Abstract
Over the last two decades, there has been increasing awareness regarding the potential impact of indoor air pollution on health. Exposure to volatile organic compounds (VOCs) or oxygenated organic compounds formed from indoor chemistry has been suggested to contribute to adverse health effects. These studies use an in vitro monitoring system called VitroCell, to assess chemicals found in the indoor air environment. The structurally similar dicarbonyls diacetyl, 4-oxopentanal (4-OPA), glyoxal, glutaraldehyde, and methyl glyoxal were selected for use in this system. The VitroCell module was used to determine whether these dicarbonyls were capable of inducing inflammatory cytokine expression by exposed pulmonary epithelial cells (A549). Increases in the relative fold change in messenger RNA expression of the inflammatory mediators, interleukin (IL)-6, IL-8, granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor alpha (TNF-α) were identified following exposure to diacetyl, 4-OPA, glyoxal, glutaraldehyde, and methyl glyoxal when compared to a clean air control. Consistent results were observed when the protein levels of these cytokines were analyzed. Exposure to 4-OPA significantly elevated IL-8, IL-6, GM-CSF, and TNF-α while glutaraldehyde caused significant elevations in IL-6, IL-8, and TNF-α. IL-6 and IL-8 were also significantly elevated after exposure to diacetyl, glyoxal, and methyl glyoxal. These studies suggest that exposure to structurally similar oxygenated reaction products may be contributing to some of the health effects associated with indoor environments and may provide an in vitro method for identification and characterization of these potential hazards.
Keywords: dicarbonyls, VOC, inflammatory cytokines, VitroCell, indoor air
It has been estimated that indoor air quality–related health issues cost businesses $20–70 billion annually due to lost productivity, decreased performance, and sick absences (Mendell et al., 2002). Modern society spends a considerable amount of time indoors, and research findings have demonstrated that some air pollutants occur more frequently and at a higher concentration in indoor air than in outdoor air and may be associated with adverse health effects (Rumchev et al., 2004). Some of the health effects suffered by exposed individuals include: dry eyes and throat, stuffy and runny nose, lethargy, headache, breathless, chest tightness, wheezing, and work-related asthma (Rios et al., 2009). While extensive research has focused on determining the underlying causes for these symptoms, it is possible that no single chemical exposure is responsible for these illnesses. More likely, a mixed exposure of chemical classes, such as particulate matter and oxygenated organic species, including biologically active oxidized volatile organic compounds (VOCs), contribute to these symptoms.
In 1989, the US Environmental Protection Agency (EPA) identified over 900 VOC (USEPA, 1989) originating from ingredients in common household products such as paints, varnishes, cleaners, and waxes. Recent reformulation of many household cleaners to include more “green” and plant-derived VOCs, such as α- and β-pinene, α-terpineol, citronellol, and β-irisone, is likely to cause increases in the concentrations of terpenes, terpene alcohols, and ethers in indoor environments. Results from epidemiological investigations have suggested a link between indoor cleaning agent exposure and increases in asthma and asthma-like symptoms among workers who use cleaning chemicals (Delclos et al., 2007). Adverse effects on skin, such as irritant and allergic dermatitis, have also been reported in these workers (Gawkrodger et al., 1986). However, in many of the situations, no specific agents or chemicals have been identified as responsible for these observed health effects.
Consumer cleaning products and air fresheners contain chemicals such as α-terpineol, which can react with ozone to form a variety of secondary pollutants that may be responsible, in part, for the symptoms described above (Singer et al., 2006; Weschler, 2004). These secondary pollutants include oxygenated organic chemicals, such as aldehydes, ketones, carboxylic acids, and dicarbonyls (Forester et al., 2007; Ham et al., 2006a; Harrison et al., 2007; Wells, 2005). Many of these oxygenated organic species have been observed from simulated indoor air chemistry using specialized detection but are not routinely detected with conventional sampling methods leading to inaccurate exposure assessments of indoor environments.
There is limited knowledge regarding exposure to complex mixtures and the potential interactions among VOCs and other indoor air pollutants. Studies exposing animals to products of chemical reactions, such as ozone with limonene, have demonstrated that the reaction products have a significant impact on the breathing rate of exposed animals when compared to animals exposed to the reactants separately (Rohr et al., 2003; Wilkins et al., 2003). A 1-h exposure of mice to the oxidation products of ozone and limonene resulted in a significant increase in upper airway irritation and airflow limitation compared to exposure to the parent compounds. These findings suggest that the oxygenated reaction products generated from indoor air chemistry may exacerbate lower airway symptoms or occupational asthma in individuals involved in industrial cleaning operations.
We have previously identified several structurally similar VOCs produced by the reaction of ozone or OH radicals with α-terpineol (Wells, 2005). All these dicarbonyls (diacetyl, 4-oxopentanal [4-OPA], glyoxal, methyl glyoxal, and glutaraldehyde) were identified as sensitizers when evaluated using a murine local lymph node assay (LLNA) (Anderson et al., 2007; Azadi et al., 2004; Franko et al., 2009). The above results suggest that these compounds may contribute to the adverse health effects associated with indoor environments. LLNA evaluation of these chemicals was based on dermal exposure to the individual products. A system that utilizes exposure to such sensitizing chemicals in their volatilized state is necessary for a thorough hazard evaluation. The purpose of these studies was to further characterize the effects of dicarbonyls on the respiratory tract by developing an in vitro indoor air exposure system. Changes in inflammatory cytokine expression were evaluated in a pulmonary epithelial cell line (A549) after exposure to the dicarbonyls (diacetyl, 4-OPA, glyoxal, methyl glyoxal, and glutaraldehyde) individually and in a reaction mixture.
MATERIALS AND METHODS
Test article.
Methyl glyoxal (CAS 78-98-8), glyoxal (CAS 107-22-2), diacetyl also known as 2,3-butanedione (CAS 431-03-8), glutaraldehyde (CAS 111-30-8), α-terpineol (CAS, 7785-53-7, 90% technical grade), and o-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine hydrochloride (PFBHA) (98+%) were all purchased from Sigma-Aldrich Chemical Company (St Louis, MO). 4-OPA was synthesized by Richman Chemical, Inc. (Lower Gwynedd, PA). Ozone was generated in the laboratory as described below.
Teflon chamber preparation.
Teflon chambers (FEP 500; American Durafilm, Hollston, MA) were constructed to facilitate cell exposure via the VitroCell apparatus to gas-phase chemicals. Chemicals were injected into a 50% relative humidity air stream through a heated ¼-inch stainless steel tee (Swagelok) into the 70- to 100-l Teflon chambers. For the cell exposure experiments, typical concentrations of the pertinent species were approximately 15–65 ppm (3.7 × 1014 to 1.6 × 1015 molecules/cm3) dicarbonyl for the gene expression studies and 65 ppm for the protein studies (Fig. 1). Previous gas-phase VOC experiments indicated that the sample preparation method above provides multi-hour concentration stability (Forester and Wells, 2009). Compressed air from the NIOSH facility was passed through anhydrous CaSO4 and molecular sieves to remove both moisture and organic contaminants. This dry air (less than 5% relative humidity) was humidified to 50% relative humidity to simulate average indoor environment conditions. For the reaction product experiments, ozone was produced by photolyzing air with a mercury pen lamp (Jelight, Irvine, CA) in a separate Teflon chamber and transferred using a gas-tight syringe. Ozone concentration (∼100 ppb) was measured with a UV photometric ozone analyzer (model 49C or 49i; Thermo Fisher Scientific, Inc., Waltham, MA). Ozone concentrations of 1–5 ppm were achieved by transferring large volumes (2–4 l) from the separate high concentration ozone chamber using an additional smaller Teflon chamber in lieu of a gas-tight syringe. Ozone was injected into the respective Teflon chamber containing ∼1, 3, or 6 ppm (2.5–15 × 1013 molecules/cm3) α-terpineol 15 min prior to the VitroCell exposure.
