Abstract
Objectives
Increased bone marrow iron levels in patients with haematological malignancies is an independent risk factor for developing invasive pulmonary aspergillosis (IPA), suggesting an important role for iron uptake in the pathogenesis of IPA. We sought to determine the potential for combination therapy with the iron chelator deferasirox + liposomal amphotericin B (LAmB) to improve the outcome of murine IPA compared with LAmB monotherapy.
Methods
In vitro MIC and minimum fungicidal concentration (MFC) values of the iron chelator, deferasirox, for Aspergillus fumigatus were determined by microdilution assay. In addition, we studied the efficacy of deferasirox alone or combined with LAmB in treating immunocompromised mice infected with A. fumigatus via inhalation.
Results
Deferasirox was cidal in vitro against A. fumigatus, with an MIC and MFC of 25 and 50 mg/L, respectively. Deferasirox monotherapy modestly prolonged survival of mice with IPA. Combination deferasirox + LAmB therapy synergistically improved survival and reduced lung fungal burden compared with either monotherapy alone.
Conclusions
Iron chelation therapy with deferasirox alone or in combination with LAmB is effective in treating experimental IPA. Further study of deferasirox is warranted as adjunctive therapy for IPA infections.
Keywords: Aspergillus fumigatus, LAmB, IPA
Introduction
A rise in the incidence of invasive pulmonary aspergillosis (IPA) in immunocompromised patients has occurred during the last two decades.1 Despite the development of new therapeutic agents, mortality with IPA remains >50%.2 Clearly new modalities are needed to prevent IPA and/or improve the clinical outcome of infected patients.
Recent laboratory studies demonstrate that iron acquisition is essential for the growth and virulence of Aspergillus.3 Evidence that invasive aspergillosis is associated with iron overload in patients with haematological malignancies has been found in case studies.4 Most recently, Kontoyiannis et al.5 reported that an increased bone marrow iron level was an independent risk factor for developing IPA in high-risk patients. Therefore, chelating host iron with an appropriate agent might improve the outcome of IPA.
Deferasirox is the first orally available iron chelator approved by the FDA, with an indication for the treatment of transfusion-related iron overload. We have found that deferasirox is highly active against Mucorales, and has significant efficacy both as monotherapy and in combination therapy with lipid polyenes for the treatment of murine mucormycosis.6 We sought to determine the potential efficacy of deferasirox against IPA.
Materials and methods
Culture conditions
A. fumigatus clinical strain AF293 was used for all experiments. To prepare the inoculum for in vitro or in vivo studies, A. fumigatus was grown on Sabouraud dextrose agar (SDA) plates for 2 weeks at 37°C. For susceptibility experiments, A. fumigatus was starved of iron by growing on SDA in the presence of 1 mM ascorbic acid and 1 mM ferrozine, to eliminate internal iron stores. Conidia were collected as described before.7
Susceptibility testing and animal model
The MIC was determined for deferasirox following the CLSI M38-A2 method8 on iron-starved conidia. The MFC was determined by spotting samples from all of the 96-well plate on SDA plates and incubating at 37°C for 5 days. The MFC was defined as the least concentration of the drug at which the organism failed to grow on the SDA plate.
BALB/c male mice were immunosuppressed by cyclophosphamide and cortisone acetate given at 250 mg/kg on day −2 relative to infection and repeated on day +3 at 200 mg/kg for cyclophosphamide and 250 mg/kg for cortisone acetate.7 This regimen resulted in a duration of leucopenia up to +7 to +8 days relative to infection.7 Mice were infected with A. fumigatus by aerosolizing 1.2 × 1010 conidia in our inhalational chamber.7 Immediately after exposure to aerosolized conidia, three mice were sacrificed, and the lungs were homogenized and quantitatively cultured to determine the infectious inoculum. Deferasirox (Novartis Pharmaceuticals) was administered at 10 mg/kg by oral gavage in 0.5% hydroxypropylcellulose (Klucel) twice daily every other day for a total of four doses.6 This treatment regimen was chosen based on previous efficacy against Rhizopus oryzae in neutropenic mice.6 Deferasirox was initiated either 24 h post-infection (delayed therapy) or 2 days prior to infection (prophylactic therapy). Liposomal amphotericin B (LAmB; Gilead Sciences) was diluted in 5% dextrose water (5DW) and administered via tail-vein injection at a dose of 3 mg/kg/day starting 24 h post-infection and continued daily for five doses. Placebo mice were given 5DW and 0.5% Klucel. All mice were randomly assigned for treatment regimens. The primary endpoint was time to death of moribund mice. As a secondary endpoint lung fungal burden was determined 96 h post-infection by homogenization using gentle rolling in Whirl-Pak bags containing 1 mL of saline, and plated on SDA for quantification of tissue fungal burden.
