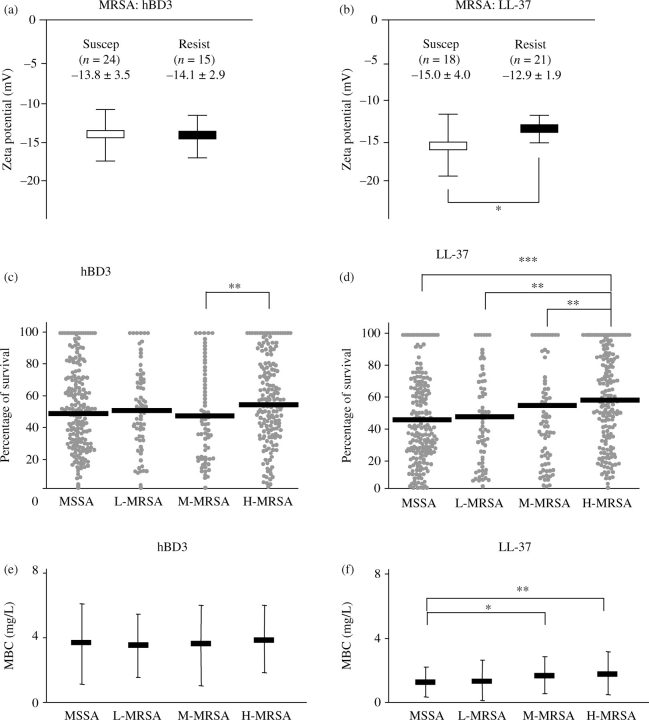
Zeta potentials of 54 randomly selected S. aureus strains (MSSA, n = 15 and MRSA, n = 39) ranged between −8 and −18 mV (−13.2 ± 2.7 mV; average ± SD). However, among these 54 S. aureus strains, there was no remarkable difference in zeta potential between MSSA and MRSA (−12.9 ± 2.8 and −13.3 ± 2.6 mV, respectively). Therefore, the zeta potentials were next compared among the isolates of the two separate groups; i.e. comparison was made among the group of 39 chosen MRSA strains or among the group of 15 chosen MSSA strains. However, to accomplish this, each group was, in turn, divided into two subgroups: (i) antimicrobial peptide-susceptible strains, as determined by percentage survival <50% and (ii) antimicrobial peptide-resistant strains, as determined by percentage survival rate equal to, or exceeding, 50% (Figure 1a and b shows MRSA subgroups). Thus, four subgroups each were established for the 39 MRSA and 15 MSSA strains, representing resistance and susceptibility to either LL-37 or hBD3. There was no significant difference in zeta potential between the hBD3-susceptible MRSA strains and the hBD3-resistant MRSA strains (Figure 1a). However, when the same group of MRSA was classified by susceptibility or resistance to LL-37, the LL-37-resistant MRSA strains showed significantly higher zeta potential than the LL-37-susceptible MRSA strains (Figure 1b). In contrast to these results, no distinct difference in either susceptibility or resistance was noted among the MSSA groups in relation to zeta potential (data not shown).
Figure 1.
Zeta potential of 39 clinical isolates of MRSA and susceptibility of 494 S. aureus strains to in vitro exposure with hBD3 and LL-37. Zeta potential was measured using the method described in the Materials and methods section. The percentage survival of MRSA to hBD3 (a) and LL-37 (b) was compared with the zeta potential of each bacterial strain. The open and filled rectangles represent the average of zeta potentials of susceptible strains that showed a percentage survival of <50% (Suscep) and resistant strains that showed a percentage survival equal to, or exceeding, 50% (Resist) for each of the antimicrobial peptides tested. Evaluation of 494 strains was conducted to determine their susceptibility to hBD3 and LL-37 using the two methods described in the Materials and methods section. MRSA strains were divided into three subgroups (L-MRSA, M-MRSA and H-MRSA). The percentage survival of S. aureus strains (MSSA and the three subgroups of MRSA) after exposure to hBD3 (2 mg/L) or LL-37 (1 mg/L) is shown (c and d). The solid bar indicates the average of percentage survival in each group. MBCs for S. aureus strains (MSSA and the three subgroups of MRSA) are shown (e and f). Filled rectangles and error bars indicate the average MBC and SD, respectively. *, ** and *** indicate significant differences between the groups shown in brackets, by Student's t-test (P < 0.05, P < 0.01 and P < 0.001, respectively).