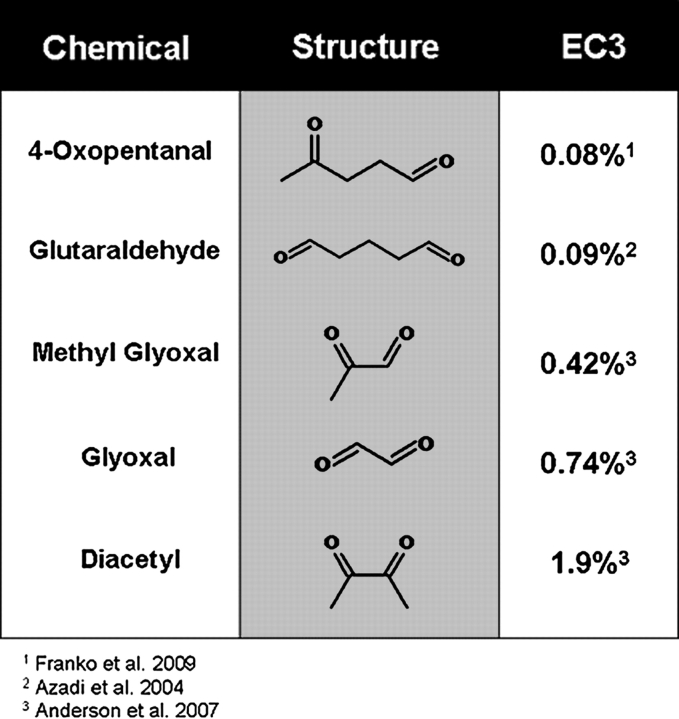
FIG. 1.
Chemical structure of evaluated dicarbonyls. Identification of the chemical and structure for each of the dicarbonyls investigated in these experiments. The sensitization potential (EC3; concentration of chemical required to induce a threefold increase in lymphocyte proliferation over control) for each of these chemicals was previously determined.
VitroCell exposures.
Human epithelial lung cells (53,533 cells/cm2), A549 (American Type Culture Collection; CCL-185), were cultured in Dulbecco's modified Eagle medium (DMEM) containing 10% heat-inactivated fetal calf serum (FCS) on removable permeable (0.4 μm) transwell inserts (Fisher Scientific) placed in a sterile 6-well plate, containing 2.5 ml of media in plate well and 1.5 ml media on insert. The media was exchanged for serum-free media 24 ± 4 h prior to VitroCell exposures. Immediately before exposures, the inserts were washed twice with 3 ml of sterile PBS and placed in the wells of the VITROCELL 6 PT-CF module (VitroCell, Waldkirch, Germany) containing 8.5 ml of serum-free media to sustain the basal surface of the cells during exposures. Two VitroCell chambers were used for simultaneous exposures. The two scenarios were clean air and dicarbonyl or α-terpineol and α-terpineol + ozone. Three inserts were placed in each chamber, and trumpets delivering test atmospheres were raised to 0.5 cm to mitigate issues of cell viability and experiment length. The chambers were attached to a circulating 37°C water bath for the duration of the experiment (Fig. 2). The contents of the prepared reaction bags were pulled via vacuum pump with attached filter across the apical surface of the cells for 2 or 4 h at a constant rate of 3.0 ml/min/trumpet. Postexposure, the Transwell inserts were placed in a new sterile six-well plate with 10% FCS DMEM (2.5 ml of media in well and 1.5 ml media on insert) and allowed recovery periods ranging from 2 to 4 h in a 37°C incubator with 5% CO2.
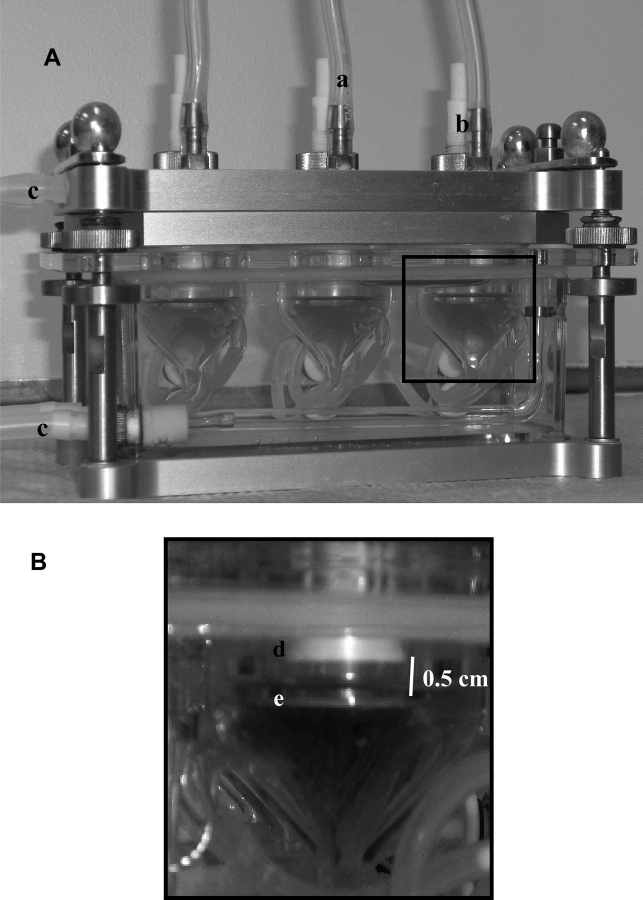
FIG. 2.
VitroCell chambers used for exposures. (A) Representation of VitroCell exposure module. Exposure atmosphere (dicarbonyls and/or reaction products) is pulled across apical surface of the cell though inlets (b) and removed from the chamber by a vacuum pump (a) at a constant flow rate of 5 ml/min for all chambers. Module is kept at a constant temperature of 37°C using a circulating water bath (c) for the duration of the exposures. (B) Enlarged section of box depicted in (A). Test atmosphere is delivered to cells grown on transmembrane insets (e) via trumpets (d). Distance between the trumpet and inserts is 0.5 cm.
Cell viability.
Cell viability was analyzed 24 h after dicarbonyl exposure using the Molecular Probes live/dead viability/cytoxicity kit, according to the manufacturer's instructions (Invitrogen). Briefly, dicarbonyl-exposed A549 cells were trypsinized, washed, and resuspended in 1 ml flow staining buffer (PBS containing 1% bovine serum albumin and 0.1% sodium azide, pH 7.4). Cells were then stained with 2 μl of calcein AM (50μM) and 4 μl of ethidium homodimer-1 (2mM), incubated for 15–20 min, and immediately analyzed by flow cytometry using a BD FacsCalibur (BD Biosciences, San Diego, CA). Incorporation of Calcein AM (FL1) into live cells was detected by measuring green fluorescence (i.e., 530/30 bandpass) while incorporation of ethidium homodimer-1 (FL3) was detected by measuring red fluorescence (i.e., 610/20 bandpass).
RNA isolation and gene expression analysis.
Prior to RNA isolation, the media was aspirated from the apical surface of the cells, and Trizol (500 μl/well) was added to each insert following a 2-h postexposure recovery. The Trizol/cell suspension was transferred to a 1.5-ml microcentrifuge tube and incubated at room temperature for 15 min. Cells were pooled from the three inserts according to exposure group. RNA was isolated from the cells according to the TRIzol protocol with QIAGEN cleanup according to the manufacturers’ instructions. RNA concentration was determined using ND-1000 spectrophotometer (NanoDrop). Reverse transcription (2 μg) was performed using the high-capacity cDNA reverse transcription kit (Applied Biosystems) according to manufacturer's instructions. RNA was quantified (chemokine [C-C motif] ligand 2 [CCL2], chemokine [C-C motif] ligand 5 [CCL5], granulocyte-macrophage colony-stimulating factor [GM-CSF], interleukin [IL]-1 alpha, IL-1β, IL-6, IL-8, tumor necrosis factor alpha [TNF-α], and transforming growth factor beta 1 [TGF-β1]) using real-time PCR on a 7500 Fast Real-Time PCR system (Applied Biosystems) with TaqMan reagents as prescribed by manufacturers.