For histopathological examination, lungs were collected 6 days post-infection, fixed in 10% zinc-buffered formalin, paraffin embedded, sectioned and stained with Gomori Methenamine Silver stain for microscopic examination. All procedures involving mice were approved by the institutional animal use and care committee, according to the National Institutes of Health guidelines for animal housing and care.
Statistical analysis
The non-parametric log-rank test was used to determine differences in survival times, whereas differences in lung fungal burden were compared by the non-parametric Mann–Whitney test.
Results
Deferasirox has a modest but significant effect against A. fumigatus in vitro and in vivo
Initially, we determined the in vitro activity of deferasirox against A. fumigatus. After a 48 h incubation period, deferasirox had an MIC of 25 mg/L and an MFC of 50 mg/L.
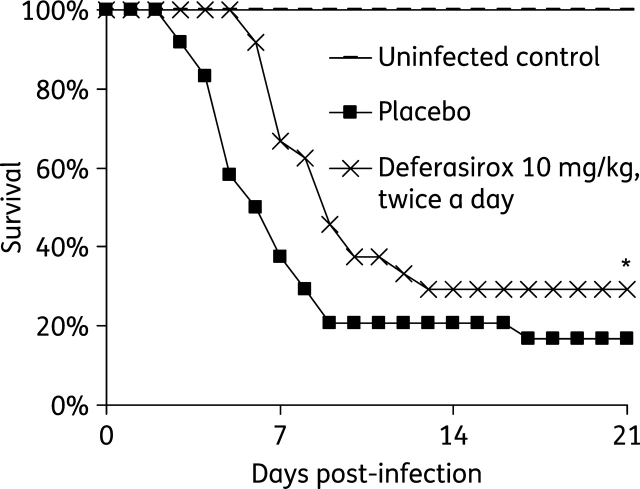
Next, mice were infected via inhalation and treated with 10 mg/kg of deferasirox alone given twice daily starting 24 h post-infection. Mice treated with deferasirox had modestly improved time to death compared with placebo-treated mice (P < 0.007 by log rank test) (Figure 1). A qualitative histopathological examination of lungs harvested from mice treated with deferasirox showed fewer fungal abscesses, which contained conidia-shaped fungal elements, compared with lungs harvested from placebo-treated mice, which had abscesses containing hyphal elements (data not shown).
Figure 1.
Efficacy of deferasirox monotherapy in a murine model of IPA. Survival of immunosuppressed uninfected control mice (n = 11) or mice with IPA treated with placebo or deferasirox at 10 mg/kg twice a day (n = 24 in each arm from three separate experiments). Deferasirox was given every other day for a total of four doses starting 24 h post-infection. A. fumigatus AF293 average inhaled inoculum was 7 × 102 conidia. *P ≤ 0.007 versus placebo by log rank test.
Deferasirox enhanced activity of LAmB in treating murine IPA
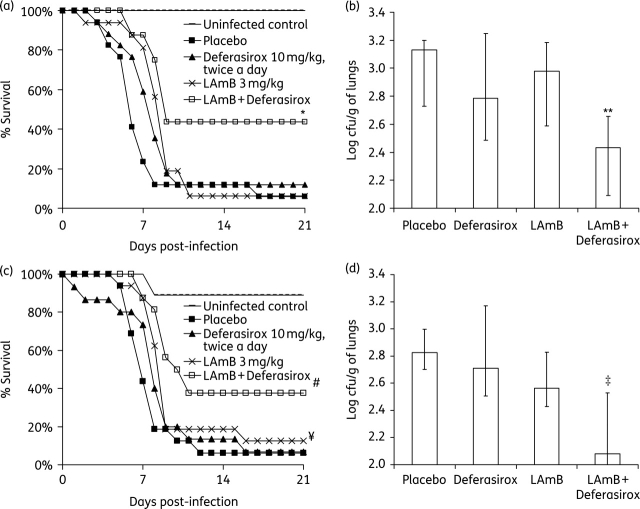
Subsequently, mice were infected with A. fumigatus as above and treated with 3 mg/kg/day LAmB, deferasirox at 10 mg/kg twice a day, a combination of both, or placebo, with treatment starting 24 h post-infection. LAmB + deferasirox significantly improved time to death compared with placebo (P = 0.006; Figure 2a). The LAmB + deferasirox combination was superior in efficacy to either drug alone (P < 0.04). LAmB monotherapy also prolonged survival of mice compared with placebo (P < 0.04). Finally, there was a strong trend for deferasirox monotherapy to improve survival compared with placebo in this set of experiments despite the average infectious inoculum being 4-fold higher than the average inoculum used in Figure 1(a) (P = 0.06).