Levels of methicillin resistance of S. aureus and their susceptibility to LL-37 or hBD3
A total of 494 S. aureus strains were divided into the following four subgroups on the basis of their level of resistance to methicillin: (i) MSSA; (ii) L-MRSA (low resistance, with an MIC of methicillin of 4–16 mg/L); (iii) M-MRSA (intermediate resistance, with an MIC of methicillin of 32–512 mg/L) and (iv) H-MRSA (high resistance, with an MIC of methicillin of ≥1024 mg/L) (Table 1). Next, bacterial percentage survival in response to hBD3 or LL-37 was cross-evaluated among the subgroups (i.e. MSSA, L-MRSA, M-MRSA and H-MRSA) (Figure 1c, hBD3 and d, LL-37). The hBD3 treatment did not show noticeable differences in percentage survival between any of the subgroups of S. aureus (Figure 1c). However, MRSA did show a trend of increasing resistance to LL-37 exposure in relation to the level of resistance to methicillin, as indicated by the subgroups listed earlier (Figure 1d). Especially, H-MRSA demonstrated a significantly higher percentage survival than MSSA, L-MRSA or M-MRSA in response to LL-37 (Figure 1d).
Table 1.
Susceptibility of S. aureus clinical strains to methicillin
| No. of isolates with MIC (mg/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | >1024 |
| MSSA (190) | 13 | 177 | |||||||||
| MRSA (304) | |||||||||||
| L-MRSA (68) | 19 | 26 | 23 | ||||||||
| M-MRSA (70) | 18 | 7 | 6 | 8 | 31 | ||||||
| H-MRSA (166) | 166 | ||||||||||
The MBC assay showed that, across all subtypes, the 494 strains of S. aureus clinical isolates examined in this study were more resistant to hBD3 than to LL-37 (mean MBC: hBD3, 3.54 ± 2.21 mg/L and LL-37, 1.14 ± 1.13 mg/L). As the concentration of antimicrobial peptides expressed in vivo in humans, especially in perspiration, is ∼2 mg/L for hBD3 and 1 mg/L for LL-37,8,9 it should be noted that the concentration for susceptibility tests determined in vitro in this study is within the physiologically meaningful range. Compared with MSSA strains, the H-MRSA and M-MRSA strains demonstrated significantly higher MBCs of LL-37, whereas the L-MRSA strains did not show significant differences in their MBCs when compared with MSSA in response to LL-37 (Figure 1f). However, in contrast to LL-37, there was no statistical difference in MBCs of hBD3 among the four subgroups of S. aureus (MSSA and the three subgroups of MRSA) (Figure 1e). Therefore, the results of both the percentage survival and MBC assays indicated that the levels of methicillin resistance of S. aureus only relate to the susceptibility of this bacterium to LL-37, not to hBD3.
Discussion
The present study demonstrated that, when compared with MSSA bacterial isolates, clinical strains of MRSA increase their resistance to LL-37 and that this increase is associated with both the level of methicillin resistance and, significantly, bacterial net charge. Although some of the clinical strains of S. aureus showed resistance to hBD3, there was no significant difference in hBD3 resistance between MRSA and MSSA. As noted earlier, the increased MRSA resistance to LL-37, but not to hBD3, appeared to partly result from the higher net charge of MRSA. To explain this, LL-37 carries a low cationic charge (calculated net peptide charge of 6), compared with hBD3 (calculated net peptide charge of 10). Therefore, it is conceivable that MRSA strains that have a high zeta potential can establish a correspondingly higher resistance to LL-37 when compared with hBD3. In other words, because of the relatively lower peptide net charge of LL-37, as opposed to that of hBD3, it is conceivable that a slight change in the bacterial net charge may be enough to attenuate the charge-dependent LL-37 attachment to the bacterial cell surface.