Protein analysis.
Cytokine levels (IL-8, IL-6, GM-CSF, and TNF-α) in supernatants collected from cultures at 8, 12, and 24 h postexposure were analyzed using OptEIA ELISA kits purchased from BD Biosciences according to the manufacturer's instructions. In brief, 96-well flat-bottom plates (Dynatec Immulon-2) were coated with the corresponding capture antibody diluted with carbonate-bicarbonate coating buffer (Sigma). Plates were sealed and incubated overnight at 4°C. After three washes, plates were blocked with Assay Diluent (PBS with 10% FCS), incubated at room temperature for 1 h, and then washed three times. Standard samples were prepared in assay diluent at concentrations specific to the ELISA kit. Supernatant samples collected from each culture (three for dicarbonyl and three for clean air) at each time point (8, 12, and 24 h) were added to the plates in triplicate along with serial dilutions of the standards. The plates were sealed and incubated at room temperature for 2 h. After five washes, the working detector (a mixture of the appropriate biotinylated anti-human cytokine and Streptavidin-horseradish peroxidase conjugate) was added to the plates, which were then sealed and incubated at room temperature for 1 h. Plates were washed seven times, and substrate solution (tetramethylbenzidine and hydrogen peroxide) was added to each well. Sealed plates were allowed to develop in the dark for 30 min at room temperature (optical density values for standards ranging from 0.77–1.93). Color development was stopped by 2N H2SO4, and plates were read at 450–570 nm using a SpectraMax M2 spectrophotometer (Molecular Devices). Cytokine concentrations were extrapolated from the standard curve.
Analysis of test atmosphere.
A sample of the α-terpineol + ozone reaction chamber was collected to identify oxygenated reaction product yields. Twenty liters of chamber contents flowing at a rate of 2.5 l/min for 8 min was pulled through an impinger containing 3.6 ml of methanol. The sample (∼1.7 ml) was removed from the impinger and 200 μl of 0.02M PFBHA in acetonitrile was added to the contents to derivatize the carbonyl reaction products to oximes (Yu et al., 1998). The samples were allowed to react in the dark for 24–48 h. The reacted solutions were gently blown to dryness with UHP N2, reconstituted with 100 μl of methanol, and then 1 μl of the reconstituted solution was injected into the Varian 3800/Saturn 2000GC/MSsystem for identification of the reaction products (Forester and Wells, 2009).
Statistics.
Gene expression data are expressed as the relative fold increase over control (clean air verses dicarbonyl), calculated by the following formula: 2ΔΔCt, where ΔΔCt = ΔCt (sample) − ΔCt (control). The ΔCt = Ct (GAPDH) − Ct (Target), where Ct = cycle threshold as defined by manufacturer's instructions. Fold changes are based on the mean value of three cultures for the indicated exposure concentrations. To determine statistically significant differences in concentrations of inflammatory proteins, a two-tailed unpaired t-test was used to compare clean air–exposed to dicarbonyl-exposed samples for each specified time point. Results are based on the mean of triplicate samples from three cultures from two independent exposures for each treatment group at each time point. Significant differences between dicarbonyl and clean air exposures are designated with ** (p < 0.01) or * (p < 0.05).
RESULTS
Increases in Inflammatory Gene Expression after Dicarbonyl Exposure
No significant changes in cell viability were detected 24 h after a 4-h glyoxal exposure (Fig. 3). For the clean air control exposure, 86% of the cells were viable postexposure while 6% were determined to be dead (A). Almost identical ratios (B) were observed after exposure to glyoxal (88% live; 5% dead). Similar trends were obtained after exposure to the other dicarbonyls with no significant differences identified between the percent viable cells for the treated verses control group (data not shown). Messenger RNA (mRNA) expression of CCL2, CCL5, GM-CSF, IL-1α, IL-1β, IL-6, IL-8, TNF-α, and TGF-β1 was analyzed from exposed A549 cells using real-time quantitative PCR. Relative fold changes in gene expression (average of two exposures) compared to clean air control are shown in Table 1. No consistent fold changes greater than 5 were observed when CCL2, CCL5, IL-1α, IL-1β, and TGF-β1 mRNA expression was analyzed (data not shown). However, consistent elevations in IL-6, IL-8, TNF-α, and GM-CSF mRNA were observed for all dicarbonyls tested (65 ppm) with glyoxal and diacetyl exposure yielding the greatest fold changes compared to the clean air control. Similar, although reduced, relative fold changes in gene expression were observed when cells were exposed to lower dicarbonyl concentrations.
FIG. 3.
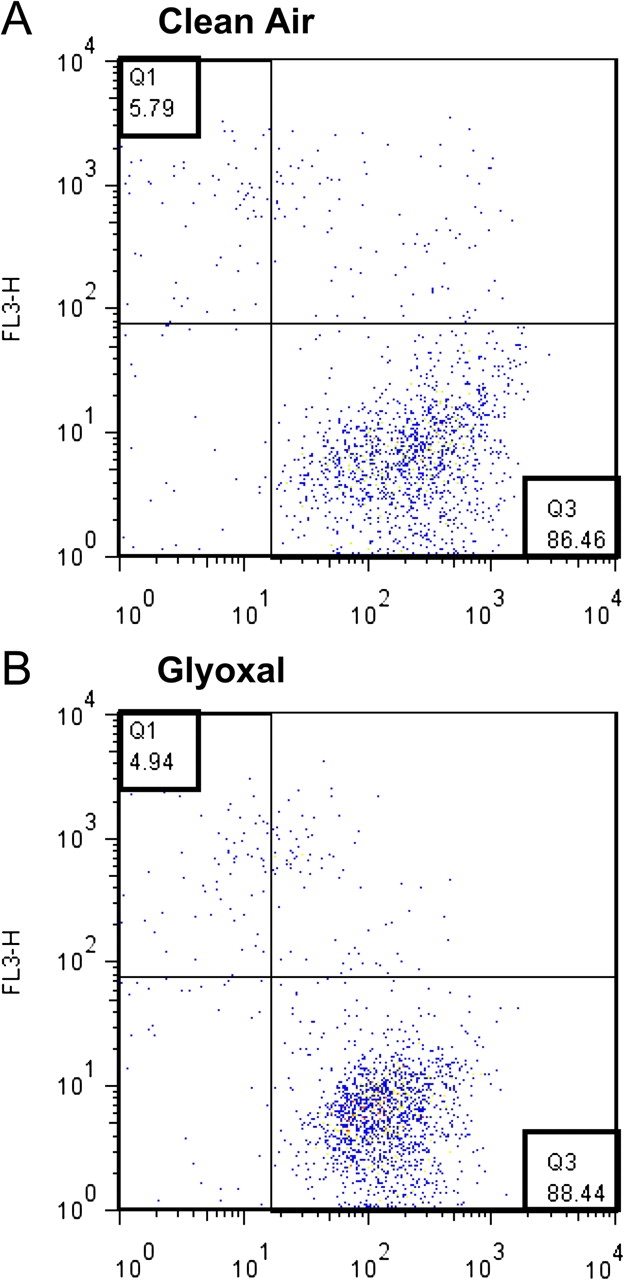
Viability of A549 cells after exposure to glyoxal and clean air. Representative dot plot showing the percent of living and dead cells 24 h after a 4-h clean air (A) or glyoxal (B) VitroCell exposure as determined by flow cytometry. The number in quadrant 1 (Q1) indicates the percent of dead cells and the number in quadrant 3 (Q3) represents the percent of viable cells. Dot plot shows FL1-calcein AM on the x-axis and FL3-ethidium homodimer-1 on y-axis.