Figure 2.
Efficacy of delayed (a and b) or prophylactic (c and d) combination deferasirox + LAmB versus monotherapy in a murine model of IPA. (a) Survival of uninfected control mice (n = 8) or of mice with IPA (A. fumigatus AF293 with average inhaled inoculum of 2.9 × 103 conidia) treated with LAmB (3 mg/kg/day, n = 16), deferasirox (10 mg/kg twice a day, n = 17), combination therapy (n = 16) or placebo (n = 17). Data are from two experiments performed on different days. *P < 0.03 for combination therapy versus placebo or monotherapy by log rank test. (b) Lung fungal burden in immunosuppressed mice (n = 13 per group) infected via inhalation with 6.7 × 102 A. fumigatus. Data are from two experiments performed on different days. **P < 0.025 versus placebo or either drug alone by the non-parametric Mann–Whitney test. (c) Survival of uninfected control mice (n = 9) or of mice with IPA (A. fumigatus AF293 with average inhaled inoculum of 1.7 × 103 conidia) treated with LAmB (3 mg/kg/day, n = 16), deferasirox (10 mg/kg twice a day, n = 15), combination therapy (n = 16) or placebo (n = 16). Data are from two experiments performed on different days. #P < 0.002 for combination therapy versus placebo or deferasirox and ¥P < 0.05 for LAmB versus placebo by log rank test. (d) Lung fungal burden in immunosuppressed mice (n = 8 per group) infected via inhalation with 1.0 × 103 A. fumigatus. ‡P < 0.05 versus placebo or monotherapy by the non-parametric Mann–Whitney test. For all cfu experiments, data are displayed as medians + interquartile ranges. The y-axes reflect lower limits of detection of the assay.
To define the impact of antifungal therapy on lung fungal burden, mice were infected and treated as above, starting 24 h after infection and continued until the morning of day 3 post-infection (three doses total). Only combination treatment of deferasirox with LAmB reduced tissue fungal burden compared with placebo or either drug alone (P < 0.03). There was no significant difference between organ fungal burden in mice treated with either monotherapy versus placebo (Figure 2b).
We next tested the efficacy of deferasirox administered prior to infection at day –2 and continued every other day after infection for a total of four doses (i.e. prophylactic treatment). LAmB was administered 24 h post-infection and continued every day for a total of five doses as above. Deferasirox did not improve time to death compared with placebo (P > 0.05), whereas LAmB monotherapy did improve time to death compared with placebo (P = 0.043). Combination therapy of LAmB with deferasirox mediated a significant improvement in time to death versus placebo (P < 0.05). Additionally, combination therapy with LAmB was superior to deferasirox monotherapy (P < 0.05), and there was a trend to improve time to death compared with LAmB monotherapy (P = 0.078) (Figure 2c).
When the experiment was repeated to determine the effect of prophylactic therapy on fungal burden (treatment continued until 3 days after infection, when mice were euthanized), combination LAmB + deferasirox therapy reduced tissue fungal burden compared with placebo or either monotherapy (P < 0.05 for all comparisons). Neither of the monotherapy arms demonstrated efficacy in reducing lung fungal burden when compared with placebo (Figure 2d).