MRSA also showed a trend of increasing resistance to LL-37 exposure in relation to the level of MRSA resistance to methicillin, as indicated by the subgroups (L-MRSA to H-MRSA) (Figure 1d and f). A gene known as mec regulates methicillin resistance, and this has raised the possibility that mec itself might be directly associated with the LL-37 resistance present in MRSA strains. To make this determination, we compared the LL-37 susceptibility between an isogenic strain of S. aureus BB255 (mec-negative MSSA) and BB270 (mec-positive mutant of BB255).10 Contrary to our expectation, no difference in susceptibility to LL-37, or to hBD3, was identified between these two strains (H. K. and K. O., unpublished results), indicating that expression of mec is not required for LL-37 resistance in MRSA. In the absence of an underlying molecular mechanism explaining LL-37 resistance in MRSA, it is plausible that there might be another bacterial gene, aside from mec, that regulates bacterial resistance to LL-37. In any case, our results did show that MRSA resistance to LL-37 does increase in proportion to the level of methicillin resistance (see the Results section) and that, although such an increase is associated with bacterial net charge, it does complement the overall resistance level of MRSA to LL-37 specifically.
In conclusion, these findings suggest that clinical strains of MRSA do possess an elevated resistance to cationic antimicrobial peptide LL-37 in association with their increased net charge and level of methicillin resistance. To the best of our knowledge, this is the first report to provide evidence that strains of MRSA possess the ability to resist host-derived antimicrobial peptides.
Funding
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan and NIH grant DE-018310.
Transparency declarations
None to declare.
References
- 1.Lehrer RI, Ganz T. Antimicrobial peptides in mammalian and insect host defence. Curr Opin Immunol. 1999;11:23–7. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 2.Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res. 2000;1:141–50. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishi H, Komatsuzawa H, Fujiwara T, et al. Reduced content of lysyl-phosphatidylglycerol in the cytoplasmic membrane affects susceptibility to moenomycin, as well as vancomycin, gentamicin, and antimicrobial peptides, in Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:4800–7. doi: 10.1128/AAC.48.12.4800-4807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouhara K, Komatsuzawa H, Yamada S, et al. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, β-defensins and LL37, produced by human epithelial cells. J Antimicrob Chemother. 2005;55:888–96. doi: 10.1093/jac/dki103. [DOI] [PubMed] [Google Scholar]
- 5.Midorikawa K, Ouhara K, Komatsuzawa H, et al. Staphylococcus aureus susceptibility to innate antimicrobial peptides, β-defensins and CAP18, expressed by human keratinocytes. Infect Immun. 2003;71:3730–9. doi: 10.1128/IAI.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harder J, Bartels J, Christophers E, et al. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 7.Komatsuzawa H, Ohta K, Sugai M, et al. Tn551-mediated insertional inactivation of the fmtB gene encoding a cell wall-associated protein abolishes methicillin resistance in Staphylococcus aureus. J Antimicrob Chemother. 2000;45:421–31. doi: 10.1093/jac/45.4.421. [DOI] [PubMed] [Google Scholar]
- 8.Murakami M, Ohtake T, Dorschner RA, et al. Cathelicidin antimicrobial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol. 2002;119:1090–5. doi: 10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- 9.Singh PK, Jia HP, Wiles K, et al. Production of β-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–6. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck WD, Berger-Bachi B, Kayser FH. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J Bacteriol. 1986;165:373–8. doi: 10.1128/jb.165.2.373-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]