TABLE 1.
Alterations in Gene Expression after Dicarbonyl Exposure
| Dicarbonyl | Concentration (ppm) | GM-CSF | IL-6 | IL-8 | TNF-α |
| Diacetyl | 65 | 359 | 48 | 29 | 109 |
| 28 | 0.2 | 15 | 10 | 0.6 | |
| 15 | 1 | 11 | 7 | 0.4 | |
| Methyl Glyoxal | 65 | 49 | 214 | 37 | 63 |
| 33 | 9 | 3 | 3 | 14 | |
| 15 | 6 | 3 | 5 | 12 | |
| Glyoxal | 65 | 338 | 325 | 80 | 312 |
| 30 | 24 | 34 | 28 | 8 | |
| 15 | 13 | 28 | 12 | 7 | |
| 4-OPA | 65 | 189 | 60 | 77 | 168 |
| 30 | 8 | 32 | 11 | 11 | |
| 15 | 7 | 32 | 14 | 16 | |
| Glutaraldehyde | 65 | 2 | 4 | 3 | 3 |
| 32 | 0.2 | 23 | 11 | 0.7 | |
| 15 | 1 | 15 | 9 | 2 |
Note. Numbers shown indicate relative fold change in gene expression of dicarbonyl-exposed A549 cells compared to clean air. A 4-h exposure with a 2-h recovery was conducted for each experiment.
Increases in Inflammatory Proteins after Dicarbonyl Exposure
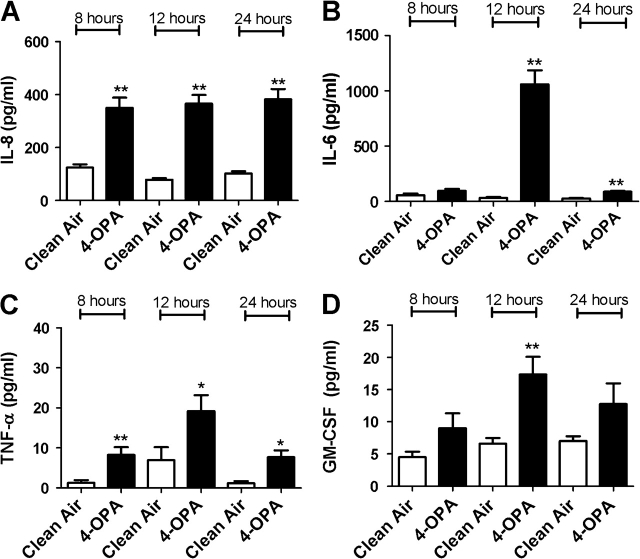
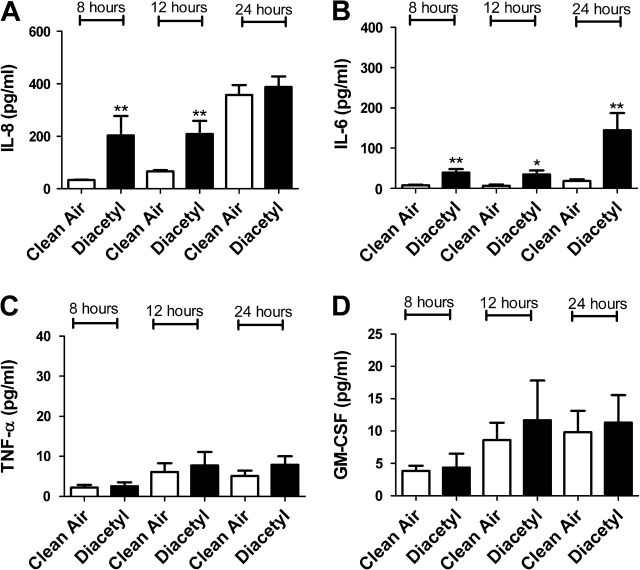
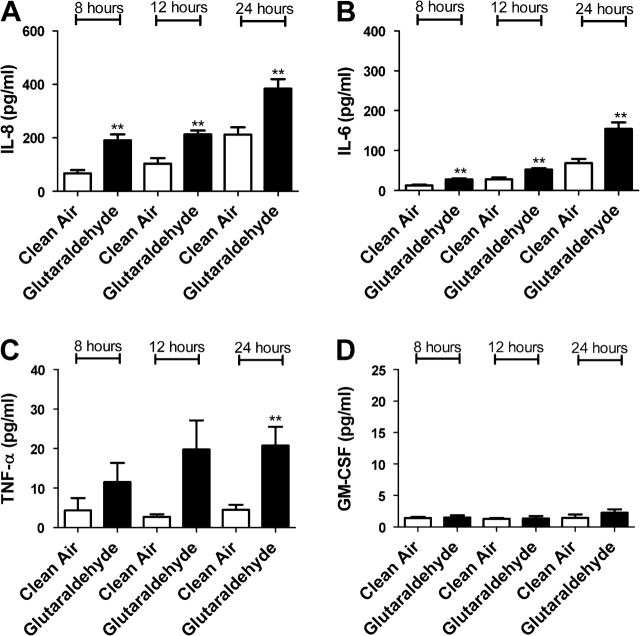
In an attempt to confirm gene expression data, protein levels for IL-6, IL-8, TNF-α, and GM-CSF were analyzed after exposure to individual dicarbonyls. Significant elevations in cytokine levels were observed in cells exposed to diacetyl, glyoxal, methyl glyoxal, 4-OPA, and glutaraldehyde (Figs. 4–8). Exposure to 4-OPA significantly elevated IL-8 and TNF-α at all time points, IL-6 at 12 and 24 h and GM-CSF at 12 h (Fig. 4). Exposure to diacetyl significantly elevated IL-6 at all time points and IL-8 at 8 and 12 h (Fig. 5) when compared to clean air control. No significant changes in GM-CSF or TNF-α were observed. Exposure to glutaraldehyde significantly elevated IL-6 and IL-8 at all time points and TNF-α at 24 h (Fig. 6) when compared to clean air control. No significant changes in GM-CSF were observed. Exposure to glyoxal significantly elevated IL-8 and IL-6 at 12 and 24 h while exposure to methyl glyoxal elevated these cytokine only at 24 h (Figs. 7 and 8). Exposure to 4-OPA generated the greatest significant increase in IL-6 (1059 pg/ml at 12 h) and GM-CSF (17 pg/ml at 12 h) production when compared to the clean air control for any of the dicarbonyls evaluated. 4-OPA was also the only dicarbonyl that significantly altered all cytokines evaluated. Exposure to glutaraldehyde produced the largest significant increase in TNF-α (21 pg/ml at 24 h), while methyl glyoxal induced the largest production of IL-8 (597 pg/ml). Significant elevations in cytokines were observed for similar time points for the evaluated dicarbonyls. At 12 h postexposure, IL-8 was increased for diacetyl, 4-OPA, glyoxal, and glutaraldehyde while IL-6 was increased for diacetyl, 4-OPA, glyoxal, and glutaraldehyde (methyl glyoxal at 24 h). More variability was observed within the results for GM-CSF and TNF-α. At 24 h, TNF-α expression was significantly elevated for glutaraldehyde and 4-OPA while GM-CSF expression was only significantly elevated after exposure to 4-OPA.
FIG. 4.