Discussion
Iron is required by virtually all microbial pathogens for growth and virulence, and the efficacy of iron chelators in treating fungal infections (other than IPA) has been shown in recent animal studies.6,9
In this study, we demonstrate that deferasirox has an in vitro activity against A. fumigatus with an MIC and MFC of 25 and 50 mg/L, respectively. We previously found deferasirox to be more active against fungi belonging to the order Mucorales in which the MIC90 and MFC90 were found to be 6.25 mg/L.6 It is unclear why higher concentrations of deferasirox are required for inhibiting the growth of A. fumigatus. It is possible that Mucorales are more sensitive to iron depravation than A. fumigatus. Alternatively, A. fumigatus might have a higher capability of trapping iron (enhanced capability to either acquire external or maintain internal stores of iron), which would result in a requirement for higher concentrations of deferasirox to deplete its iron stores. The decreased susceptibility of A. fumigatus to deferasirox in vitro was also seen in vivo since deferasirox only modestly enhanced survival time of mice infected with A. fumigatus when compared with placebo-treated mice. The enhanced survival time of mice treated with deferasirox is probably due to chelation of iron since we already demonstrated that administration of free iron reverses the protective effects of deferasirox6 and another iron chelator (deferiprone)9 in mice infected with R. oryzae.
There are no published data on deferasirox pharmacology in rodents. In previous experience with deferasirox in our neutropenic mouse model we have found evidence that higher doses of deferasirox (>10 mg/kg twice per day given every other day) are toxic, resulting in inferior efficacy in mice infected with R. oryzae.6 Therefore, we selected the dose based on this prior experience.
A recent study demonstrated synergy between iron chelators including lactoferrin, ciclopirox and deferiprone in inhibiting A. fumigatus conidial growth in vitro when combined with amphotericin B, ketoconazole or fluconazole.10 Therefore, we sought to evaluate the benefit of combination deferasirox + LAmB therapy for IPA. We found both drugs to act synergistically against IPA. In contrast to lactoferrin, ciclopirox and deferiprone, deferasirox is already approved for use in humans by the FDA, as well as by the European Medicines Agency. Given the poor outcomes of IPA with current treatments, continued investigation of the potential for combination deferasirox–polyene therapy to improve survival in IPA is warranted.
Funding
This work was supported by Public Health Service grants R01 AI063503 and R21 AI064716, and research and educational grants from Novartis and Gilead Sciences Inc. to A. S. I.
Transparency declarations
None to declare.
Acknowledgements
This work was presented in part at the Seventeenth Congress of The International Society for Human and Animal Mycology, Tokyo, Japan (Abstract PP-07-17). Research described in this manuscript was conducted at the research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
References
- 1.Kontoyiannis DP, Bodey GP. Invasive aspergillosis in 2002: an update. Eur J Clin Microbiol Infect Dis. 2002;21:161–72. doi: 10.1007/s10096-002-0699-z. [DOI] [PubMed] [Google Scholar]
- 2.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–15. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 3.Hissen AH, Wan AN, Warwas ML, et al. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding l-pornithine N5-oxygenase, is required for virulence. Infect Immun. 2005;73:5493–503. doi: 10.1128/IAI.73.9.5493-5503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altes A, Remacha AF, Sarda P, et al. Frequent severe liver iron overload after stem cell transplantation and its possible association with invasive aspergillosis. Bone Marrow Transplant. 2004;34:505–9. doi: 10.1038/sj.bmt.1704628. [DOI] [PubMed] [Google Scholar]
- 5.Kontoyiannis DP, Chamilos G, Lewis RE, et al. Increased bone marrow iron stores is an independent risk factor for invasive aspergillosis in patients with high-risk hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation. Cancer. 2007;110:1303–6. doi: 10.1002/cncr.22909. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim AS, Gebermariam T, Fu Y, et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117:2649–57. doi: 10.1172/JCI32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheppard DC, Rieg G, Chiang LY, et al. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2004;48:1908–11. doi: 10.1128/AAC.48.5.1908-1911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi M38-A2. CLSI, Wayne, PA, USA; 2008. [Google Scholar]
- 9.Ibrahim AS, Edwards JE, Jr, Fu Y, et al. Deferiprone iron chelation as a novel therapy for experimental mucormycosis. J Antimicrob Chemother. 2006;58:1070–3. doi: 10.1093/jac/dkl350. [DOI] [PubMed] [Google Scholar]
- 10.Zarember KA, Cruz AR, Huang CY, et al. Antifungal activities of natural and synthetic iron chelators alone and in combination with azole and polyene antibiotics against Aspergillus fumigatus. Antimicrob Agents Chemother. 2009;53:2654–6. doi: 10.1128/AAC.01547-08. [DOI] [PMC free article] [PubMed] [Google Scholar]