The effect of 4-OPA exposure on concentrations of inflammatory cytokines. Bars represent the mean protein concentration ± SE determined by ELISA present in supernatants of six A549 cell cultures from two independent exposures. Samples were collected at 8, 12, and 24 h after VitroCell exposure and evaluated for IL-8 (A), IL-6 (B), TNF-α (C), and GM-CSF (D) protein levels. Significant differences between dicarbonyl and clean air exposure are designated with **(p ≤ 0.01) or *(p ≤ 0.05).
FIG. 5.
The effect of diacetyl exposure on concentrations of inflammatory cytokines. Bars represent the mean protein concentration ± SE determined by ELISA present in supernatants of three A549 cell cultures from two independent exposures. Samples were collected at 8, 12, and 24 h after VitroCell exposure and evaluated for IL-8 (A), IL-6 (B), TNF-α (C), and GM-CSF (D) protein levels. Significant differences between dicarbonyl and clean air exposure are designated with **(p ≤ 0.01) or *(p ≤ 0.05).
FIG. 6.
The effect of glutaraldehyde exposure on concentrations of inflammatory cytokines. Bars represent the mean protein concentration ± SE determined by ELISA present in supernatants of three A549 cell cultures from two independent exposures. Samples were collected at 8, 12, and 24 h after VitroCell exposure and evaluated for IL-8 (A), IL-6 (B), TNF-α (C), and GM-CSF (D) protein levels. Significant differences between dicarbonyl and clean air exposure are designated with **(p ≤ 0.01) or *(p ≤ 0.05).
FIG. 7.
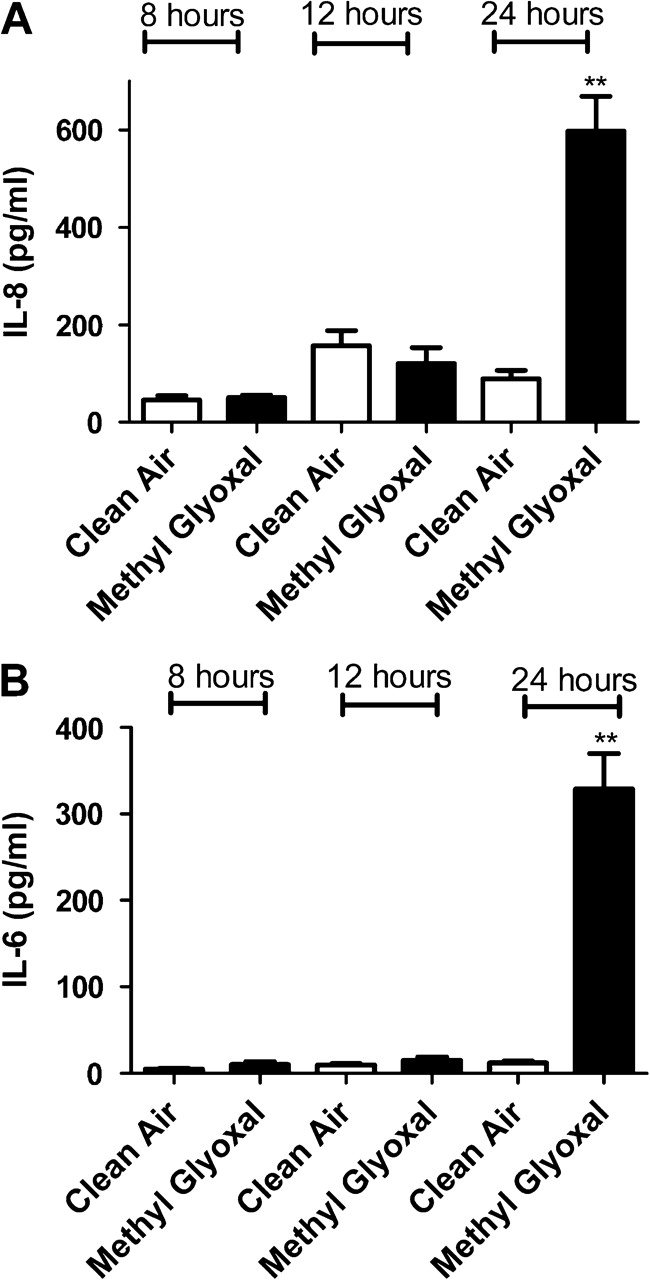
The effect of methyl glyoxal exposure on concentrations of inflammatory cytokines. Bars represent the mean protein concentration ± SE determined by ELISA present in supernatants of three A549 cell cultures from two independent exposures. Samples were collected at 8, 12, and 24 h after VitroCell exposure and evaluated for IL-8 (A) and IL-6 (B). Significant differences between dicarbonyl and clean air exposure are designated with ** (p ≤ 0.01) or *(p ≤ 0.05).
FIG. 8.
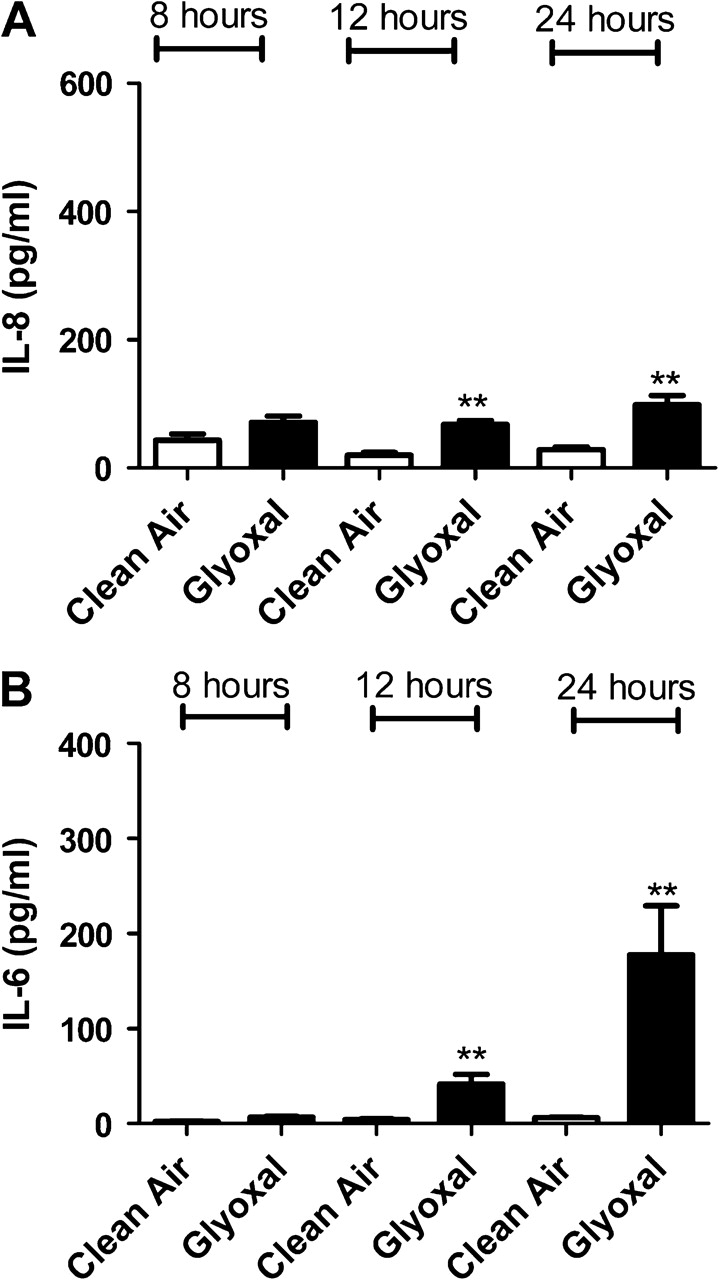
The effect of glyoxal exposure on concentrations of inflammatory cytokines. Bars represent the mean protein concentration ± SE determined by ELISA present in supernatants of three A549 cell cultures from two independent exposures. Samples were collected at 8, 12, and 24 h after VitroCell exposure and evaluated for IL-8 (A) and IL-6 (B). Significant differences between dicarbonyl and clean air exposure are designated with **(p ≤ 0.01) or *(p ≤ 0.05).
No Alterations in Inflammatory Cytokines after Exposure to α-Terpineol and Ozone Reaction Products
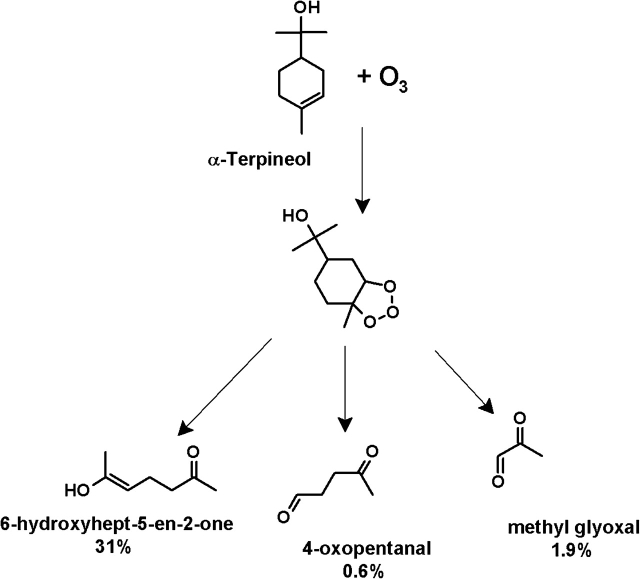
In an attempt to simulate the generation of dicarbonyls in an indoor air environment, α-terpineol was reacted with ozone and exposed to A549 cells as previously described for the individual dicarbonyls. In the α-terpineol versus α-terpineol + ozone system, α-terpineol was in excess (1–6 ppm [2.5–15] × 1013 molecules/cm3) with ozone (100–5000 ppb [2.5–123 × 1012 molecules/cm3]) being the limiting reagent which reacts completely. The reaction generated (Fig. 9) 31–1550 ppb (7.6–382 × 1011 molecules/cm), 1.9–95 ppb (4.7–234 × 1010 molecules/cm3), and 0.6–30 ppb (1.5–74 × 1010 molecules/cm3) of 6-hydroxyhept-5-en-2-one, methyl glyoxal and 4-OPA, respectively (Forester and Wells, 2009). No change in cell viability was detected after the VitroCell exposure (data not shown). No changes in gene or protein expression were identified for any of the inflammatory cytokines examined based on the exposure conditions described above when compared to α-terpineol alone. Longer exposure periods (up to 8 h) and multiple exposures (6 h/day for 3 days) were also examined, but no differences were detected (data not shown). In a separate control experiment, changes in gene expression were observed for α-terpineol and ozone individually when compared to clean air control (Table 2).
FIG. 9.
α-Terpineol + ozone products and yields. Reaction mechanism for α-terpineol + ozone showing formation and yield (in percentages) of 6-hydroxyhept-5-en-2-one, 4-OPA, and methyl glyoxal.
TABLE 2.
Alterations in Gene Expression after Individual Exposures to Ozone and α-Terpineol
| Exposure | Concentration | GM-CSF | IL-6 | IL-8 | TNF-α |
| Ozone | 100 ppb | 29 | 4 | 10 | 25 |
| 5 ppm | 46 | 7 | 14 | 43 | |
| α-Terpineol | 1 ppm | 9 | 1 | 5 | 17 |
| 6 ppm | 38 | 2 | 16 | 35 |
Note. Numbers shown indicate relative fold change in gene expression of exposed A549 cells compared to clean air. A 4-h exposure with a 2-h recovery was conducted.
DISCUSSION
The term “sick building syndrome” (SBS) has been used to describe situations in which no specific illness or cause, aside from time spent indoors, explains adverse health effects experienced by building occupants; however, the specific cause of the related symptoms has yet to be identified (USEPA, 1991). Increasing evidence suggests that some of these health effects may be attributed to reactive indoor air chemistry, such as ozone-terpene reactions (Weschler, 2006).
The dicarbonyls tested in these studies, with the exception of diacetyl, are oxygenation reaction products detected in indoor chemistry experiments. There is extensive literature available on the adverse health effects associated with glutaraldehyde (a dicarbonyl) exposure (Gannon et al., 1995; Rideout et al., 2005; Waters et al., 2003), supporting its selection for testing in this system. Likewise, diacetyl is a known sensitizer, and epidemiological studies have found that exposed workers have twice the expected rates of physician-diagnosed asthma compared to the general population (Anderson et al., 2007; Kreiss et al., 2002; Mendell et al., 2002). Additionally, diacetyl has received considerable attention in the investigations of worker exposure to butter flavorings (Hubbs et al., 2008). 4-OPA has not been investigated as extensively as glutaraldehyde and diacetyl; however, recent data suggest that exposure may be responsible for certain health complaints including increased lip and skin dryness (Strom-Tejsen et al., 2007). Research investigating the exposure-related health effects for glyoxal and methyl glyoxal is lacking.
This work describes a novel in vitro exposure method, utilizing the VitroCell module, for the analysis of oxygenated reaction products found in the indoor environment. The system has many advantages over what has previously been described in the literature, which lack a realistic exposure atmosphere and require the addition of chemicals directly to the cell media or the evaporation of a volatile liquid (Bakand et al., 2006; Wichmann et al., 2005). The VitroCell exposure systems allows for continuous chemical exposures while maintaining cell viability and avoiding dehydration of the cells. Airway epithelial cells were chosen for these studies since they would be expected to be the first cells exposed to these volatized compounds in the respiratory tract and are known to produce inflammatory cytokines capable of modulating immune cell activation.
These studies investigated high concentrations (15–65 ppm) of individual dicarbonyls to establish if exposure to these compounds generated a biological effect. Because these compounds have not been measured by conventional indoor air sampling methods, a “typical” concentration is not truly known. However, based on simulated indoor chemistry data, concentrations of 10–100’s of ppb (∼2.5–25 × 1011 molecules/cm) are anticipated. Results demonstrated that exposure to the structurally similar dicarbonyls caused alterations in both inflammatory cytokine mRNA and protein expression in A549 cells. There also appears to be a trend between the sensitization potential and inflammatory cytokine expression for these chemicals. Of the five dicarbonyls tested, 4-OPA is considered to be the strongest sensitizer based on the lowest EC3 value (Fig. 1) in the LLNA. In these studies 4-OPA was also the only chemical to significantly alter all cytokines investigated and generated the highest expression levels for IL-6 and GM-CSF. Glutaraldehye, considered to be the second strongest sensitizer, significantly altered three of the four cytokines, inducing the highest expression of TNF-α.
IL-8, IL-6, TNF-α, and GM-CSF are proinflammatory mediators produced by macrophages and epithelial cells with suggested roles in asthma pathogenesis (Bautista et al., 2009; Macedo et al., 2009; Saha et al., 2009). Epidemiological studies have associated occupational asthma with increases in IL-8 (Zhang et al., 2009), IL-6 (Piirila et al., 2008), GM-CSF, and TNF-α (Ogawa et al., 2006).
The structurally similar dicarbonyls evaluated in this system have the potential to be oxygenated reaction products. Methyl glyoxal, 6-hydroxyhept-5-en-2-one (not synthesized), and 4-OPA are generated through the reaction of ozone + α-terpineol; glyoxal is generated by reactions with geraniol, β-ionone, and citronellol (Forester et al., 2007; Ham et al., 2006b); and glutaraldehyde is generated through indoor air reactions of cyclohexene + ozone (Aschmann et al., 2003; Harrison et al., 2007). Although not described in the literature as a reaction product based on its structure, diacetyl could be a reaction product of ozone reacting with a compound having a carbon-carbon double bond connected to a methyl group and a methyl ketone (-C(=O)CH3) group. The frequent incidence of ozone in indoor ventilated air along with the ubiquitous presence of terpenes in the indoor environment favors the occurrence of ozone-terpene indoor chemistry, which generates these types of reaction products. Ozone-terpene reactions are extremely complex and can generate a variety of products such as dicarboxylic acid, dicarbonyls, and oligomers. Ozone-terpene reactions also produce the hydroxyl radical (OH), which reacts with terpenes in addition to a wide range of hydrocarbons, possibly further contributing to the formation of indoor oxygenated reaction products.
In order to more closely simulate an indoor environment, the products of an α-terpineol + ozone reaction were also evaluated in these studies. However, the trend of increased inflammatory cytokines did not carry over when the cells were exposed to the α-terpineol + ozone reaction. One possible explanation for the noncongruent results is dicarbonyl exposure concentrations as the α-terpineol + ozone reaction used in the latter studies generated much lower concentrations of 4-OPA and methyl glyoxal than those used in initial experiments. However, consistent with results described in the literature, changes in gene expression were observed after individual exposures to terpenes and ozone (Wilkins et al., 2003). It is important to note that this work is not focused on the known effects caused by exposure to the parent compounds, but rather seeks to identify enhanced alterations in inflammatory responses, caused by oxygenated reaction products.
While the results from the individual dicarbonyl and ozone/α-terpineol reaction product studies were not consistent, limited exposure conditions and complexity of the ozone-terpene reaction may also be important factors that can influence the effects of dicarbonyl exposure in an actual indoor environment. While individuals often spend the majority of a 24-h day indoors, a 6-h exposure was the longest duration the cells would tolerate in this in vitro system. In addition, for these studies, cells were exposed only to the oxygenated reaction products generated from the α-terpineol + ozone reaction. In an indoor environment, individuals are exposed to numerous potential oxygenated reaction products generated from ozone-initiated reactions. While there is a likely possibility that products can be generated from these types of reactions, the identification can be challenging. For example, the dicarbonyls generated from the reaction of ozone and α-terpineol only account for ∼34% of the total reaction products. The “missing” chemicals generated from these indoor air reactions (Fig. 9) along with the detailed oxidation pathways still need to be identified and investigated to advance our knowledge of indoor air exposure-related health effects.
While the concentrations of the individual chemicals in the Teflon chambers are higher than those likely to be found in most indoor environments, it is important to consider the cumulative effect of these structurally similar indoor contaminants. Comparable results were observed in these studies for all dicarbonyls tested suggesting the exposure-related health effects and biological mode of action may also be similar. Given the abundance of oxygenated reaction products possible in the indoor environment along with the potential for similar types of health effects, exposure to small amounts of individual chemicals could cause amplified responses when present in a mixture. Structure-activity relationships (SARs), which utilizes a combination of computational biology and statistics, have been developed to identify a link between structure and biological activity. There has been increased attention and promising evidence, focused on the development of models to predict respiratory sensitizers based on chemical structure (Jarvis et al., 2005).
There has been an unexplained increase in the incidence of asthma and allergies in the developed world over the last 30–40 years. More air-tight buildings, a wide range of new building materials, association of VOCs with respiratory conditions, and a greater amount of time spent indoors suggest the possibility that exposure to VOCs may contribute to this increase of adverse health effects. Attempts have been made to regulate VOCs exposures based on chemical class, mixtures, and suspected health effects by organizations including the Health Council of the Netherlands (2000), Commission of the European Communities (1992), and the World Health Organization (2000) Air Quality Guidelines for Europe, but given the complexities and variability of the indoor environment along with the large number of potential VOCs and reaction products, this has proven to be a problematic task.
These results show that dicarbonyls stimulate the release of proinflammatory mediators from lung epithelial cells and suggest that oxygenated reaction products may be contributing to the adverse health effects associated with indoor air exposure. In addition, consistent elevations in inflammatory cytokines obtained for the structurally similar compounds suggest that the effects may be caused by exposure to the summation of small amounts of reaction products. The novel system described here may provide an in vitro method based on the expression of inflammatory cytokine by airway epithelial cells to screen contaminated indoor environments and help to clarify the culprits responsible for the symptoms associated with SBS and the other diverse health complaints of workers in those environments.
FUNDING
Inter-Agency Agreement (NIEHS Y1-ES0001-06).
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
References
- Anderson SE, Wells JR, Fedorowicz A, Butterworth LF, Meade BJ, Munson AE. Evaluation of the contact and respiratory sensitization potential of volatile organic compounds generated by simulated indoor air chemistry. Toxicol. Sci. 2007;97:355–363. doi: 10.1093/toxsci/kfm043. [DOI] [PubMed] [Google Scholar]
- Aschmann SM, Tuazon EC, Arey J, Atkinson R. Products of the gas-phase reaction of O-3 with cyclohexene. J. Phys. Chem. 2003;107:2247–2255. [Google Scholar]
- Azadi S, Klink KJ, Meade BJ. Divergent immunological responses following glutaraldehyde exposure. Toxicol. Appl. Pharmacol. 2004;197:1–8. doi: 10.1016/j.taap.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Bakand S, Winder C, Khalil C, Hayes A. A novel in vitro exposure technique for toxicity testing of selected volatile organic compounds. J. Environ. Monit. 2006;8:100–105. doi: 10.1039/b509812b. [DOI] [PubMed] [Google Scholar]
- Bautista MV, Chen Y, Ivanova VS, Rahimi MK, Watson AM, Rose MC. IL-8 regulates mucin gene expression at the posttranscriptional level in lung epithelial cells. J. Immunol. 2009;183:2159–2166. doi: 10.4049/jimmunol.0803022. [DOI] [PubMed] [Google Scholar]
- Commission of the European Communities. Guidelines for Ventilation Requirements in Building. Report No. 11. European Concerted Action: Indoor Air Quality and Its Impact on Man. 1992. EUR 14449 EN. Office for Publication of the European Communities, Luxembourg, Germany. [Google Scholar]
- Delclos GL, Gimeno D, Arif AA, Burau KD, Carson A, Lusk C, Stock T, Symanski E, Whitehead LW, Zock JP, et al. Occupational risk factors and asthma among health care professionals. Am. J. Respir. Crit. Care Med. 2007;175:667–675. doi: 10.1164/rccm.200609-1331OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester CD, Ham JE, Wells JR. Geraniol (2,6-dimethyl-2,6-octadien-8-ol) reactions with ozone and OH radical: rate constants and gas-phase products. Atmos. Environ. 2007;41:1188–1199. [Google Scholar]
- Forester CD, Wells JR. Yields of carbonyl products from gas-phase reactions of fragrance compounds with OH radical and ozone. Environ. Sci. Technol. 2009;43:3561–3568. doi: 10.1021/es803465v. [DOI] [PubMed] [Google Scholar]
- Franko J, Munson AE, Ham J, Jackson LG, Wells JR, Butterworth L, Anderson SE. 4-Oxopentanal identified as a potential indoor air irritant and allergen. Toxicologist. 2009;108:313. (A1509: Abstract) [Google Scholar]
- Gannon PF, Bright P, Campbell M, O'Hickey SP, Burge PS. Occupational asthma due to glutaraldehyde and formaldehyde in endoscopy and x ray departments. Thorax. 1995;50:156–159. doi: 10.1136/thx.50.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawkrodger DJ, Lloyd MH, Hunter JA. Occupational skin disease in hospital cleaning and kitchen workers. Contact Derm. 1986;15:132–135. doi: 10.1111/j.1600-0536.1986.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Ham JE, Proper SP, Wells JR. The gas-phase chemistry of citronellol with ozone and OH radical: Rate constants and products. Atmos. Environ. 2006a;40:726–735. [Google Scholar]
- Ham JE, Proper SP, Wells JR. Gas-phase chemistry of citronellol with ozone and OH radical: rate constants and products. Atmos. Environ. 2006b;40:726–735. [Google Scholar]
- Harrison JC, Ham JE, Wells JR. Citronellal reactions with ozone and OH radical: rate constants and gas-phase products detected using PFBHA derivatization. Atmos. Environ. 2007;41:4482–4491. [Google Scholar]
- Health Council of the Netherlands. Volatile Organic Compounds in Indoor Environments. 2000 Health Council of the Netherlands, The Hague, The Netherlands. Publication no. 2000/10. [Google Scholar]
- Hubbs AF, Goldsmith WT, Kashon ML, Frazer D, Mercer RR, Battelli LA, Kullman GJ, Schwegler-Berry D, Friend S, Castranova V. Respiratory toxicologic pathology of inhaled diacetyl in Sprague-Dawley rats. Toxicol. Pathol. 2008;36:330–344. doi: 10.1177/0192623307312694. [DOI] [PubMed] [Google Scholar]
- Jarvis J, Seed MJ, Elton RA, Sawyer L, Agius RM. Relationship between chemical structure and the occupational asthma hazard of low molecular weight organic compounds. Occup. Environ. Med. 2005;62:243–250. doi: 10.1136/oem.2004.016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N. Engl. J. Med. 2002;347:330–338. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- Macedo P, Hew M, Torrego A, Jouneau S, Oates T, Durham A, Chung KF. Inflammatory biomarkers in airways of patients with severe asthma compared with non-severe asthma. Clin. Exp. Allergy. 2009;39:1630–1632. doi: 10.1111/j.1365-2222.2009.03319.x. [DOI] [PubMed] [Google Scholar]
- Mendell MJ, Fisk WJ, Kreiss K, Levin H, Alexander D, Cain WS, Girman JR, Hines CJ, Jensen PA, Milton DK, et al. Improving the health of workers in indoor environments: priority research needs for a national occupational research agenda. Am. J. Public Health. 2002;92:1430–1440. doi: 10.2105/ajph.92.9.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Inoue S, Ogushi F, Ogura H, Nakamura Y. Toluene diisocyanate (TDI) induces production of inflammatory cytokines and chemokines by bronchial epithelial cells via the epidermal growth factor receptor and p38 mitogen-activated protein kinase pathways. Exp. Lung Res. 2006;32:245–262. doi: 10.1080/01902140600817515. [DOI] [PubMed] [Google Scholar]
- Piirila PL, Meuronen A, Majuri ML, Luukkonen R, Mantyla T, Wolff HJ, Nordman H, Alenius H, Laitinen A. Inflammation and functional outcome in diisocyanate-induced asthma after cessation of exposure. Allergy. 2008;63:583–591. doi: 10.1111/j.1398-9995.2007.01606.x. [DOI] [PubMed] [Google Scholar]
- Rideout K, Teschke K, Dimich-Ward H, Kennedy SM. Considering risks to healthcare workers from glutaraldehyde alternatives in high-level disinfection. J. Hosp. Infect. 2005;59:4–11. doi: 10.1016/j.jhin.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Rios JL, Boechat JL, Gioda A, Santos CY, Aquino Neto FR, Lapa ESJR. Symptoms prevalence among office workers of a sealed versus a non-sealed building: associations to indoor air quality. Environ. Int. 2009;35:1136–1141. doi: 10.1016/j.envint.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Rohr AC, Shore SA, Spengler JD. Repeated exposure to isoprene oxidation products causes enhanced respiratory tract effects in multiple murine strains. Inhal. Toxicol. 2003;15:1191–1207. doi: 10.1080/08958370390229870. [DOI] [PubMed] [Google Scholar]
- Rumchev K, Spickett J, Bulsara M, Phillips M, Stick S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax. 2004;59:746–751. doi: 10.1136/thx.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Doe C, Mistry V, Siddiqui S, Parker D, Sleeman M, Cohen ES, Brightling CE. Granulocyte-macrophage colony-stimulating factor expression in induced sputum and bronchial mucosa in asthma and COPD. Thorax. 2009;64:671–676. doi: 10.1136/thx.2008.108290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BC, Destaillats H, Hodgson AT, Nazaroff WW. Cleaning products and air fresheners: emissions and resulting concentrations of glycol ethers and terpenoids. Indoor Air. 2006;16:179–191. doi: 10.1111/j.1600-0668.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- Strom-Tejsen P, Weschler CJ, Wargocki P, Myskow D, Zarzycka J. The influence of ozone on self-evaluation of symptoms in a simulated aircraft cabin. J Expo Sci Environ Epidemiol. 2007;18:272–281. doi: 10.1038/sj.jes.7500586. [DOI] [PubMed] [Google Scholar]
- U. S. Environmental Protection Agency (USEPA) Indoor Air Facts No. 4, Sick Building Syndrome. 1991 Research and Development (MD-56). Available at: http://www.lchd.org/environhealth/aq/pdfs/EPA%20Sick%20Building.pdf. [Google Scholar]
- U. S. Environmental Protection Agency (USEPA) Report to Congress on Indoor Air Quality. Assessment and Control of Indoor Air Pollution Report, 1989. Volume II: Assessment and Control of Indoor Air Pollution, pp. I, 4–14. USEPA, Washington, DC. [Google Scholar]
- Waters A, Beach J, Abramson M. Symptoms and lung function in health care personnel exposed to glutaraldehyde. Am. J. Ind. Med. 2003;43:196–203. doi: 10.1002/ajim.10172. [DOI] [PubMed] [Google Scholar]
- Wells JR. Gas-phase chemistry of alpha-terpineol with ozone and OH radical: Rate constants and products. Environ. Sci. Technol. 2005;39:6937–6943. doi: 10.1021/es0481676. [DOI] [PubMed] [Google Scholar]
- Weschler CJ. Chemical reactions among indoor pollutants: what we've learned in the new millennium. Indoor Air. 2004;14:184–194. doi: 10.1111/j.1600-0668.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- Weschler CJ. Ozone's impact on public health: contributions from indoor exposures to ozone and products of ozone-initiated chemistry. Environ. Health Perspect. 2006;124:1489–1496. doi: 10.1289/ehp.9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann G, Muhlenberg J, Fischader G, Kulla C, Rehwagen M, Herbarth O, Lehmann I. An experimental model for the determination of immunomodulating effects by volatile compounds. Toxicol. In Vitro. 2005;19:685–693. doi: 10.1016/j.tiv.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Wilkins CK, Wolkoff P, Clausen PA, Hammer M, Nielsen GD. Upper airway irritation of terpene/ozone oxidation products (TOPS). Dependence on reaction time, relative humidity and initial ozone concentration. Toxicol. Lett. 2003;143:109–114. doi: 10.1016/s0378-4274(03)00115-2. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Air Quality Guidelines for Europe, European Series, No. 91. 2nd ed. Geneva, Switzerland: WHO Regional Publications; 2000. [PubMed] [Google Scholar]
- Yu JZ, Flagan RC, Seinfeld JH. Identification of products containing -COOH, -OH, and -C=O in atmospheric oxidation of hydrocarbons. Environ. Sci. Technol. 1998;32:2357–2370. [Google Scholar]
- Zhang JJ, McCreanor JE, Cullinan P, Chung KF, Ohman-Strickland P, Han IK, Jarup L, Nieuwenhuijsen MJ. Health effects of real-world exposure to diesel exhaust in persons with asthma. Res. Rep. Health Eff. Inst. 2009 5–109 discussion 111–123. [PubMed] [Google Scholar]